Abstract
Purpose:
Obesity is a heterogeneous condition, and distinct adiposity subtypes may differentially affect type 2 diabetes risk. We assessed relations between genetically determined subtypes of adiposity and changes in glycemic traits in a dietary intervention trial.
Methods:
The four genetic subtypes of adiposity including waist hip ratio-increase only (WHRonly+), body mass index-increase only (BMIonly+), WHR-increase and BMI-increase (BMI+WHR+), and WHR-decrease and BMI-increase (BMI+WHR−), were assessed by polygenetic scores (PGSs), calculated based on 159 single nucleotide polymorphisms related to BMI and/or WHR. We examined the associations between the four PGSs and changes in fasting glucose, insulin, β-cell function (HOMA-B) and insulin resistance (HOMA-IR) in 692 overweight participants (84% white Americans) who were randomly assigned to one of four weight-loss diets in a 2-year intervention trial.
Results:
Higher BMI+WHR− PGS was associated with greater decrease on 2-year changes in waist circumference in white participants (P=0.002). We also found significant interactions between WHRonly+ PGS and dietary protein on 2-year changes in fasting glucose and HOMA-B (P=0.0007 and <0.0001, respectively). When consuming an average-protein diet, participants with higher WHRonly+ PGS showed less increased fasting glucose (β=−0.46, P = 0.006) and less reduction in HOMA-B (β=0.02, P = 0.005) compared with lower WHRonly+ PGS. Conversely, eating high-protein diet was associated with less decreased HOMA-B among individuals with lower than higher WHRonly+ PGS (β=−0.02, P = 0.006).
Conclusions:
Distinct genetically determined adiposity subtypes may differentially modify the effects of weight-loss diets on improving glucose metabolism in white Americans. This trial was registered at clinicaltrials.gov as NCT00072995.
Keywords: adiposity subtypes, body mass index, waist-hip ratio, polygenetic score, gene-diet interaction, glycemic traits
Introduction
Obesity has been related to pre-diabetes and type 2 diabetes [1–3]. Weight-loss interventions through modifications on diets and lifestyle have shown beneficial effects on improving glycemic control and reducing diabetes risk [4–6]. However, obesity is a phenotypically and metabolically-diverse entity determined by discrete patterns of body fat distribution [7]. Growing evidence has shown that various subtypes of obesity, which are determined by amount and distribution of body fat, may differentially affect cardiometabolic risk and may distinctly respond to diet or lifestyle interventions [8]. Such heterogeneous obesity subtypes are partly determined by genetic variations [9, 10].
In the past ten years, genetic studies have identified hundreds of genetic variations associated with body mass index (BMI) and waist-hip ratio (WHR) [11–14], which are correlated but represent two distinct aspects of obesity − overall fat mass and fat distribution, respectively. At the population level, both BMI and WHR are independently and positively associated with type 2 diabetes [15, 16], and such associations are likely to be causal [17]. However, some studies proved the existence of “favorable adiposity” alleles which were associated with higher adiposity but lower risk of type 2 diabetes [18]. A recent study classified 159 SNPs associated with BMI, WHR, or WHR adjusted for BMI (WHRadjBMI) at genome-wide significance level into four subtypes based on the direction of their associations with these adiposity phenotypes: (1) WHR-decrease only (WHR only effects, WHRonly+), (2) BMI-increase only (BMI only effects, BMIonly+), (3) WHR-increase and BMI-increase (BMI and WHR effects in the same direction, BMI+WHR+), (4) WHR-decrease and BMI-increase (BMI and WHR effects to the opposite directions, BMI+WHR−), which showed distinct associations with type 2 diabetes risk [19]. Thus, we hypothesized that individuals with varying genetic subtypes of adiposity, assessed by polygenetic scores (PGS), might show divergent changes in glycemic traits in response to weight-loss diets.
The Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial is a 2-year weight-loss diet intervention trial. A total of 811 overweight participants were assigned to four reduced-calorie diets with different compositions of macronutrients. The participants who completed the trial had reduced body weight and improved fasting insulin levels [4].
In this study, we examined the associations between four genetic subtypes of adiposity [19], evaluated by the PGSs, and 2-year changes in fasting glucose, fasting insulin, β-cell function (HOMA-B) and insulin resistance (HOMA-IR) in response to weight-loss diets among participants from the POUNDS Lost trial. In particular, we also assessed the interactions between PGSs and diet interventions on changes in the glycemic traits.
Methods
Study participants
The POUNDS Lost trial was a 2-year randomized clinical trial, conducted at Harvard School of Public Health and Brigham and Women’s Hospital, Boston, MA, and Pennington Biomedical Research Center of Louisiana State University System, Baton Rouge, LA from October 2004 to December 2007. The details of the study including design and methods have been described previously [4]. Briefly, a total of 811 participants with obesity or overweight [BMI (in kg/m2): 25–40], aged 30–70 y were enrolled. Among all the participants, 643 of them were white Americans while others were black Americans. Major exclusion criteria were the presence of diabetes treated with oral medications or insulin, unstable or recent onset of cardiovascular disease, use of medications that affect body weight, or insufficient motivation, which were assessed by interview and questionnaire. All participants gave written informed consent. The study was approved by the human subjects committee at each institution and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute.
Subjects were randomly assigned to one of the four weight-loss diets to compare their effects on weight change in the 2-year follow-up. The percentages of energy derived from three macronutrient in the four diets were: (1) 20% fat, 15% protein, and 65% carbohydrate; (2) 20% fat, 25% protein, and 55% carbohydrate; (3) 40% fat, 15% protein, and 45% carbohydrate; and (4) 40% fat, 25% and 35% carbohydrate. Two diets were low-fat (20%), or high-fat (40%), and two were average-protein (15%), or high-protein (25%), which formed a 2-by-2 factorial design. Similar foods for each diet was used to maintain blinding. Participants were provided daily meal plans in 2-week blocks and they were instructed to record their food and beverage intake. After 2 years, 645 (80%) of the participants completed the trial.
In the main results, we analyzed data among 583 white participants (mean age 52.1±9.0 y) with SNPs data available at baseline. Black participants were not included because the classification of 159 SNPs into four subtypes were analyzed in the Europeans. Of the participants included, 328 (56.3%) were women. Data on fasting glucose and insulin level were available for 583 and 577 participants, respectively at baseline; 517 and 497 participants, respectively at 6 months; 446 and 433 participants, respectively at 2 years. The results of the whole population (692 participants) were presented in the supplemental material as a sensitivity analysis.
Measurements
Fasting blood and 24-hour urine were collected at baseline, 6 months and 2 years. Serum glucose, insulin, and urinary nitrogen (as a biomarker of diet adherence) were measured at the clinical laboratory at the Pennington Biomedical Research Center. Homeostasis model assessment (HOMA) models were used to estimate insulin resistance (HOMA-IR), and β-cell function (HOMA-B), which were calculated by the following equations: HOMA-IR= (fasting insulin (μU/mL)×fasting glucose (mmol/L))/22.5, and HOMA-B=(20× fasting insulin (μU/mL))/(fasting glucose (mmol/L) −3.5).
Body weight and waist circumference were measured in the morning before breakfast on 2 separate days, and averaged at baseline, 6 months, and 2 years. Body weight was measured by calibrated hospital scales, clothed in a hospital gown. Waist circumference was measured by using a non-stretchable tape measure, 4 cm above the iliac crest. Height was measured at baseline. BMI was calculated as weight (kg) divided by the square of height (m2). Baseline dietary intake was assessed in a random sample of 50% of the total participants by a 5-day diet record. It was also determined through 24-hour recalls by telephone interview on 3 nonconsecutive days at 6 months and 2 years. Changes in biomarkers (respiratory quotient and urinary nitrogen) confirmed that differences among the groups in macronutrient intake were consistent with those recorded in the dietary reports [4].
Genotyping and PGS calculation
DNA was extracted from the buffy coat fraction of centrifuged blood with the QIAmp Blood Kit (Qiagen). We selected 152 SNPs from the 159 SNPs classified for the analysis of white participants [19]: (1) 27 WHRonly+ SNPs (PBMI ≥ 0.05, PWHR <0.05), 23 BMIonly+ SNPs (PBMI < 0.05, PWHR ≥ 0.05), 79 BMI+WHR+ SNPs (PBMI < 0.05, PWHR <0.05), 23 BMI+WHR− SNPs (PBMI < 0.05, PWHR < 0.05) (Supplemental Table 1), with 4 SNPs excluded for not in Hardy-Weinberg equilibrium in the white population (all P<0.05) and 3 SNPs excluded for related to type 2 diabetes. For the sensitive analysis in the whole population, 131 SNPs were selected with 25 SNPs excluded for not in Hardy-Weinberg equilibrium in the whole population (all P<0.05) and 3 SNPs excluded for related to type 2 diabetes.
We assumed each SNP in the panel would act independently in an additive manner, and the PGS was calculated by summing risk allele’s numbers, and weighted by their effect sizes (β-coefficients) derived from the reported genome-wide association study (GWAS) data (WHRonly+ SNPs were weighted by WHRadjBMI-effect; BMIonly+, BMI+WHR+, BMI+WHR− SNPs were weighted by the BMI-effect) [19]. By using the same method for the previously reported PGS of BMI and WHR [20, 21], the PGS was computed using the equation: PGS = (β1 × SNP1+β2 × SNP2+… +βn × SNPn) × (n/ sum of the β-coefficients), where β is the β coefficient for each individual SNP, and n is number of SNPs. The SNPs were genotyped successfully in 692 of 811 total participants (583 of 643 whites) using the OpenArray SNP Genotyping System (BioTrove). The success rate of genotyping was 99%. Replicate quality control samples (10%) were included and genotyped with >99% concordance [22]. The mean (range) of PGS of WHRonly+, BMIonly+, BMI+WHR+, BMI+WHR− are 23.2 (13.5–34.3), 20.2 (13.1–28.1), 84.4 (64.1–105.0) and 26.3 (16.6–36.1) respectively, among the white participants. A higher PGS of WHRonly+, BMIonly+, BMI+WHR+, and BMI+WHR− indicated a higher genetic risk of higher only in WHR, higher only in BMI, higher in both BMI and WHR, higher in BMI but lower in WHR subtypes, respectively.
Statistical analysis
The primary endpoints in this study were changes in fasting glucose, fasting insulin, HOMA-B and HOMA-IR. Levels of insulin, HOMA-B and HOMA-IR were log-transformed before analysis to improve the normality of the distributions. General linear models for continuous variables and chi-square test for categorical variables were applied to compare characteristics among tertiles of the PGS at baseline. Nutrient intakes and biomarkers of diet adherence across tertiles of the PGS at baseline, 6 months and 2 years were compared using general linear models. For gene-diet interaction, we examined the PGS, dietary protein or dietary fat, and PGS-by-diet interaction as independent predictors of changes in primary outcomes adjusted for age, sex, race (whole populations only), baseline BMI, weight change, and the baseline value for the respective outcome trait in general linear models. We also analyzed data in the whole population as a sensitivity analysis. A Bonferroni correction was applied to adjust for multiple testing. P< 0.003 (0.05 ÷ 16) was considered significant for the main effect and P< 0.0015 (0.05 ÷ 32) was considered significant for the PGS-diet interaction. Secondly, linear mixed models were used to test the PGS effect on the trajectory of changes in primary outcomes among the participants who provide measurements at baseline, 6 months, and 2 years in each of diet groups over the 2-year intervention by including PGS-by-time interaction terms. P < 0.05 was considered significant. All statistical analyses were performed with SAS (version 9.4, SAS Institute Inc.).
Results
Baseline characteristics
Baseline characteristics of the participants according to tertiles of one of the polygenetic scores, WHRonly+, which showed significant interaction with diets (see below), are presented in Table 1. Mean age, sex and diet group distributions were similar (all P>0.05). Higher WHRonly+ PGS was associated with shorter height (P=0.03) and higher fasting glucose (P=0.0002), fasting insulin (P=0.04), and HOMA-IR (P=0.01) after adjustment for age and sex.
Table 1.
Baseline characteristics of white participants according to WHRonly+ PGS in the POUNDS Lost Trial
| Characteristics | Tertiles of the WHRonly+ PGS | Pa | ||
|---|---|---|---|---|
| T1(n=192) | T2(n=193) | T3(n=198) | ||
| Age, y | 51.7±9.1 | 52.6±8.7 | 52.0±9.1 | 0.75 |
| Female, n (%) | 104(54.2) | 108(56.0) | 116(58.6) | 0.68 |
| Height, cm | 170.2±8.9 | 169.5±8.8 | 168.2±8.7 | 0.03 |
| Weight, kg | 93.7±15.6 | 94.9±15.6 | 92.4±16.6 | 0.75 |
| BMI, kg/m2 | 32.2±3.9 | 32.9±4.0 | 32.5±3.9 | 0.43 |
| Waist, cm | 104.1±12.6 | 105±13.6 | 103.7±14.1 | 0.78 |
| Glucose, mg/dL | 90.5±10.1 | 91.9±10.6 | 94.7±14.1 | 0.0002 |
| Insulin, μU/mL | 9.5(6.4, 14.0) | 10.5(7.1, 15.3) | 10.9(7.3, 16.4) | 0.04 |
| HOMA-IR | 2.1(1.4, 3.1) | 2.4(1.5,3.5) | 2.5(1.6, 3.9) | 0.01 |
| HOMA-B | 127.2(96.4, 186.9) | 130.3(103.2, 186.4) | 131.5(87.5, 201.2) | 0.75 |
| HbAlc, % | 5.3±0.3 | 5.4±0.4 | 5.4±0.4 | 0.16 |
| Diet group, n (%) | 0.36 | |||
| High fat, high protein | 53(27.6) | 54(28.0) | 37(18.7) | |
| High fat, average protein | 49(25.5) | 44(22.8) | 58(29.3) | |
| Low fat, high protein | 44(22.9) | 48(24.9) | 51(25.8) | |
| Low fat, average protein | 46(24.0) | 47(24.4) | 52(26.3) | |
| Dietary intake per day2 | ||||
| Energy, kcal | 2005±607 | 1992±522 | 1958±523 | 0.79 |
| Carbohydrate, % of energy | 43.4±8.0 | 44.9±7.5 | 44.3±7.7 | 0.55 |
| Fat, % of energy | 37.5±6.4 | 36.9±6.1 | 37.0±5.7 | 0.62 |
| Protein, % of energy | 18.7±3.5 | 17.7±3.1 | 18.3±3.6 | 0.45 |
| Biomarkers of adherence | ||||
| Respiratory quotient | 0.84±0.04 | 0.84±0.04 | 0.84±0.04 | 0.52 |
| Urinary nitrogen, g/d | 12.6±4.0 | 12.4±4.2 | 12.6±5.3 | 0.56 |
Values are means±SDs, median (25th, 75th), or n (%). Insulin, HOMA-IR, HOMA-B were log-transformed before analysis.
P values were calculated by using the chi-square test for categorical variables and general linear regression test after adjusting for age (except age) and sex for continuous variables.
Regarding the baseline characteristics of the other three PGSs (Supplemental Table 2–4), higher BMIonly+ PGS was associated with higher baseline HbA1c and higher fat intake (both P=0.02) after adjustment for age and sex. No associations were observed between the four PGSs and baseline BMI or waist circumference (all P>0.1). No other significant associations between the four PGSs and baseline characteristics were observed.
Nutrient intake and biomarkers of adherence
The dietary intake (total energy, carbohydrate, fat, and protein) and biomarkers of adherence (respiratory quotient and urinary nitrogen) were used to evaluate the dietary adherence across the intervention. Nutrient intake and biomarkers of adherence according to tertiles of one of the polygenetic scores, WHRonly+, which showed significant interaction with diets (see below), are presented in Table 2. It was confirmed that participants modified their intake of macronutrients in the direction of the intervention goals, although the targets were not fully achieved [4]. At 6 month, higher WHRonly+ PGS was associated with higher carbohydrate and lower protein intake (both P=0.04), however, no other significant differences were observed at 6 months or 2 years (all P>0.1).
Table 2.
Nutrient intakes and biomarkers of weight-loss diet adherence in white participants according to WHRonly+ PGS at 6 months and 2 years
| Characteristics | 6 months | 2 years | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P | T1 | T2 | T3 | P | |
| Dietary intake per daya | ||||||||
| Energy, kcal | 1589±498 | 1662±467 | 1648±498 | 0.18 | 1561±500 | 1563±502 | 1560±509 | 0.72 |
| Carbohydrate, % of energy | 49.1±10.3 | 48.5±9.7 | 52.1±10.5 | 0.04 | 48.1±10.0 | 47.6±9.5 | 50.0±10.6 | 0.32 |
| Fat, % of energy | 30.5±8.0 | 31.7±8.3 | 29.6±8.4 | 0.44 | 30.5±8.7 | 31.5±6.8 | 31.1±9.9 | 0.74 |
| Protein, % of energy | 20.8±4.7 | 20.1±4.0 | 19.5±4.6 | 0.04 | 20.9±4.7 | 20.6±4.7 | 20.0±4.5 | 0.31 |
| Biomarkers of adherence | ||||||||
| Respiratory quotientb | 0.84±0.04 | 0.84±0.04 | 0.84±0.04 | 0.33 | 0.83±0.04 | 0.83±0.05 | 0.84±0.04 | 0.19 |
| Urinary nitrogen, g/dc | 11.7±4.8 | 11.9±4.8 | 11.3±4.2 | 0.63 | 11.7±4.2 | 12.2±4.8 | 12.0±4.7 | 0.84 |
Values are means±SDs. P values were calculated by general linear regression test after adjusting for sex and age.
Data for dietary intake per day were available for 280 individuals at 6 months: T1, n = 87; T2, n = 95; T3, n=98; 142 individuals at 2 years: T1, n = 48; T2, n = 44; T3, n=50.
Data for respiratory quotient were available for 488 individuals at 6 months: T1, n = 167; T2, n = 159; T3, n = 162. 387 individuals at 2 years: T1, n = 128; T2, n = 128; T3, =131.
Data for urinary nitrogen were available for 451 individuals at 6 months: T1, n = 151; T2, n = 148; T3, n=152. 320 individuals at 2 years: T1, n = 104; T2, n = 104; T3, n=112.
No significant differences across tertiles of the other three PGSs in nutrient intake and biomarkers of adherence were found at 6-month or 2-year intervention (all P>0.05, data not shown).
Associations between the PGSs and 2-year changes in glycemic traits
The associations between the four PGSs and 2-year changes in BMI, waist circumference, and glycemic traits in the white participants are presented in Table 3. Higher BMIonly+ and BMI+WHR− PGS was associated with greater decrease in 2-year changes in BMI (P=0.01) and waist circumference (P=0.002) respectively after adjustment for age, sex, diet group, baseline BMI, 2-year weight change (only waist circumference) and baseline waist circumference (only waist circumference). No other significant associations between the four PGSs and 2-year changes in glycemic traits including glucose, insulin, HOMA-B, and HOMA-IR were observed (all P≥0.02). In the sensitive analysis, we also found similar association between BMIonly+, BMI+WHR− PGS and 2-year changes in BMI (P=0.04) and waist circumference (P=0.001) respectively in the whole population.
Table 3.
Associations between the four polygenetic scores and 2-year changes in glycemic traits in white participants
| Outcomes | WHRonly+ | BMIonly+ | BMI+WHR+ | BMI+WHR− | ||||
|---|---|---|---|---|---|---|---|---|
| β(SE) | P | β(SE) | P | β(SE) | P | β(SE) | P | |
| Δ BMI, kg/m2 | −0.05(0.04) | 0.13 | −0.12(0.05) | 0.01 | −0.03(0.02) | 0.19 | −0.01(0.04) | 0.69 |
| Δ Waist, cm | 0.01(0.05) | 0.89 | −0.05(0.07) | 0.48 | −0.01(0.03) | 0.80 | −0.16(0.05) | 0.002 |
| Δ Glucose, mg/dL | −0.05(0.12) | 0.69 | −0.03(0.16) | 0.87 | 0.01(0.07) | 0.87 | −0.09(0.12) | 0.46 |
| Δ Insulin, μU/mL | −0.001(0.005) | 0.87 | −0.0003(0.007) | 0.96 | −0.0002(0.003) | 0.95 | −0.006(0.005) | 0.27 |
| Δ HOMA-B | −0.002(0.005) | 0.75 | −0.0003(0.007) | 0.97 | −0.001(0.003) | 0.76 | −0.001(0.005) | 0.84 |
| Δ HOMA-IR | −0.0005(0.006) | 0.93 | −0.0005(0.007) | 0.95 | −0.00005(0.003) | 0.99 | −0.007(0.006) | 0.23 |
β represents changes in each outcome per increment of 1 PGS. P for trend between the four PGSs and changes in BMI, waist circumference and glycemic traits was calculated by applying general linear models, after adjustment for age, sex, diet group, baseline BMI, 2-year weight change (except BMI change), and baseline values for respective phenotypes (except BMI change). Data on insulin, HOMA-B, HOMA-IR were log-transformed before analysis. HOMA-B, homeostasis model assessment of β cell function; HOMA-IR, insulin resistance.
Gene-diet interactions on 2-year changes in glycemic traits
We further tested interactions between the four PGSs and diets on 2-year changes in glycemic traits. We found that dietary protein significantly modified the associations between WHRonly+ PGS and 2-year changes in fasting glucose (P=0.0007) and HOMA-B (P<0.0001) after adjusting for age, sex, baseline BMI, weight change at 2 years and the baseline value for the respective outcome trait (Table 4, Fig. 1). In the average-protein diet group, higher PGS was associated with less increase in fasting glucose level (P-trend=0.006) and less decrease in HOMA-B (P-trend=0.005) compared with the participants with lower PGS. Opposite associations were observed in the high-protein diet group where lower PGS was related to a less reduction in HOMA-B (P-trend=0.006) than higher WHRonly+ PGS. Similar interaction between dietary protein and WHRonly+ PGS were found on changes in fasting glucose (P=0.0009), while the interaction on changes in HOMA-B became insignificant (P=0.003) when the total population were analyzed (Supplemental Fig. 1). No significant interactions were observed of WHRonly+ PGS with dietary fat or carbohydrates (all P>0.5, data not shown); and no interaction was observed between diets and other three PGSs on changes in glycemic traits (all P>0.1, data not shown).
Table 4.
Effects of the WHRonly+ PGS and weight-loss diets on 2-year changes in glycemic traits in white participants
| Outcomes | Average protein diet | High protein diet | Pinteraction | ||
|---|---|---|---|---|---|
| β(SE) | Ptrend | β(SE) | Ptrend | ||
| Δ Glucose, mg/dL | −0.46(0.17) | 0.006 | 0.29(0.16) | 0.08 | 0.0007 |
| Δ Insulin, μU/mL | 0.010(0.007) | 0.19 | −0.011(0.007) | 0.12 | 0.05 |
| Δ HOMA-B | 0.021(0.007) | 0.005 | −0.020(0.007) | 0.006 | <0.0001 |
| Δ HOMA-IR | 0.006(0.008) | 0.49 | −0.007(0.007) | 0.34 | 0.26 |
β represents changes in each outcome per increment of 1 PGS. P for trend between WHRonly+ PGS and changes in glycemic traits was calculated by applying general linear models, after adjustment for age, sex, baseline BMI, 2-year weight change, and baseline values for respective phenotypes. Data on insulin, HOMA-B, HOMA-IR were log-transformed before analysis. HOMA-B, homeostasis model assessment of β cell function; HOMA-IR, insulin resistance.
Fig. 1.
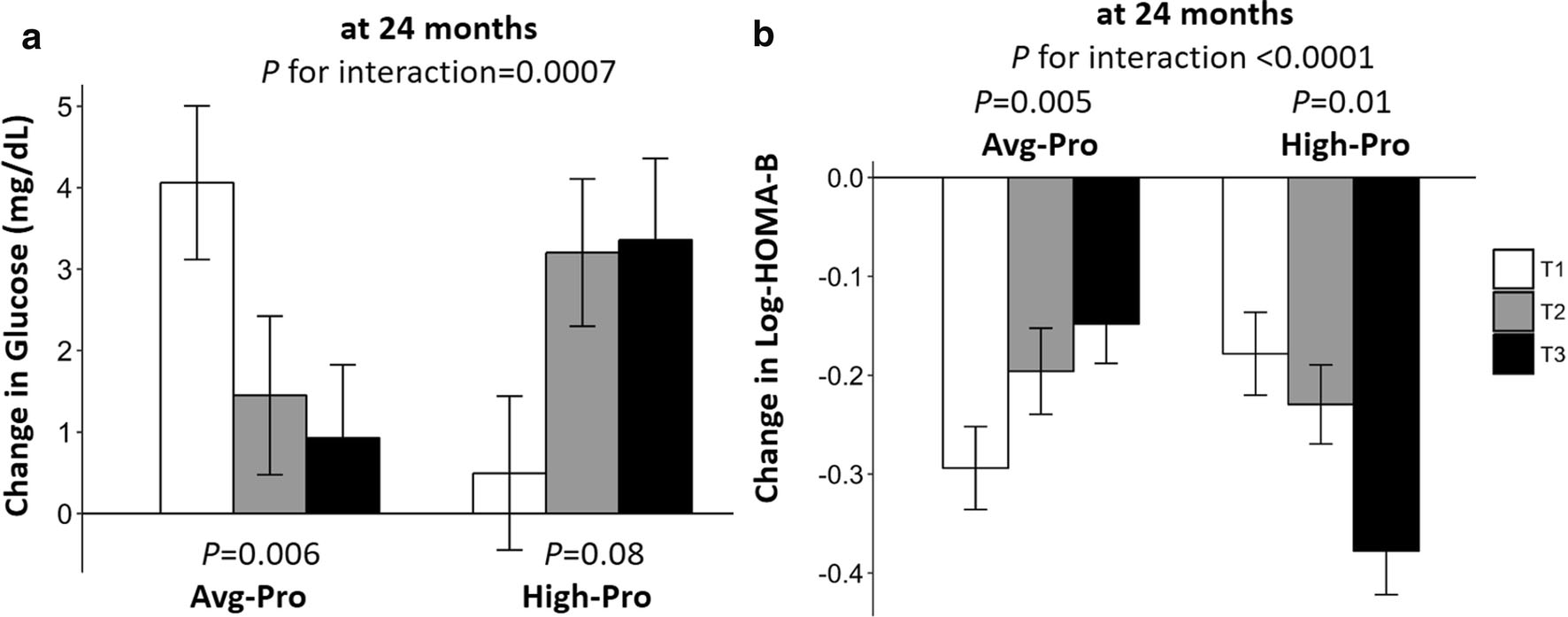
Effects of the WHRonly+ PGS and weight-loss diets on changes in fasting glucose and HOMA-B during the 2-year intervention in white participants. Values were means±SEs, adjusted for age, sex, baseline BMI, weight loss at 2 years, and baseline values of the respective outcomes in the general linear models. HOMA-B were log-transformed before analysis. High-Pro, high protein; Avg-Pro, average protein; HOMA-B, homeostasis model assessment of β cell function; T, tertile. Panel a: changes in glucose; panel b: changes in HOMA-B.
Trajectory of changes in fasting glucose and β cell function
The 2-year trajectory of the changes in fasting glucose and β cell function is shown in Fig. 2. Linear mixed models were used to examine the genetic effect on glycemic traits by time effect during the 2-year intervention. We found significant interactions between WHRonly+ PGS and intervention time on changes in fasting glucose among the white participants in the average-protein diet group (P=0.02) and for HOMA-B in the high-protein diet group (P=0.0007). From baseline to 6 months, the levels of glucose and HOMA-B were decreased in all participants, whereas, from 6 months to 2 years, glucose level increased and HOMA-B was continuously decreased [4]. Individuals with higher WHRonly+ PGS had a greater decrease in glucose from baseline to 6 months and less increase of glucose when assigned to the average-protein diet compared with individuals who had lower WHRonly+ PGS. Among participants, those with lower WHRonly+ PGS had less decrease in HOMA-B from baseline to 2 years in HOMA-B from 6 months to 2 years when choosing a high protein diet. We also found similar gene-time interactions for changes in fasting glucose (Paverage-protein=0.002, Phigh-protein=0.21) and HOMA-B (Paverage-protein=0.23, Phigh-protein=0.02) in the sensitivity analysis (Supplemental Fig. 2).
Fig. 2.
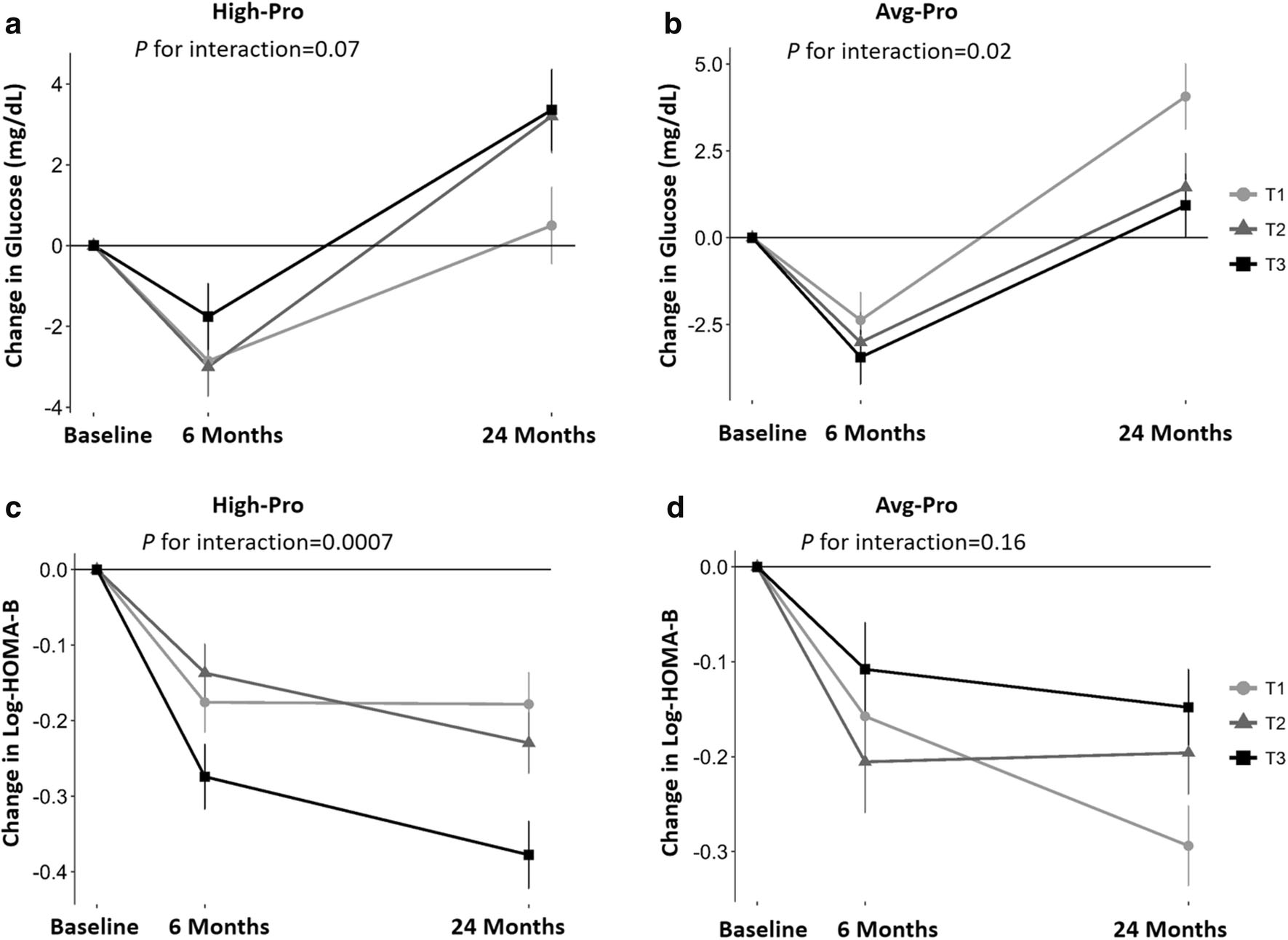
WHRonly+ PGS with 2-year trajectory of changes in fasting glucose and HOMA-B in response to weight-loss diet in white participants. Values were means±SEs after adjustment for age, sex, baseline BMI, weight change, and baseline values for respective phenotypes. P values were tested for the interaction between PGS and intervention time. HOMA-B was log-transformed before analysis. HOMA-B, homeostasis model assessment of β cell function; T, tertile. Panel a: changes in glucose in high protein group; panel b: changes in glucose in average protein group; panel c: changes in HOMA-B in high protein group; panel d: changes in HOMA-B in average protein group.
Discussion
In the present study, we observed positive associations between WHRonly+ PGS and baseline fasting glucose, fasting insulin, and HOMA-IR in white Americans. No association was observed with the other three PGSs. During the 2-year intervention, we found that a higher BMI+WHR− PGS was associated with greater decrease in 2-year changes in waist circumference. In addition, we found that dietary protein significantly modified the associations between WHRonly+ PGS and 2-year changes in fasting glucose and HOMA-B. Participants with higher WHRonly+ PGS showed less increase in glucose and less reduction in HOMA-B than those with lower WHRonly+ PGS when assigned to the average-protein diet. Conversely, lower WHRonly+ PGS had less decreased HOMA-B than higher WHRonly+ PGS when consuming the high-protein diet.
Obesity is a phenotypically and metabolically-diverse entity determined by discrete patterns of body fat distribution. For example, a subgroup of individuals with obesity, known as metabolically healthy obesity (MHO), may not have increased cardiometabolic risk. Visceral abdominal fat accumulation may convert MHO to metabolically unhealthy obesity (MUO) [23]. A number of GWAS studies found thousands of SNPs related to BMI, WHR, or WHRadjBMI and a recent study classified these SNPs into four adiposity subtypes based on the direction of their effects on BMI and WHR (WHRonly+, BMIonly+, BMI+WHR+ and BMI+WHR−) and the four adiposity subtypes were also classified into three metabolic subtypes: metabolically unfavorable (BMI+WHR+), metabolically neutral or inconclusive (BMIonly+ and WHRonly+), and metabolically rather favorable (BMI+WHR−) subtypes [19].
At baseline, we found that WHRonly+ PGSs was associated with insulin and insulin resistance, while such associations were not observed in the other three subtypes. In our study, all WHRonly+ variants were derived from WHRadjBMI [19]. The associations between genetic variation of WHR and glycemic traits are supported by numerous lines of evidence [16, 20, 24] and are likely to be causal [3, 16, 17]. To our knowledge, most previous studies investigating genetic variation of WHR in relation to type 2 diabetes focused on WHRadjBMI [3, 16, 17, 20, 24]. However, some of the WHRadjBMI variants, such as BMI+WHR− variants, were not completely independent of BMI and showed effect on BMI in the unexpected direction [25]. BMI+WHR− PGS is related to both BMI and WHR but in opposite direction, i.e. higher BMI+WHR− PGS was associated with lower WHR but higher BMI. WHRonly+ PGS is only related to WHR, i.e. higher WHRonly+ PGS was associated with higher WHR. Our results indicated that the WHRadjBMI variants only related to WHR were associated with insulin and insulin resistance, suggesting abdominal fat is more important in determining insulin and insulin resistance.
Among the four PGSs, we found that only the BMI+WHR− PGS was negatively associated with 2-year changes of waist circumference independent of concurrent weight change. Notably, this PGS was not associated with 2- year BMI change, indicating that participants with higher BMI+WHR− PGS (genetically higher risk for general obesity but lower risk for abdominal obesity) might benefit from reducing central obesity rather than overall obesity by taking weight-loss diets. Our data also lend support to the classification that BMI+WHR− PGS is metabolically rather favorable and the most healthy adiposity subtype [19]. Besides, BMIonly+ PGS was negatively associated with 2-year changes of BMI, indicating that participants who had higher BMIonly+ PGS might benefit from reducing overall obesity rather than central obesity by taking weight-loss diets.
Interestingly, we found that WHRonly+ PGS modified the effect of dietary protein on changes in glucose and β cell function. The relations between dietary protein intakes and glycemic metabolism have been inconsistently reported [26]: Some studies found that high protein intake was associated with higher levels of fasting glucose [27], better β cell function [28] and increased risk of type 2 diabetes [29, 30], while others revealed that there was no relation between dietary protein and fasting glucose [31], β cell function [32] or type 2 diabetes risk [32, 33]. Our data suggest that the conflicting findings might be partly due to the individual genetic differences.
From the trajectory of changes in glycemic traits during the 2-year visit, we observed that the diet intervention on lowering fasting glucose was more effective at 6 months than 2 years, which is consistent with the trajectory of changes in body weight [4]. Another 10-week dietary intervention study also observed reduction in both fasting glucose and HOMA-B [34]. Similar to other diet or lifestyle intervention trials, the diminished adherence that occurred between 6 months and 2 years may partially explain the regain of fasting glucose [35, 36]. In our study, interaction patterns between WHRonly+ PGS and dietary protein on glycemic traits appeared to be similar at 6 months and 2 years, thought the modification effects were less significant at 6 months.
To the best of our knowledge, this is the first study to investigate the association and interaction between genetically determined subtypes of adiposity and weight-loss diet interventions on changes in glycemic traits in a long-term randomized clinical trial. However, several limitations in our study should also be addressed. Firstly, instead of gold standard (hyperinsulinemic-euglycemic clamp and the hyperglycemic clamp), we used the homeostatic model to assess β cell function and insulin resistance, however, this model can also yield valuable data when primary input data are robust, and the result is interpreted carefully [37]. Secondly, hip circumference was not measured in this trial, so the association between WHR phenotype and changes in glycemic traits during weight loss could not be assessed. Thirdly, although we found similar interactions in both white population and whole population, further replications in other races are needed to generalize our findings.
In conclusion, our study suggests that distinct genetically determined adiposity subtypes may differentially modify the effects of weight-loss diets on improving glucose metabolism, and dietary protein may modify the genetic effects of WHRonly+ PGS on glycemic changes in white Americans. Our results may provide novel information to develop diet interventions by considering various genetic subtypes of obesity.
Supplementary Material
Acknowledgments
We thank all of the participants in the trial for their dedication and contribution to the research.
Funding sources
The work was supported by the National Heart, Lung, and Blood Institute [grant numbers HL071981, HL034594, HL126024]; the National Institute of Diabetes and Digestive and Kidney Diseases [grant numbers DK091718, DK100383, DK078616, DK115679]; the Boston Obesity Nutrition Research Center [grant number DK46200]; and United States - Israel Binational Science Foundation [grant number 2011036]. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). Yuhang Chen is a recipient of a scholarship under the China Scholarship Council to pursue her study in the United States of America (201706240060). The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- 1.Kopelman PG (2000) Obesity as a medical problem. Nature 404:635–643. 10.1038/35007508 [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. (2003) Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA 289(1):76–79. 10.1001/jama.289.1.76 [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Zhang R, Ma X, Wang S, He Z, Huang Y, et al. (2018) Causal Association of Overall Obesity and Abdominal Obesity with Type 2 Diabetes: A Mendelian Randomization Analysis. Obesity 26(5):934–942. 10.1002/oby.22167 [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH (2009) Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N Engl J Med 360(9):859–873. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler William C., Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santilli F, Simeone PG, Guagnano MT, Leo M, Maccarone MT, Castelnuovo A Di, et al. (2017) Effects of liraglutide on weight loss, fat distribution, and b-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care 40(11):1556–1564. 10.2337/dc17-0589 [DOI] [PubMed] [Google Scholar]
- 7.Phillips CM (2016) Metabolically Healthy Obesity: Personalised and Public Health Implications. Trends Endocrinol Metab 27(4):189–191. 10.1016/j.tem.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Phillips CM (2013) Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev Endocr Metab Disord 14(3):219–227. 10.1007/s11154-013-9252-x [DOI] [PubMed] [Google Scholar]
- 9.Heianza Y, Qi L (2017) Gene-diet interaction and precision nutrition in obesity. Int J Mol Sci 18(4):787. 10.3390/ijms18040787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi L (2014) Personalized nutrition and obesity. Ann Med 46(5):247–252. 10.1007/s10741-014-9462-7.Natural [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538):197–206. 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518(7538):187–196. 10.1038/nature14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama M, Okada Y, Kanai M, Takahashi A, Momozawa Y, Ikeda M, et al. (2017) Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet 49(10):1458–1467. 10.1038/ng.3951 [DOI] [PubMed] [Google Scholar]
- 14.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Ssteinthorsdottir V, et al. (2010) Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42(11):949–960. 10.1038/ng.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. (2008) General and Abdominal Adiposity and Risk of Death in Europe. N Engl J Med 359(13):2105–2120. 10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]
- 16.Emdin CA, Khera AV, Natarajan P, Klarin D, Zekavat SM, Hsiao AJ, et al. (2017) Genetic Association of Waist-to-Hip Ratio With Cardiometabolic Traits, Type 2 Diabetes, and Coronary Heart Disease. Jama 317(6):626–634. 10.1001/jama.2016.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D (2017) Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation 135:2373–2388. 10.1161/CIRCULATIONAHA.116.026560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaghootkar H, Lotta LA, Tyrrell J, Smit RAJ, Jones SE, Donnelly L, et al. (2016) Genetic Evidence for a Link Between Favorable Adiposity and Lower Risk of Type 2 Diabetes, Hypertension, and Heart Disease. Diabetes 65(August):2448–2460. 10.2337/db15-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler TW, Günther F, Höllerer S, Zimmermann M, Loos RJ, Kutalik Z, et al. (2018) A joint view on genetic variants for adiposity differentiates subtypes with distinct metabolic implications. Nat Commun 9:1946. 10.1038/s41467-018-04124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T, Qi Q, Zheng Y, Ley SH, Manson JAE, Hu FB, et al. (2015) Genetic predisposition to central obesity and risk of type 2 diabetes: Two independent cohort studies. Diabetes Care 38(7):1306–1311. 10.2337/dc14-3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, et al. (2014) Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ 348:g1610. 10.1136/bmj.g1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L (2011) Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial the preventing overweight using novel dietary strategies (POUNDS LOST) Trial. Circulation 124(5):563–571. 10.1161/CIRCULATIONAHA.111.025767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. (2015) Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes 39(9):1365–1370. 10.1038/ijo.2015.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Q-Y, Meng X-R, Hinney A, Song J-Y, Huang T, Ma J, et al. (2018) Waist-hip ratio related genetic loci are associated with risk of impaired fasting glucose in Chinese children: a case control study. Nutr Metab (Lond) 15:34. 10.1186/s12986-018-0270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschard H, Vilhjálmsson BJ, Joshi AD, Price AL, Kraft P (2015) Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet 96(2):329–339. 10.1016/j.ajhg.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rietman A, Schwarz J, Tomé D, Kok FJ, Mensink M (2014) High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 68(9):973–979. 10.1038/ejcn.2014.123 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Gan W, Gao C, Qi X, Liao X, Lin J, et al. (2018) The independent associations of protein consumption with body fat and glycaemic control in adult Chinese. Eur J Nutr 58(5):1981–1990. 10.1007/s00394-018-1751-9 [DOI] [PubMed] [Google Scholar]
- 28.Kitabchi AE, Mcdaniel KA, Wan JY, Tylavsky FA, Jacovino CA, Sands CW, et al. (2013) Effects of High-Protein Versus High-Carbohydrate Diets on Markers of β-Cell Function, Oxidative Stress, Lipid Peroxidation, Proinflammatory Cytokines, and Adipokines in Obese, Premenopausal Women Without Diabetes: a randomized controlled trial. Diabetes Care 36(7):1919–1925. 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sluijs I, Beulens JWJ, Van Der A DL, Annemieke MW Spijkerman DEG, Schouw Van Der YT (2010) Dietary Intake of Total, Animal, and Vegetable Protein and Risk of Type 2 Diabetes in the European Prospective. Diabetes Care 33(1):43–48. 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Sun C, Liu S, Li Y (2016) Dietary Protein Intake and Type 2 Diabetes among Women and Men in Northeast China. Sci Rep 6:37604. 10.1038/srep37604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwingshackl L, Hoffmann G (2013) Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors : a systematic review and meta-analysis. Nutr J 12:48. 10.1186/1475-2891-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Biggelaar LJCJ., Eussen SJPM, Sep SJS, Mari A, Ferrannini E, van Greevenbroek MM, et al. (2018) Prospective associations of dietary carbohydrate, fat, and protein intake with β-cell function in the CODAM study. Eur J Nutr 58(2):597–608. 10.1007/s00394-018-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halton TL, Liu S, Manson JE, Hu FB (2008) Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr 87:339–346. 10.3945/ajcn.110.004333.INTRODUCTION [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goni L, Qi L, Cuervo M, Milagro FI, Saris WH, MacDonald IA, et al. (2017) Effect of the interaction between diet composition and the PPM1K genetic variant on insulin resistance and β cell function markers during weight loss: results from the Nutrient Gene Interactions in Human Obesity: implications for dietary guidelines (NUGEN. Am J Clin Nutr 106:902–908. 10.3945/ajcn.117.156281 [DOI] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. (2002) Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med 346(6):393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock CL, Flatt SW, Pakiz B, Taylor KS, Leone AF, Brelje K, et al. (2014) Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: A randomized controlled trial. Diabetes Care 37(6):1573–1580. 10.2337/dc13-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27(6):1487–1495. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


