Abstract
Objectives
Standard 6-week and hypofractionated 3-week courses of adjuvant radiation therapy (RT) are both options for older patients with glioblastoma (GBM), but deciding the optimal regimen can be challenging. This analysis explores clinical factors associated with selection of RT course, completion of RT, and outcomes following RT.
Materials and Methods
This IRB-approved retrospective analysis identified patients ≥70 years old with GBM who initiated adjuvant RT at our institution between 2004 and 2016. We identified factors associated with standard or hypofractionated RT using the Cochran-Armitage trend test, estimated time-to-event endpoints using the Kaplan-Meier method, and found predictors of overall survival (OS) using Cox proportional hazards models.
Results
Sixty-two patients with a median age of 74 (range 70–90) initiated adjuvant RT, with 43 (69%) receiving standard RT and 19 (31%) receiving hypofractionated RT. Selection of short-course RT was associated with older age (p = 0.04) and poor KPS (p = 0.03). Eight (13%) patients did not complete RT, primarily for hospice care due to worsening symptoms. After a median follow-up of 37 months, median OS was 12.3 months (95% CI 9.0–15.1). Increased age (p < 0.05), poor KPS (p < 0.0001), lack of MGMT methylation (p < 0.05), and lack of RT completion (p < 0.0001) were associated with worse OS on multivariate analysis. In this small cohort, GTV size and receipt of standard or hypofractionated RT were not associated with OS.
Conclusions
In this cohort of older patients with GBM, age and KPS was associated with selection of short-course or standard RT. These regimens had similar OS, though a subset of patients experienced worsening symptoms during RT and discontinued treatment. Further investigation into predictors of RT completion and survival may help guide adjuvant therapies and supportive care for older patients.
Keywords: glioblastoma, frail elderly, aged, radiotherapy, radiation dose hypofractionation, radiation oncology
Introduction
Glioblastoma (GBM) is a malignancy of older adults. The median age at diagnosis is 65 years old, and the incidence increases with age, peaking in the 75–84 years old age group (1). The Stupp trial established the current standard treatment of maximal safe resection followed by adjuvant radiation therapy (RT) for 6 weeks with concurrent and adjuvant temozolomide (2). However, this trial excluded patients >70 years old, and as age is both a negative prognostic factor and predictor of response to RT, other randomized studies have investigated radiation or temozolomide alone for older adults (3–5). The Canadian trial found that in patients ≥60 years old, 40 Gy in 15 fractions was non-inferior to 60 Gy in 30 fractions, with median survival of 5.1 and 5.6 months, respectively (6). The Nordic trial found that in patients >70 years old, 34 Gy in 10 fractions or temozolomide alone both had improved survival compared to 60 Gy in 30 fractions, though the latter group had more patients discontinue treatment (7). NOA-08 found that in patients >65 years old, temozolomide was non-inferior to 60 Gy in 30 fractions (8). In both the Nordic and NOA-08 trials, O6-methylguanine DNA methyltransferase (MGMT) promoter methylation predicted a survival benefit from temozolomide (7, 8). More recently, a randomized study of patients ≥65 years old found that addition of temozolomide to the 40 Gy regimen did improve survival from 7.6 to 9.3 months (9).
Based on the above studies, temozolomide with standard or hypofractionated RT are both options for patients >70 years old with good performance status (10). The optimal RT regimen is not clear, though individualized treatment decisions may take into account factors such as age, performance status, and MGMT methylation (11). Standardized geriatric assessments have also been proposed to help guide treatment decisions (12). Overall, utilization of hypofractionated RT in the United States remains low. In several National Cancer Database (NCDB) analyses of older patients with GBM receiving adjuvant RT, only 2.5–20% received a hypofractionated regimen (13–17).
Here, we report our institutional experience with older patients initiating adjuvant RT, focusing on factors affecting the selection of standard or hypofractionated regimens and clinical outcomes.
Materials and Methods
Following institutional review board approval, we identified patients with GBM who were ≥70 years old at time of pathologic diagnosis and initiated adjuvant RT in our radiation oncology department between 2004 and 2016.
Patient characteristics including age, sex, Karnofsky performance status (KPS), and MGMT methylation status were obtained from the medical record. KPS was documented following maximal resection at the time of radiation oncology consultation. Treatment details including radiation technique, gross tumor volume (GTV), planning target volume (PTV), and receipt of concurrent temozolomide or bevacizumab were also obtained. Standard radiation therapy to primary and boost volumes was delivered per Radiation Therapy Oncology Group (RTOG) guidelines (18, 19). Specifically, the primary PTV consisted of the pre-operative T2-hyperintense GTV plus a 2 cm margin and received 45–50.4 Gy at 1.8–2 Gy/fraction. The boost PTV was the post-operative T1 contrast-enhancing GTV plus a 1.5 cm margin and received a total dose of 59.4–60 Gy at 1.8–2 Gy/fraction. For hypofractionated radiation therapy, the PTV comprised of T1 contrast-enhancing GTV plus a 1.5 cm margin and received 40.05 Gy in 2.67 Gy/fraction. As we used frequent image guidance and stereotactic radiosurgery-capable, custom-molded head immobilization, there was no further expansion for set-up error. PTVs were trimmed where they extended across anatomic boundaries such as the falx, into non-target tissues such as the orbits or outer table of the skull or the scalp. Boost PTVs were also trimmed where they extended into critical organs at risk such as the brainstem and anterior visual pathways. Temozolomide was administered to all patients where possible and dosed per the Stupp trial, and bevacizumab was administered at the discretion of the treating oncologist (2, 20, 21).
Statistics
Association between clinical characteristics and selection of standard or hypofractionated RT was assessed using the Cochran-Armitage test for trend. Overall survival, measured from date of pathologic diagnosis, was estimated by the Kaplan-Meier method and compared via log-rank test. Clinical factors associated with overall survival were evaluated using Cox proportional hazards models. Significance was assumed if p < 0.05. Statistical analyses were performed using SAS 9.4.
Results
Between 2004 and 2016, 62 older patients initiated adjuvant RT. Baseline patient characteristics are shown in Table 1 . Overall, patients had a median age of 74 years old, and 34 (55%) were male. Most patients received a resection; 33 (53%) had a gross total resection (GTR) and 10 (16%) had a subtotal resection, while 19 (31%) underwent biopsy only. Forty-four (71%) patients had a Karnofsky performance status (KPS) of ≥70 prior to starting adjuvant RT. MGMT methylation status was known for 46 (74%) patients, and 20 (32%) had MGMT methylation. In patients receiving standard RT, the median initial GTV was 98 cm3 and the median boost GTV was 31 cm3. In patients receiving hypofractionated RT, the median GTV was 27 cm3. Fifty-eight (94%) and 20 (32%) patients received concurrent temozolomide and bevacizumab, respectively.
Table 1.
Baseline demographics and treatment details.
| Characteristic | Standard (N = 43) | Hypofractionated (N = 19) | All (N = 62) | |
|---|---|---|---|---|
| Age (years) | 74 (70–88) | 77 (71–91) | 74 (70–91) | |
| Sex | Female | 17 (40) | 11 (58) | 28 (45) |
| Male | 26 (61) | 8 (42) | 34 (55) | |
| Maximal resection | GTR | 26 (61) | 7 (37) | 33 (53) |
| STR | 7 (16) | 3 (16) | 10 (16) | |
| Biopsy only | 10 (23) | 9 (47) | 19 (31) | |
| KPS | ≥70 | 32 (74) | 12 (63) | 44 (71) |
| <70 | 6 (14) | 4 (22) | 10 (16) | |
| Unknown | 5 (12) | 3 (16) | 8 (13) | |
| MGMT | Methylated | 13 (30) | 7 (37) | 20 (32) |
| Unmethylated | 18 (42) | 8 (42) | 26 (42) | |
| Unknown | 12 (28) | 4 (21) | 16 (26) | |
| Radiation technique | 3D | 4 (9) | 9 (47) | 13 (21) |
| IMRT | 39 (91) | 10 (53) | 49 (79) | |
| GTV initial (cm3) | 98 (8–283) | 27 (6–137) | ||
| GTV boost (cm3) | 31 (7–165) | |||
| PTV initial (cm3) | 459 (121–1,049) | 238 (85–505) | ||
| PTV boost (cm3) | 181 (78–410) | |||
| Concurrent TMZ | Yes | 43 (100) | 15 (79) | 58 (94) |
| No | 0 (0) | 4 (21) | 4 (6) | |
| Concurrent bevacizumab | Yes | 13 (30) | 7 (37) | 20 (32) |
| No | 30 (70) | 12 (63) | 42 (68) | |
| Completed RT | Yes | 37 (86) | 17 (90) | 54 (87) |
| No | 6 (14) | 2 (10) | 8 (13) |
KPS, Karnofsky Performance Status; MGMT, O6-methylguanine DNA methyltransferase; GTV, gross target volume; PTV, planning target volume; TMZ, temozolomide; RT, radiation therapy.
Data show number (%) or median (range).
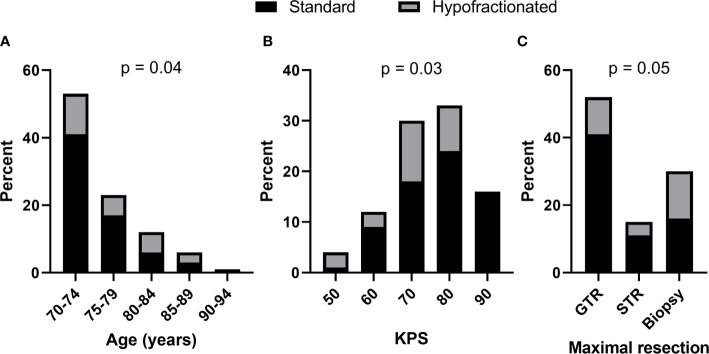
Forty-three (69%) patients received standard RT while 19 (31%) of patients received hypofractionated RT. As shown in Figure 1 , increased age (p = 0.04, Cochran Armitage test for trend) and decreased KPS (p = 0.03, Cochran Armitage test for trend) were both significantly associated with receipt of hypofractionated RT rather than standard RT. Patients who underwent biopsy only compared to gross or subtotal resection appeared to receive hypofractionated RT more frequently as well, but the association was not significant. RT regimen was not associated with MGMT methylation status or the volume of enhancing tumor, as approximated by the GTV size.
Figure 1.
Increased age and decreased KPS were significantly associated with hypofractionated (gray) vs. standard (black) adjuvant radiation therapy. Bars show percent of total patients categorized by (A) age, (B) KPS, and (C) extent of maximal resection. Cochran-Armitage test for trend p values shown.
During RT, 13 patients had unscheduled interruptions, and RT was ultimately discontinued early in six receiving standard RT and two receiving hypofractionated RT. Patients who stopped RT early had a median age of 78 (range 71–85), median pre-RT KPS of 80 (range 50–90), and received a median of 66% (range 3–94%) of the prescribed dose. The most common reason for discontinuation was worsening symptoms prompting transition to hospice. Within this small sample, RT discontinuation was not significantly associated with age, pre-RT KPS, extent of maximal resection, RT dose-fractionation, or size of treatment volumes. Following RT, 41 (66%) patients received adjuvant temozolomide for a median of five cycles (range 1–12), and there was no significant association between receipt of adjuvant temozolomide and RT regimen in this series.
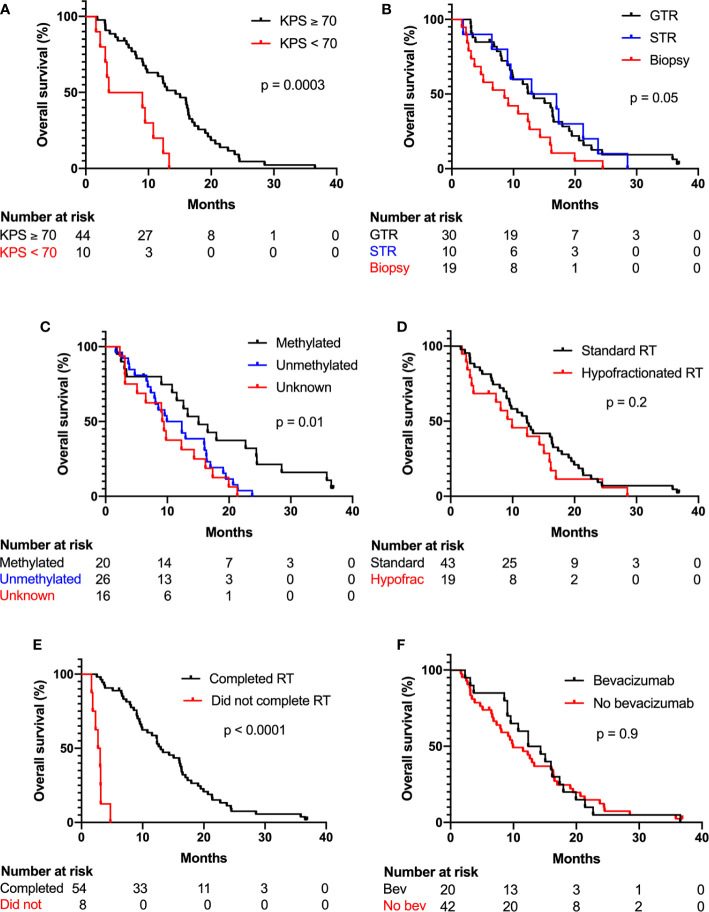
Median follow-up time was 37 months, and two patients were alive at last follow-up. Median overall survival was 12.3 months (95% CI 9.0–15.1 months) across all patients. Median overall survival in patients receiving standard RT and hypofractionated RT was 12.4 months (95% CI 9.0–16.4 months) and 9.9 months (95% CI 3.4–15.1 months), respectively. Kaplan-Meier curves of overall survival categorized by KPS, extent of maximal resection, methylation status, hypofractionated vs. standard RT, RT completion, and receipt of concurrent bevacizumab are shown in Figure 2 . On univariate Cox regression analysis, increased age, KPS <70, biopsy vs. GTR, unmethylated MGMT vs. methylated MGMT, unknown MGMT status vs. methylated MGMT, and early RT discontinuation were significantly associated worse survival, as shown in Table 2 . STR vs. biopsy, use of hypofractionated or standard RT, GTV size, and use of concurrent bevacizumab were not significantly associated with survival. On multivariate analysis with the above covariates, only age (HR 1.09, 95% CI 1.01–1.18), KPS <70 (HR 9.29, 95% CI 3.27–26.38), unmethylated MGMT (HR 2.48, 95% CI 1.09–5.64) or unknown MGMT status (HR 3.58, 95% CI 1.31–9.79), and early RT discontinuation (HR 71.76, 95% CI 13.32–386.6) were significantly associated with decreased survival.
Figure 2.
KPS, MGMT methylation status, and RT completion were significantly associated with overall survival. Kaplan-Meier plots show percent overall survival categorized by (A) KPS, (B) extent of maximal resection, (C) methylation status, (D) standard or hypofractionated RT, (E) completion of RT, and (F) receipt of concurrent bevacizumab. Log-rank test p values shown.
Table 2.
Univariate and multivariate Cox proportional hazards models of overall survival.
| Risk factor | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | 1.08 | 1.02–1.15 | 0.01 | 1.09 | 1.01–1.18 | 0.02 | |
| KPS <70 vs. KPS ≥70 | 3.74 | 1.74–8.06 | <0.001 | 9.29 | 3.27–26.38 | <0.0001 | |
| Maximal resection | |||||||
| GTR vs. biopsy | 0.51 | 0.28–0.91 | 0.02 | 1.72 | 0.67–4.41 | 0.26 | |
| STR vs. biopsy | 0.51 | 0.23–1.11 | 0.09 | 1.01 | 0.38–2.67 | 0.98 | |
| MGMT methylation | |||||||
| Unmethylated vs. methylated | 2.37 | 1.19–4.72 | 0.01 | 2.48 | 1.09–5.64 | 0.03 | |
| Unknown vs. methylated | 2.95 | 1.37–6.34 | 0.01 | 3.58 | 1.31–9.79 | 0.01 | |
| Hypofractionated vs. standard RT | 1.39 | 0.79–2.44 | 0.25 | 1.69 | 0.68–4.18 | 0.26 | |
| Did not complete vs. completed RT | 32.36 | 9.85–106.3 | <0.0001 | 71.76 | 13.32–386.6 | <0.0001 | |
| GTV size | 1.01 | 1.00–1.01 | 0.06 | 1.00 | 0.99–1.01 | 0.59 | |
| Bevacizumab yes vs. no | 1.04 | 0.56–1.64 | 0.88 | 0.76 | 0.38–1.53 | 0.45 | |
KPS, Karnofsky Performance Status; GTR, gross total resection; STR, subtotal resection; MGMT, O6-methylguanine DNA methyltransferase; RT, radiation therapy; GTV, gross target volume.
Data show hazard ratios (HR) and 95% confidence intervals (CI).
Discussion
Current National Comprehensive Cancer Network (NCCN) guidelines in the United states allow for a range of adjuvant therapies for older GBM patients, including clinical trial, standard RT with temozolomide, hypofractionated RT with temozolomide, temozolomide alone for MGMT methylated patients, or hypofractionated RT alone (10). In the temozolomide era, direct comparisons between standard and hypofractionated RT are limited to retrospective studies, as no randomized data are available. Most retrospective analyses have report similar survival between standard 6-week and hypofractionated 3-week courses of RT (22–27). However, 2 larger series from Italy and California with 129 and 239 patients, respectively, did observe significantly increased survival with standard fractionation (28, 29). A 2019 meta-analysis of 917 patients also detected a significant difference in outcomes, with median OS 13.5 months (95% CI 10.0–16.9) after standard RT and 9.9 months (95% CI 6.5–13.3) after hypofractionated RT both with temozolomide (30).
The present study builds on existing literature and also examines RT details such as GTV size and early RT discontinuation. Similar to prior studies, increased age and poor KPS were significantly associated with selection of hypofractionated rather than standard RT with temozolomide. Median survival following standard and hypofractionated RT was not significantly different at 12.4 and 9.9 months, respectively. Instead, other clinical factors including increased age and poor KPS were associated with decreased survival. Both unmethylated and unknown MGMT status were also associated with poor outcomes, as the latter group likely contained mostly unmethylated patients. Eight (13%) patients discontinued adjuvant RT in the present study due to functional decline, with significantly diminished survival. The majority of these patients had already completed at least half of their RT courses. In this small cohort, no clinical factors were significantly associated with RT discontinuation, and the median pre-RT age was 78 and KPS was 80.
Limitations of the present study as well as other institutional retrospective series include small sample sizes as well as biases in patient and treatment selection. This study includes a highly selected patient population receiving treatment at a tertiary referral center, which may not reflect the patients seen in the community, especially those with limited functional status. This study also included patients ≥70 years old in accordance with NCCN guidelines, however, generally studies of older patients use cutoffs ranging from 65 to 75, making comparison across studies somewhat more challenging (10).
Further investigation into predictors of functional decline may help identify patients where shorter RT courses, palliative care, and other supportive interventions may be more appropriate (31). As noted above, the patients who discontinued RT had similar age and KPS compared to the larger cohort. Thus, in addition to age and KPS, additional measures such as geriatric screening tools and assessments may be helpful to guide selection of adjuvant RT fractionation. For example, the G8 screening tool has been validated in oncology patients >70 years old and more recently in GBM patients ≥65 years old (32, 33). In GBM patients, the G8 score was as stronger predictor of overall survival than age and receipt of radiation or chemotherapy (32). The G8 score also correlated with receipt of standard chemoradiation rather than more radiation alone, chemotherapy alone, or no medical treatment, though all chemoradiation in this study was given per the 6-week Stupp protocol (32). These geriatric screening tools and assessments are also useful for identifying baseline nutrition, mobility, and other functional vulnerabilities that may benefit from early intervention and perhaps even prevent functional decline during RT as well (34).
Conclusions
In this retrospective single-institution study of 62 GBM patients ≥70 years old who initiated adjuvant RT, median OS was 12.3 months. Age, KPS, MGMT methylation, and RT discontinuation were significantly associated with OS on multivariate analysis, while extent of maximal resection, use of standard or hypofractionated RT, and GTV size were not. Future investigation into factors associated with RT discontinuation and survival may help guide clinical decision-making on RT dose-fractionation and supportive care.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Duke University Medical Center Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JL: data collection, data analysis, and writing. JK: supervision. FM: formal data analysis. JH: formal data analysis. EL: data curation. AD: supervision. DR: supervision. HF: supervision. DA: supervision. KP: conceptualization, supervision, and project administration. MJ: data collection, writing, conceptualization, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JK reports grants from Varian Medical Systems, others from Clearsight RT Products, LLP, outside the submitted work. AD also receives research funding from Triphase Accelerator Corp., Orbus Therapeutics and Symphogen. AD serves as advisor/speaker/consultant for Istari Oncology and Orbus Therapeutics. AD holds stock/ownership interest with Istari Oncology. AD holds a letter of patent for Oncolytic Polio virus for the treatment of human tumors. HF received compensation for serving as Chief Medical Officer with Istari Oncology. HF holds stock/ownership interest with Istarti Oncology, Diverse Biotech, and Cancer Expert. HF serves as advisor/speaker/consultant for Cancer Expert. HF holds a letter of patent for Oncolytic Polio virus for the treatment of human tumors. KP receives research funding from Agios, BioMimetix, and Novocure. KP serves as advisor/speaker/consultant for Agios and Bayer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Wendy Gentry for assistance with editing and submission of this manuscript.
Abbreviations
RT, radiation therapy; GBM, glioblastoma; IRB, institutional review board; KPS, Karnofsky performance status; OS, overall survival; MGMT, O6-methylguanine DNA methyltransferase MGMT.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncology (2018) 20(suppl_4):iv1–iv86. 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352(10):987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3. Barker FG,2, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, et al. Age and radiation response in glioblastoma multiforme. Neurosurgery (2001) 49(6):1288–97; discussion 97-8. 10.1097/00006123-200112000-00002 [DOI] [PubMed] [Google Scholar]
- 4. Kita D, Ciernik IF, Vaccarella S, Franceschi S, Kleihues P, Lutolf UM, et al. Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology (2009) 33(1):17–22. 10.1159/000210017 [DOI] [PubMed] [Google Scholar]
- 5. Lutterbach J, Bartelt S, Momm F, Becker G, Frommhold H, Ostertag C. Is older age associated with a worse prognosis due to different patterns of care? A long-term study of 1346 patients with glioblastomas or brain metastases. Cancer (2005) 103(6):1234–44. 10.1002/cncr.20895 [DOI] [PubMed] [Google Scholar]
- 6. Roa W, Brasher PMA, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated Course of Radiation Therapy in Older Patients With Glioblastoma Multiforme: A Prospective Randomized Clinical Trial. J Clin Oncol (2004) 22(9):1583–8. 10.1200/JCO.2004.06.082 [DOI] [PubMed] [Google Scholar]
- 7. Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol (2012) 13(9):916–26. 10.1016/S1470-2045(12)70265-6 [DOI] [PubMed] [Google Scholar]
- 8. Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol (2012) 13(7):707–15. 10.1016/S1470-2045(12)70164-X [DOI] [PubMed] [Google Scholar]
- 9. Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. New Engl J Med (2017) 376(11):1027–37. 10.1056/NEJMoa1611977 [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network Inc . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Central Nervous System Cancers 2020 [V.1.2020]. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 11. Palmer JD, Bhamidipati D, Mehta M, Williams NL, Dicker AP, Werner-Wasik M, et al. Treatment recommendations for elderly patients with newly diagnosed glioblastoma lack worldwide consensus. J Neurooncol (2018) 140(2):421–6. 10.1007/s11060-018-2969-3 [DOI] [PubMed] [Google Scholar]
- 12. Lorimer CF, Saran F, Chalmers AJ, Brock J. Glioblastoma in the elderly - How do we choose who to treat? J Geriatr Oncol (2016) 7(6):453–6. 10.1016/j.jgo.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 13. Glaser SM, Dohopolski MJ, Balasubramani GK, Flickinger JC, Beriwal S. Glioblastoma multiforme (GBM) in the elderly: initial treatment strategy and overall survival. J Neurooncol (2017) 134(1):107–18. 10.1007/s11060-017-2493-x [DOI] [PubMed] [Google Scholar]
- 14. Bingham B, Patel CG, Shinohara ET, Attia A. Utilization of hypofractionated radiotherapy in treatment of glioblastoma multiforme in elderly patients not receiving adjuvant chemoradiotherapy: A National Cancer Database Analysis. J Neurooncol (2018) 136(2):385–94. 10.1007/s11060-017-2665-8 [DOI] [PubMed] [Google Scholar]
- 15. Haque W, Verma V, Butler EB, Teh BS. Patterns of Care and Outcomes of Hypofractionated Chemoradiation Versus Conventionally Fractionated Chemoradiation for Glioblastoma in the Elderly Population. Am J Clin Oncol (2018) 41(2):167–72. 10.1097/COC.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 16. Nead KT, Swisher-McClure S. Utilization of hypofractionated radiation therapy in older glioblastoma patients. J Geriatr Oncol (2019) 10(1):155–8. 10.1016/j.jgo.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 17. Mak KS, Agarwal A, Qureshi MM, Truong MT. Hypofractionated short-course radiotherapy in elderly patients with glioblastoma multiforme: an analysis of the National Cancer Database. Cancer Med (2017) 6(6):1192–200. 10.1002/cam4.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol (2013) 31(32):4085–91. 10.1200/JCO.2013.49.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabrera AR, Kirkpatrick JP, Fiveash JB, Shih HA, Koay EJ, Lutz S, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol (2016) 6(4):217–25. 10.1016/j.prro.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 20. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):709–22. 10.1056/NEJMoa1308345 [DOI] [PubMed] [Google Scholar]
- 22. Arvold ND, Tanguturi SK, Aizer AA, Wen PY, Reardon DA, Lee EQ, et al. Hypofractionated versus standard radiation therapy with or without temozolomide for older glioblastoma patients. Int J Radiat Oncol Biol Phys (2015) 92(2):384–9. 10.1016/j.ijrobp.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 23. Minniti G, Scaringi C, Lanzetta G, Terrenato I, Esposito V, Arcella A, et al. Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: a propensity-matched analysis. Int J Radiat Oncol Biol Phys (2015) 91(1):109–15. 10.1016/j.ijrobp.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 24. Wang TJC, Wu CC, Jani A, Estrada J, Ung T, Chow DS, et al. Hypofractionated radiation therapy versus standard fractionated radiation therapy with concurrent temozolomide in elderly patients with newly diagnosed glioblastoma. Pract Radiat Oncol (2016) 6(5):306–14. 10.1016/j.prro.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 25. Biau J, Chautard E, De Schlichting E, Dupic G, Pereira B, Fogli A, et al. Radiotherapy plus temozolomide in elderly patients with glioblastoma: a “real-life” report. Radiat Oncol (2017) 12(1):197. 10.1186/s13014-017-0929-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris G, Jayamanne D, Wheeler H, Gzell C, Kastelan M, Schembri G, et al. Survival Outcomes of Elderly Patients With Glioblastoma Multiforme in Their 75th Year or Older Treated With Adjuvant Therapy. Int J Radiat Oncol Biol Phys (2017) 98(4):802–10. 10.1016/j.ijrobp.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 27. Lapointe S, Florescu M, Simonyan D, Michaud K. Impact of standard care on elderly glioblastoma patients. Neurooncol Pract (2017) 4(1):4–14. 10.1093/nop/npw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang-Halpenny CN, Yeh J, Lien WW. Elderly patients with glioblastoma multiforme treated with concurrent temozolomide and standard- versus abbreviated-course radiotherapy. Perm J (2015) 19(1):15–20. 10.7812/TPP/14-083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lombardi G, Pace A, Pasqualetti F, Rizzato S, Faedi M, Anghileri E, et al. Predictors of survival and effect of short (40 Gy) or standard-course (60 Gy) irradiation plus concomitant temozolomide in elderly patients with glioblastoma: a multicenter retrospective study of AINO (Italian Association of Neuro-Oncology). J Neurooncol (2015) 125(2):359–67. 10.1007/s11060-015-1923-x [DOI] [PubMed] [Google Scholar]
- 30. Lu VM, Kerezoudis P, Brown DA, Burns TC, Quinones-Hinojosa A, Chaichana KL. Hypofractionated versus standard radiation therapy in combination with temozolomide for glioblastoma in the elderly: a meta-analysis. J Neurooncol (2019) 143(2):177–85. 10.1007/s11060-019-03155-6 [DOI] [PubMed] [Google Scholar]
- 31. Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D, et al. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol (2015) 33(35):4145–50. 10.1200/JCO.2015.62.6606 [DOI] [PubMed] [Google Scholar]
- 32. Deluche E, Leobon S, Lamarche F, Tubiana-Mathieu N. First validation of the G-8 geriatric screening tool in older patients with glioblastoma. J Geriatr Oncol (2019) 10(1):159–63. 10.1016/j.jgo.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 33. Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PloS One (2014) 9(12):e115060. 10.1371/journal.pone.0115060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szumacher E, Sattar S, Neve M, Do K, Ayala AP, Gray M, et al. Use of Comprehensive Geriatric Assessment and Geriatric Screening for Older Adults in the Radiation Oncology Setting: A Systematic Review. Clin Oncol (R Coll Radiol) (2018) 30(9):578–88. 10.1016/j.clon.2018.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.