Abstract
γδT cells are an unconventional population of T lymphocytes that play an indispensable role in host defense, immune surveillance, and homeostasis of the immune system. They display unique developmental, distributional, and functional patterns and rapidly respond to various insults and contribute to diverse diseases. Although γδT cells make up only a small portion of the total T cell pool, emerging evidence suggest that aberrantly activated γδT cells may play a role in the pathogenesis of psoriasis. Dermal γδT cells are the major IL-17-producing cells in the skin that respond to IL-23 stimulation. Furthermore, γδT cells exhibit memory-cell-like characteristics that mediate repeated episodes of psoriatic inflammation. This review discusses the differentiation, development, distribution, and biological function of γδT cells and the mechanisms by which they contribute to psoriasis. Potential therapeutic approaches targeting these cells in psoriasis have also been detailed.
Keywords: psoriasis, γδT, IL-17, skin inflammation, biological function
Introduction
Gamma delta T cells (γδ T cells) are T cells that have a distinctive T-cell receptor (TCR) on their surface. Most T cells are αβ (alpha beta) T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, gamma delta (γδ) T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain (1). This group of T cells is usually less common than αβ T cells, but significantly enriched in mucosal and epithelial sites, such as the skin and respiratory, digestive, and reproductive tracts. γδT cells are major histocompatibility complex (MHC)-unrestricted innate-like lymphocytes with more unique antigen receptors compared to αβT cells (2). They produce cytokines such as IL-17/IFN-γ/IL-22 (3–5). Although they constitute a small portion of the total T cell pool, γδT cells bridge the innate and adaptive immune system and contribute to various physiological and pathological processes (2). Relative to αβT cells, γδT cells have been less studied and characterized. It is becoming clear that γδT cells are heterogeneous populations of cells with multifunctional capacities in repairing host tissue (6), pathogen clearance (7), tumor surveillance (8, 9), and proinflammatory effects (10).
Psoriasis is a chronic inflammatory skin disease with an autoimmune component and a strong genetic basis. Plaque psoriasis is characterized by well-defined, raised, chronic erythematous plaques with silver patches observed commonly in the elbows, knees, scalp, umbilicus, and lumbar area (11–13). The worldwide reported prevalence of psoriasis ranges from 0.09% to 11.43% and results in a severe economic burden to patients and a significant challenge to public health (14, 15). Multiple comorbidities and other autoimmune disorders have been correlated with psoriasis, which includes arthritis, cardiovascular disease, obesity, diabetes mellitus, and inflammatory bowel disease, indicate common cellular mediators that drive the pathogenesis of these diseases (16). Increasing evidence has demonstrated that aberrantly activated γδT cells may direct the pathogenesis of autoimmune disorders, such as psoriasis (17–19). To understand what they do in psoriasis, it is important to understand their the differentiation, development, distribution, and biological function.
In this review, we expound on the properties of γδT cells and review the effects of γδT cells in psoriasis. We hope that this review provides insights into its pathogenesis, especially in disease recurrence, and sheds light on potentially novel therapies targeting γδ T cell function.
Differentiation and Development of γδT Cells in the Thymus
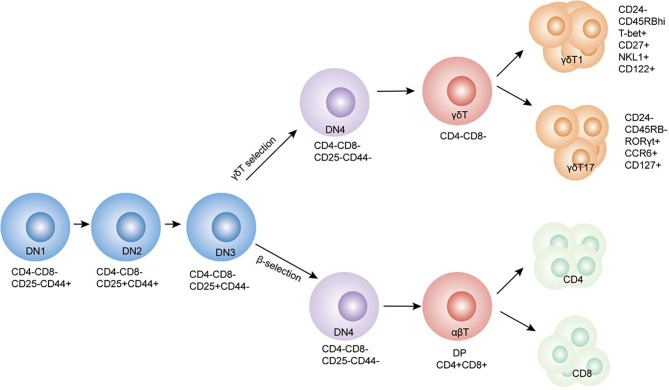
γδT cells were first discovered and reported 30 years ago during the manufacture of antibodies using the TCRγ gene sequence (20). αβT and γδT cell lineages originate from common T precursor cells that lack CD4 and CD8 coreceptors (CD4-CD8-), also known as double-negative (DN) thymocytes. Based on their differential CD44 and CD25 expression, DN cells can be further subdivided into DN1 (CD44+CD25-), DN2 (CD44+CD25+), DN3 (CD44-CD25+), and DN4 (CD44-CD25-) stages, as indicated in Figure 1 . Clonal assays for determining DNT cell progenitors permit the identification of the branch-point of αβT and γδT cell lineages at the late DN2 to DN3 developmental stages (21, 22). DN3 is the critical selection stage that determines the fate of γδ or αβ cell lineages (22). Rearrangements at the Tcrd, Tcrg, and Tcrb loci are initiated at the DN2 stage, and αβ and γδ lineage divergence occur at the DN3 stage (23, 24). Successful rearrangement of the TCRβ chain is achieved with the assembly of the constant pTα and CD3 subunits to form the pre-TCR complex. Commitment to the αβT cell lineage and differentiation of DN3 cells into DN4 (CD44-CD25-) cells transpires in a ligand-independent manner. This process is termed β-selection and is a checkpoint for the generation of a functional TCRβ chain (25, 26). TCRγ and δ chains rearrange during the DN stages and express γδTCR/CD3 on the plasma membrane. ‘γδ selection’ is associated with increased extracellular signal-related kinase 1/2(ERK1/2) phosphorylation and early growth response gene (Egr) protein expression. Ectopic expression of Egr proteins promotes the selection of the γδ T cells. Inhibitor of DNA binding 3 (Id3) is an essential target by which Egr proteins regulate αβ/γδ lineages (26–29).
Figure 1.
Schematic depicting the development of γδ and αβ T cells in the thymus. γδ and αβ cells develop from the same ancestral DN (CD4-CD8-) cells. Lineage changes in γδ and αβ cells mainly occur at the DN3 stage. Cell surface markers and transcription factors of the cells have been labeled alongside.
What determines cell fate specification and differentiation from precursors to αβT or γδT lineage? Two models have been proposed, an instructive model and a stochastic model. In the instructive model, pre-TCR or γδ TCR signaling intensities decide the fate of αβT/γδT cell lineage (30). The instructive model is based on several lines of evidence showing that the strong TCR signals are inclined to γδT cells, while the relatively weak TCR signals are inclined to generate abundant αβT lineage cells (27, 31, 32). The stronger signals that promote adoption of the γδ-fate involve activation of the ERK-Egr-Id3 pathway (29, 33). Sang-Yun et al. demonstrated that ERK signaling promotes γδT cell maturation. ERK signaling that promotes γδT cell fate depends not only on conventional substrate targeting through the D-domain but also through an alternate mode of ERK action mediated by its DBP. This induces molecular effectors responsible for the execution of ERK-mediated developmental outcomes post-transcriptionally (34). E proteins are helix-loop-helix transcription factors that bind DNA at E-box motifs (CANNTG). It acts as a downstream focal point for TCR and plays an essential role in thymocyte development (35). Strong TCR signals could selectively restrain αβT cell development by phenocopying E protein insufficiency and increasing ERK activation. This induces early growth response (EGR1, EGR3) transcription factors and targets DNA-binding inhibitors (ID3). ID3 has been shown to interact with and suppress E protein targets (33, 36, 37).
Under stochastic conditions, other signals dominate this differentiation before TCR expression, hence pre-committing cell fate and allowing them to mature further. Increasing evidence has presented that progenitor T cells are heterogeneous in their developmental potential prior to TCR gene rearrangement. Their development potential has been associated with IL-7R expression (pre-T cells) and was independent of TCR-mediated signals (30). High mobility group box transcription factor 13 (Sox13) that modulate Wnt/TCF1 signaling has also been reported to regulate the T cell-fate decision process, while Sox13 expression has been shown to promote γδT cell development and restrain αβT cell development (38, 39). Nevertheless, γδT cell development has been observed in Sox13-deficient mice, suggesting that it is dispensable for γδT cell development. This is contrary to what has been suggested in the stochastic model (38).
Distribution of γδT
Human γδT Cells
Humans γδT cells can be distinguished based on δ chain expression, which includes the Vδ1, Vδ2, and Vδ3 subtypes (40) ( Table 1 ). Vδ1 cells are mainly found in the gut epithelium, skin, spleen, and liver, and are involved in maintaining epithelial tissue integrity. They constitute approximately 30% of the γδT cells in the peripheral blood (PB). Typically, the Vδ1 chain is associated with different VγI family members (Vγ2/3/4/5/8/9) (41–43). Vδ1 cells exert their effector function through TCR recognition of stress molecules on epithelial cells. Furthermore, Vδ1 cells express natural killer receptors (NKG2C, NKG2D, NKp30), Toll-like receptors, CD8, and the β-glucan receptor, dectin-1 (44–48). Activated Vδ1T cells release IL-10, IL-2, IL-4, IL-17, IFN-γ, TNF-α, and chemokines (CCL3, CCL4, and CCL5). Vδ1T cells play an essential role in maintaining barrier tissue integrity and establishing antiviral immunity (49–51). Studies have demonstrated that Vδ1 cells are involved in several diseases, such as malaria (52, 53), human immune deficiency virus (HIV) (54, 55), cytomegalovirus (CMV) (56), inflammatory bowel disease, and Crohn’s disease by exerting their cytotoxic effects and secreting cytokines (57). Notably, activated Vδ1T cells recognize B7-H6 via NKp30. B7-H6 is a B7 family member exclusively expressed on tumor cells and is involved in the antitumor effect (58).
Table 1.
Characteristics of human and murine γδT cell subsets.
| Classify | Common pairs | Tissue resident | Production of cytokines | |
|---|---|---|---|---|
| Human | Vδ1 | Vγ2+/3+/4+/5+/8+/9+ | gut, skin, liver PB (peripheral blood) |
IL-10,IL-2,IL-4,IL-17,IFN-γ, TNF-α |
| Vδ2 | Vγ9-/9+ | PB, skin | IFN-γ,TNF-α,IL-17,IL-21,IL-24 | |
| Vδ3 | Vγ2+/3+/4+ | PB, liver | IL-10,IL-4,IL-17,IFN-γ,TNF-α | |
| Murine | Vγ1 | Vγ6.3/6.4 | skin, lung, colon, liver, PB | IL-4,IFN-γ |
| Vγ2 | Vδ4 | skin, lung, colon, liver, PB | IL-17 | |
| Vγ3 | Vδ1 | skin | ? | |
| Vγ4 | Vδ4 | skin, lung, colon, liver, PB, joint |
IL-17,IFN-γ | |
| Vγ5 | Vδ1 | skin, liver | IL-17,IFN-γ | |
| Vγ6 | Vδ1 | genital tract, togue, lung, colon, skin, adipose tissue |
IL-17,IFN-γ,IL-22 | |
| Vγ7 | Vδ4/5/6 | IEL (Intraepithelial lymphocytes) | IFN-γ |
Heilig and Tonegawa nomenclature used for classification.
Vδ2T cells are primarily distributed in the blood and the lymphoid system and are the main subset found in healthy humans. It accounts for 50%–90% of the γδT cell population in peripheral blood (59). Vδ2T cells are divided into the innate-like (Vγ9+) and adaptive (Vγ9-) subsets, with the majority of Vδ2T cells being Vδ2Vγ9+T cells (60). Vδ2Vγ9+T cells are responsive to cytokines, such as CCR1, CCR2, CCR5, and CXCR6 ligands and IL-12, and produce proinflammatory factors, such as IFN-γ, TNF-α, IL-17, IL-21, and IL-24 (61, 62). Vδ2Vγ9+T cells can be divided into naive γδT (CD45RA+CD27+Vδ2Vγ9+), central memory γδT (TCM, CD45RA-CD27+Vδ2Vγ9), effector memory γδT (TEM, CD45RA-CD27-Vδ2 Vγ9+), and CD45RA+ effector memory γδT (TEMRA, CD45RA+CD27-Vδ2Vγ9+) based on their surface expression of CD45RA and CD27. Naive γδT cells comprise of the Vδ2Vγ9+T cell subset in the lymph nodes and express CCR7 and CD62L. However, CCR2, CCR5, CCR6, and CXCR3 are only expressed and activated in the presence of high concentrations of isopentenyl pyrophosphate (IPP) but do not produce IFN-γ. CM cells express CCR7 and CD62L and are activated at low IPP concentrations and produce some IFN-γ. TEM cells are present in the blood and inflammatory sites and are CCR7-CD62L-. However, they are positive for the chemokine receptors CCR2, CCR5, CCR6, and CXCR3. TEM cells secrete abundant IFN-γ and tumor necrosis factor-alpha (TNF-a) when activated with IPP+IL-2. TEMRA cells are CCR7-CD62L- but express CCR5 and CXCR3, and have a cytotoxic effect. TEMRA cells also secrete abundant perforin, granulysin, and N-a-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT)-esterase, but do not produce IFN-γ. In addition, they are terminally differentiated and are no longer able to respond to TCR stimulation, and have poor proliferative ability (63–66). Vδ2 specifically recognizes (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) and isopentenyl pyrophosphate (IPP) and rapidly respond to exogenous infections or endogenous transformed cells (67, 68). Furthermore, activated Vδ2Vγ9+T cells acquire antigen-presenting cell (APC) characteristics and display a strong ability to secrete cytokines, such as Th1/Th2/Th17-type cytokines. These induce the maturation of dendritic cells (DCs) into APCs (69–71).
Vδ3T cells are the smallest subsets of the peripheral blood lymphocytes, accounting for 0.2% of circulating cells. They express CD56, NKG2D, CD28, HLA-DR, CD161, and T cell activation marker CD69, but not CD25, NKG2A, or NKG2C (72). Vδ3T cells are abundant in the liver and gut and are involved in chronic viral infections and leukemia (73, 74). Expanded Vδ3T cells only recognize CD1d and release Th1, Th2, and Th17 cytokines to induce the maturation of dendritic cells into APCs. They do not recognize CD1a, CD1b, or CD1c (72). Vδ3 T cells and B cells reciprocally regulate the expression of maturation markers, CD40, CD86, and HLA-DR, and promote IgM release by B cells (75).
Interestingly, Vδ4, Vδ6, Vδ7, and Vδ8 T cells have been observed in the PB of lymphoma patients, however, their roles are yet to be deciphered (76).
Murine γδ T Cells
Murine γδT cells can be distinguished based on their γ chain expression. Two nomenclature methods have been commonly reported in the literature, i.e., the Heilig and Tonegawa, and the Garman classification (77, 78). This review uses the Heilig and Tonegawa nomenclature and is used for the Vγ1–Vγ7 subtypes (79) ( Table 1 ).
The development of the γδT subsets begins during the fetal period. First are the Vγ5+cells that are produced between embryonic day13 (E13) to approximately E17, followed by Vγ6+ cells from E14 to around birth, and the last are the Vγ1+, Vγ2+, and Vγ4+ cells from E16 onward (25, 80, 81). Vγ5+ cells, also known as dendritic epidermal T cells (DETCs), are involved in innate body barrier defense. The increased expression of sphingosine-1-phosphate receptor 1 (S1P1), E and P selectin ligands, and chemokines CCR10 and CCR4 in mature Vγ5+ cells, and the decreased expression of CCR6, CCR9, CCR7, and CD62L allow the egression of Vγ5+ cells from the thymus to the epidermis (82, 83). In normal healthy skin, DETC secretes IL-15 and IGF-1 to maintain skin homeostasis and promote wound healing (84, 85). After skin trauma, DETCs undergo morphological changes accompanied by the upregulation of the activation marker, CD69. It then releases soluble factors that regulate various aspects of tissue repair (85). DETCs produce CCL3 and CCL4 chemokines that are important for macrophage homing. Furthermore, DETCs promotes macrophage recruitment by regulating hyaluronan production through DETC-derived keratinocyte growth factor (KGF) (86, 87). Vγ5+Vδ1+cells produce IFN-γ by activating the Egr3-mediated pathway while suppressing the γδT cell lineage factor, Sox13, and the RORγt transcription factor associated with IL-17 production (39). However, some studies have shown that DETCs produce IL-17, promote keratinocyte proliferation, and participate in skin inflammation (88).
The second γδT subsets produced are the Vγ6 cells. They pair with the Vδ1 subsets of γδ TCR (Vγ6Jγ1 and Vδ1Dδ2Jδ2) and migrate to the genital tract, tongue, lungs, peritoneal cavity (PEC), dermis, colon, and adipose tissues (89). Vγ6+Vδ1+ γδT cells that produce IL-17 and other effector molecules drive inflammation and tumor cell proliferation (90).
Typically, Vγ1+, Vγ2+, and Vγ4+ cells migrate to the dermis, lungs, colon, liver, and peripheral lymphoid organs (91). Both Vγ1+ and Vγ4+ cells can secrete IFN-γ, TNF-a, TGF-β, and IL-10 upon activation. However, Vγ1+γδT cells are predisposed to produced IL-4 and IL-5, while Vγ4+γδT cells preferentially produce IL-17 (92). Vγ1+γδT cells occur mainly in the form of Vγ1Vδ6.3/6.4 TCR cells and secrete IL-4 and IFN-γ (93). Upon acute infection with Coxsackievirus B3 (CVB3), Vγ1+γδT cells are the early and primary producers of IL-4 and play a protective role in CVB3 myocarditis (94). Vγ4+ γδT cells express high levels of Rorc, Sox13, Scart, Bclaf1, and Atf2 and secrete abundant levels of IL-17A and IL-17F (92) (95). IL-17A-producing Vγ4+γδT cells also express high levels of CCR6 on their surface and are chemoattracted by CCL20 that are secreted by keratinocytes to inflammatory sites, which in turn facilitates keratinocytes to secrete IL-1β and IL-23 (96). In addition, IL-17 secreted by Vγ4+γδT cells inhibits the production of IGF-1, thereby delaying skin wound healing (84, 97). Studies have shown that Vγ2+ T cells recruit neutrophils and aggravate liver fibrosis by secreting IL-17A (98, 99). It has also been demonstrated that Vγ7+T cells are the main components of the murine intestinal intraepithelial T cell compartment. Consequently, the selective maturation and expansion of Vγ7+T cells are driven by both Btnl1 and Btnl6 (100).
Biological Effects of γδT Cells
γδT cells have strong plasticity and secrete different cytokines and chemokines. They exhibit diverse functions similar to Th1, Th2, Tregs, and Th17 cells in different microenvironments (2). Some γδ T cells generate growth factors such as VEGF, FGF-2, and IGF-1, suggesting that these cells have the capacity to maintain epithelial integrity and wound repair (101). Nonetheless, some γδ T cells have been reported to induce the production of antimicrobial peptides, including β-defensin 2, S100A7, and S100A8 in keratinocytes to exert a protective function in local epithelial defense (101). γδT cells secrete interleukin-10 (IL-10), control CD8+ T cell expansion, and regulate and reduce TNF-a secretion by activated CD8+ T cells (102). The role of IL-17-producing γδT cells has been investigated in various models of infection and autoimmunity (103, 104). IL-17-producing γδT cells robustly direct the recruitment of neutrophils and monocytes to increase the inflammatory response.
γδ T cells are involved in the regulation of macrophage homeostasis and recruitment. In patients suffering from listeriosis (a serious infection caused by the germ Listeria monocytogenes), γδT cells play a critical role in neutrophil replacement by producing chemokines such as macrophage chemoattractant protein1 (MCP-1) (105). Additional evidence has shown that γδT cells facilitate differentiation of the monocyte/macrophage lineage. Remarkably, monocytes differentiate into inflammatory macrophages during bacterial infections but fail to undergo maturation in mice lacking γδT cells (106). In contrast, the role of Vγ4 has been demonstrated to enhance macrophage activity and the production of specific pro-inflammatory and immunoregulatory cytokines by macrophages. Different subsets of γδT cells have opposing roles in macrophage homeostasis, indicating the complexity and plasticity of γδT cells (107). γδT cells present antigens to αβT cells, while Vδ2+ T cells display characteristics similar to professional APCs. Once activated, these cells efficiently process and present antigens and prime co-stimulatory signals for potent induction of αβT cell proliferation and differentiation (108). Receptors associated with DC, such as antigen presentation molecules (MHC class II), co-stimulatory receptors (CD40, CD80, and CD86), maturation markers (CD83), and adhesion receptors (CD11a, CD11b, CD11c, CD18, CD50, and CD54) have been found to be expressed on the surface of activated γδT cells (109, 110).
Activated γδT cells exhibit a broad range of cytotoxic activity, especially against a wide variety of tumor cells that utilize death receptor/ligand (Fas/Fas-ligand)-dependent and perforin/granzyme or granulysin-dependent pathways. Exogenous IL-18 promotes the expansion of γδT cells in human peripheral blood mononuclear cells (PBMCs) stimulated by Zoledronate (Zol) and IL-2 (109). The expansion of γδT cells is inhibited by neutralizing anti-IL-18 receptor antibodies, indicating that IL-18 efficiently promotes the expansion of γδT cells with potent antitumor activity (110). Furthermore, studies have shown that γδT cells directly kill activated hepatic stellate cells (HSCs) and increase NK cell-mediated cytotoxicity against activated HSCs in liver fibrosis (10).
γδT cells are highly efficient in promoting B cell maturation and producing IgM, IgG, and IgA antibodies. Vδ2Vγ9 T cells express IL-21R on their surface, which is enhanced upon HMB-PP induced irritation (111, 112). Activated Vδ2Vγ9T cells express CXCL13, CXCR5, and ICOS and upregulate the expression of B cell surface markers CD25, CD69, CD40, and CD86. This suggests that CXCR5+ Vδ2Vγ9 T cells are a distinct memory T cell subset with B cell helper function (111, 113).
γδT in Psoriasis
Dysregulation of the immune system and T cell activation has been well demonstrated to play an essential role in psoriasis development. Several studies have attributed T cell function in the skin to αβT cells, while γδT cells have been often overlooked. IFN-γ-producing T helper (Th) 1 cells were initially thought to be primary drivers of psoriasis. However, substantial clinical and basic research findings in the past decade have proved that the interleukin (IL)-23/Th17 axis plays an important role in the pathogenesis of psoriasis (114, 115). Psoriatic inflammation was found to be impaired in IL-23- and IL-17-deficient mice, thereby confirming the involvement of the IL-23/IL-17 axis (116, 117). Th17 cells and their downstream effector molecules, including IL-17A, IL-17F, IL-22, and tumor necrosis factor (TNF-α), were found to be increased in the sera and psoriatic skin lesion (118). Recently, Th17 cells were found not to be the primary source of these pathogenic cytokines in psoriasis. Instead, IL-17A, IL-17F, and IL-22 were found to be produced by γδT cells (115). Injecting IL-23 into the skin of mice or applying a topical dose of imiquimod cream (5%) induced a typical psoriasis-like phenotype, i.e., epidermal thickness, erythema, and inflammation. These two models were demonstrated to mimic psoriasis-like inflammation and have been used to evaluate the efficacy of different treatment methods (119). Epidermal hyperplasia and inflammation response induced by IL-23/IMQ was observed to be significantly reduced in T cell receptor δ deficient (Tcrd−/−) mice, however, no significant changes were observed in T cell receptor β deficient Tcrb−/− mice (120). In addition, Cai et al. demonstrated that upon IL-23 stimulation, IL-17 produced in Tcrd−/− mice was significantly lower compared to WT or Tcra−/− mice (121). These data further suggested that dermal γδT cells were the major IL-17-producing cells in the skin in response to IL-23 stimulation.
The production of IL-17 by dermal γδ T cells requires endogenous IL-1β (121). Mechanistically, IL-1β activates the mammalian target of rapamycin (mTOR) signaling pathway via IL-1R-MyD88, whereas IL-23 activates the STAT3 pathway. Transcription factor IRF-4 links the IL-1R and IL-23R pathways to induce enhanced IL-17 production in dermal γδ T cells (122). Both Vγ4 and Vγ6 dermal T cells produce IL-17, however, dermal Vγ4 T cells expand and produce significantly more IL-17 compared to Vγ6 (123). Dermal Vγ4 and Vγ6T cells have different effector signaling requirements. Dermal Vγ4 T cell proliferation and IL-17 production are dependent on STAT3, whereas dermal Vγ6 T cells may be activated through the STAT3-independent RelA/NF-kB pathway (122). Thus, dermal Vγ4 T cells appear to have a critical role in IMQ-induced psoriasis-like dermatitis (123).
Dermal γδT cells constitutively express IL-23R, IL-17R, RORγt, and the chemokine receptors CCR1, CCR2, CCR4, CCR5, CCR6, CXCR3, and CXCR4 (120, 121). CCL20, which is a unique CCR6 ligand, mediates skin infiltration of IL-17-producing γδT-cells and DCs. Numerous studies have shown that CCL20/CCR6 regulates T migration from the dermis to the epidermis, promotes neutrophil aggregation, and exacerbates inflammation (124). In IL-23-injected WT mice, CCL20 was highly upregulated with numerous CCR6+γδT cells observed in the epidermis (125). Anti-CCL20-neutralizing antibodies or engineered CCL20 variants with minimal chemotactic activity prevented the infiltration of IL-17-producing γδT-cell into the skin of IL-23-injected mice. This lead to IL-17 and IL-22 downregulation, blocked γδT cell recruitment to the epidermis, and reduced psoriasiform dermatitis (126, 127). In CCR6-knockout (KO) mice, γδT cells failed to migrate and accumulate in the epidermis after IL-23 treatment. Keratinocytes secrete CCL20, bind and activate CCR6, and regulate the migration of γδT cell subsets into the skin. This suggests the potential relevance of CCR6/CCL20 as a therapeutic target for psoriasis (126, 128, 129).
Psoriasis recurs frequently and relapse occurs in the same area after treatment discontinuation. Hence, recurrent psoriasis is a major problem that needs to be solved. TNF-a, IL-12/23, and IL-17 inhibitors have been shown to exhibit potent and rapid therapeutic efficacy (130, 131). However, these biological agents have been associated with several adverse events, the most common being susceptibility to infections (130). In addition to infections, biological inhibitors have been associated with demyelinating diseases, nasopharyngitis, upper respiratory infection, headaches, lupus, or lupus-like syndromes, mucocutaneous candidiasis, mild neutropenia, and new-onset or worsening of heart failure. The long-term safety concerns and high cost hamper the extensive use of these agents (130, 132, 133).
Psoriasis relapses around the original lesion area suggest these manifestations have an “immune memory.” Adaptive immune responses by memory T cells are not limited to foreign antigens, and relapses in autoimmune diseases are typically driven by auto-aggressive memory lymphocytes. There have been published reports regarding the adaptive-type memory responses in γδT cells. The response of human Vγ9Vδ2+ T cells to phospho-antigens is increased after initial Mycobacterium bovis BCG vaccinations (134). In macaques, a memory-type response and rapid expansion of Vγ9Vδ2 T cells have been observed after a secondary challenge with Bacillus Calmette-Guerin (135). Mouse “memory-like” Vγ6+ γδT cells were found to be retained for more than five months in the mesenteric lymph nodes after Listeria monocytogenes infection (136).
Memory-like γδT has been seen in psoriasiform mouse model, IL-17A-producing Vγ2Vδ4+ T cells initially derive from the neonatal thymus where they are instructed with tissue tropism. These Vγ2Vδ4+ T cells were phenotypically memory-like with a CD44hi CD62Llo CD27- expression pattern (137). After exposure to IMQ, Vγ4+γδT17 cells in the skin have been shown to rapidly expand in the draining lymph nodes (LNs) and then release from the LNs. They then migrate via the action of the chemokine, CCR2, to accumulate at sites of both inflamed and uninflamed skin in a S1P1-dependent manner. This in turn exacerbates the inflammatory response and recruitment of neutrophils. They have also been shown to migrate via the blood and persist in normal skin and peripheral LNs for a minimum of three months. Importantly, when subjected to the same second challenge at a distant skin site, memory-like Vγ4+γδT17 cells expand at a faster rate and produce more IL-17 compared to that after exposure to the first challenge, leading to a rapid and severe skin inflammatory response (19) ( Figure 2 ). Sensitized mice showed elevated skin inflammation, significant cell proliferation, and IL-17 production by Vγ4+γδT cells upon IMQ challenge. Adoptive transfer experiments have confirmed that memory-like Vγ4+γδT17 cells respond rapidly, and their memory drives their involvement in the psoriasis recurrence (19, 138, 139).
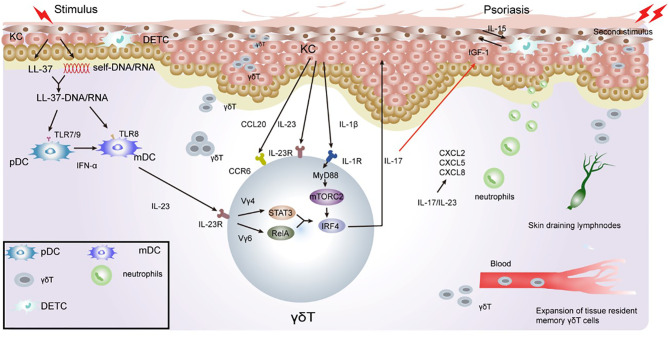
Figure 2.
Role of γδT cells in the immune pathogenesis of psoriasis. Keratinocytes in the epidermis undergo apoptosis, necrosis, or death when exposed to certain external stimulation. With the release of cell contents, such as DNA and RNA, keratinocytes release antimicrobial peptides, such as LL-37. LL-37 binds with DNA and RNA to form a complex, promote immature DC activation, and secrete IFN-γ/IL-23 through the TLR7/8/9 pathway. IL-23 activates RORγt+γδT cells to secrete IL-17. γδT cell-derived IL-17 directly inhibits IGF-1 production in DETCs by increasing epidermal IL-23/IL-1β expression. During excessive keratinocyte proliferation, the secretion of TNF-α and chemokine ligand 20 (CCL20) increases, which consequently recruits CCR6+γδT cells to the inflammatory site of the epidermis. IL-17 cytokines produced by γδT cells potently upregulate the chemokine, CCL20, in keratinocytes, which chemoattracts IL-17A-producing CCR6+ immune cells to the inflamed site, thus forming a positive feedback loop. Il-23/IL-17 also promotes the recruitment of neutrophils to inflammatory sites, leading to excessive proliferation of the stratum corneum to form psoriatic inflammatory lesions. γδT cells have memory properties and can migrate rapidly to inflammatory sites through the blood and skin when subjected to a secondary stimulation. This consequently gives rise to severe inflammatory manifestations.
γδT cells are rarely found in healthy human skin (140), however, they are easily generated from the skin of psoriatic patients. γδT cells have different adhesion properties compared to αβT cell subsets (141). A higher frequency of sequence sharing of the γ-chain has been found in psoriatic lesions from different individuals compared to those without psoriasis, suggesting that although the T cell response in psoriasis is highly polyclonal, particular γδT cell subsets could be associated with this disease (142). Following study demonstrated that an increased level of Vγ9Vδ2 T cells was present in psoriatic skin compared to healthy controls, while a significant reduction in Vγ9Vδ2 cells was observed in the blood of psoriasis patients. The number of circulating Vγ9Vδ2 T cells returned to normal levels after successful psoriasis-targeted treatment. These findings demonstrated the redistribution of Vγ9Vδ2 T cells from the blood to the skin of psoriasis patients (101). The recruitment of specific monoclonal population of γδT cells to psoriatic skin suggests local expression or modification of a cognate TCR ligand that is recognized by this population of memory-like γδT cells (143). Consistently, Zheng group found the higher expression of Vγ9 in psoriasis lesion than that in healthy individuals, indicating that Vγ9 γδT cells may be the main pathogenic cell (144). Additionally, Vγ9Vδ2 T cells have been shown to produce psoriasis-relevant cytokines, such as IFN-γ, TNF-α, and IL-17A and chemokines such as IL-8, CCL3, CCL4, CCL5, and CCR6. These cytokines and chemokines are responsible for recruiting crucial immune effector cells to the skin to activate keratinocytes (63, 145).
Targeting γδT Cells for Psoriasis Therapy
The important role of dermal immobilized γδT cells in the pathogenesis of psoriasis has been elucidated in the past years. Hence, dermal γδT cells and their associated molecules have become attractive targets for drug development. Adiponectin, a metabolic mediator of insulin sensitivity, plays a crucial role in metabolic regulation and inflammatory/anti-inflammatory processes. Studies have demonstrated that in psoriasiform skin, inflammation, and infiltration of dermal γδT cells producing IL-17 were significantly enhanced in the absence of adiponectin. The negative regulation of adiponectin on IL-17 production from dermal γδT cells is mainly mediated through AdipoR1. This suggests that increasing adiponectin levels may be effective for improving psoriasis as well as metabolic disorders (146, 147). BTLA belongs to the immunoglobulin superfamily and has been reported to play a role in the homeostasis of γδT cells/ILCs in lymphoid tissues and controls the production of IL-17 in mature lymph node γδT cells. BTLA-deficient animal models have been shown to have a dysregulated proportion of inflammatory γδT cells and were susceptible to psoriasis and severe skin inflammation. BTLA agonism was found to limit the progression of these phenotypes. Activation of BTLA may restore the balance of γδT cell subsets to control autoimmune pathogenesis (148, 149). The agonistic anti-BTLA antibody (clone 6A6) was demonstrated to suppress γδT cell expansion and IL-17 production within the lymph nodes and skin induced by IMQ (149, 150). Thus, BTLA may be a potential target for the treatment of psoriasis. Dermal γδT cells constitutively express CCR6. CCR6KO or anti-CCL20 monoclonal antibodies administered to mice resulted in a decline in psoriatic dermatitis in IL-23-induced skin inflammation mouse models. This demonstrates that CCL20, together with its receptor, CCR6, are potential targets for the treatment of psoriasis (129, 151). CCL20 S64C is a CCL20 variant that binds to CCR6 and inhibits CCR6-mediated T cell migration. Previous studies have shown that CCL20 S64C alleviates the inflammatory response in psoriasis-like models induced by IL-23, and have been associated with reduced accumulation of CCR6+ IL-17-producing γδT cells in the epidermis (127). FTY720 is an FDA-approved immunomodulatory drug for the treatment of multiple sclerosis. It reduces lymphocyte egress from lymphoid tissues by inhibiting the sphingosine-1 phosphate receptor (S1PR). FTY720 inhibits the migration of Vγ4+VγT4+ T17 cells from the lymph nodes to the skin, suggesting its potential as a treatment for psoriasis (152). Indirubin (IR) is a bisindole compound extracted from the leaves of the Chinese herb Indigo naturalis. It has been demonstrated to alleviate IMQ-induced psoriasis-like dermatitis by primarily reducing the inflammatory responses mediated by IL-17 A-producing γδT cells through Jak3/Stat3 activation (153). Dashlkhumbe et al. reported a newly formulated methotrexate (MTX, a chemical conjugate of MTX with a cell-permeable peptide) for the treatment of psoriasis. Topically applied skin-penetrating (SP)-MTX reduced the psoriasiform skin phenomenon and epidermal thickness by reducing CD11c+, CD4+, and IL-17-producing γδT cell-containing infiltrate of immune cells in the skin (154).
Conclusions and Future Directions
Psoriasis has a complex and varied pathogenesis. During disease development, γδT cells secrete proinflammatory cytokines, such as IL-17 and IFN-γ, which induce and aggravate psoriasis. Notably, γδT cells have memory cell properties that rapidly respond to secondary stimulation. This contributes to the recurrence of psoriasis.
Future studies should investigate whether γδT cells that reside in skin lesions have resident memory cell properties, how long they persist, how often they turn over, and what environmental niches within peripheral tissues support their long-term survival. Studies have shown that metabolism and immune function are tightly linked (155, 156). Nutrient availability and cellular metabolism tightly control the differentiation, survival, and function of immune cells (157). However, whether cellular metabolism regulates γδT fate decisions remains to be deciphered. Additional studies are necessary to identify the mechanisms that reduce γδT cells to prevent the recurrence of psoriasis.
Author Contributions
CQ drafted and edited the manuscript. CQ drafted and edited the figures and figure legends. YW and PL edited the manuscript. JZ edited and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81673989 and No. 82074434) and the Beijing municipal health system high-level health technology talent team construction project (No. 2015-3-116).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Holtmeier W, Kabelitz D. gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy (2005) 86:151–83. 10.1159/000086659 [DOI] [PubMed] [Google Scholar]
- 2. Lu H, Li DJ, Jin LP. gammadeltaT Cells and Related Diseases. Am J Reprod Immunol (2016) 75:609–18. 10.1111/aji.12495 [DOI] [PubMed] [Google Scholar]
- 3. Ness-Schwickerath KJ, Morita CT. Regulation and function of IL-17A- and IL-22-producing gammadelta T cells. Cell Mol Life Sci (2011) 68:2371–90. 10.1007/s00018-011-0700-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reinhardt A, Prinz I. Whodunit? The Contribution of Interleukin (IL)-17/IL-22-Producing gammadelta T Cells, alphabeta T Cells, and Innate Lymphoid Cells to the Pathogenesis of Spondyloarthritis. Front Immunol (2018) 9:885. 10.3389/fimmu.2018.00885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raverdeau M, Cunningham SP, Harmon C, Lynch L. gammadelta T cells in cancer: a small population of lymphocytes with big implications. Clin Transl Immunol (2019) 8:e01080. 10.1002/cti2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu P, Fu X, Xiao N, Guo Y, Pei Q, Peng Y, et al. Involvements of gammadeltaT Lymphocytes in Acute and Chronic Skin Wound Repair. Inflammation (2017) 40:1416–27. 10.1007/s10753-017-0585-6 [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Kan X, Li Y, Li P, Zhang C, Li G, et al. Deficiency of gammadeltaT cells protects against abdominal aortic aneurysms by regulating phosphoinositide 3-kinase/AKT signaling. J Vasc Surg (2018) 67:899–908.e1. 10.1016/j.jvs.2016.03.474 [DOI] [PubMed] [Google Scholar]
- 8. Girard P, Charles J, Cluzel C, Degeorges E, Manches O, Plumas J, et al. The features of circulating and tumor-infiltrating gammadelta T cells in melanoma patients display critical perturbations with prognostic impact on clinical outcome. Oncoimmunology (2019) 8:1601483. 10.1080/2162402X.2019.1601483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kierkels GJJ, Scheper W, Meringa AD, Johanna I, Beringer DX, Janssen A, et al. Identification of a tumor-specific allo-HLA-restricted gammadeltaTCR. Blood Adv (2019) 3:2870–82. 10.1182/bloodadvances.2019032409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. gammadeltaT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front Immunol (2019) 10:477. 10.3389/fimmu.2019.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schadler ED, Ortel B, Mehlis SL. Biologics for the primary care physician: Review and treatment of psoriasis. Dis Mon (2019) 65:51–90. 10.1016/j.disamonth.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 12. Arnone M, Takahashi MDF, Carvalho AVE, Bernardo WM, Bressan AL, Ramos AMC, et al. Diagnostic and therapeutic guidelines for plaque psoriasis - Brazilian Society of Dermatology. Bras Dermatol (2019) 94:76–107. 10.1590/abd1806-4841.2019940211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee EB, Wu KK, Lee MP, Bhutani T, Wu JJ. Psoriasis risk factors and triggers. Cutis (2018) 102:18–20. [PubMed] [Google Scholar]
- 14. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol (2017) 31:205–12. 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 15. Boehncke WH, Schon MP. Psoriasis. Lancet (2015) 386:983–94. 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 16. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol (2017) 140:645–53. 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mo WX, Yin SS, Chen H, Zhang X. Amino-bisphosphonates, gammadeltaT cells, and their roles in Rheumatoid Arthritis. Ann Rheum Dis (2018) 77:e58. 10.1136/annrheumdis-2017-212569 [DOI] [PubMed] [Google Scholar]
- 18. Ma H, Yuan Y, Zhao L, Ye Z, Xu J, Li M, et al. Association of gammadelta T Cell Compartment Size to Disease Activity and Response to Therapy in SLE. PLoS One (2016) 11:e0157772. 10.1371/journal.pone.0157772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A (2015) 112:8046–51. 10.1073/pnas.1508990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, et al. Identification of a putative second T-cell receptor. Nature (1986) 322:145–9. 10.1038/322145a0 [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Dong X, Wang H, Li J, Yang B, Zhang J, et al. FADD regulates thymocyte development at the beta-selection checkpoint by modulating Notch signaling. Cell Death Dis (2014) 5:e1273. 10.1038/cddis.2014.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol (2002) 14:311–23. 10.1016/S1044-5323(02)00064-7 [DOI] [PubMed] [Google Scholar]
- 23. Fahl SP, Coffey F, Wiest DL. Origins of gammadelta T cell effector subsets: a riddle wrapped in an enigma. J Immunol (2014) 193:4289–94. 10.4049/jimmunol.1401813 [DOI] [PubMed] [Google Scholar]
- 24. Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J Immunol (1999) 162:2575–80. [PubMed] [Google Scholar]
- 25. Muro R, Takayanagi H, Nitta T. T cell receptor signaling for gammadeltaT cell development. Inflammation Regener (2019) 39:6. 10.1186/s41232-019-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chann AS, Russell SM. An integrated transcriptional switch at the beta-selection checkpoint determines T cell survival, development and leukaemogenesis. Biochem Soc Trans (2019) 47:1077–89. 10.1042/BST20180414 [DOI] [PubMed] [Google Scholar]
- 27. Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity (2005) 22:595–606. 10.1016/j.immuni.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 28. Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity (2006) 24:53–64. 10.1016/j.immuni.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 29. Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol (2010) 11:666–73. 10.1038/ni.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang J, Volkmann A, Raulet DH. Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med (2001) 193:689–98. 10.1084/jem.193.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med (2008) 205:1173–86. 10.1084/jem.20072425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zarin P, Wong GW, Mohtashami M, Wiest DL, Zuniga-Pflucker JC. Enforcement of gammadelta-lineage commitment by the pre-T-cell receptor in precursors with weak gammadelta-TCR signals. Proc Natl Acad Sci U S A (2014) 111:5658–63. 10.1073/pnas.1312872111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, et al. Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity (2009) 31:565–75. 10.1016/j.immuni.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee SY, Coffey F, Fahl SP, Peri S, Rhodes M, Cai KQ, et al. Noncanonical mode of ERK action controls alternative alphabeta and gammadelta T cell lineage fates. Immunity (2014) 41:934–46. 10.1016/j.immuni.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Engel I, Murre C. The function of E- and Id proteins in lymphocyte development. Nat Rev Immunol (2001) 1:193–9. 10.1038/35105060 [DOI] [PubMed] [Google Scholar]
- 36. Ciofani M, Zuniga-Pflucker JC. Determining gammadelta versus alphass T cell development. Nat Rev Immunol (2010) 10:657–63. 10.1038/nri2820 [DOI] [PubMed] [Google Scholar]
- 37. Zarin P, Chen EL, In TS, Anderson MK, Zuniga-Pflucker JC. Gamma delta T-cell differentiation and effector function programming, TCR signal strength, when and how much? Cell Immunol (2015) 296:70–5. 10.1016/j.cellimm.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 38. Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science (2007) 315:230–3. 10.1126/science.1135344 [DOI] [PubMed] [Google Scholar]
- 39. Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity (2011) 35:59–68. 10.1016/j.immuni.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 40. Fichtner AS, Ravens S, Prinz I. Human gammadelta TCR Repertoires in Health and Disease. Cells (2020) 9:800. 10.3390/cells9040800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol (2010) 10:467–78. 10.1038/nri2781 [DOI] [PubMed] [Google Scholar]
- 42. Wesch D, Hinz T, Kabelitz D. Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. Int Immunol (1998) 10:1067–75. 10.1093/intimm/10.8.1067 [DOI] [PubMed] [Google Scholar]
- 43. Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat Commun (2017) 8:14760. 10.1038/ncomms14760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maher CO, Dunne K, Comerford R, O’Dea S, Loy A, Woo J, et al. Candida albicans stimulates IL-23 release by human dendritic cells and downstream IL-17 secretion by Vdelta1 T cells. J Immunol (2015) 194:5953–60. 10.4049/jimmunol.1403066 [DOI] [PubMed] [Google Scholar]
- 45. Fausther-Bovendo H, Wauquier N, Cherfils-Vicini J, Cremer I, Debre P, Vieillard V. NKG2C is a major triggering receptor involved in the V[delta]1 T cell-mediated cytotoxicity against HIV-infected CD4 T cells. AIDS (2008) 22:217–26. 10.1097/QAD.0b013e3282f46e7c [DOI] [PubMed] [Google Scholar]
- 46. Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of gammadelta T cell responses by TLR ligands. Cell Mol Life Sci (2011) 68:2357–70. 10.1007/s00018-011-0699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hudspeth K, Fogli M, Correia DV, Mikulak J, Roberto A, Della Bella S, et al. Engagement of NKp30 on Vdelta1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood (2012) 119:4013–6. 10.1182/blood-2011-11-390153 [DOI] [PubMed] [Google Scholar]
- 48. Kuroda H, Saito H, Ikeguchi M. Decreased number and reduced NKG2D expression of Vdelta1 gammadelta T cells are involved in the impaired function of Vdelta1 gammadelta T cells in the tissue of gastric cancer. Gastric Cancer (2012) 15:433–9. 10.1007/s10120-011-0138-x [DOI] [PubMed] [Google Scholar]
- 49. Mao Y, Yin S, Zhang J, Hu Y, Huang B, Cui L, et al. A new effect of IL-4 on human gammadelta T cells: promoting regulatory Vdelta1 T cells via IL-10 production and inhibiting function of Vdelta2 T cells. Cell Mol Immunol (2016) 13:217–28. 10.1038/cmi.2015.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chabab G, Barjon C, Abdellaoui N, Salvador-Prince L, Dejou C, Michaud HA, et al. Identification of a regulatory Vdelta1 gamma delta T cell subpopulation expressing CD73 in human breast cancer. J Leukoc Biol (2020) 107:1057–67. 10.1002/JLB.3MA0420-278RR [DOI] [PubMed] [Google Scholar]
- 51. Singh AK, Novakova L, Axelsson M, Malmestrom C, Zetterberg H, Lycke J, et al. High Interferon-gamma Uniquely in Vdelta1 T Cells Correlates with Markers of Inflammation and Axonal Damage in Early Multiple Sclerosis. Front Immunol (2017) 8:260. 10.3389/fimmu.2017.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hviid L, Smith-Togobo C, Willcox BE. Human Vdelta1(+) T Cells in the Immune Response to Plasmodium falciparum Infection. Front Immunol (2019) 10:259. 10.3389/fimmu.2019.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rutishauser T, Lepore M, Di Blasi D, Dangy JP, Abdulla S, Jongo S, et al. Activation of TCR Vdelta1(+) and Vdelta1(-)Vdelta2(-) gammadelta T Cells upon Controlled Infection with Plasmodium falciparum in Tanzanian Volunteers. J Immunol (2020) 204:180–91. 10.4049/jimmunol.1900669 [DOI] [PubMed] [Google Scholar]
- 54. Dunne PJ, Maher CO, Freeley M, Dunne K, Petrasca A, Orikiiriza J, et al. CD3epsilon Expression Defines Functionally Distinct Subsets of Vdelta1 T Cells in Patients With Human Immunodeficiency Virus Infection. Front Immunol (2018) 9:940. 10.3389/fimmu.2018.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S. gammadelta T Cells in HIV Disease: Past, Present, and Future. Front Immunol (2014) 5:687. 10.3389/fimmu.2014.00687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knight A, Arnouk H, Britt W, Gillespie GY, Cloud GA, Harkins L, et al. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vdelta1+ gammadelta T cells. PLoS One (2013) 8:e68729. 10.1371/journal.pone.0068729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lo Presti E, Di Mitri R, Mocciaro F, Di Stefano AB, Scibetta N, Unti E, et al. Characterization of gammadelta T Cells in Intestinal Mucosa From Patients With Early-Onset or Long-Standing Inflammatory Bowel Disease and Their Correlation With Clinical Status. J Crohns Colitis (2019) 13:873–83. 10.1093/ecco-jcc/jjz015 [DOI] [PubMed] [Google Scholar]
- 58. Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood (2011) 118:992–1001. 10.1182/blood-2011-02-339135 [DOI] [PubMed] [Google Scholar]
- 59. Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, et al. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A (2015) 112:E556–65. 10.1073/pnas.1412058112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat Commun (2018) 9:1760. 10.1038/s41467-018-04076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kress E, Hedges JF, Jutila MA. Distinct gene expression in human Vdelta1 and Vdelta2 gammadelta T cells following non-TCR agonist stimulation. Mol Immunol (2006) 43:2002–11. 10.1016/j.molimm.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 62. Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. J Leukoc Biol (2011) 89:743–52. 10.1189/jlb.0910501 [DOI] [PubMed] [Google Scholar]
- 63. Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology (2012) 136:283–90. 10.1111/j.1365-2567.2012.03582.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G, Poccia F, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol (2005) 35:1764–72. 10.1002/eji.200525983 [DOI] [PubMed] [Google Scholar]
- 65. Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med (2003) 198:391–7. 10.1084/jem.20030235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA, et al. Ontogeny of gamma delta T cells in humans. J Immunol (2004) 172:1637–45. 10.4049/jimmunol.172.3.1637 [DOI] [PubMed] [Google Scholar]
- 67. Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett (2001) 509:317–22. 10.1016/S0014-5793(01)03191-X [DOI] [PubMed] [Google Scholar]
- 68. Wang H, Sarikonda G, Puan KJ, Tanaka Y, Feng J, Giner JL, et al. Indirect stimulation of human Vgamma2Vdelta2 T cells through alterations in isoprenoid metabolism. J Immunol (2011) 187:5099–113. 10.4049/jimmunol.1002697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dunne MR, Mangan BA, Madrigal-Estebas L, Doherty DG. Preferential Th1 cytokine profile of phosphoantigen-stimulated human Vgamma9Vdelta2 T cells. Mediators Inflamm (2010) 2010:704941. 10.1155/2010/704941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petrasca A, Doherty DG. Human Vdelta2(+) gammadelta T Cells Differentially Induce Maturation, Cytokine Production, and Alloreactive T Cell Stimulation by Dendritic Cells and B Cells. Front Immunol (2014) 5:650. 10.3389/fimmu.2014.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Devilder MC, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol (2006) 176:1386–93. 10.4049/jimmunol.176.3.1386 [DOI] [PubMed] [Google Scholar]
- 72. Mangan BA, Dunne MR, O’Reilly VP, Dunne PJ, Exley MA, O’Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J Immunol (2013) 191:30–4. 10.4049/jimmunol.1300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kenna T, Golden-Mason L, Norris S, Hegarty JE, O’Farrelly C, Doherty DG. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin Immunol (2004) 113:56–63. 10.1016/j.clim.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 74. Dunne MR, Elliott L, Hussey S, Mahmud N, Kelly J, Doherty DG, et al. Persistent changes in circulating and intestinal gammadelta T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS One (2013) 8:e76008. 10.1371/journal.pone.0076008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Petrasca A, Melo AM, Breen EP, Doherty DG. Human Vdelta3(+) gammadelta T cells induce maturation and IgM secretion by B cells. Immunol Lett (2018) 196:126–34. 10.1016/j.imlet.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 76. Wang L, Xu M, Wang C, Zhu L, Hu J, Chen S, et al. The feature of distribution and clonality of TCR gamma/delta subfamilies T cells in patients with B-cell non-Hodgkin lymphoma. J Immunol Res (2014) 2014:241246. 10.1155/2014/241246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature (1986) 322:836–40. 10.1038/322836a0 [DOI] [PubMed] [Google Scholar]
- 78. Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell (1986) 45:733–42. 10.1016/0092-8674(86)90787-7 [DOI] [PubMed] [Google Scholar]
- 79. Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B. Thymic Determinants of gammadelta T Cell Differentiation. Trends Immunol (2017) 38:336–44. 10.1016/j.it.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 80. Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur J Immunol (2013) 43:1988–94. 10.1002/eji.201343759 [DOI] [PubMed] [Google Scholar]
- 81. Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol (2002) 2:336–45. 10.1038/nri797 [DOI] [PubMed] [Google Scholar]
- 82. Jiang X, Campbell JJ, Kupper TS. Embryonic trafficking of gammadelta T cells to skin is dependent on E/P selectin ligands and CCR4. Proc Natl Acad Sci U S A (2010) 107:7443–8. 10.1073/pnas.0912943107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nakamura K, White AJ, Parnell SM, Lane PJ, Jenkinson EJ, Jenkinson WE, et al. Differential requirement for CCR4 in the maintenance but not establishment of the invariant Vgamma5(+) dendritic epidermal T-cell pool. PLoS One (2013) 8:e74019. 10.1371/journal.pone.0074019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li Y, Wu J, Luo G, He W. Functions of Vgamma4 T Cells and Dendritic Epidermal T Cells on Skin Wound Healing. Front Immunol (2018) 9:1099. 10.3389/fimmu.2018.01099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol (2010) 184:5423–8. 10.4049/jimmunol.0902733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med (2005) 201:1269–79. 10.1084/jem.20042057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ramirez K, Witherden DA, Havran WL. All hands on DE(T)C: Epithelial-resident gammadelta T cells respond to tissue injury. Cell Immunol (2015) 296:57–61. 10.1016/j.cellimm.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nielsen MM, Lovato P, MacLeod AS, Witherden DA, Skov L, Dyring-Andersen B, et al. IL-1beta-dependent activation of dendritic epidermal T cells in contact hypersensitivity. J Immunol (2014) 192:2975–83. 10.4049/jimmunol.1301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hatano S, Tun X, Noguchi N, Yue D, Yamada H, Sun X, et al. Development of a new monoclonal antibody specific to mouse Vgamma6 chain. Life Sci Alliance (2019) 2. 10.26508/lsa.201900363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell (2019) 176:998–1013.e16. 10.1016/j.cell.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Buus TB, Schmidt JD, Bonefeld CM, Geisler C, Lauritsen JP. Development of interleukin-17-producing Vgamma2+ gammadelta T cells is reduced by ICOS signaling in the thymus. Oncotarget (2016) 7:19341–54. 10.18632/oncotarget.8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dong P, Zhang S, Cai M, Kang N, Hu Y, Cui L, et al. Global characterization of differential gene expression profiles in mouse Vgamma1+ and Vgamma4+ gammadelta T cells. PLoS One (2014) 9:e112964. 10.1371/journal.pone.0112964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pereira P, Berthault C, Burlen-Defranoux O, Boucontet L. Critical role of TCR specificity in the development of Vgamma1Vdelta6.3+ innate NKTgammadelta cells. J Immunol (2013) 191:1716–23. 10.4049/jimmunol.1203168 [DOI] [PubMed] [Google Scholar]
- 94. Wan F, Yan K, Xu D, Qian Q, Liu H, Li M, et al. Vgamma1(+)gammadeltaT, early cardiac infiltrated innate population dominantly producing IL-4, protect mice against CVB3 myocarditis by modulating IFNgamma(+) T response. Mol Immunol (2017) 81:16–25. 10.1016/j.molimm.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 95. Papotto PH, Ribot JC, Silva-Santos B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat Immunol (2017) 18:604–11. 10.1038/ni.3726 [DOI] [PubMed] [Google Scholar]
- 96. Li Y, Huang Z, Yan R, Liu M, Bai Y, Liang G, et al. Vgamma4 gammadelta T Cells Provide an Early Source of IL-17A and Accelerate Skin Graft Rejection. J Invest Dermatol (2017) 137:2513–22. 10.1016/j.jid.2017.03.043 [DOI] [PubMed] [Google Scholar]
- 97. Li Y, Wang Y, Zhou L, Liu M, Liang G, Yan R, et al. Vgamma4 T Cells Inhibit the Pro-healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17A. Front Immunol (2018) 9:240. 10.3389/fimmu.2018.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Spidale NA, Sylvia K, Narayan K, Miu B, Frascoli M, Melichar HJ, et al. Interleukin-17-Producing gammadelta T Cells Originate from SOX13(+) Progenitors that Are Independent of gammadeltaTCR Signaling. Immunity (2018) 49:857–72.e5. 10.1016/j.immuni.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zheng L, Hu Y, Wang Y, Huang X, Xu Y, Shen Y, et al. Recruitment of Neutrophils Mediated by Vgamma2 gammadelta T Cells Deteriorates Liver Fibrosis Induced by Schistosoma japonicum Infection in C57BL/6 Mice. Infect Immun (2017) 85. 10.1128/IAI.01020-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell (2016) 167:203–18.e17. 10.1016/j.cell.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol (2011) 187:2783–93. 10.4049/jimmunol.1100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur J Immunol (2008) 38:2274–83. 10.1002/eji.200838354 [DOI] [PubMed] [Google Scholar]
- 103. Patil RS, Bhat SA, Dar AA, Chiplunkar SV. The Jekyll and Hyde story of IL17-Producing gammadeltaT Cells. Front Immunol (2015) 6:37. 10.3389/fimmu.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Palomino-Segura M, Latino I, Farsakoglu Y, Gonzalez SF. Early production of IL-17A by gammadelta T cells in the trachea promotes viral clearance during influenza infection in mice. Eur J Immunol (2020) 50:97–109. 10.1002/eji.201948157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. DiTirro J, Rhoades ER, Roberts AD, Burke JM, Mukasa A, Cooper AM, et al. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun (1998) 66:2284–9. 10.1128/IAI.66.5.2284-2289.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Skeen MJ, Freeman MM, Ziegler HK. Changes in peritoneal myeloid populations and their proinflammatory cytokine expression during infection with Listeria monocytogenes are altered in the absence of gamma/delta T cells. J Leukoc Biol (2004) 76:104–15. 10.1189/jlb.1103574 [DOI] [PubMed] [Google Scholar]
- 107. Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR. Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis. Eur J Immunol (2006) 36:1729–38. 10.1002/eji.200635959 [DOI] [PubMed] [Google Scholar]
- 108. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science (2005) 309:264–8. 10.1126/science.1110267 [DOI] [PubMed] [Google Scholar]
- 109. D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol (2010) 184:3260–8. 10.4049/jimmunol.0903454 [DOI] [PubMed] [Google Scholar]
- 110. Li W, Kubo S, Okuda A, Yamamoto H, Ueda H, Tanaka T, et al. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J Immunother (2010) 33:287–96. 10.1097/CJI.0b013e3181c80ffa [DOI] [PubMed] [Google Scholar]
- 111. Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human gammadelta T cells to provide B-cell help. Eur J Immunol (2012) 42:110–9. 10.1002/eji.201142017 [DOI] [PubMed] [Google Scholar]
- 112. Wu K, Zhao H, Xiu Y, Li Z, Zhao J, Xie S, et al. IL-21-mediated expansion of Vgamma9Vdelta2 T cells is limited by the Tim-3 pathway. Int Immunopharmacol (2019) 69:136–42. 10.1016/j.intimp.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 113. Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol (2006) 177:5290–5. 10.4049/jimmunol.177.8.5290 [DOI] [PubMed] [Google Scholar]
- 114. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol (2018) 201:1605–13. 10.4049/jimmunol.1800013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol (2018) 45:264–72. 10.1111/1346-8138.14139 [DOI] [PubMed] [Google Scholar]
- 116. Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol (2011) 186:1495–502. 10.4049/jimmunol.1001001 [DOI] [PubMed] [Google Scholar]
- 117. Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest (2012) 122:3965–76. 10.1172/JCI63451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol (2018) 9:1682. 10.3389/fimmu.2018.01682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Singh TP, Zhang HH, Hwang ST, Farber JM. IL-23- and Imiquimod-Induced Models of Experimental Psoriasis in Mice. Curr Protoc Immunol (2019) 125:e71. 10.1002/cpim.71 [DOI] [PubMed] [Google Scholar]
- 120. Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest (2012) 122:2252–6. 10.1172/JCI61862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity (2011) 35:596–610. 10.1016/j.immuni.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cai Y, Xue F, Qin H, Chen X, Liu N, Fleming C, et al. Differential Roles of the mTOR-STAT3 Signaling in Dermal gammadelta T Cell Effector Function in Skin Inflammation. Cell Rep (2019) 27:3034–48.e5. 10.1016/j.celrep.2019.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun (2014) 5:3986. 10.1038/ncomms4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Katayama H. Development of psoriasis by continuous neutrophil infiltration into the epidermis. Exp Dermatol (2018) 27:1084–91. 10.1111/exd.13746 [DOI] [PubMed] [Google Scholar]
- 125. Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol (2011) 187:5026–31. 10.4049/jimmunol.1101817 [DOI] [PubMed] [Google Scholar]
- 126. Mabuchi T, Singh TP, Takekoshi T, Jia GF, Wu X, Kao MC, et al. CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis. J Invest Dermatol (2013) 133:164–71. 10.1038/jid.2012.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Getschman AE, Imai Y, Larsen O, Peterson FC, Wu X, Rosenkilde MM, et al. Protein engineering of the chemokine CCL20 prevents psoriasiform dermatitis in an IL-23-dependent murine model. Proc Natl Acad Sci U S A (2017) 114:12460–5. 10.1073/pnas.1704958114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hedrick MN, Lonsdorf AS, Hwang ST, Farber JM. CCR6 as a possible therapeutic target in psoriasis. Expert Opin Ther Targets (2010) 14:911–22. 10.1517/14728222.2010.504716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Furue K, Ito T, Tsuji G, Nakahara T, Furue M. The CCL20 and CCR6 axis in psoriasis. Scand J Immunol (2020) 91:e12846. 10.1111/sji.12846 [DOI] [PubMed] [Google Scholar]
- 130. Gisondi P, Geat D, Pizzolato M, Girolomoni G. State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis. Curr Opin Pharmacol (2019) 46:90–9. 10.1016/j.coph.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 131. Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev (2020) 1:CD011535. 10.1002/14651858.CD011535.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Conrad C, Gilliet M. Psoriasis: from Pathogenesis to Targeted Therapies. Clin Rev Allergy Immunol (2018) 54:102–13. 10.1007/s12016-018-8668-1 [DOI] [PubMed] [Google Scholar]
- 133. Mrowietz U, Barker J, Boehncke WH, Iversen L, Kirby B, Naldi L, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol (2018) 32(Suppl 3):3–14. 10.1111/jdv.15218 [DOI] [PubMed] [Google Scholar]
- 134. Dieli F, Sireci G, Di Sano C, Romano A, Titone L, Di Carlo P, et al. Ligand-specific alphabeta and gammadelta T cell responses in childhood tuberculosis. J Infect Dis (2000) 181:294–301. 10.1086/315180 [DOI] [PubMed] [Google Scholar]
- 135. Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood (2008) 112:1317–24. 10.1182/blood-2008-01-136713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F,3, Schubert WD, et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity (2013) 39:184–95. 10.1016/j.immuni.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Khairallah C, Chu TH, Sheridan BS. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front Immunol (2018) 9:2636. 10.3389/fimmu.2018.02636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhu R, Cai X, Zhou C, Li Y, Zhang X, Li Y, et al. Dermal Vgamma4(+)T cells enhance the IMQ-induced psoriasis-like skin inflammatidon in re-challenged mice. Am J Transl Res (2017) 9:5347–60. [PMC free article] [PubMed] [Google Scholar]
- 139. Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing gammadelta T cells establish long-lived memory in the skin. Eur J Immunol (2015) 45:3022–33. 10.1002/eji.201545883 [DOI] [PubMed] [Google Scholar]
- 140. Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR-delta repertoire in normal human skin is restricted and distinct from the TCR-delta repertoire in the peripheral blood. J Invest Dermatol (2001) 116:275–80. 10.1046/j.1523-1747.2001.01250.x [DOI] [PubMed] [Google Scholar]
- 141. de Boer OJ, Verhagen CE, Visser A, Bos JD, Das PK. Cellular interactions and adhesion molecules in psoriatic skin. Acta Derm Venereol Suppl (Stockh) (1994) 186:15–8. [PubMed] [Google Scholar]
- 142. Harden JL, Hamm D, Gulati N, Lowes MA, Krueger JG. Deep Sequencing of the T-cell Receptor Repertoire Demonstrates Polyclonal T-cell Infiltrates in Psoriasis. F1000Res (2015) 4:460. 10.12688/f1000research.6756.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lalor SJ, McLoughlin RM. Memory gammadelta T Cells-Newly Appreciated Protagonists in Infection and Immunity. Trends Immunol (2016) 37:690–702. 10.1016/j.it.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 144. Liu JT, Xue F, Li X, Shi RF, Zheng J. Preliminary Study of T Cell Receptor Vγ Gene Usage of γδT Cells in Psoriatic Skin Lesions. Chin J Dermatovenereol (2012) 26:571–4. [Google Scholar]
- 145. Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol (2010) 184:7268–80. 10.4049/jimmunol.1000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ruan H, Dong LQ. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol (2016) 8:101–9. 10.1093/jmcb/mjw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gammadelta-T cells. Nat Commun (2015) 6:7687. 10.1038/ncomms8687 [DOI] [PubMed] [Google Scholar]
- 148. Bekiaris V, Sedy JR, Macauley MG, Rhode-Kurnow A, Ware CF. The inhibitory receptor BTLA controls gammadelta T cell homeostasis and inflammatory responses. Immunity (2013) 39:1082–94. 10.1016/j.immuni.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Youssef RM, El-Ramly AZ, Hussien MF, Shoukry NM, Amr K. Expression of B and T lymphocyte attenuator, retinoid-related orphan receptor gamma-isoform-t and interleukin 7 in psoriasis vulgaris. Australas J Dermatol (2019) 60:e132–7. 10.1111/ajd.12965 [DOI] [PubMed] [Google Scholar]
- 150. Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM, et al. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol (2005) 174:3377–85. 10.4049/jimmunol.174.6.3377 [DOI] [PubMed] [Google Scholar]
- 151. Cochez PM, Michiels C, Hendrickx E, Dauguet N, Warnier G, Renauld JC, et al. Ccr6 Is Dispensable for the Development of Skin Lesions Induced by Imiquimod despite its Effect on Epidermal Homing of IL-22-Producing Cells. J Invest Dermatol (2017) 137:1094–103. 10.1016/j.jid.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 152. Maeda Y, Seki N, Kataoka H, Takemoto K, Utsumi H, Fukunari A, et al. IL-17-Producing Vgamma4+ gammadelta T Cells Require Sphingosine 1-Phosphate Receptor 1 for Their Egress from the Lymph Nodes under Homeostatic and Inflammatory Conditions. J Immunol (2015) 195:1408–16. 10.4049/jimmunol.1500599 [DOI] [PubMed] [Google Scholar]
- 153. Xie XJ, Di TT, Wang Y, Wang MX, Meng YJ, Lin Y, et al. Indirubin ameliorates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting inflammatory responses mediated by IL-17A-producing gammadelta T cells. Mol Immunol (2018) 101:386–95. 10.1016/j.molimm.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 154. Byamba D, Kim DY, Kim DS, Kim TG, Jee H, Kim SH, et al. Skin-penetrating methotrexate alleviates imiquimod-induced psoriasiform dermatitis via decreasing IL-17-producing gamma delta T cells. Exp Dermatol (2014) 23:492–6. 10.1111/exd.12448 [DOI] [PubMed] [Google Scholar]
- 155. Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic Instruction of Immunity. Cell (2017) 169:570–86. 10.1016/j.cell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shehata HM, Murphy AJ, Lee MKS, Gardiner CM, Crowe SM, Sanjabi S, et al. Sugar or Fat?-Metabolic Requirements for Immunity to Viral Infections. Front Immunol (2017) 8:1311. 10.3389/fimmu.2017.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dimeloe S, Burgener AV, Grahlert J, Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology (2017) 150:35–44. 10.1111/imm.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]