Significance
Upon enteropathogenic bacterial infection, cytokines orchestrate innate and adaptive immune activation that promotes barrier protection and pathogen control. Using Citrobacter rodentium, we defined a critical role for IL-36R signaling in driving early IL-23- and late IL-6-mediated IL-22 production, antimicrobial activity, and host protection. Our data show that the IL-36/IL-36R axis integrates innate and adaptive immune responses to afford host protection to enteropathogenic bacterial infection. These findings provide mechanistic insight into how the proinflammatory IL-36 pathway coordinates immunity and highlight the potential for targeting IL-36 cytokines and IL-36R in treating enteropathogenic bacterial infection.
Keywords: innate immunity, adaptive immunity, bacterial infection, interleukin
Abstract
Enteropathogenic bacterial infections are a global health issue associated with high mortality, particularly in developing countries. Efficient host protection against enteropathogenic bacterial infection is characterized by coordinated responses between immune and nonimmune cells. In response to infection in mice, innate immune cells are activated to produce interleukin (IL)-23 and IL-22, which promote antimicrobial peptide (AMP) production and bacterial clearance. IL-36 cytokines are proinflammatory IL-1 superfamily members, yet their role in enteropathogenic bacterial infection remains poorly defined. Using the enteric mouse pathogen, C. rodentium, we demonstrate that signaling via IL-36 receptor (IL-36R) orchestrates a crucial innate-adaptive immune link to control bacterial infection. IL-36R-deficient mice (Il1rl2−/−) exhibited significant impairment in expression of IL-22 and AMPs, increased intestinal damage, and failed to contain C. rodentium compared to controls. These defects were associated with failure to induce IL-23 and IL-6, two key IL-22 inducers in the early and late phases of infection, respectively. Treatment of Il1rl2−/− mice with IL-23 during the early phase of C. rodentium infection rescued IL-22 production from group 3 innate lymphoid cells (ILCs), whereas IL-6 administration during the late phase rescued IL-22-mediated production from CD4+ T cell, and both treatments protected Il1rl2−/− mice from uncontained infection. Furthermore, IL-36R-mediated IL-22 production by CD4+ T cells was dependent upon NFκB-p65 and IL-6 expression in dendritic cells (DCs), as well as aryl hydrocarbon receptor (AhR) expression by CD4+ T cells. Collectively, these data demonstrate that the IL-36 signaling pathway integrates innate and adaptive immunity leading to host defense against enteropathogenic bacterial infection.
Enteric bacterial infection of the gastrointestinal tract represents a significant cause of mortality worldwide. Human enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) are the two common attaching/effacing bacterial infections and have been associated with a high infant mortality rate in developing nations (1). To date, much of our knowledge on host immune responses to enteric pathogens is derived from experimental studies with Citrobacter rodentium, a gram-negative mouse-restricted bacterium. C. rodentium colonizes the intestinal mucosal layer via the formation of attaching and effacing lesions that result in a breach of the intestinal epithelial barrier, leading to colitis (2–4).
Effective host protection against C. rodentium colonization of mice is characterized by the combined responses of innate and adaptive immune cells, as well as nonimmune cells. Studies using C. rodentium infection of mice have defined the crucial role for both innate and adaptive immunity with group 3 innate lymphoid cells (ILCs) and CD4+ T cells being essential for controlling bacterial growth and resolving infection (5, 6). Central cytokines secreted from innate and adaptive immune cells in response to C. rodentium infection include interleukin (IL)-23, IL-6, and IL-22, which coordinately regulate host defense (5–8). IL-23 is induced early following C. rodentium infection and potently induces IL-22 expression by group 3 ILCs. Consequently, mice lacking IL-23 or IL-22 rapidly succumb to C. rodentium infection (5, 6, 9). During the later phase of C. rodentium infection, CD4+ T cells produce IL-22 in response to IL-6 to further control bacterial expansion and pathogenesis. The mechanisms via which early and late IL-22 production limit C. rodentium infection appear to involve stimulating intestinal epithelial cell proliferation and secretion of antimicrobial peptides.
The IL-1 family of cytokines is central to immunity and host defense. IL-36 cytokines are members of the IL-1 superfamily and include three agonists and two antagonists (10). IL-36 agonists (IL-36α, IL-36β, and IL-36γ) bind to IL-36R and triggers proinflammatory immune responses, including cytokine/chemokine production, dendritic cell (DC) maturation, and T cell differentiation (11, 12). IL-36R is widely expressed on numerous cell types, including DCs, CD4+ T cells, and epithelial cells of tissues such as the skin, intestine, and lung. Similar to IL-36R, IL-36 ligands are expressed by a variety of cells dependent on the tissues and physiological condition (11). Recently, signaling via IL-36R has been demonstrated to be protective or pathogenic for intestinal inflammation, depending on the model system (13–19). However, the mechanistic role of the IL-36/IL-36R axis in controlling immunity to enteric bacterial infections is still emerging (16).
In this study, we examined the contribution of the IL-36/IL-36R axis to shaping host immunity in the murine intestine following infection with bioluminescent C. rodentium. We found that IL-36α and IL-36γ were expressed in the large intestine early after C. rodentium infection and that signaling via IL-36R controlled proinflammatory cytokine expression, bacterial expansion, and intestinal inflammation. Moreover, we identified a critical role for IL-36R in linking host protective innate and adaptive immune responses during C. rodentium infection. Specifically signaling via IL-36R promoted IL-23 production, which subsequently regulated IL-22 expression by group 3 ILCs in the early phase of infection. During the late phase of infection, IL-36R signaling induced host defense associated with the IL-6 expression and the differentiation of IL-22 producing CD4+ T cells. Mice deficient in IL-36R (Il1rl2−/−) exhibited a profound impairment of IL-23, IL-6, and IL-22 production and succumbed to C. rodentium infection, as compared to control mice. Administration of exogenous IL-23 in the early phase, or IL-6 in the late phase, rescued IL-22 production and host protection in Il1rl2−/− mice. We further defined that IL-36R-mediated IL-22 production by CD4+ T cells was dependent upon NFκB-p65 and IL-6 expression by DCs, as well as aryl hydrocarbon receptor (AhR) by CD4+ T cells. Collectively, these data demonstrate that IL-36/IL-36R signaling provides critical integration of innate and adaptive immunity and host defense against enteropathogenic bacterial infection.
Results
IL-36R Signaling Controls C. rodentium Infection.
To investigate the role of IL-36R signaling in response to intestinal bacterial infection, we first assessed whether IL-36 agonists are expressed during C. rodentium infection. To do so, wild-type C57BL/6 mice were inoculated with 5 to 6 × 109 colony forming units (CFUs) of C. rodentium, and the expression of Il1f6 (IL-36α), Il1f8 (IL-36β), and Il1f9 (IL-36γ) mRNA was measured in the large intestine over 12 d. IL-36α mRNA began to be induced on day 2 and peaking at day 12, while IL-36β was undetectable (SI Appendix, Fig. S1 A and B). IL-36γ mRNA was expressed beginning at day 1 and peaking at day 6 (SI Appendix, Fig. S1C). Given that IL-36γ was highly expressed early following C. rodentium infection, we next analyzed IL-36γ mRNA expression in colonic immune cell subsets at day 6. CD11b+Ly6C+ inflammatory monocytes expressed the highest levels of IL-36γ, followed by Ly6G+ neutrophils and intestinal epithelial cells (IECs), while T, B, and natural killer (NK) cells did not express detectable levels of IL-36γ mRNA (SI Appendix, Fig. S1D). Moreover, in vitro stimulation of bone-marrow-derived macrophages (BMDMs) with heat-killed C. rodentium resulted in robust induction of IL-36γ mRNA (SI Appendix, Fig. S1E). Collectively, these results demonstrate that IL-36 agonists, particularly IL-36γ, are induced early following infection with C. rodentium.
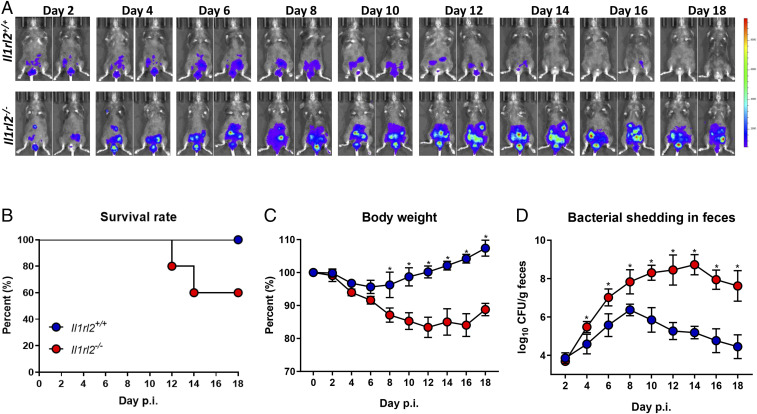
Recently, IL-36R signaling has been shown to play a role in host protection against microbial infections, as well as during skin and mucosal inflammation (11, 12). To examine the contribution of IL-36R signaling during enteropathogenic bacterial infection, we infected wild-type (Il1rl2+/+) and IL-36R-deficient (Il1rl2−/−) mice with 5 to 6 × 109 CFUs of bioluminescent C. rodentium (20) and monitored survival rate, body weight, and bacterial shedding in feces. Consistent with a previous report (5) bioluminescent imaging revealed that Il1rl2+/+ mice controlled C. rodentium infection by 14 d postinfection (p.i.), while Il1rl2−/− mice still exhibited robust infection, leading to ∼40% mortality by day 18 (Fig. 1 A and B and SI Appendix, Fig. S2A). Il1r2−/− mice also displayed substantial body weight loss and had significantly higher bacterial shedding in feces (Fig. 1 C and D). Bioluminescence imaging of internal organs at day 10 postinfection revealed that while the C. rodentium load was high in the large intestine and the cecum of Il1rl2−/− mice, there was no evidence of dissemination to the spleen, liver, or mesenteric lymph nodes (SI Appendix, Fig. S2 B–F). These data suggest that IL-36R signaling is crucial in controlling C. rodentium infection.
Fig. 1.
IL-36 signaling controls C. rodentium infection. Il1rl2+/+ and Il1rl2−/− mice infected with bioluminescent C. rodentium (5 to 6 × 109 CFU) by gastric gavage. (A) Serial whole-body imaging at indicated time points. Images are representative of two independent experiments with n = 5 mice per experiment (B–D) (B) Survival rate; (C) average body weight changes; and (D) bacterial shedding in feces of infected Il1rl2+/+ and Il1rl2−/− mice at indicated time points. Data are representative of three independent experiments with five mice per group. All data presented as mean ± SEM (multiple t tests, one per row, corrected for multiple comparisons using the Holm–Sidak method; *P < 0.5).
IL-36R Deficiency Results in Diminished Colonic IL-23, IL-6, and IL-22 during C. rodentium Infection.
Mice with defective IL-36R signaling have compromised mucosal healing in response to DSS (dextran sodium sulfate)-induced colonic damage (13–15). To explore potential mechanisms for how IL-36R signaling conferred host protection against enteropathogenic bacterial infection, we performed PCR array analysis on total colonic tissues isolated from Il1rl2+/+ and Il1rl2−/− mice at the early (day 4) and late (day 8) phase of C. rodentium infection (Fig. 2A). In the early phase of infection IFNγ, IL-22, and IL-23 mRNA expression was significantly higher in Il1rl2+/+ mice compared to Il1rl2−/− mice, while during the late phase of infection IFNγ, IL-6, and IL-22 mRNA expression were higher in Il1rl2+/+ mice as compared to Il1rl2−/− mice (Fig. 2 B–E). Since the complex role of IFNγ in C. rodentium infection has previously been investigated with various outcomes (5, 21), we focused our study on IL-23, IL-6, and IL-22. ELISA analysis of IL-22, IL-23, and IL-6 protein expression over the course of 12 d postinfection further demonstrated that these cytokines were significantly elevated in colonic tissues from Il1rl2+/+ mice compared to Il1rl2−/− mice (Fig. 2 F–H).
Fig. 2.
IL-36R deficiency results in diminished IL-23, IL-6, and IL-22 expression in the large intestine during C. rodentium infection. (A) Experimental schematic of intestinal bacterial infection by oral infected Il1rl2+/+ and Il1rl2−/− mice with 5 to 6 × 109 CFU of C. rodentium. (B and C) PCR array gene expression analyses from large intestine tissues isolated at indicated time points (p.i.) of Il1rl2+/+ and Il1rl2−/− mice. (D and E) The top 10 significantly expressed genes with highest up-regulation were obtained from PCR array analysis between C. rodentium-infected Il1rl2+/+ and Il1rl2−/− mice at day 4 p.i. (D), and day 8 p.i. (E). (F–H) The time course of (F) IL-22, (G) IL-23, and (H) IL-6 protein expression from colonic tissues isolated from infected mice. Data are representative of two independent experiments with four to five mice per group. All data are presented as mean ± SEM (multiple t tests, one per row, corrected for multiple comparisons using the Holm–Sidak method; *P < 0.5).
We next examined whether loss of IL-36R could affect the recruitment of innate immune cells to the large intestine during C. rodentium infection. Comparative analysis of colonic innate immune subsets between Il1rl2+/+ and Il1rl2−/− mice revealed no differences in the percentage of inflammatory monocytes (CD11b+Ly6C+) or neutrophils (CD11b+Ly6G+) during the early (day 4) phase of infection. However, these two innate immune cell subsets were dramatically elevated during the late (day 8) phase in Il1rl2+/+ mice, but not Il1rl2−/− mice (SI Appendix, Fig. S3 A–C). The significantly reduced accumulation of inflammatory monocytes and neutrophils in Il1rl2−/− mice corresponded with decreased mRNA expression of the neutrophil chemoattractant cxcl1, cxcl2, and cxcl5, as well as the monocyte chemoattractant cxcl3. Interestingly, at day 16 postinfection cxcl1, cxcl2, and cxcl3 expression increased in Il1rl2−/− mice compared to infected Il1rl2+/+ mice and coincided with increased inflammation, likely as a result of defective bacterial clearance (SI Appendix, Fig. S3 D–G).
Early IL-23 Administration Rescues Protective Immunity in C. rodentium-Infected Il1rl2−/− Mice.
In light of Il1rl2−/− mice displaying reduced IL-23 expression during the early phase of C. rodentium infection, we examined whether IL-23 administration could rescue this defect. C. rodentium-infected Il1rl2−/− mice received either PBS (phosphate buffered saline) or rIL-23 (0.5 μg) at days 0 and 2 postinfection (Fig. 3A). This systemic administration of IL-23 to C. rodentium-infected Il1rl2−/− mice was sufficient to promote bacterial clearance, improve survival rate, prevent body weight loss, reduce bacterial shedding in feces, and reduce colonic histology scores (Fig. 3 B–G), similar to that observed in Il1rl2+/+ mice. Further, at day 4 postinfection, Il1rl2−/− mice exhibited significantly diminished total colonic IL-22 production (Fig. 3H); IL-22 production by group 3 ILCs (Fig. 3 I and J); as well as reduced claudin-2 (Fig. 3K), AMPs including RegIIIβ, RegIIIγ, and S100A9 (Fig. 3L); and mucin-2 (Fig. 3M), when compared to Il1rl2+/+ mice; and these defects were reversible by delivery of IL-23.
Fig. 3.
Early IL-23 administration rescues protective immunity in C. rodentium-infected Il1rl2−/− mice. (A) Experimental schematic of C. rodentium infection (5 to 6 × 109 CFU) by gastric gavage into Il1rl2+/+ and Il1rl2−/− mice, in the presence or absence of IL-23. (B) Serial whole-body imaging of infected mice at indicated time points. Images are representative of two independent experiments with at least five mice/group. (C–E) (C) Survival rate; (D) average body weight change; and (E) bacterial shedding in feces of infected mice at indicated time points. (F and G) (F) The H&E staining and histology scoring of (G) colon sections from infected mice as in A are shown. (H) IL-22 protein expression in the colon at 10 days post C. rodentium infection from Il1rl2+/+ and Il1rl2−/− mice. (I) Colonic lamina propria cells of C. rodentium-infected mice at day 4 p.i. were isolated and analyzed by FACS for expression of intracellular IL-22 by Thy1+RORγt+ gated cells. (J) FACS frequency data of Thy1+RORγt+IL-22+ gated cells of infected mice generated as in I. (K–M) (K) Claudin-2, (L) antimicrobial peptides, and (M) Mucin-2 mRNA expression in colon from infected mice at day 4 p.i. (N) Serial whole-body imaging of C. rodentium-infected Il1rl2−/− mice in the presence or absence of IL-23 and neutralization antibodies, αCD90 or αCD4 as indicated. Images are representative of two independent experiments. (O–P) (O) Survival rate; (P) average body weight changes; and (Q) bacterial shedding in feces of infected mice as in N at indicated time points. (R) IL-22 protein determined by ELISA of colon of infected mice as in N at indicated time points. Data are representative of three independent experiments with five mice per group. All data are presented as mean ± SEM (one-way ANOVA with Tukey’s multiple comparison test. *P < 0.5; **P < 0.05; ****P < 0.0001, ns, not significant).
We next explored which immune cell populations in Il1rl2−/− mice were associated with host protection afforded by delivery of exogenous IL-23. To do so, Il1rl2−/− mice were infected with C. rodentium and treated with PBS or rIL-23 at days 0 and 2 postinfection. Some mice also received either αCD90 antibody to deplete primarily T cells and group 3 ILCs or αCD4 antibody to deplete primarily CD4+ T cells. While Il1rl2−/− mice treated with IL-23 successfully cleared C. rodentium, Il1rl2−/− mice treated with IL-23 and with αCD90 antibody or αCD4 antibody failed to fully reverse defective host protection, similar to untreated Il1rl2−/− mice, although αCD90-treated mice displayed the most profound impairment (Fig. 3 N–Q). In addition, the depletion of ILCs with αCD90 antibody also nullified the ability of IL-23 to induce IL-22 in infected Il1rl2−/− mice (Fig. 3R). Interestingly, and unlike Il1rl2−/− mice treated with IL-23 and αCD90 antibody, Il1rl2−/− mice treated with IL-23 and αCD4 antibody had normal IL-22 levels at day 4 postinfection and appeared to control C. rodentium expansion early but not late following infection (Fig. 3 N–R). These data suggest that IL-23 can rescue defective control of C. rodentium in Il1rl2−/− mice via inducing IL-22 production primarily from group 3 ILCs at day 4 postinfection (p.i.) (SI Appendix, Fig. S4 A and B). Of note, we observed that the frequencies and cell numbers of group 3 ILCs (Thy1+Lin−RORγt+) and groups 1 and 2 ILCs (Thy1+Lin−RORgt−) in Il1rl2−/− mice were comparable to Il1rl2+/+ mice post C. rodentium infection (SI Appendix, Fig. S4 C–E). Thus, the main difference in group 3 ILCs between Il1rl2+/+ and Il1rl2−/− mice appears to be in their ability to generate IL-22; however, we do not exclude a role for groups 1 and 2 ILCs in IL-36R-mediated C. rodentium clearance, as group 1 ILCs can respond to IL-12/IL-18 to produce IFNγ, a cytokine that has been implicated in control of C. rodentium (21).
IL-36R Regulates CD4+ T Cell IL-22 Production via AhR- and IL-6-Mediated Signaling.
Having observed that Il1rl2−/− mice display significantly reduced IL-6 expression during the late phase of C. rodentium infection, combined with the fact that IL-6 has been reported to be involved in Th22 generation and host protection to C. rodentium (5), we next sought to explore whether signaling via IL-36R regulates naïve CD4+ T cell differentiation into IL-22-producing cells and if this process involves IL-6. To do so, naïve CD4+CD25− T cells and CD11c+ DCs were FACS (fluorescence-activated cell sorting)-sorted from spleens of wild-type (WT) mice and cultured either alone or together (coculture) and stimulated with or without IL-36γ in the presence of αCD3. While T cells and DCs cultured alone failed to induce IL-22 irrespective of the addition of IL-36γ, T/DC cocultures induced robust expression of IL-22 only in the presence of IL-36γ (Fig. 4A). We next examined whether IL-36γ was signaling via IL-36R on CD4+ T cells or DCs to stimulate the induction of IL-22. Interestingly, the expression of IL-36R by DCs was found to be indispensable for the induction of IL-22 in a T/DC coculture system since IL-22 was only induced when DCs from either splenic, mesenteric lymph node (mLN) or large intestine (LI) lamina propria were from Il1rl2+/+ mice (Fig. 4B and SI Appendix, Fig. S5 A and B).
Fig. 4.
IL-36R regulates CD4+ T cell IL-22 production via AhR- and IL-6-mediated signaling. (A) DCs and CD4+ T cells from spleen of WT mice were FACS sorted and were cultured either alone or cocultured for 72 h in the presence or absence of IL-36γ. Supernatant were analyzed for IL-22 by ELISA. (B) FACS-sorted naïve CD4+ T cells and DCs were cocultured using indicated cells from Il1rl2+/+ and/or Il1rl2−/− mice with ± IL-36γ for 72 h. IL-22 protein in supernatant was determined by ELISA. (C) IL-22 protein expression by FACS-sorted coculture DCs and naïve CD4+ T cells from ahr+/+ or ahr-/− mice in the presence of IL-36γ for 72 h. (D) FACS-sorted naïve CD4+ T cells and DCs were cocultured using indicated cells from ahr+/+ and/or ahr-/− mice with ± IL-36γ for 72 h. IL-22 protein in supernatant was determined by ELISA. (E) FACS-sorted DCs and CD4+ T cells from WT mice were cocultured and stimulated with IL-36γ and αIL-6 antibody for 72 h. IL-22 protein was assessed by ELISA. (F) FACS-sorted naïve CD4+ T cells and DCs were cocultured using indicated cells from Il1rl2+/+ and/or Il1rl2−/− mice with ± IL-36γ for 72 h. IL-6 protein in supernatant was determined by ELISA. Data are representative of three independent experiments with four to five replicates. (G) BMDCs were generated from WT mice and cultured in the presence or absence of IL-36γ for 24 h, and IL-6 was assessed by ELISA; some cultures were pretreated with NFκB inhibitor, or c-Rel inhibitor, or p50 inhibitor, or p65 inhibitor or with vehicle control for 1 h. (H) ChIP assays for p50, p65, and c-Rel binding to Il6 promoter in BMDCs treated with ± IL-36γ for 8 h. Data are representative of three independent experiments with four to five replicates. All data are presented as mean ± SEM (one-way ANOVA with Tukey’s multiple comparison test. **P < 0.01, ***P < 0.001; ****P < 0.0001; ns, not significant).
AhR has been implicated as a key transcription factor involved in generating IL-22-producing CD4+ T cells. Therefore, we next examined whether IL-36R signals through AhR to promote differentiation of naïve CD4+ T cells into IL-22-producing CD4+ T cells. In the presence of IL-36γ, T/DC cocultures with cells obtained from ahr+/+ mice robustly induced IL-22 and this effect was abolished when using T cells and DCs from ahr−/− mice (Fig. 4C). Unlike IL-6 expression, which required IL-36R on DCs for optimal IL-36γ-induced IL-22 production in T/DC cocultures, the expression of AhR by CD4+ T cells was critical for the induction of IL-22 in this T/DC coculture system since IL-22 was only induced when T cells were from ahr+/+ mice and abrogated when CD4+ T cells were from ahr−/− mice (Fig. 4D). Furthermore, CH-223191, a pharmacological inhibitor of AhR, blocked the ability of IL-36γ to induce IL-22 expression in T/DC cocultures from ahr+/+ mice, while not interfering with IL-2 levels (SI Appendix, Fig. S5 C and D). Of note, our data demonstrating that IL-36R signaling can induce CD4+ T cells to produce IL-22 did not distinguish the relative contribution from Th22 cells versus Th17 cells. We observed, however, that TGFβ mRNA expression, which is involved in Th17 development, is expressed at normal level in infected Il1rl2−/− mice compared to Il1rl2+/+-infected mice (SI Appendix, Fig. S5E) and previous studies have shown that signaling via IL-36R does not induce Th17 cells (16, 19).
We next investigated whether IL-36γ was acting to induce IL-22 expression from CD4+ T cells in vitro via the induction of IL-6. To do so, we performed antibody-mediated blockade of numerous inflammatory cytokines to test whether they played a role in IL-36γ-induced IL-22 expression in T/DC cocultures. Interestingly, antibody-mediated blockade of IL-6 significantly decreased the effect of IL-36γ-induced IL-22 production in T/DC cocultures, while blockade of IL-1β, IL-4, TGFβ, IFNγ, or TNFα had no significant effects on IL-36γ-induced IL-22 expression (Fig. 4E and SI Appendix, Fig. S6A). Notably, antibody-mediated blockade of IL-23p19 and IL-12/23p40, as well as genetic deficiency of IL-12/23p40 by using Il12b−/− bone-marrow-derived DCs (BMDCs) in T/DC cocultures, had only a modest reduction in IL-36γ-induced IL-22 expression (SI Appendix, Fig. S6 A and B).
Since blockade of IL-6 significantly reduced the ability of IL-36γ to induce IL-22 in T/DC cocultures, we directly assessed the ability of IL-36γ to induce IL-6 expression. We observed that IL-36R expression on DCs is indispensable for IL-36γ to stimulate IL-6 secretion in the T/DC coculture system (Fig. 4F). We next explored the molecular cascade from IL-36γ signaling via IL-36R to IL-6 induction. We first generated BMDCs from myd88+/+ and myd88−/− mice and cultured them with IL-36γ. In the presence of IL-36γ, myd88+/+ BMDCs induced a more robust expression of IL-6 compared to myd88−/− BMDCs (SI Appendix, Fig. S7A).
We then probed the role of NFκB in IL-36γ-induced IL-6 in DCs by using small molecule inhibitors of NFκB, p50, p65, or c-Rel. The NFκB inhibitor (Bay 11-7082) as well as the p65 peptide inhibitor partially blocked IL-36γ-induced IL-6 expression, while the p50 inhibitory peptide or c-Rel inhibitor (IT-603) showed no significant effects (Fig. 4G). Likewise, following stimulation with IL-36γ, we observed no significant reduction in IL-6 from BMDCs generated from p50−/− or crel−/− mice (SI Appendix, Fig. S7 B and C). To further explore how signaling via IL-36R induced IL-6 expression in DCs, we performed a chromatin immunoprecipitation (ChIP) assay to determined p50, c-Rel, and p65 binding to the IL-6 promoter in BMDCs treated with IL-36γ. As shown in Fig. 4H, there was a significant increase in p65, but not p50 or c-Rel, binding to the IL-6 promoter in response to treatment of BMDCs with IL-36γ for 6 h. Together, these findings demonstrate that MyD88 and NFκB-p65 are part of the signaling cascade downstream of IL-36R that is involved in IL-6 expression in DCs.
Since in vitro neutralization of IL-6 did not completely inhibit the ability of IL-36γ to induce IL-22 in the T/DCs cocultures, involvement of the Notch pathway was assessed by coculturing CD4+ T cells and DCs in the presence of GSI, a pan-Notch inhibitor. The secretion of IL-36γ-induced IL-22 in T/DC cocultures was reduced by ∼40% in the presence of GSI, while FACS and qPCR analysis revealed that IL-36γ induced dll1 and dll4 among the five Notch ligands (SI Appendix, Fig. S8 A–I). Further, while antibody-mediated neutralization of DLL1 showed minimal effect on IL-22, blockade of DLL4 drastically diminished IL-22 (∼30%), and neutralization of both IL-6 and DLL-1/4 abolished the effect of IL-36γ to induce IL-22 in T/DC cocultures (SI Appendix, Fig. S8J). Collectively, these data demonstrate that signaling via IL-36R induces IL-22-producing CD4+ T cells in T/DC cocultures via AhR, NFκB-p65, and IL-6-dependent pathways.
IL-6 Administration Restores Protective Immunity in C. rodentium-Infected Il1rl2−/− Mice.
We next investigated whether IL-6 administration could induce containment of C. rodentium in Il1rl2−/− mice during the late phase of infection via induction of CD4+ T cell-dependent IL-22 production. To do so, C. rodentium-infected Il1rl2−/− mice received either vehicle control (PBS) or IL-6 (1 μg) at days 4, 6, and 8 and were monitored for 20 d (Fig. 5A). While C. rodentium-infected Il1rl2−/− mice failed to control bacterial expansion in the first few days p.i., they were able to accelerate bacterial clearance quickly following delivery of exogenous IL-6, and this protective effect was abrogated when using αCD4 antibody to deplete CD4+ T cells (Fig. 5B). Further, systemic administration of IL-6 to C. rodentium-infected Il1rl2−/− mice was able to improve survival rate, prevent body weight loss, reduce bacterial shedding in feces, and reduce colon histology scores, and all of these beneficial effects were rendered ineffective by treatment with αCD4 antibody (Fig. 5 C–G).
Fig. 5.
IL-6 administration accelerates bacterial clearance and restores IL-22 production in C. rodentium-infected Il1rl2−/− mice. (A) Experimental schematic of C. rodentium infection, in the presence or absence of recombinant IL-6 and/or αCD4. (B) Serial whole-body imaging of infected mice at indicated time points. Images are representative of two independent experiments with at least five mice/group. (C–E) (C) Survival rate; (D) average body weight changes; and (E) bacterial shedding in feces of infected mice at indicated time points. (F and G) (F) H&E staining, and (G) histology scoring of colon sections from infected mice as in A are shown. (H) IL-22 protein expression in colons from infected mice at day 10 p.i. (I) Colonic lamina propria cells of C. rodentium-infected mice as shown in A were isolated on day 10 p.i. and analyzed by FACS for expression of intracellular IL-22 by TCRβ+CD4+ gated cells. (J) FACS frequency data from TCRβ+CD4+IL-22+ gated cells of C. rodentium-infected mice generated based on I. (K–M) (K) Claudin-2, (L) antimicrobial peptides, and (M) Mucin-2 mRNA expression in colon from infected mice at day 10 p.i. Data are representative of three independent experiments with five mice per group. All data are presented as mean ± SEM (one-way ANOVA with Tukey’s multiple comparison test. *P < 0.5; **P < 0.05; ***P < 0.001; ns, not significant).
We next examined if defective IL-22, AMPs, claudin-2, and mucin-2 expression following C. rodentium infection in Il1rl2−/− mice could be reversed by IL-6 administration. At day 10 postinfection, colonic tissue from Il1rl2−/− mice expressed markedly reduced IL-22 mRNA expression, CD4+ T cells expressing IL-22, as well as impaired expression of RegIIIβ, RegIIIγ, S100A9, claudin-2, and mucin-2, when compared to Il1rl2+/+ mice, and these defects could be reversed by IL-6 administration. Further, the ability of IL-6 administration to induce these factors in Il1rl2−/− mice was negated when using αCD4 antibody (Fig. 5 H–M). Of note, anti-C. rodentium IgG and IgA were comparable at days 4, 8, and 18 postinfection, suggesting that IL-36R signaling was dispensable for mounting antibody responses to C. rodentium (SI Appendix, Fig. S9 A–F).
Contribution of Hematopoietic and Nonhematopoietic Compartments during C. rodentium Infection of Il1rl2−/− Mice.
Hematopoietic and nonhematopoietic cells are involved in host protection against C. rodentium (22). However, the relative contribution of IL-36R signaling in these compartments is unclear, so we generated bone marrow chimeras that were subsequently infected with C. rodentium. Using these mice, we observed that both the hematopoietic and nonhematopoietic compartments were required for optimal host defense against C. rodentium (SI Appendix, Fig. S10 A–E).
Among nonhematopoietic cells, intestinal fibroblasts have been shown to respond to IL-36 agonist stimulation to produce IL-6 (13). Thus, we explored whether IL-6 derived from fibroblasts can stimulate the differentiation of IL-22-producing CD4+ T cells. Intestinal fibroblasts and naïve CD4+ T cells were purified and cultured either alone or together in Transwell plates in the presence of IL-36γ. While T cells and fibroblasts cultured alone failed to induce IL-22, T cell/fibroblast cocultures induced IL-22 expression in the presence of IL-36γ and this induction was blocked by αIL-6 antibody (SI Appendix, Fig. S10F). Furthermore, CD4+ T cells treated with supernatant from IL-36γ-stimulated fibroblasts produced IL-22, and this effect was also blocked by αIL-6 antibody (SI Appendix, Fig. S10G). Of note, using CD4+ T cells and fibroblasts from Il1rl2+/+ and Il1rl2−/− mice, we observed that IL-36R-expressing fibroblasts were required for IL-6 and subsequent IL-22 production (SI Appendix, Fig. S10 H and I). Collectively, based on the data presented in this report, we propose a model for the role of IL-36/IL-36R signaling in host protection against C. rodentium infection (SI Appendix, Fig. S11).
Discussion
Here we provide evidence that IL-36R signaling is critical for control of C. rodentium. Mice deficient in IL-36R exhibited decreased IL-22 and AMPs, increased intestinal damage, and impaired resistance to bacterial colonization. These defects were associated with diminished IL-23 and IL-6—the respective early and late inducers of IL-22. Consistent with these data, exogenous IL-23 administration during the early phase of infection in Il1rl2−/− mice induced IL-22 production by group 3 ILCs, whereas administration of IL-6 during the late phase induced IL-22 expression by CD4+ T cells. Our data also demonstrate that IL-36γ induces IL-22 from CD4+ T cells via NFκB-p65, IL-6, and AhR-dependent mechanisms. Overall, these data highlight a fundamental contribution of IL-36/IL-36R axis signaling to IL-23/IL-22/AMP- and IL-6/IL-22/AMP-mediated control of enteric bacterial infection.
Previous data have established that group 3 ILC-mediated production of IL-22 is instrumental in driving early host protection against C. rodentium. Mice deficient in IL-22 were unable to control C. rodentium expansion and rapidly succumbed to infection (6). In addition, late expression of IL-22 by CD4+ T cells is also critical for protection against C. rodentium (5). Interestingly, while early IL-22 production by group 3 ILCs is IL-23 dependent, the late IL-22 production by CD4+ T cells is IL-6 dependent (5). Therefore, unique cytokines induce IL-22 during distinct windows of enteric bacterial infection; however, we do not exclude the contribution of other cytokines such as IFNγ (16, 21).
We and others have previously reported that IL-36 agonists were induced in various models of experimental colitis and in human inflammatory bowel disease (IBD) (13–16, 18, 19, 23–26). In the absence of signaling via IL-36R, the induction of IL-23 and IL-6 were dramatically reduced, as was early and late IL-22 expression. Importantly, IL-36R-deficient mice could be rescued from uncontrolled C. rodentium infection by administration of IL-23 during the early phase or IL-6 during the late phase of infection. Consistent with the DSS model of colitis, IL-36R signaling afforded critical host protective effects against C. rodentium associated with the induction of IL-22 and AMPs, although other barrier protective factors such as matrix metalloproteinase 9 and neutrophil gelatinase-associated lipocalin cannot be excluded (27). Thus, the inflammatory role of IL-36R signaling is instrumental in providing barrier protection from acute intestinal damage as well as enteric bacterial infection. This role of IL-36R is distinct from the pathogenic role during chronic intestinal inflammation, as observed during chronic DSS colitis and oxazolone colitis (18, 19).
IL-36R can be expressed on immune cells such as DCs and T cells (14, 19, 28–30), as well as nonimmune cells, including endothelial cells (31), keratinocytes (32, 33), intestinal epithelial cells (13), and intestinal fibroblasts (13, 18). While we have focused on IL-36R expression by DCs and CD4+ T cells in this study, it is important to note that the relative contribution of IL-36R by specific cell lineages during intestinal inflammation remains unclear. Use of IL-36R-floxed mice will allow cell lineage-specific contributions to be defined, as has recently been shown for keratinocyte-specific IL-36R expression in skin inflammation (32, 33). Likewise, IL-36 agonists can be expressed by various cell types, including macrophages (13, 15, 18, 25, 34), DCs (23), keratinocytes (34–37), and intestinal epithelial cells (13), among others. Our data indicate that colonic Ly6C+ monocytes/macrophages are one source of IL-36γ following C. rodentium infection, yet do not exclude the potentially important contributions of other sources such as Ly6G+ neutrophils and/or intestinal epithelial cells. Additionally, while IL-36γ peaked at day 6 postinfection and decreased afterward, bacterial clearance was around day 16. One possible explanation for this is that early IL-36γ primes subsequent both early and late IL-36/IL-23/IL-22 and IL-36/IL-6/IL-22 responses, and thus IL-36γ expression is not required in the later phase. We also observed that IL-36α mRNA is expressed later compared to IL-36γ and continued to peak at day 12 postinfection. Therefore, it is possible that IL-36γ could play an important role in the early phase of infection and IL-36α more so in the later phase. Future studies using mice deficient in specific IL-36 ligands should aid in addressing the relative contributions of individual ligands.
Overall, our study indicates that therapeutic targeting of IL-36R in inflammatory processes, such as IBD, may be optimized by a clear understanding of the dual roles this pathway plays in acute and chronic inflammatory conditions and during infections. It is attractive to envisage blockade of IL-36R while concomitantly delivering IL-22 and/or AMPs to ameliorate pathogenic inflammation while promoting barrier recovery in situations where barrier damage is a relevant pathophysiological factor. This concept may not apply only to the IL-36 pathway, but also to other IL-1 family members, and even other inflammatory cytokines such as IL-17A that have both inflammatory and barrier protective functions.
Materials and Methods
Mice.
The following mice were purchased from The Jackson Laboratory: WT C57BL/6 (B6 WT), B6.129P-Nfkb1tm1Bal/J (p50−/−), B6.129P2(SJL)-Myd88tm1.1Defr/J (myd88−/−), and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1). Il1rl2−/− (IL-36R−/−) mice on the C57BL/6 background (backcrossed more than nine generations) were originally provided by Amgen. Ahr−/− mice were kindly provided by A.T.G. Unless otherwise stated, mice were used at 6 wk of age and experiments were carried out using age- and gender-matched groups. Animal studies were approved by the Institutional Animal Care and Use Committee of Georgia State University.
C. rodentium Infection Model.
C. rodentium strain ICC180 (derived from DBS100) was originally generated by Gad Frankel and Fiouxsie Wiles (Imperial College London) and generously provided by Casey Weaver, University of Alabama, Birmingham, AL, with permission. Mice were inoculated with 5 to 6 ×109 CFUs in a volume of 200 µL PBS via gastric gavage and monitored daily.
Bioluminescence Imaging.
Mice were anesthetized with isoflurane and imaged with an IVIS-100 system and Living Image Software (Xenogen, Inc.). Images were taken at binning of four over 3 to 10 min at the indicated times during infection.
CD4+ T Cell Differentiation.
FACS-sorted CD4+CD25− T cells were cocultured with FACS-sorted CD45+MHCII+CD11c+ DCs for 72 to 80 h ± 100 ng/mL IL-36γ. In some experiments, monoclonal antibodies and/or pharmaceutical inhibitors were added. Unless otherwise stated, monoclonal antibodies were purchased from R&D and inhibitors from Cayman Chemicals.
ELISA.
IL-22 and IL-6 was measured in culture supernatants using IL-22 ELISA kit (R&D Systems) and IL-6 ELISA kit (eBiosciences) according to the manufacturer’s protocol.
In Vivo Administration of Cytokines.
Recombinant mouse IL-23 and IL-6 were purchased from R&D Systems and delivered via i.p. injection. Il1rl2−/− mice received either PBS or 0.5 μg of IL-23 at day 0 and day 2 p.i., or 1 μg of IL-6 at days 4, 6, and 8 p.i.
Histology.
Colon tissues were fixed in 10% neutral formalin buffer. Paraffin embedding, sectioning, and hematoxylin/eosin staining, and slide scanning were performed at HistoWiz, Inc.
Statistical Analysis.
All statistical analyses were done with GraphPad Prism software, version 8.0. One-way ANOVA, Tukey’s multiple comparison, Holm–Sidak, and Student’s t test were used to determine significance (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, no significance).
Supplementary Material
Acknowledgments
We thank Dr. Gad Frankel and Dr. Casey Weaver for providing bioluminescent C. rodentium. This work was supported by NIH Grant 1R01DK120907 (to T.L.D.) and 1R01DK107739 (to D.M. and T.L.D.). D.M. is a recipient of a Veterans Affairs Research Career Science Award.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004484117/-/DCSupplemental.
Data Availability.
All of the data and reagents that support the findings of this study are available within the main text or the SI Appendix.
References
- 1.Rojas-Lopez M., Monterio R., Pizza M., Desvaux M., Rosini R., Intestinal pathogenic Escherichia coli: Insights for vaccine development. Front. Microbiol. 9, 440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins J. W.et al., Citrobacter rodentium: Infection, inflammation and the microbiota. Nat. Rev. Microbiol. 12, 612–623 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Silberger D. J., Zindl C. L., Weaver C. T., Citrobacter rodentium: A model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol. 10, 1108–1117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullineaux-Sanders C.et al., Citrobacter rodentium-host-microbiota interactions: Immunity, bioenergetics and metabolism. Nat. Rev. Microbiol. 17, 701–715 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Basu R.et al., Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y.et al., Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Li L.et al., Cytokine IL-6 is required in Citrobacter rodentium infection-induced intestinal Th17 responses and promotes IL-22 expression in inflammatory bowel disease. Mol. Med. Rep. 9, 831–836 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Dann S. M.et al., IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180, 6816–6826 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlfors H.et al., IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello C. A., Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassoy E. Y., Towne J. E., Gabay C., Regulation and function of interleukin-36 cytokines. Immunol. Rev. 281, 169–178 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Gabay C., Towne J. E., Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J. Leukoc. Biol. 97, 645–652 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Scheibe K.et al., IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 66, 823–838 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Ngo V. L.et al., A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc. Natl. Acad. Sci. U.S.A. 115, E5076–E5085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina-Contreras O.et al., Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J. Immunol. 196, 34–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell S. E.et al., IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 9, 1193–1204 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Walsh P. T., Fallon P. G., The emergence of the IL-36 cytokine family as novel targets for inflammatory diseases. Ann. N. Y. Acad. Sci. 1417, 23–34 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Scheibe K.et al., Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 156, 1082–1097.e11 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Harusato A.et al., IL-36γ signaling controls the induced regulatory T cell-Th9 cell balance via NFκB activation and STAT transcription factors. Mucosal Immunol. 10, 1455–1467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiles S.et al., Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 6, 963–972 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Simmons C. P.et al., Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168, 1804–1812 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Kim Y. G.et al., The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca-Camarillo G., Furuzawa-Carballeda J., Iturriaga-Goyon E., Yamamoto-Furusho J. K., Differential expression of IL-36 family members and IL-38 by immune and nonimmune cells in patients with active inflammatory bowel disease. BioMed Res. Int. 2018, 5140691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida A.et al., Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm. Bowel Dis. 22, 303–314 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Boutet M. A.et al., Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 184, 159–173 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich M., Tillack C., Wollenberg A., Schauber J., Brand S., IL-36γ sustains a proinflammatory self-amplifying loop with IL-17C in anti-TNF-induced psoriasiform skin lesions of patients with Crohn’s disease. Inflamm. Bowel Dis. 20, 1891–1901 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Heath J. E., Scholz G. M., Veith P. D., Reynolds E. C., IL-36γ regulates mediators of tissue homeostasis in epithelial cells. Cytokine 119, 24–31 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Vigne S.et al., IL-36R ligands are potent regulators of dendritic and T cells. Blood 118, 5813–5823 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Foster A. M.et al., IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 192, 6053–6061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigne S.et al., IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood 120, 3478–3487 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Bridgewood C.et al., IL-36γ is a strong inducer of IL-23 in psoriatic cells and activates angiogenesis. Front. Immunol. 9, 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Santana Y. E., Leon G., St Leger D., Fallon P. G., Walsh P. T., Keratinocyte interleukin-36 receptor expression orchestrates psoriasiform inflammation in mice. Life Sci. Alliance 3, e201900586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein J. D.et al., IL-36 signaling in keratinocytes controls early IL-23 production in psoriasis-like dermatitis. Life Sci. Alliance 3, e202000688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio L.et al., IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 9, 1104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller A.et al., IκBζ is a key transcriptional regulator of IL-36-driven psoriasis-related gene expression in keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 115, 10088–10093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashiguchi Y.et al., IL-36α from skin-resident cells plays an important role in the pathogenesis of imiquimod-induced psoriasiform dermatitis by forming a local autoamplification loop. J. Immunol. 201, 167–182 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Franzke C. W.et al., Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J. Exp. Med. 209, 1105–1119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data and reagents that support the findings of this study are available within the main text or the SI Appendix.