Abstract
Background and objectives
An excessive inflammatory response in patients with coronavirus disease 2019 (COVID-19) is associated with high disease severity and mortality. Specific acute phase reactants might be useful for risk stratification. A systematic review and meta-analysis was conducted of studies on serum amyloid A (SAA) in patients with COVID-19.
Methods
The PubMed, Web of Science, and Scopus databases were searched, covering the period January 2020 to December 2020, for studies reporting SAA concentrations, COVID-19 severity, and survival status.
Results
Nineteen studies involving 5617 COVID-19 patients were included in the meta-analysis. Pooled results showed that SAA concentrations were significantly higher in patients with severe disease and non-survivors (standard mean difference (SMD) 1.20, 95% confidence interval 0.91–1.49, P < 0.001). Extreme between-study heterogeneity was observed (I2 = 92.4%, P < 0.001). In the sensitivity analysis, the effect size was not significantly affected when each study was removed in turn (range 1.10–1.29). The Begg test (P = 0.030), but not the Egger test (P = 0.385), revealed the presence of publication bias. Pooled SMD values were significantly and positively associated with sex (t = 2.20, P = 0.047) and aspartate aminotransferase (t = 3.44, P = 0.014).
Conclusions
SAA concentrations were significantly and positively associated with higher COVID-19 severity and mortality. This acute phase reactant might assist with risk stratification and monitoring in this group.
Keywords: Serum amyloid A, COVID-19, Disease severity, Mortality
Introduction
A state of excessive local and systemic inflammation and immune activation are strongly associated with oxidative stress, coagulation abnormalities, and multi-organ dysfunction in patients with coronavirus disease 2019 (COVID-19) (Fajgenbaum and June, 2020, Hojyo et al., 2020). While safe and effective vaccines have been developed and are currently being rolled out, effective therapies to mitigate the clinical manifestations of COVID-19, e.g., repurposed antiviral and immunosuppressant agents, remain limited (Siemieniuk et al., 2020). In this context, the use of biomarkers of disease severity and clinical progression would facilitate the early identification of patients requiring aggressive management and monitoring and assist with the judicious use of healthcare resources. Given the key pathophysiological role of inflammation and immunity in the clinical progress of COVID-19, markers that reflect the activation of these pathways might be particularly useful for risk stratification and effective management.
Serum amyloid A (SAA) genes and proteins are significantly activated during the acute phase response, which comprises a number of phenomena that occur in the presence of inflammation and infection, e.g., increased temperature and hormonal and metabolic alterations (Yoo and Desiderio, 2003). Circulating SAA concentrations, typically low under physiological circumstances (20–50 mg/l), can increase up to 1000-fold within the first 24–48 h of an acute phase response. This is the consequence of increased synthesis in the liver that is triggered by several stimuli, including tumour necrosis factor (TNF), interleukin (IL)-1β, IL-6, and interferon gamma (IFN-γ) (Morrow et al., 1981, Uhlar and Whitehead, 1999). SAA, in turn, can activate the complement system and the nucleotide-binding domain leucine-rich repeat-containing family pyrin-domain containing 3 (NLRP3) inflammasome, further increase the synthesis of TNF, IL-1β, and IL-6, and activate other proinflammatory cytokines such as IL-1α and IL-23 (Ather et al., 2011, De Buck et al., 2016, Yuste et al., 2007). Notably, these mediators have been shown to contribute significantly to the onset of the cytokine storm and its adverse clinical consequences in COVID-19 (Fajgenbaum and June, 2020). Therefore, it is plausible that the acute increase in SAA concentrations in patients with COVID-19 might not only reflect the presence of an acute phase response, but also herald the development of a cytokine storm and, consequently, multi-organ failure and an increased risk of adverse outcomes.
Two systematic reviews and meta-analyses on a relatively limited number of studies, three and five, respectively, have reported a significant and positive association between SAA concentrations and COVID-19 severity (Akbari et al., 2020, Zeng et al., 2020a). Following the publication of several additional studies, an updated systematic review and meta-analysis was conducted of the available evidence on the clinical implications of SAA concentrations in patients with COVID-19.
Materials and methods
Search strategy, eligibility criteria, and study selection
A systematic search was conducted using the terms “serum amyloid A” OR “SAA” AND “coronavirus disease 19” OR “COVID-19”, in the electronic databases PubMed, Web of Science, and Scopus, covering the period January 2020 to December 2020, to identify studies investigating SAA concentrations in COVID-19 patients according to disease severity or survival status. The references of the retrieved articles were also searched to identify additional studies. The following inclusion criteria were applied: (1) studies reporting continuous data on SAA concentrations in COVID-19 patients, (2) articles investigating COVID-19 patients with different disease severity or survival status, (3) adult patients, (4) ≥10 participants, (5) English language, and (6) full text available. Two investigators independently screened the abstracts. If relevant, the two investigators independently reviewed the full articles. The Newcastle–Ottawa scale was used to assess the quality of each study, with a score ≥6 indicating high quality (Wells et al., 2013).
Statistical analysis
Standardized mean differences (SMD) were calculated to build forest plots of continuous data and to evaluate differences in SAA concentrations between COVID-19 patients with low versus high disease severity or survivors versus non-survivors during follow-up. A P-value of less than 0.05 was considered statistically significant, and 95% confidence intervals (CI) were reported. When studies reported concentrations as the median and interquartile range (IQR), the mean and standard deviation were estimated as described previously (Wan et al., 2014). The Q-statistic was used to test the heterogeneity of the SMD across studies (the significance level was set at P < 0.10). Inconsistency across studies was evaluated using the I 2 statistic where I 2 < 25% indicated no heterogeneity, I 2 between 25% and 50% moderate heterogeneity, I 2 between 50% and 75% large heterogeneity, and I 2 > 75% extreme heterogeneity (Bowden et al., 2011, Higgins and Thompson, 2002). A random-effects model was used to calculate the pooled SMD and the corresponding 95% CI in the presence of significant heterogeneity. Sensitivity analyses were conducted to assess the influence of individual studies on the overall effect size using the leave-one-out method (Tobias, 1999). The presence of publication bias was assessed using the Begg adjusted rank correlation test and the Egger regression asymmetry test at the P < 0.05 level of significance (Begg and Mazumdar, 1994, Sterne and Egger, 2001). The Duval and Tweedie ‘trim-and-fill’ procedure was also used to investigate the effect of publication bias. This method recalculates a pooled SMD by incorporating the hypothetical missing studies as though they actually existed, to augment the observed data so that the funnel plot is more symmetric (Duval and Tweedie, 2000). The statistical analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA). The study was fully compliant with the PRISMA statement regarding the reporting of systematic reviews and meta-analyses (Liberati et al., 2009).
Results
Literature search and study selection
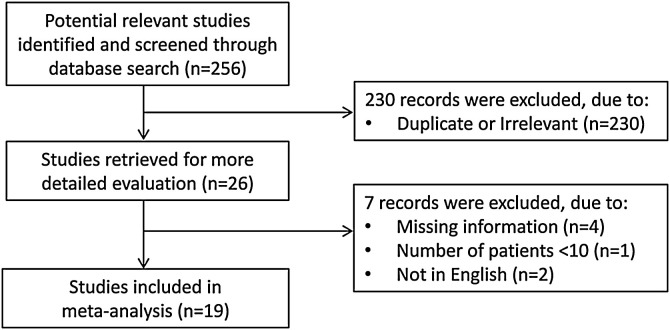
A flow chart describing the screening process is presented in Figure 1. A total of 256 studies were initially identified. Of these, 230 studies were excluded because they were either duplicates or irrelevant. After full-text review of the remaining 26 articles, seven were excluded because they did not meet the inclusion criteria. Thus, 19 studies, all conducted in China, were included in the meta-analysis (Table 1 ) (Chen et al., 2020a, Chen et al., 2020b, Cheng et al., 2020, Dong et al., 2020, Fu et al., 2020, Li et al., 2020, Li and Chen, 2020, Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Mo et al., 2020, Wang et al., 2020, Xu et al., 2020, Yang et al., 2020, Yu et al., 2020, Zeng et al., 2020b, Zhang et al., 2020a, Zhang et al., 2020b, Zhao et al., 2020).
Figure 1.
Flow chart of study selection.
Table 1.
Summary of the studies included in the meta-analysis.
| First author | Study design | Endpoint | NOS (stars) | Mild disease and survivors |
Severe disease and non-survivors |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, years (Mean) | Sex, M/F | SAA, mg/l (Mean ± SD) | n | Age, years (Mean) | Sex, M/F | SAA, mg/l (Mean ± SD) | ||||
| Chen et al. (2020a) | NR | ARDS | 6 | 47 | 42 | 24/23 | 110 ± 86 | 24 | 57 | 20/4 | 170 ± 67 |
| Non-ARDS | |||||||||||
| Chen et al. (2020b) | R | Survivor | 7 | 445 | 64 | 244/201 | 162 ± 140 | 103 | 65 | 69/34 | 201 ± 62 |
| Non-survivor | |||||||||||
| Cheng et al. (2020) | R | Survivor | 6 | 53 | 54 | 29/24 | 162 ± 83 | 36 | 69 | 20/16 | 132 ± 101 |
| Non-survivor | |||||||||||
| Dong et al. (2020) | R | Severe | 7 | 94 | 40 | 34/60 | 144 ± 240 | 53 | 60 | 29/24 | 554 ± 332 |
| Non-severe | |||||||||||
| Fu et al. (2020) | R | Severe | 7 | 22 | 41 | 11/11 | 90 ± 55 | 13 | 60 | 2/11 | 144 ± 57 |
| Non-severe | |||||||||||
| Li et al. (2020) | R | Severe | 7 | 60 | 57 | 28/32 | 124 ± 76 | 72 | 66 | 47/25 | 174 ± 53 |
| Non-severe | |||||||||||
| Li and Chen (2020) | P | Severe | 5 | 60 | 52 | NR | 130 ± 46 | 12 | 45 | NR | 261 ± 43 |
| Non-severe | |||||||||||
| Liu et al. (2020a) | R | Severe | 7 | 27 | 43 | 8/19 | 67 ± 84 | 13 | 60 | 7/6 | 558 ± 225 |
| Non-severe | |||||||||||
| Liu et al. (2020b) | R | Severe | 6 | 59 | 49 | 31/28 | 17 ± 16 | 25 | 52 | 14/11 | 41 ± 24 |
| Non-severe | |||||||||||
| Liu et al. (2020c) | R | Severe | 6 | 194 | 43 | 103/91 | 8 ± 14 | 31 | 64 | 14/17 | 26 ± 38 |
| Non-severe | |||||||||||
| Mo et al. (2020) | NR | Severe | 5 | 102 | NR | NR | 40 ± 53 | 16 | NR | NR | 198 ± 55 |
| Non-severe | |||||||||||
| Wang et al. (2020) | R | Severe | 6 | 72 | 44 | 29/43 | 65 ± 94 | 71 | 65 | 44/27 | 561 ± 583 |
| Non-severe | |||||||||||
| Xu et al. (2020) | R | Severe | 7 | 80 | 56 | 30/50 | 99 ± 141 | 107 | 66 | 73/34 | 271 ± 64 |
| Non-severe | |||||||||||
| Yang et al. (2020) | R | Severe | 6 | 109 | NR | NR | 48 ± 33 | 11 | NR | NR | 155 ± 153 |
| Non-severe | |||||||||||
| Yu et al. (2020) | R | Severe | 7 | 2115 | NR | NR | 40 ± 71 | 1150 | NR | NR | 120 ± 92 |
| Non-severe | |||||||||||
| Zeng et al. (2020b) | R | Severe | 6 | 36 | 54 | 20/16 | 114 ± 174 | 41 | 62 | 28/13 | 127 ± 171 |
| Non-severe | |||||||||||
| Zhang et al. (2020a) | NR | Severe | 6 | 82 | 52 | 38/44 | 93 ± 102 | 58 | 64 | 33/25 | 108 ± 80 |
| Non-severe | |||||||||||
| Zhang et al. (2020b) | R | Severe | 7 | 47 | 61 | 18/29 | 24 ± 36 | 27 | 72 | 18/9 | 109 ± 86 |
| Non-severe | |||||||||||
| Zhao et al. (2020) | R | Severe | 6 | 19 | 49 | 7/12 | 68 ± 70 | 31 | 60 | 23/8 | 808 ± 578 |
| Non-severe | |||||||||||
ARDS, acute respiratory distress syndrome; F, female; M, male; Non-severe, patients with mild or moderate disease; NOS, Newcastle–Ottawa quality assessment scale for case–control studies; NR, not reported; P, prospective; R, retrospective; SAA, serum amyloid A; SD, standard deviation; Severe, patients with severe or critical disease.
A total of 5617 COVID-19 patients were studied, 3723 (49% male, mean age 53 years) with low disease severity or alive during follow-up and 1894 (63% male, mean age 64 years) with high severity or not surviving during follow-up. Fifteen studies were retrospective (Chen et al., 2020b, Cheng et al., 2020, Dong et al., 2020, Fu et al., 2020, Li et al., 2020, Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Wang et al., 2020, Xu et al., 2020, Yang et al., 2020, Yu et al., 2020, Zeng et al., 2020b, Zhang et al., 2020b, Zhao et al., 2020) and one was prospective (Li and Chen, 2020); no information was available for the remaining three (Chen et al., 2020a, Mo et al., 2020, Zhang et al., 2020a). Endpoints included disease severity based on current clinical guidelines (16 studies) (Dong et al., 2020, Fu et al., 2020, Li et al., 2020, Li and Chen, 2020, Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Mo et al., 2020, Wang et al., 2020, Xu et al., 2020, Yang et al., 2020, Yu et al., 2020, Zeng et al., 2020b, Zhang et al., 2020a, Zhang et al., 2020b, Zhao et al., 2020), the occurrence of acute respiratory distress syndrome (ARDS, one study) (Chen et al., 2020a), and survival status (two studies) (Chen et al., 2020b, Cheng et al., 2020).
Meta-analysis
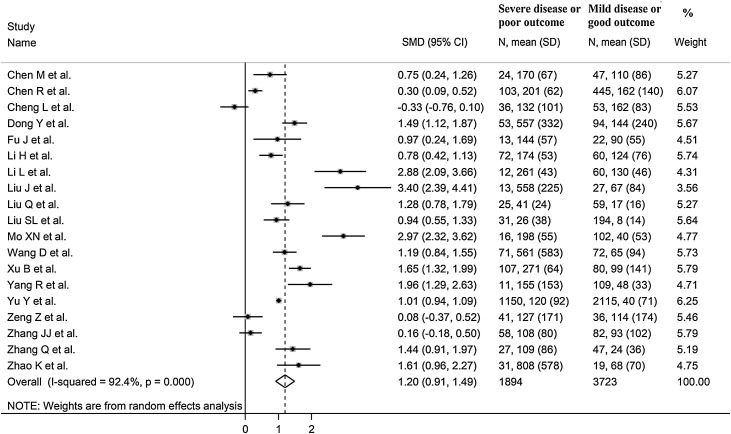
The overall SMD in SAA concentrations between COVID-19 patients with low versus high severity or survivors versus non-survivors is shown in Figure 2 . In 18 studies, patients with high severity or non-surviving status had higher SAA concentrations when compared to those with low severity or alive during follow-up (mean difference range 0.08–3.40) (Chen et al., 2020a, Chen et al., 2020b, Dong et al., 2020, Fu et al., 2020, Li et al., 2020, Li and Chen, 2020, Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Mo et al., 2020, Wang et al., 2020, Xu et al., 2020, Yang et al., 2020, Yu et al., 2020, Zeng et al., 2020b, Zhang et al., 2020a, Zhang et al., 2020b, Zhao et al., 2020), although the difference was non statistically significant in two studies (Zeng et al., 2020b, Zhang et al., 2020a). By contrast, in the remaining study, SAA concentrations were slightly higher in patients with low severity or alive during follow up (mean difference −0.33) (Cheng et al., 2020). The pooled results confirmed that SAA concentrations were significantly higher in patients with high severity or non-surviving status (SMD 1.20, 95% CI 0.91–1.49, P < 0.001) (Figure 2). Extreme heterogeneity between studies was observed (I 2 = 92.4%, P < 0.001). SAA concentrations remained significantly higher (SMD 1.25, 95% CI 0.87–1.63, P = 0.001; I 2 = 92.7%, P < 0.001) in patients with high severity or non-survival status after excluding the large study by Yu et al. (Yu et al., 2020), which accounted for nearly 58% of the overall sample size.
Figure 2.
Forest plot of studies examining serum amyloid A concentrations in patients with COVID-19.
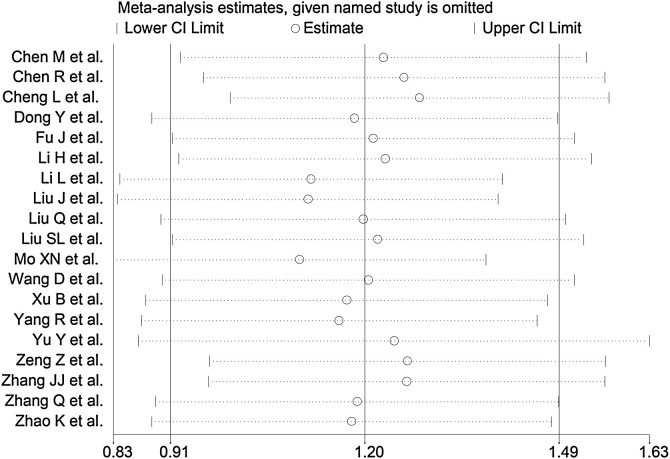
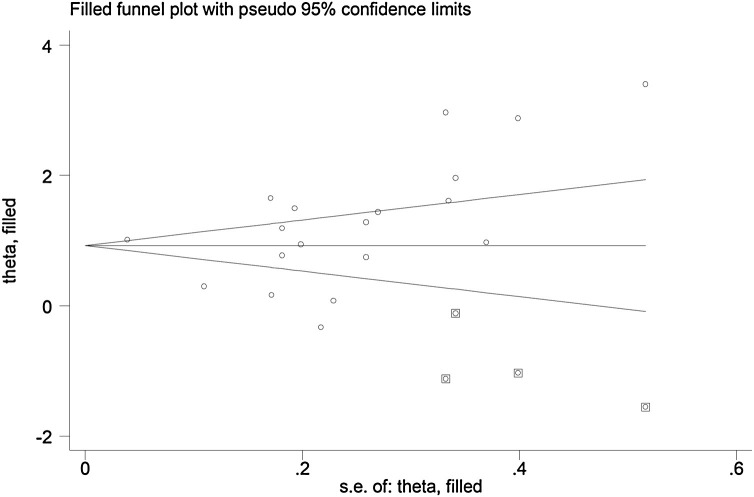
The sensitivity analysis, performed by removing each study in turn and re-assessing the pooled estimates, showed that the magnitude and direction of the effect size were not affected (effect size ranged between 1.10 and 1.29) (Figure 3). The Begg test ( P = 0.03), but not the Egger test (P = 0.385), revealed the presence of publication bias. Accordingly, the trim-and-fill method identified four potential missing studies to add on the left side of the funnel plot to ensure symmetry (Figure 4 ). The adjusted SMD was attenuated but remained significant (SMD 0.89, 95% CI 0.59–1.20, P = 0.001).
Figure 3.
Sensitivity analysis of the association between serum amyloid A concentrations and COVID-19 disease. The influence of individual studies on the overall standardized mean difference (SMD) is shown. The middle vertical axis indicates the overall SMD and the two vertical axes indicate the 95% confidence interval (CI). The hollow circles represent the pooled SMD when the remaining study is omitted from the meta-analysis. The two ends of each broken line represent the 95% CI.
Figure 4.
Funnel plot of studies investigating low versus high severity or survivor versus non-survivor status after trimming and filling. Dummy studies are represented by enclosed circles and genuine studies by free circles.
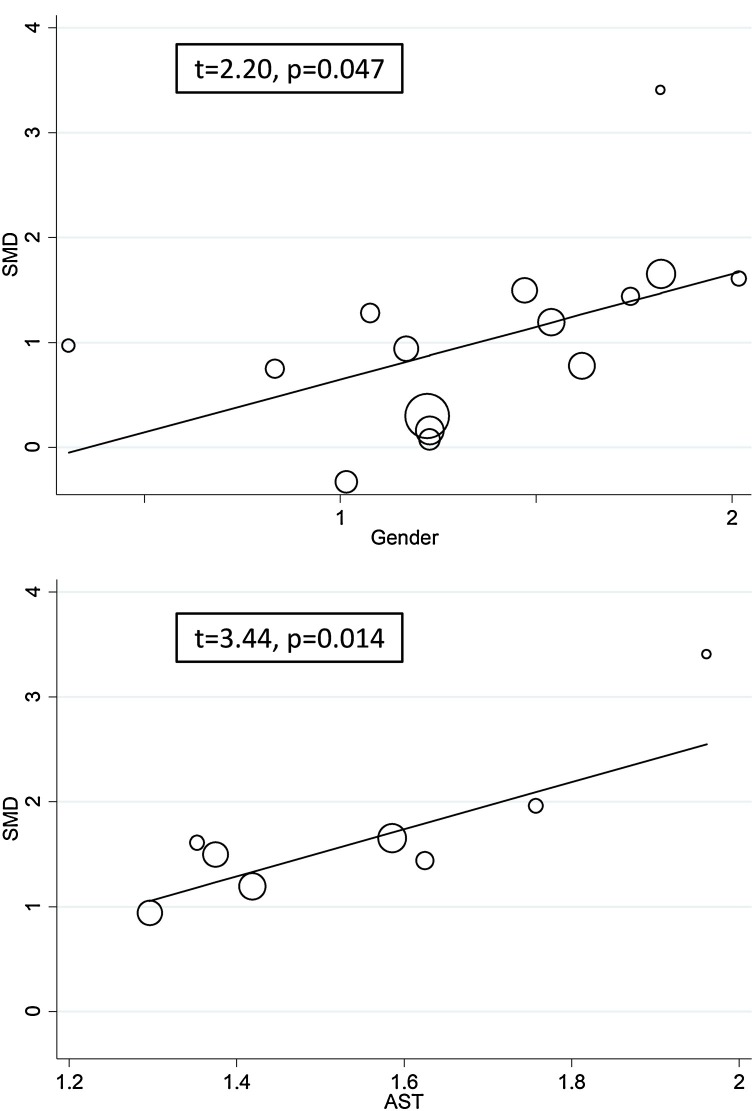
To explore possible contributors to the between-study variance, the effects on the SMD of age, sex, end point studied, study design (retrospective or prospective), aspartate aminotransferase (AST), alanine aminotransferase (ALT), d-dimer (DD), serum creatinine (Cr), prothrombin time (PT), and the inflammation biomarkers C-reactive protein (CRP) and white blood cell (WBC) count were investigated by univariate meta-regression analysis. Both sex (t = 2.20, P = 0.047) and AST values (t = 3.44, P = 0.014) were significantly and positively associated with the pooled SMD (Figure 5 ). By contrast, no significant correlations were observed between SMD and age (t = 0.58, P = 0.57), end point (t = −1.73, P = 0.10), study design (t = 0.87, P = 0.40), ALT (t = −0.15, P = 0.88), DD (t = −0.45, P = 0.47), Cr (t = −0.31, P = 0.76), PT (t = 0.46, P = 0.67), CRP (t = 0.86, P = 0.40), or WBC count (t = −0.53, P = 0.61).
Figure 5.
Univariate meta-regression analysis between sex and effect size, and between aspartate aminotransferase and effect size.
Subgroup analysis showed that, in the 16 studies in which patients were characterized according to disease severity, the SMD was significantly higher in severe versus mild disease patients (SMD 1.39, 95% CI 1.09–1.69, P = 0.001), albeit with extreme between-study variance (I 2 = 90.3%, P < 0.001). Similar results were obtained when considering the 15 retrospective studies (SMD 1.10, 95% CI 0.81–1.39, P = 0.001), again in the presence of extreme heterogeneity (I 2 = 90.9%; P < 0.001).
Discussion
In this systematic review and meta-analysis, SAA concentrations were significantly higher in COVID-19 patients with more severe disease, assessed on clinical grounds or based on the presence of ARDS, and in those who did not survive during follow-up when compared to patients with milder forms of the disease or those who survived during follow-up. The observed SMD of 1.20 suggests an effect size that is both biologically and clinically relevant (Cohen, 1988). There was extreme between-study heterogeneity, however in the sensitivity analysis the overall effect size was not significantly influenced when individual studies were removed in turn. The analyses based on the Begg test, but not the Egger test, revealed the presence of publication bias. Although the trim-and-fill method identified four potential missing studies to add on the left side of the funnel plot to ensure symmetry, the adjusted SMD was attenuated but remained significant. The SMD was significantly and positively associated with sex and AST, but not with age, end point studied (disease severity, ARDS, or survival status), study design (retrospective or prospective), ALT, DD, Cr, PT, CRP, and WBC count.
Two previously published systematic reviews and meta-analyses identified a relatively low number of studies investigating SSA in COVID-19 patients. The first, including three studies, reported a weighted mean difference (WMD) between severe and non-severe patients of 43.35 (95% CI 5.85–80.85, P = 0.020; I 2 = 66.7%, P = 0.050) (Zeng et al., 2020a). The second, including five studies, reported a WMD of 90.45 (95% CI 28.69–152.21; I 2 = 90.6%, P < 0.0001) between severe and non-severe patients (Akbari et al., 2020). In both meta-analyses, the small number of studies prevented the use of a meta-regression analysis to identify clinical and demographic factors potentially accounting for the between-study variance. It is important to highlight that, in contrast with these meta-analyses, we evaluated the effect size by using the SMD instead of the WMD. As highlighted previously by other authors, the SMD is particularly appropriate when different studies investigate separate end points, in our case disease severity and mortality (Faraone, 2008).
Increases in SAA concentrations can be useful to diagnose inflammatory processes and monitor the response to therapeutic interventions (Zhang et al., 2019). The magnitude and the speed of the increase in SAA concentrations during the acute phase response is greater than that observed with other inflammatory markers, particularly CRP. Moreover, SAA concentrations generally return to baseline levels more quickly than CRP due to the shorter half-life of the former. These characteristics suggest a specific pathophysiological role of SAA, which might complement that provided by other biomarkers, e.g., CRP, in clinical practice (Maury, 1985, Nakayama et al., 1993, Takata et al., 2011). This proposition is further confirmed by the lack of significant associations between the SMD and CRP concentrations in the meta-regression, indicating the potential complementary role of SAA and CRP in informing clinical decisions.
An increase in SAA concentrations has been observed in several proinflammatory conditions, including infections, particularly viral (Kajiya et al., 2008), liver disease (Siegmund et al., 2016), autoimmune disease (O’Hara et al., 2000), diabetes (Kumon et al., 1994), obesity (O’Brien et al., 2005), atherosclerotic cardiovascular disease (King et al., 2011), amyloidosis (Real de Asua et al., 2014), and cancer (Biran et al., 1986). Notably, most of these conditions, particularly obesity, diabetes, cardiovascular disease, liver disease, and cancer, have also been independently associated with significantly worse outcomes in patients with COVID-19 (Zhou et al., 2020).
In addition to its potential role in the pathogenesis of the cytokine storm, it has been reported recently that SAA might also exert pro-coagulant effects that are mediated by an increase in fibrinogen and a concomitant platelet activation to generate a prothrombotic state (Page et al., 2019). Therefore, pending further research, acute increases in SAA concentrations might represent an important factor linking proinflammatory and prothrombotic pathways. The interplay between inflammation and thrombosis has also been observed in COVID-19, a condition that is often characterized by significant alterations in coagulation and a prothrombotic state, particularly in patients with the more severe form of the disease (Al-Samkari et al., 2020).
The extreme between-study heterogeneity and the presence of publication bias represent potential limitations of this study. However, the overall effect size was not significantly influenced in the sensitivity analyses. The lack of significant associations between clinical and demographic characteristics and the SMD (with the exception of sex and AST) and the persistently high heterogeneity observed in subgroup analyses suggest that other unreported factors might contribute to the heterogeneity. Few studies have investigated the association between SAA concentrations and sex. No significant differences between males and females have been reported in healthy populations (Carbone et al., 2020). In another study, the percentage fat mass and the waist-to-hip ratio explained the highest percentage of the variability in SAA concentrations in females and males, respectively (Thorand et al., 2006). While no studies have specifically investigated the associations between SAA concentrations and AST, this relationship might be explained, at least in part, by the involvement of this acute phase reactant in several conditions associated with liver injury (Yuan et al., 2019).
Potential unreported methodological factors contributing to the observed between-study heterogeneity include the use of different SAA detection methods based on immuno-based assays, different antibodies against various SAA components, and different calibrators in individual studies (Zhang et al., 2019). While some of these methodological inconsistencies might have been mitigated by the fact that all of the identified studies were conducted in the same country, this issue should be addressed when planning future multicentre studies on the clinical use of SAA in patients with COVID-19 or other disease states.
Another limitation is represented by the paucity of data in the selected studies on the dynamic changes in SAA concentrations in hospitalized COVID-19 patients, and their associations with disease severity and/or mortality. Notably, in the five studies that addressed this issue, serial SAA concentrations remained high, or increased during hospitalization, in COVID-19 patients who experienced adverse clinical outcomes (Chen et al., 2020b, Cheng et al., 2020, Fu et al., 2020, Liu et al., 2020c, Yu et al., 2020). However, the different time-points for SAA measurement used in individual studies precluded their meta-analysis. Further research is warranted to better characterize and justify the routine clinical use of serial SAA assessments in COVID-19.
In conclusion, this updated systematic review and meta-analysis has shown that the presence of relatively high SAA concentrations is significantly associated with more severe disease, based on clinical assessment or the presence of ARDS, and an increased risk of mortality in patients with COVID-19. The measurement of this acute phase reactant, alone or in combination with other clinical and demographic parameters, might be useful for risk stratification and clinical monitoring in this group.
Ethical approval
This study did not require ethical approval as it was a systematic review and meta-analysis of published studies.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interest.
References
- Akbari H., Tabrizi R., Lankarani K.B., Aria H., Vakili S., Asadian F., et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather J.L., Ckless K., Martin R., Foley K.L., Suratt B.T., Boyson J.E., et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187(1):64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- Biran H., Friedman N., Neumann L., Pras M., Shainkin-Kestenbaum R. Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol. 1986;39(7):794–797. doi: 10.1136/jcp.39.7.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone T., Pafundi V., Schievano C., Assunta D., Padula M.C., Giordano M., et al. Serum amyloid A in healthy subjects: assessment of reference value using ELISA method. J Immunoassay Immunochem. 2020:1–9. doi: 10.1080/15321819.2020.1837160. [DOI] [PubMed] [Google Scholar]
- Chen M., Wu Y., Jia W., Yin M., Hu Z., Wang R., et al. The predictive value of serum amyloid A and C-reactive protein levels for the severity of coronavirus disease 2019. Am J Transl Res. 2020;12(8):4569–4575. [PMC free article] [PubMed] [Google Scholar]
- Chen R., Sang L., Jiang M., Yang Z., Jia N., Fu W., et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Yang J.Z., Bai W.H., Li Z.Y., Sun L.F., Yan J.J., et al. Prognostic value of serum amyloid A in patients with COVID-19. Infection. 2020;48(5):715–722. doi: 10.1007/s15010-020-01468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Erlbaum; Hillsdale, NJ, USA: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- De Buck M., Gouwy M., Wang J.M., Van Snick J., Proost P., Struyf S., et al. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016;30:55–69. doi: 10.1016/j.cytogfr.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Zhou H., Li M., Zhang Z., Guo W., Yu T., et al. A novel simple scoring model for predicting severity of patients with SARS-CoV-2 infection. Transbound Emerg Dis. 2020;67(6):2823–2829. doi: 10.1111/tbed.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- Fu J., Huang P.P., Zhang S., Yao Q.D., Han R., Liu H.F., et al. The value of serum amyloid A for predicting the severity and recovery of COVID-19. Exp Ther Med. 2020;20(4):3571–3577. doi: 10.3892/etm.2020.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya T., Orihara K., Hamasaki S., Oba R., Hirai H., Nagata K., et al. Toll-like receptor 2 expression level on monocytes in patients with viral infections: monitoring infection severity. J Infect. 2008;57(3):249–259. doi: 10.1016/j.jinf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- King V.L., Thompson J., Tannock L.R. Serum amyloid A in atherosclerosis. Curr Opin Lipidol. 2011;22(4):302–307. doi: 10.1097/MOL.0b013e3283488c39. [DOI] [PubMed] [Google Scholar]
- Kumon Y., Suehiro T., Itahara T., Ikeda Y., Hashimoto K. Serum amyloid A protein in patients with non-insulin-dependent diabetes mellitus. Clin Biochem. 1994;27(6):469–473. doi: 10.1016/0009-9120(94)00044-v. [DOI] [PubMed] [Google Scholar]
- Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., et al. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chen C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19) Epidemiol Infect. 2020;148:e164. doi: 10.1017/S095026882000165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Dai Y., Feng M., Wang X., Liang W., Yang F. Associations between serum amyloid A, interleukin-6, and COVID-19: a cross-sectional study. J Clin Lab Anal. 2020;34(10) doi: 10.1002/jcla.23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.L., Wang S.Y., Sun Y.F., Jia Q.Y., Yang C.L., Cai P.J., et al. Expressions of SAA, CRP, and FERR in different severities of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(21):11386–11394. doi: 10.26355/eurrev_202011_23631. [DOI] [PubMed] [Google Scholar]
- Maury C.P. Comparative study of serum amyloid A protein and C-reactive protein in disease. Clin Sci (Lond) 1985;68(2):233–238. doi: 10.1042/cs0680233. [DOI] [PubMed] [Google Scholar]
- Mo X.N., Su Z.Q., Lei C.L., Chen D.F., Peng H., Chen R.C., et al. Serum amyloid A is a predictor for prognosis of COVID-19. Respirology. 2020;25(7):764–765. doi: 10.1111/resp.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J.F., Stearman R.S., Peltzman C.G., Potter D.A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Sonoda S., Urano T., Yamada T., Okada M. Monitoring both serum amyloid protein A and C-reactive protein as inflammatory markers in infectious diseases. Clin Chem. 1993;39(2):293–297. [PubMed] [Google Scholar]
- O’Brien K.D., Brehm B.J., Seeley R.J., Bean J., Wener M.H., Daniels S., et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005;90(4):2244–2249. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- O’Hara R., Murphy E.P., Whitehead A.S., FitzGerald O., Bresnihan B. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res. 2000;2(2):142–144. doi: 10.1186/ar78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., Thomson G.J.A., Nunes J.M., Engelbrecht A.M., Nell T.A., de Villiers W.J.S., et al. Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-39056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real de Asua D., Costa R., Galvan J.M., Filigheddu M.T., Trujillo D., Cadinanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol. 2014;6:369–377. doi: 10.2147/CLEP.S39981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S.V., Schlosser M., Schildberg F.A., Seki E., De Minicis S., Uchinami H., et al. Serum amyloid a induces inflammation, proliferation and cell death in activated hepatic stellate cells. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Takata S., Wada H., Tamura M., Koide T., Higaki M., Mikura S.I., et al. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community-acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers. 2011;16(6):530–535. doi: 10.3109/1354750X.2011.607189. [DOI] [PubMed] [Google Scholar]
- Thorand B., Baumert J., Doring A., Herder C., Kolb H., Rathmann W., et al. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;47:15–17. [Google Scholar]
- Uhlar C.M., Whitehead A.S. The kinetics and magnitude of the synergistic activation of the serum amyloid A promoter by IL-1 beta and IL-6 is determined by the order of cytokine addition. Scand J Immunol. 1999;49(4):399–404. doi: 10.1046/j.1365-3083.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Li R., Wang J., Jiang Q., Gao C., Yang J., et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. 2020;20(1):519. doi: 10.1186/s12879-020-05242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., et al. 2013. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from: [Google Scholar]
- Xu B., Fan C.Y., Wang A.L., Zou Y.L., Yu YH He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan. China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Gui X., Gao S., Ke H., Xiong Y. Clinical progression and changes of chest CT findings among asymptomatic and pre-symptomatic patients with SARS-CoV-2 infection in Wuhan, China. Expert Rev Respir Med. 2020:1–7. doi: 10.1080/17476348.2021.1840358. [DOI] [PubMed] [Google Scholar]
- Yoo J.Y., Desiderio S. Innate and acquired immunity intersect in a global view of the acute-phase response. Proc Natl Acad Sci U S A. 2003;100(3):1157–1162. doi: 10.1073/pnas.0336385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Liu T., Shao L., Li X., He C.K., Jamal M., et al. Novel biomarkers for the prediction of COVID-19 progression a retrospective, multi-center cohort study. Virulence. 2020;11(1):1569–1581. doi: 10.1080/21505594.2020.1840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.Y., Zhang X.X., Wu Y.J., Zeng Z.P., She W.M., Chen S.Y., et al. Serum amyloid A levels in patients with liver diseases. World J Gastroenterol. 2019;25(43):6440–6450. doi: 10.3748/wjg.v25.i43.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste J., Botto M., Bottoms S.E., Brown J.S. Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog. 2007;3(9):1208–1219. doi: 10.1371/journal.ppat.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Hong X.Y., Li Y., Chen W., Ye G., Li Y., et al. Serum-soluble ST2 as a novel biomarker reflecting inflammatory status and illness severity in patients with COVID-19. Biomark Med. 2020;14(17):1619–1629. doi: 10.2217/bmm-2020-0410. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wei Y., Chen M., Wan Q., Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabetes Complicat. 2020;34(10) doi: 10.1016/j.jdiacomp.2020.107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Sheng H., Li H., Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv Clin Chem. 2019;90:25–80. doi: 10.1016/bs.acc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7) doi: 10.1093/ofid/ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L., et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]