Introduction
The Moderna mRNA-1273 vaccine was authorized by the Food and Drug Administration for emergency use during the ongoing COVID-19 pandemic in December 2020.1 Out of 15,185 participants in the phase III Coronavirus Efficacy (COVE) trials who received at least one dose of the Moderna vaccine, 228 (1.5%) reported hypersensitivity adverse events, including injection site rash and urticaria.2 Although we have seen an eruptive, morbilliform rash that manifests 48 hours postvaccination that is similar to a drug rash (Fig 1), we report 4 cases of “COVID arm”: a localized erythematous rash surrounding the injection site that manifests days after the first dose of the Moderna COVID-19 vaccine.
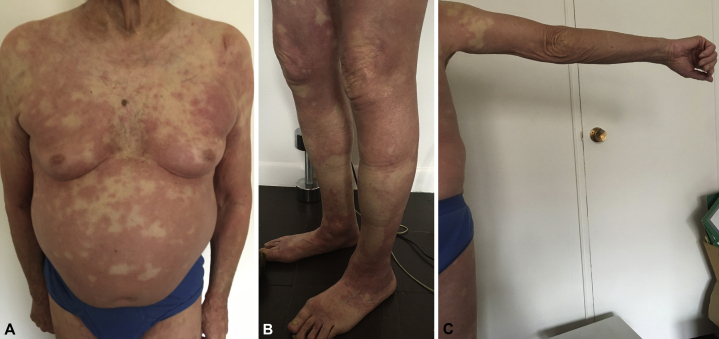
Fig 1.
Generalized erythrodermic reaction observed 48 hours after the first dose of the Moderna vaccine in an 82-year-old man. A, Torso, anterior view. B Bilateral lower extremity involvement. C Right upper extremity.
Case 1
A 74-year-old woman with no history of medical conditions or allergies developed a pruritic, erythematous plaque with mild scaling on her left upper arm 8 days after the first Moderna vaccination (Fig 2). There was no edema, calor, tenderness, or systemic symptoms. The rash spread to 15 cm in diameter over 10 days. After 1 week of treatment with topical clobetasol propionate 0.05% cream and oral cetirizine 10 mg, the rash decreased in rubor and size but did not completely resolve.
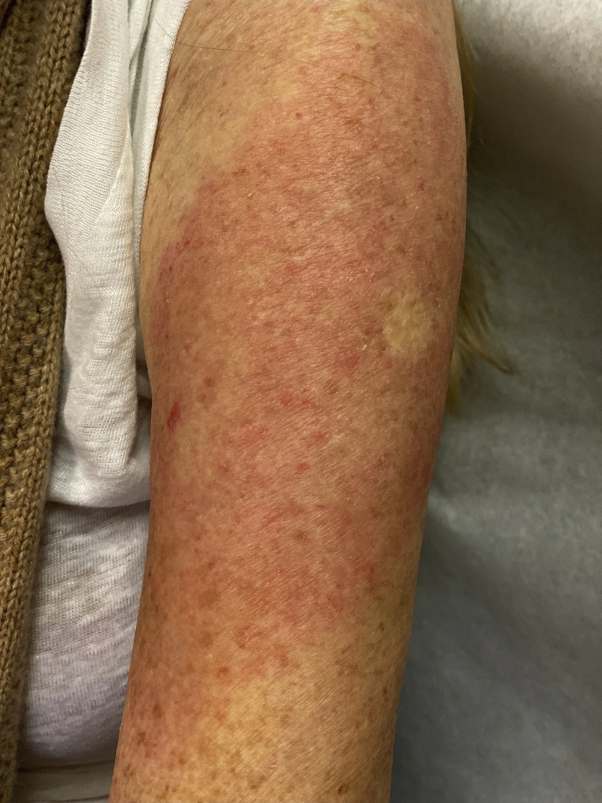
Fig 2.
Pruritic rash of the left upper arm observed 8 days after the first dose of the Moderna vaccine in a 74-year-old woman.
Case 2
A 62-year-old woman with no significant medical history presented with a pruritic erythematous rash on her left deltoid 8 days after receiving the Moderna vaccine (Fig 3). The rash began as a maculopapular eruption over the injection site and progressed in 3 days to an erythematous indurated plaque that was mildly edematous and warm to the touch. There was no associated pain, fevers, chills, or other systemic symptoms. The rash gradually receded after 3 days of treatment with mometasone furoate 0.01% ointment, diphenhydramine hydrochloride 1% cream, and oral loratadine 10 mg. The patient noted 2 recurrences lasting a few hours over the next 2 days that resolved with oral loratadine.
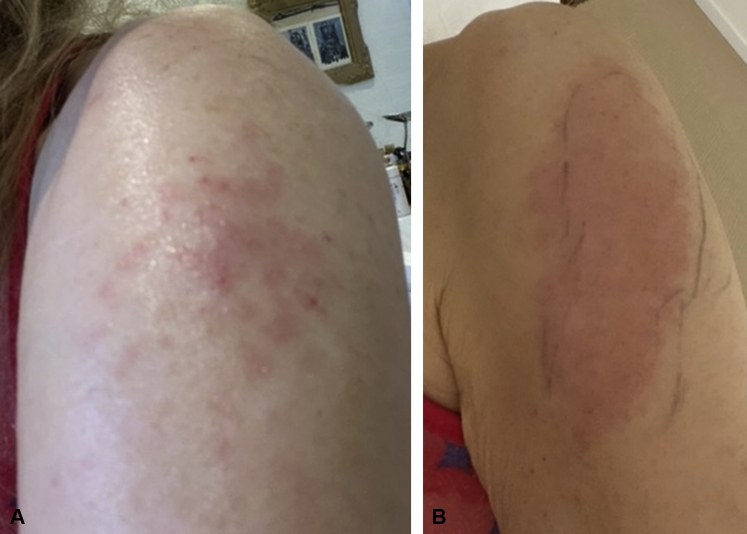
Fig 3.
Pruritic rash of the left deltoid observed 8 days after the first dose of the Moderna vaccine in a 62-year-old woman. A, Initial maculopapular appearance. B, 3 days later.
Case 3
A 54-year-old woman with no medical conditions presented with an erythematous, nonscaly patch on her left upper arm 7 days after the first dose of the Moderna vaccine (Fig 4). The rash was nonpruritic and nonpainful and lasted for 4 days before resolving spontaneously.
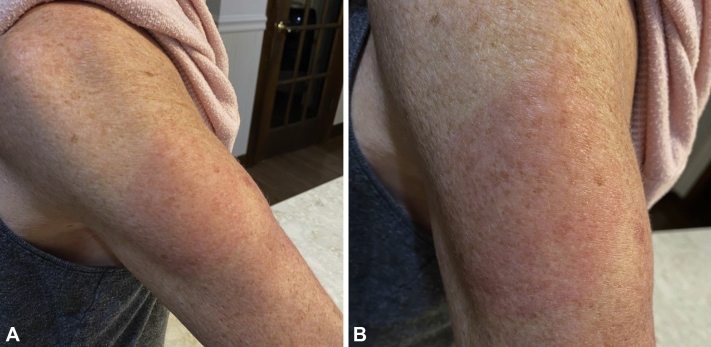
Fig 4.
Nonpruritic rash of the left upper arm observed 7 days after the first dose of the Moderna vaccine in a 54-year-old woman. A, Erythmatous patch at site of vaccination. B, Close up view.
Case 4
A 72-year-old woman with a history of psoriasis, atrial fibrillation, hypercholesterolemia, and hypothyroidism developed an erythematous patch on her left deltoid surrounding the injection site 10 days after the first dose of the Moderna vaccine. The rash was pruritic, warm to the touch, and measured 14 cm in diameter. After 2 days, the rash gradually decreased in size and color and resolved without intervention.
Discussion
Photographs and reports of the “COVID arm” phenomenon several days after the first Moderna vaccine have recently appeared in social and public media. Consistent with our findings, patients experience a transient, pruritic rash that can present either as an urticarial welt or a macular erythematous patch in close proximity to the vaccination site 7 to 10 days after the first dose.3 Two of our patients' rashes resolved without intervention, and the other 2 cleared with the use of topical steroids and/or oral antihistamines. There was no relation between development of the rash and patients' medical histories or allergies. Although the rash might be attributable to an immunologic response to the mRNA vaccine, it has not been reported as a reaction to other COVID-19 mRNA vaccines.4 Both the Pfizer and the Moderna vaccines include polyethylene glycol, a known allergen, as well as other proprietary nonactive ingredients.5, 6, 7 It is not known why this reaction has only been observed in recipients of the Moderna vaccine.
In a nationally representative survey of 1676 US adults conducted in December 2020 by the Kaiser Family Foundation, 27% stated they would not get a COVID-10 vaccine even if it were available for free and deemed safe by scientists.8 Among these, 59% cited worries about possible adverse events as a major reason for vaccine hesitancy. It is thus important for providers to reassure patients that “COVID arm” is a benign possible side effect that should not dissuade them from obtaining the Moderna vaccine.
Conclusions
“COVID arm” is an uncommon adverse effect that can present as a localized, transient erythematous rash several days following the first dose of the Moderna COVID-19 vaccine. Although most cases resolve spontaneously, topical steroids and oral histamines have proven to be successful in clearing the rash and controlling symptoms. Providers should inform patients that COVID arm is a benign possible side effect that should not deter them from obtaining a second dose of the Moderna vaccine.
Conflicts of interest
Dr Lebwohl is an employee of Mount Sinai Dermatology and receives research funds from Abbvie, Amgen, Eli Lilly, Janssen Research & Development, LLC, Novartis, Ortho Dermatologics, Regeneron-Sanofi, and UCB, Inc. He has been the principal investigator in numerous clinical trials but has no personal financial gain. Authors Wei, Fishman, Wattenberg, and Gordon have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical Trial Data Moderna COVID-19 Vaccine (EUA) https://www.modernatx.com/covid19vaccine-eua/providers/clinical-trial-data Available at:
- 3.Usatoday.com. 2021. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001 Available at:
- 4.Cdc.gov. 2021. Reactions and adverse events of the Pfizer-BioNTech COVID-19 vaccine | CDC. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html Available at:
- 5.Moderna COVID-19 vaccine EUA fact sheet for recipients and caregivers (n.d.) https://www.fda.gov/media/144638/download Available at:
- 6.Pfizer COVID-19 Vaccine EUA fact sheet for recipients and caregivers (n.d.) https://www.fda.gov/media/144414/download Available at:
- 7.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 8.KFF KFF COVID-19 vaccine monitor: December 2020. 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/ Available at: