Dear Editor,
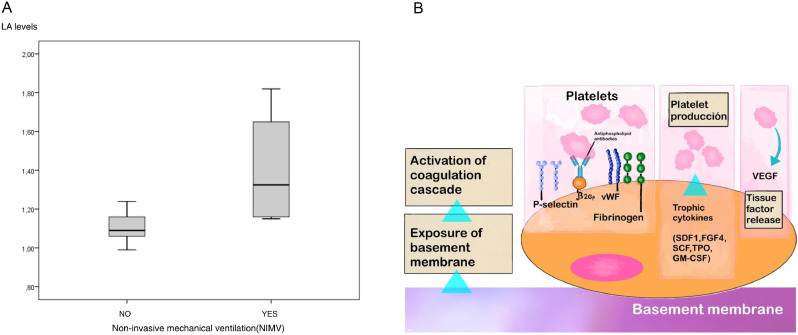
We read with interest the excellent letter of Falcinelli et at.1 entitled “Role of endothelial dysfunction in the thrombotic complications of COVID-19 patients” recently published in the Journal. By the way of having being cited in this letter,2 we would like to present some practical considerations based on our experience in a small series of patients with severe SARS-CoV-2 pneumonia. Currently, there is a better understanding of the pathophysiological mechanisms underlying disseminated microthrombosis at the endothelial level, reactive chronic endotheliitis,3 release of von Willebrand (vWF) multimers4 and the coagulopathy as a hallmark of severe COVID-19, mainly characterized by a pro-coagulative state with high D-dimer and thrombin levels. Measurement of increasing D-dimer and thrombin levels have prognostic value since all genetic variants of SARS-CoV-2 show a high thrombogenic potential, particularly in patients of advanced age. In our limited group of 35 patients with severe SARS-CoV-2 pneumonia, 20 men with a mean age of 76.7 ± 12.8 years, the levels of activity of vWF were randomly determined in 17 patients, and in all of which were above 200% (upper limit of normal 150%). This is in agreement with the results reported by Falcinelli et al.1 In addition, lupus anticoagulant (LA) testing was performed, with significantly higher values in patients requiring non-invasive mechanical ventilation (NIMV) as compared to those requiring conventional oxygen therapy only (1.41 ± 0.32 vs. 1.1 ± 0.07 IU; P = 0.024) (Fig. 1a ). In 8 patients, LA values were higher than 1.1 IU and 4 of them needed NIMV, whereas none of the patients with LA levels < 1.1 IU required ventilatory support.
Fig. 1.
(a) Box-plot of serum levels of lupus anticoagulant (LA) according to the need of non-invasive mechanical ventilation. (b) Role of lupus anticoagulant (LA) and von Willebrand factor (vWF) in thrombogenesis associated with SARS-CoV-2 infection (modified from Teuwen et al., reference #4).
These data indicate that in addition to vWF and other molecules, such as fibrinogen, thrombin, and beta-2 glycoprotein I (beta-2 GPI), LA plays a crucial role in thrombogenesis induced by SARS-CoV-2 infection. Beta-2 GPI promotes LA activity, with platelet adhesion, aggregation, release of tissue factor and activation of the coagulation cascade (Fig. 1b), leading to a hypercoagulable state, which may be frequently irreversible in advanced stages.5 On the other hand, the cytokine storm can amplify platelets’ thrombotic response by increasing platelet production and development of disseminated microthrombi in different territories of the vasculature, with direct involvement in the pathogenesis of thrombosis in COVID-19. Another consequence is that high LA levels are directly related to a higher risk for prothrombotic complications, progression to the need of mechanical ventilation, and poor clinical outcome. The combination of both parameters, vWF and LA, would be a very useful predictive tool to assess the risk of hospitalization as well as to assess which patients would be candidates for mechanical ventilation or even to evaluate the risk of death.
Therefore, it would be advisable to evaluate in prospective studies the possibility of routinely measuring serum levels of vWF and LA as prognostic predictive markers of severe COVID19 and at risk of acute respiratory distress syndrome.6 Assessment of these parameters may contribute to determine which of the patients that recover from the acute phase of the disease will present chronic or permanent endothelial damage, and in some cases, for predicting reactivation in different microvasculature territories, such as vasa vasorum of the peripheral nerves and cerebral microvasculature causing a proinflammatory status (endotheliitis)1 leading to chronic neuropathic or neuropsychiatric late sequelae.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Funding statement
None to be declared.
Acknowledgments
The contribution of Drs Hermitas García Quiroga, Victor Manuel López Mouriño, and Enrique Álvarez Asensio with data collection, and Mrs. Charo Ibáñez González with the process database depuration is kindly acknowledged.
References
- 1.Falcinelli E., Petito E., Becattini C., De Robertis E., Paliani U., Sebastiano M., Vaudo G., Guglielmini G., Paciullo F., Cerotto V., Malvestiti M., Gori F., Bury L., Lazzarini T., Gresele P. Role of endotelial dysfunction in the thrombotic complications of COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.11.041. Dec 2:S0163-4453(20)30760-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López Castro J. COVID-19 and thrombosis: beyond a casual association. Med Clin. 2020;155(1):44. doi: 10.1016/j.medcle.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López Castro J. Post-COVID-19 syndrome (PC19S): chronic reactive endotheliitis and disseminated vascular disease. Acta Med Port. 2020;33:855. doi: 10.20344/amp.14612. [DOI] [PubMed] [Google Scholar]
- 4.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoi S., Kakkar A.M., Golowa Y., Grushko M., Coyle C.M., Elrafei T., Langston M.D., Faillace R.T., Bangalore S., Sokol S.I. Saddle pulmonary embolism and clot in transit in COVID-19 infection: a case report of catastrophic venous thromboembolism. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieiro Santos C., López Castro J. Post-coronavirus disease syndrome and disseminated microthrombosis: the role of von Willebrand factor and antiphospholipid antibodies. Clinics. 2021;76:e2784. doi: 10.6061/clinics/2021/e2784. [DOI] [PMC free article] [PubMed] [Google Scholar]