Abstract
Background.
In 2007, the American Heart Association recommended that antibiotic prophylaxis (AP) be restricted to those at high risk of developing complications due to infective endocarditis (IE) undergoing invasive dental procedures. The authors aimed to estimate the appropriateness of AP prescribing according to type of dental procedure performed in patients at high risk, moderate risk, or low or unknown risk of developing IE complications.
Methods.
Eighty patients at high risk, 40 patients at moderate risk, and 40 patients at low or unknown risk of developing IE complications were randomly selected from patients with linked dental care, health care, and prescription benefits data in the IBM MarketScan Databases, one of the largest US health care convenience data samples. Two clinicians independently analyzed prescription and dental procedure data to determine whether AP prescribing was likely, possible, or unlikely for each dental visit.
Results.
In patients at high risk of developing IE complications, 64% were unlikely to have received AP for invasive dental procedures, and in 32 of 80 high-risk patients (40%) there was no evidence of AP for any dental visit. When AP was prescribed, several different strategies were used to provide coverage for multiple dental visits, including multiday courses, multidose prescriptions, and refills, which sometimes led to an oversupply of antibiotics.
Conclusions.
AP prescribing practices were inconsistent, did not always meet the highest antibiotic stewardship standards, and made retrospective evaluation difficult. For those at high risk of developing IE complications, there appears to be a concerning level of underprescribing of AP for invasive dental procedures.
Practical Implications.
Some dentists might be failing to fully comply with American Heart Association recommendations to provide AP cover for all invasive dental procedures in those at high risk of developing IE complications.
Keywords: Infective endocarditis, antibiotic prophylaxis, dental procedures, guidelines, prevention, antibiotic stewardship, risk
In 1955, the American Heart Association (AHA) issued the first guidelines on the use of antibiotic prophylaxis (AP) before invasive dental procedures to prevent infective endocarditis (IE).1 However, there has never been a randomized controlled trial to prove the efficacy of AP in prevention of IE,2 and it has been argued that daily activities, such as toothbrushing, flossing, and mastication, pose a greater risk of developing IE than invasive dental procedures.3 This, along with concerns about the risk of developing adverse drug reactions and antibiotic resistance, has led guideline committees to limit those for whom AP is recommended. As a result, in 2007 the AHA recommended that AP be restricted to patients undergoing invasive dental procedures who are at highest risk of developing IE complications.4
Although researchers have investigated the impact of this guideline change on the incidence of IE, in only 2 studies have they examined the impact on AP prescribing.5,6 Researchers in both of these studies identified a large reduction in AP prescribing for those at moderate risk of developing IE, for whom AP was no longer recommended. However, these researchers also identified a considerable reduction in AP prescribing for those at high risk of developing IE, for whom AP is recommended.5,6 Investigators in neither study evaluated whether AP was prescribed appropriately.
Researchers in only 1 US study attempted to quantify inappropriate AP prescribing. This group used a large administrative database to determine that 81% of AP prescribing for dental visits was inappropriate.7 However, these data combined the analysis of inappropriate prescribing of AP to prevent prosthetic joint infections and IE. Furthermore, the study was not designed to investigate whether AP was appropriately prescribed to those who should receive it.7
Our aim in conducting this study was to estimate the appropriateness of AP prescribing before dental procedures to prevent IE in a random sample of patients at high risk, moderate risk, or low or unknown risk of developing complications from IE selected from a large commercial claims database. A secondary aim was to evaluate the sensitivity and specificity of different protocols for automatic identification of when AP was prescribed to cover a dental procedure in large data sets, such as the IBM MarketScan Databases, to facilitate subsequent large-scale studies.
METHODS
Data source
The IBM MarketScan Databases8,9 are a collection of Health Insurance Portability and Accountability Act10–compliant data sets that integrate deidentified patient-level health data across the different databases. Because data are statistically deidentified in a Health Insurance Portability and Accountability Act–compliant manner to protect patient privacy, studies using the data are exempt from institutional review board review. The databases provide one of the largest convenience US health care data samples, with more than 255 million unique patients since 1995, including 41.2 million patients in the last full data year.8,9 For our study, we linked data from the IBM MarketScan Commercial, Medicare Supplemental, Dental and Prescription Benefits data sets.8,9 Enrollees in these health care plans older than 18 years with linked data for the period January 1, 2000 through August 31, 2015, were included in our study. These data provided a large nationally representative data sample of the US population with employer-provided health insurance.8,9 Data were included up through August 31, 2015; after this date, medical diagnosis and medical and dental procedure coding changed from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)11 to ICD-10,12 and codes were not always transferrable or equivalent.
IE risk stratification and identification of IE admissions
The database was queried from January 2000 to identify any ICD-9-CM or Current Procedural Terminology13 diagnosis or procedure codes that would have placed a patient at high or moderate risk of developing complications from IE defined according to the 2007 AHA guidelines (eBox 1, eTables 1–2 available online at the end of this article).4,14 Patients not identified as being at moderate or high risk were considered to be at low or unknown risk of developing IE.
IE hospital admissions were identified using ICD-9-CM diagnosis codes 421.0, 421.1, or 421.9, primary or secondary discharge diagnoses. Previously described methods were used to ensure that single continuous episodes of IE were counted only once.15 After enrollees had an IE-related hospital admission, they were considered at high risk of developing complications from future episodes of IE.
Selection of cases
From all patients who had fully linked dental, prescription benefits, and commercial medical or Medicare Supplemental data for January 1, 2011 through August 31, 2014, a computer algorithm randomly selected 80 patients at high risk, 40 at moderate risk, and 40 at low or unknown risk of developing complications from IE. Selecting patients from these dates ensured that there was an 11-year period to determine whether patients had diagnoses or procedures that would put them at high or moderate risk of developing complications from IE, and that there was at least 1 year to study AP coverage of dental procedures.
Invasive dental procedures
AHA guidelines provide a definition of dental procedures that should be covered with AP in those at highest risk of developing IE complications (eBox 2, available online at the end of this article).4 The American Dental Association Code on Dental Procedures and Nomenclature (CDT) codes16 and ICD-9-CM procedure codes11 were used to identify the types of dental procedure performed. Four dentists scrutinized these codes and divided them into the following 3 categories: codes identifying “red procedures” (invasive procedures that “should” be covered with AP for those at high risk of developing IE complications)4; codes identifying “yellow procedures” (invasive dental procedures that “may” be covered with AP on some occasions and not on others); and codes identifying “green procedures” (noninvasive dental procedures for which there is no recommendation for AP cover). When there were differences in opinion about grading of CDT and ICD-9-CM codes, agreement was reached through consensus discussion. Grading of CDT and ICD-9-CM codes is described in eTables 3 through 5 (available online at the end of this article). When a dental visit included multiple procedures, the most invasive procedure was ascribed to that visit, that is, red dental procedures took precedence, followed by yellow and then green procedures.
Prescribing data and manual identification of when a dental visit was covered by means of AP
Prescribing data available for each case included, the name of the prescribed drug, fill date, number of days’ supply, metric quantity, strength, dose prescribed, and refill number for all antibiotic prescriptions. Two clinicians, blinded to a patient’s risk status and nature of the dental procedure performed, independently evaluated each dental visit to determine whether it was likely, possible, or unlikely that visit had been covered by means of AP. Where there was disagreement, a third clinician arbitrated the case to achieve consensus. For AP to be considered likely or possible, a prescription had to be an oral antibiotic at a dose, or multiple of the dose, recommended for AP purposes according to the AHA guidelines; that is, 2 grams of amoxicillin, 600 milligrams of clindamycin, 2 g of cephalexin, 500 mg of azithromycin, or 500 mg of clarithromycin. All prescriptions were considered invalid after 1 year, and all prescriptions for more than a 5-day supply of antibiotic were considered unlikely to be for AP purposes. Both the number of AP prescriptions and, because some prescriptions covered multiple courses of AP (by providing several days’ supply or multiples of the recommended dose), the number of courses of AP prescribed were examined.
Automated identification of AP cover of a dental visit using computer algorithms
We also evaluated the performance of 3 AP algorithms (Table 1) against manual chart review (reference standard). Because the reference standard outcome was likely, possible, or unlikely covered by means of AP, but the algorithms produced a binary (covered versus not covered) outcome, we calculated the sensitivity of each algorithm against 2 alternative reference standard binary outcomes, that is, likely versus possible or unlikely; or likely or possible versus unlikely.
Table 1.
The definitions of the different algorithms used for identifying when antibiotic prophylaxis has been prescribed in large administrative data sets.
| PARAMETER* | ALGORITHM A | ALGORITHM B | ALGORITHM C |
|---|---|---|---|
| Antibiotics | Any systemic antibiotic | Oral amoxicillin, clindamycin, cephalexin azithromycin, or clarithromycin | Oral amoxicillin, clindamycin, cephalexin azithromycin, or clarithromycin |
| Dosage† | Any | 2 grams for amoxicillin, 600 milligrams for clindamycin, 2 g for cephalexin, 500 mg for azithromycin, or 500 mg for clarithromycin | 2 g for amoxicillin, 600 mg for clindamycin, 2 g for cephalexin, 500 mg for azithromycin, or 500 mg for clarithromycin |
| Days’ Supply | ≤ 2 | ≤ 3 | ≤ 5 |
| Time (Days) Between | ≤ 7 | ≤ 30 when days’ supply = 1 | ≤ 73 when days’ supply = 1 |
| Fill Date and Visit Date | ≤ 60 when days’ supply = 2 | ≤ 146 when days’ supply = 2 | |
| ≤ 90 when days’ supply = 3 | ≤ 219 when days’ supply = 3 | ||
| ≤ 292 when days’ supply = 4 | |||
| ≤ 365 when days’ supply = 5 |
Data parameters available for each prescription included drug name, fill date, number of days’ supply, metric quantity, strength, and refill number.
Dosage = metric quantity / 1-day supply × strength.
RESULTS
Patient characteristics
Our study comprised 160 cases: 80 high risk, 40 medium risk, and 40 low or unknown risk. The high-risk group was 63% men and the mean age (65 years) was significantly higher than the mean age of all patients (59 years). In contrast, the low or unknown risk group was 60% women and the mean age (47 years) was significantly lower than that for all patients (Table 2).
Table 2.
Patient characteristics.
| CHARACTERISTIC | HIGH-RISK PATIENTS | MODERATE-RISK PATIENTS | LOW- OR UNKNOWN-RISK PATIENTS | ALL PATIENTS |
|---|---|---|---|---|
| Patients, No. | 80 | 40 | 40 | 160 |
| Female Sex, % | 37.5 | 47.5 | 60 | 45.6 |
| Age, y | ||||
| Mean (SD*) | 65.1 (13.4)† | 60.1 (15.11) | 46.9 (15.0)† | 59.3 (16.0) |
| Median (IQR‡) | 67.0 (59.0–76.0) | 60.0 (49.0–71.0) | 49.0 (32.0–58.0) | 61.0 (49.0–70.0) |
| Study Duration for Each Patient, d | ||||
| Mean (SD) | 1,104 (473) | 1,199 (337) | 1,015 (437) | 1,105 (436) |
| Median (IQR) | 1,173 (696–1,394) | 1,255 (932–1,451) | 1,078 (703–1,361) | 1,196 (830–1,392) |
| Total Dental Visits/Patient Studied, No. | ||||
| Mean (SD) | 9.8 (5.3) | 10.1 (4.1) | 9.4 (5.7) | 9.8 (5.1) |
| Median (IQR) | 10 (6–13) | 10 (8–13) | 9 (5–12) | 9 (6–13) |
| Red§ Dental Visits/Patient, No. | ||||
| Mean (SD) | 5.8 (3.0) | 7.3 (2.6) | 5.8 (3.0) | 6.2 (3.5) |
| Median (IQR) | 6 (4–8) | 7 (5–9) | 6 (3–8) | 6 (4–8) |
| Yellow¶ Dental Visits/Patient, No. | ||||
| Mean (SD) | 1.7 (2.1) | 1.5 (1.5) | 1.6 (1.5) | 1.6 (1.8) |
| Median (IQR) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 1 (0–2) |
| Green# Dental Visits/Patient, No. | ||||
| Mean (SD) | 2.3(2.4) | 1.4 (1.9) | 1.6 (2.0) | 1.9 (2.2) |
| Median (IQR) | 2 (0–3) | 1 (0–2) | 1 (0–2) | 1 (0–3) |
SD: Standard deviation.
Significantly different from the mean for all cases (P < .05).
IQR: Interquartile range.
Invasive procedures that “should” be covered with antibiotic prophylaxis for those at high risk of developing infective endocarditis complications.
Invasive dental procedures that “may” be covered with antibiotic prophylaxis on some occasions and not on others.
Noninvasive dental procedures for which there is no recommendation for antibiotic prophylaxis cover.
There was no significant difference in the number of days of study or number of dental visits per patient available for us to examine among the different risk groups. The number of red dental visits studied was significantly higher for the moderate-risk group than for all patients, but there were no other significant differences in the number of different types of dental visit (red, yellow, and green) among the patient risk groups.
The most common factor for being high risk was prior heart valve replacement in 69 of 80 patients (86%), of which 45 (56%) were men, followed by previous IE in 11 of 80 patients (14%). Native valve abnormalities accounted for 37 of 40 patients (93%) at moderate risk of developing IE complications. In addition, there was 1 case each of rheumatic heart disease (in a female patient), hypertrophic cardiomyopathy (in a female patient), and congenital valve disease (in a male patient).
AP
Despite each case containing several red procedure visits, there was no evidence that a dental visit was covered in 32 of 80 patients (40%) at high risk of developing IE complications. This included 13 of 30 women (43%) and 19 of 80 men (24%). In the remaining 48 of 80 high-risk patients, there was evidence that AP was likely to have been given on at least 1 occasion. In all 80 high-risk patients, only 125 of 468 red dental visits (27%) were likely to have been (Figure 1). An additional 44 of 468 (9%) were possibly covered and 299 of 468 (64%) were unlikely to have been covered.
Figure 1.
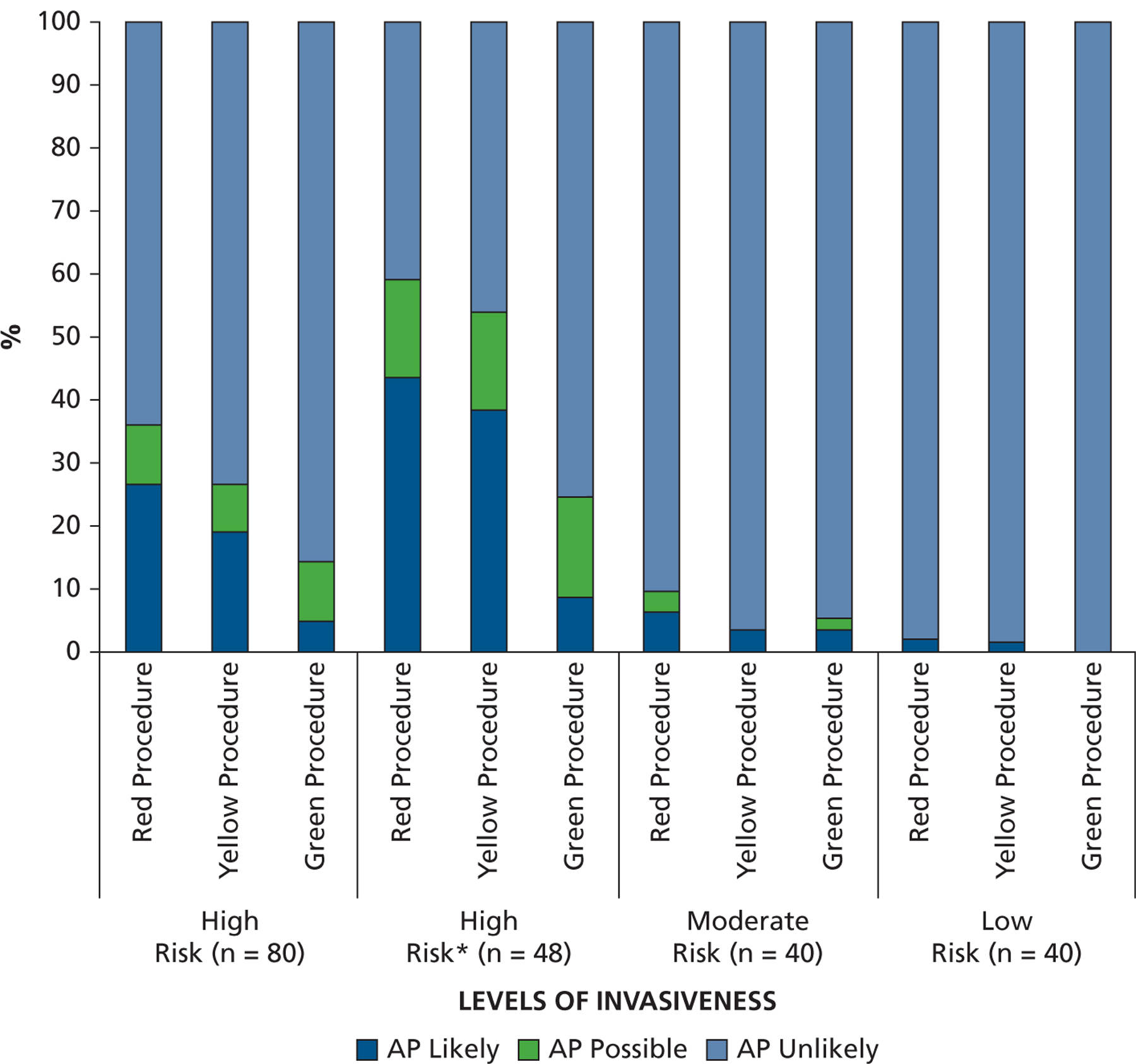
Percentage of different levels of invasiveness of dental procedures for which antibiotic prophylaxis coverage was likely, possible, or unlikely for those at high-risk, moderate-risk, or low or unknown risk of developing complications from infective endocarditis. * Excluding the 32 high-risk patients for whom there was no evidence of antibiotic prophylaxis coverage for any dental visit or procedure.
When analysis was confined to the 48 high-risk cases with evidence of AP being prescribed on at least 1 occasion, it was likely that only 125 of 290 red dental visits (43%) were covered with AP. An additional 44 of 290 (15%) were possibly covered and 121 of 290 (42%) were unlikely to have been covered.
For yellow dental visits, for which dentists have some discretion about when AP should be given, 19% of visits were likely to have been covered with AP, 7% possibly covered, and 74% were unlikely to have been covered in the entire high-risk group of 80 patients. In the 48-patient group, when those with no evidence of AP prescribing were excluded, 39% of yellow procedure visits were likely to have been covered, 15% possibly covered, and 46% were unlikely to have been covered. Although there is no recommendation to cover green procedure dental visits, 5% were likely and 9% possibly covered in the entire high-risk group. In the restricted high-risk group, 9% were likely and 16% possibly covered by means of AP.
In those at moderate risk of developing IE complications, for whom AP is not recommended, only 22 of 410 dental visits (5%) were likely and 11 of 410 (3%) possibly covered by means of AP. Of 376 dental visits recorded in those at low or unknown risk, only 5 were likely to have been covered by means of AP, all of which were for the same patient (Figure 1).
IE development after invasive dental procedures
Two patients, 1 man and 1 woman, were admitted to a hospital with an IE diagnosis after a dental visit. In both cases, the patients had not been identified as high risk or moderate risk of developing complications before IE developed. Both, however, had a record of undergoing a red D1110 (American Dental Association Code on Dental Procedures and Nomenclature) dental prophylaxis (supragingival scaling) procedure that was unlikely to have been covered by means of AP in the 4 months preceding the IE diagnosis.16 In 1 case, IE admission occurred 8 days after dental prophylaxis and 11 weeks after in the other case. Having become high risk as a result of IE, both patients continued to receive dental treatment that was tracked. In 1 patient, this continued for 41 months after IE diagnosis, during which 8 red, 2 yellow, and 10 green dental visits occurred, of which only 1 red and 1 green visit were likely to have been covered by means of AP; both of these visits occurred more than 3 years after the IE diagnosis. In the other patient, dental visit data continued for 13 months after the IE diagnosis, during which there were 3 red and 1 yellow dental visit. On each occasion, there was no evidence that AP was likely to have been prescribed.
Types of dental procedures performed
Across the different risk groups, scaling accounted for most (80%–90%) of the red procedures performed (Figure 2). Extractions were the next most common, followed by endodontic treatments. Figure 3 provides the percentages of the different types of red procedure that were likely, possibly, or unlikely to have been covered with AP. In those at high risk, the percentage of visits likely to have been covered with AP was highest (50%) for oral surgery procedures, implant procedures, and periodontal probing. It was lower for extractions (38% likely and 7% possible), endodontic treatment (31% likely and 4% possible), and all types of scaling (25% likely and 10% possible). However, coverage of subgingival scaling procedures was higher (34% likely and 10% possible) than for supragingival scaling (24% likely and 10% possible).
Figure 2.
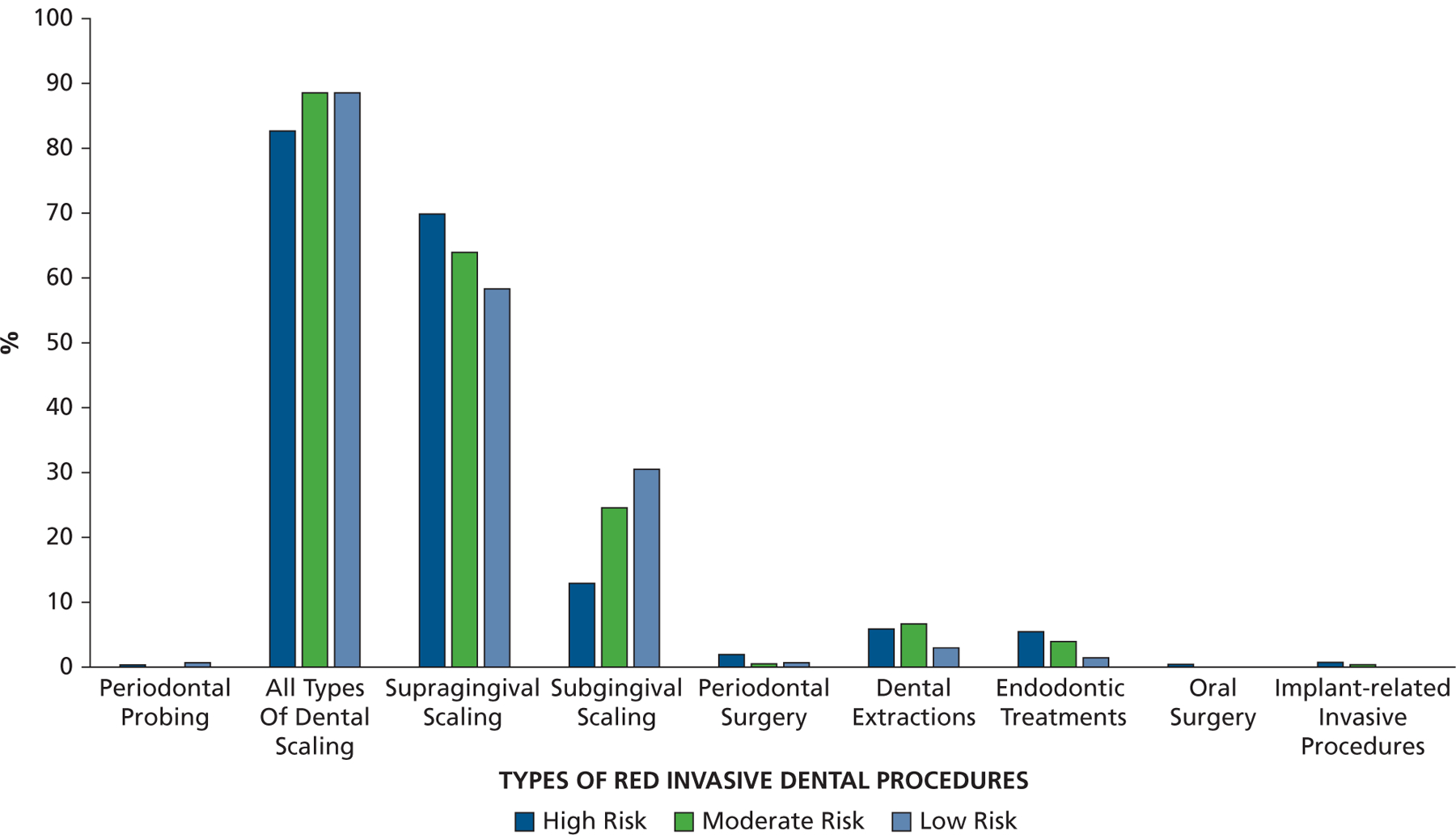
The proportion of all red invasive dental procedures characterized as periodontal probing, all types of dental scaling (subdivided via codes representing mainly supragingival scaling and those in which subgingival scaling is also required), periodontal surgery, dental extractions, endodontic treatments, oral surgery procedures, and implant-related invasive procedures.
Figure 3.
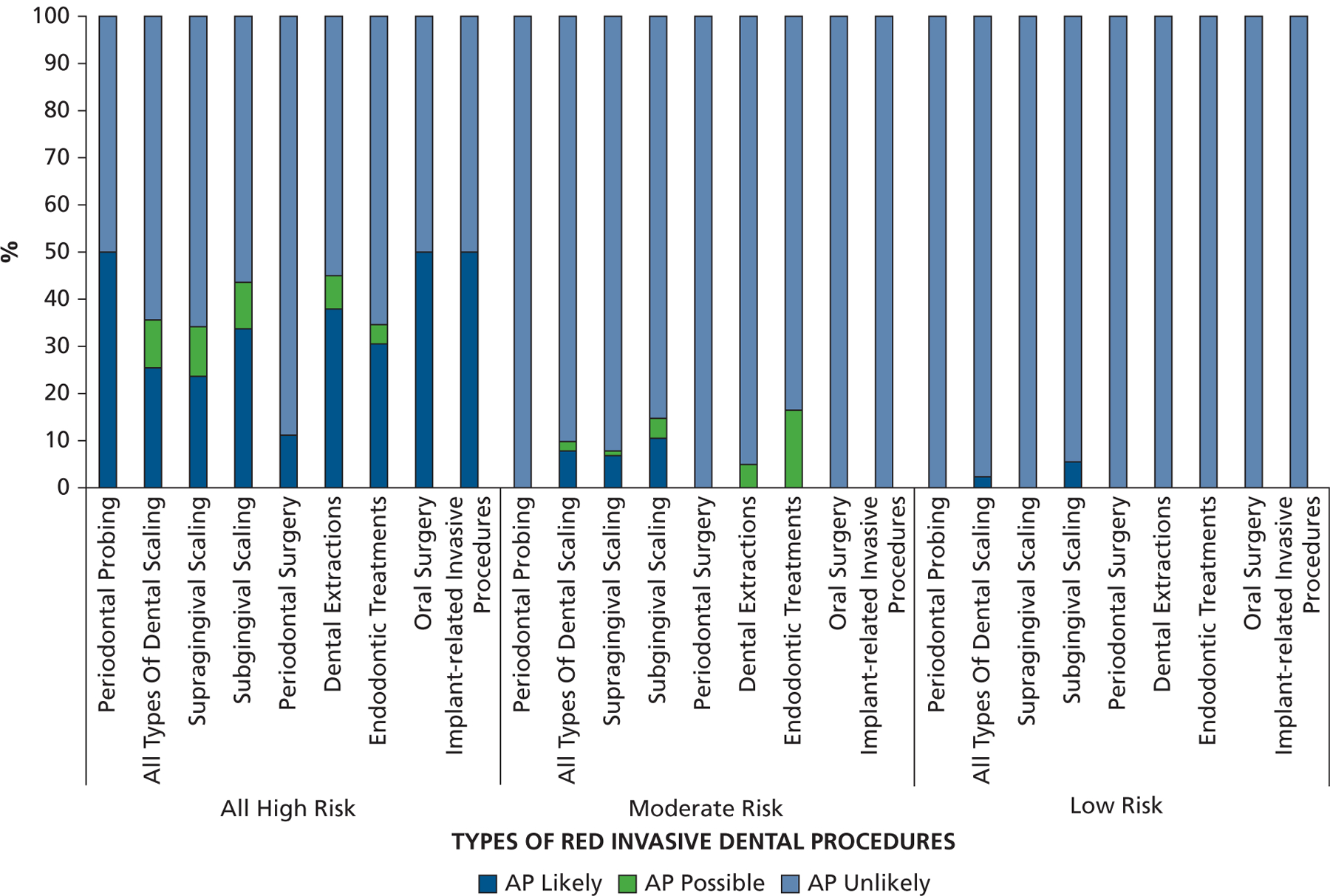
The proportion of different red invasive dental procedures, that is, periodontal probing, all types of dental scaling (subdivided via codes representing mainly supragingival scaling and those in which subgingival scaling is also required), periodontal surgery, dental extractions, endodontic treatments, oral surgery procedures, and implant-related invasive procedures for which antibiotic prophylaxis coverage was likely, possible, or unlikely for those at high risk, moderate risk, or low or unknown risk of developing complications from infective endocarditis. AP: Antibiotic prophylaxis.
In those at moderate risk, that is, patients for whom the AHA recommended AP should have ceased, 5 of 40 (13%) were likely to have been given AP and 2 of 40 (5%) possibly received AP. In those at low or unknown risk, for whom AP has never been recommended, only 1 patient received AP (for 5 D1110 dental prophylaxis visits and 1 visit for a 2-surface composite restoration of a posterior tooth [D2392] [American Dental Association Code on Dental Procedures and Nomenclature]).16
Types of AP prescription issued
Amoxicillin was the most frequently prescribed antibiotic, accounting for 147 of 196 (75%) of all AP prescriptions and 359 of 489 (73%) of all AP courses prescribed (Figure 4). This was followed by clindamycin (17% of prescriptions), clarithromycin (4%), azithromycin (3%), and cephalexin (1%).
Figure 4.
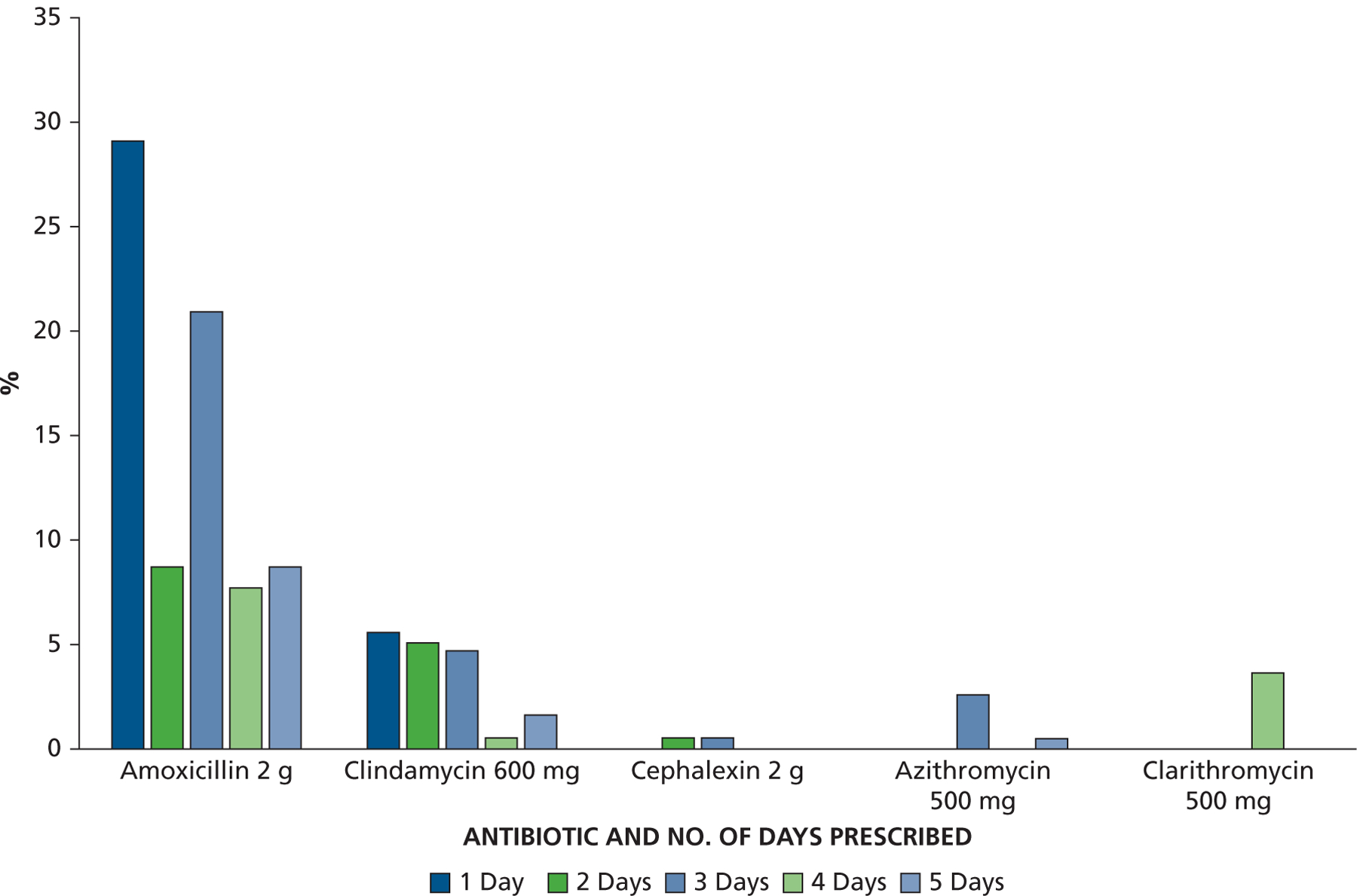
Antibiotic type and number of days prescribed as a percentage of all antibiotic prophylaxis prescriptions. g: Grams. mg: Milligrams.
Although, a prescription for a 1-day supply of AP was the most common, it accounted for only 35% of all AP prescriptions. A 3-day supply (3 courses) was the next most common, accounting for 29% of AP prescriptions, but prescriptions for 2, 4, and 5 days were also common. Multiday prescriptions were the norm for cephalexin, azithromycin, and clarithromycin. Figure 4 provides the proportion of all AP prescriptions according to antibiotic and number of days’ supply prescribed.
Sensitivity and specificity of automated protocols for detecting AP cover of dental visits
The performance of the 3 automated algorithms is displayed in Table 3. Of note, algorithm A has already been used in a “big data” study of the appropriateness of AP prescriptions before dental procedures.7 This algorithm had high specificity (100%) but very low sensitivity (25%) for correctly identifying when AP had been prescribed. That is, the prescriptions it identified were highly likely to be for AP, but it also frequently missed situations in which AP had been prescribed correctly. The other 2 protocols were developed for future studies to try to improve sensitivity while retaining good specificity. Although there was improved sensitivity (54%) in algorithm B compared with algorithm A, while retaining good specificity (99%), sensitivity was still poor. Algorithm C, however, improved sensitivity considerably (88%), with only a small additional reduction in specificity (96%).
Table 3.
The performance of different algorithms for identifying when antibiotic prophylaxis has been prescribed in large administrative data sets.
| VARIABLE | AP* LIKELY VERSUS AP POSSIBLE OR AP UNLIKELY | AP LIKELY OR AP POSSIBLE VERSUS AP UNLIKELY |
|---|---|---|
| Algorithm A | ||
| Sensitivity, % (confidence interval) | 25.4 (19.4 to 32.1) | 18.0 (13.6 to 23.0) |
| Specificity, % (confidence interval) | 100 (99.7 to 100) | 100 (99.7 to 100) |
| PPV,† % | 100 | 100 |
| NPV,‡ % (confidence interval) | 90.6 (89.8 to 91.2) | 85.3 (84.6 to 86.0) |
| Positive likelihood ratio | NA§ | NA |
| Negative likelihood ratio (confidence interval) | 0.75 (0.69 to 0.81) | 0.82 (0.78 to 19.31) |
| Algorithm B | ||
| Sensitivity, % (confidence interval) | 53.9 (46.6 to 61.1) | 42.5 (36.6 to 48.6) |
| Specificity, % (confidence interval) | 99.0 (98.3 to 99.4) | 99.9 (99.5 to 100) |
| PPV, % (confidence interval) | 88.1 (81.3 to 92.7) | 98.3 (93.5 to 99.6) |
| NPV, % (confidence interval) | 93.9 (93.0 to 94.7) | 89.2 (88.2 to 90.2) |
| Positive likelihood ratio (confidence interval) | 53.15 (31.09 to 90.96) | 276.40 (68.73 to 111.63) |
| Negative likelihood ratio (confidence interval) | 0.47 (0.40 to 0.54) | 0.58 (0.52 to 0.64) |
| Algorithm C | ||
| Sensitivity, % (confidence interval) | 87.6 (82.1 to 91.9) | 75.8 (70.3 to 80.8) |
| Specificity, % (confidence interval) | 95.5 (94.3 to 96.5) | 98.2 (97.3 to 98.8) |
| PPV, % (confidence interval) | 73.2 (68.0 to 77.8) | 89.6 (85.2 to 92.8) |
| NPV, % (confidence interval) | 98.2 (97.4 to 98.8) | 95.1 (94.0 to 96.0) |
| Positive likelihood ratio (confidence interval) | 19.50 (15.21 to 25.02) | 41.10 (27.50 to 61.44) |
| Negative likelihood ratio (confidence interval) | 0.13 (0.09 to 0.19) | 0.25 (0.20 to 0.30) |
AP: Antibiotic prophylaxis.
PPV: Positive predictive value.
NPV: Negative predictive value.
NA: Not applicable.
DISCUSSION
The finding that there was no evidence of AP being prescribed before any dental procedure in 40% of high-risk patients is high. When all high-risk patients were considered, 64% of red dental procedures were unlikely to have been covered with AP. This suggests significant underprescribing of AP. It is unclear whether this lack of compliance with the AHA recommendations is consistent with continued widespread confusion about AP in general, a lack of awareness of patient comorbidities, prescriber inertia, patient nonadherence, or limitations with the use of administrative data.17,18
Our findings are consistent with those of researchers from a large French study who reported only 50% of invasive dental procedures were covered by means of AP in patients at high risk of developing complications from IE.19 However, that study accepted all antibiotic prescriptions that might have activity against oral streptococci in the 21 days before an invasive dental procedure as evidence of AP coverage, regardless of duration or dose prescribed. The actual level of AP prescribing was likely lower. These findings are also consistent with the 15% to 20% decline in AP prescribing for those at high risk of developing IE complications after the 2007 changes in AHA recommendations.5,6
The underprescribing and fall in AP observed in those at high risk might reflect difficulties experienced by dentists in distinguishing between high-risk and moderate-risk cardiac conditions, as identified in a 2020 systematic review17 and questionnaire survey of US dentists.18 However, high-risk patients in our study had either a replacement heart valve or a history of IE and should not have been difficult to identify.
Survey data suggest that delayed adoption of evidence-based practices and confusion might be contributing to our observations.17,18,20 A 2010 survey of 878 US dentists reported that 70% had 1 or more patients who continued to receive AP before invasive dental procedures, although it was no longer recommended,20 and results of a 2020 survey of US dentists showed confusion about which patients should receive AP.18
Scaling procedures accounted for most of the dental procedures that might require AP in dental offices. These were divided into those mainly involving supragingival scaling (eTable 5, available online at the end of this article) and those that involved subgingival scaling or root planning. The ratio of supragingival to subgingival procedures was highest in those at high risk, less in those at moderate risk, and lowest in those at low or unknown risk. This might suggest that dentists were attempting to be less invasive with dental prophylaxis in those at highest risk. In addition, supragingival scaling was less likely to be covered by means of AP than subgingival scaling and root planning, which suggests that dentists might regard supragingival scaling as less invasive. In a study published in 2018, however, researchers found that there was no significant difference in size or magnitude of bacteremia that characterizes supragingival scaling and dental extractions,21 suggesting supragingival scaling is of considerable invasiveness. The IE that developed in 2 patients after an invasive dental procedure in our investigation did so after uncovered supragingival scaling.
A 2 g dose of amoxicillin accounted for most of all AP prescriptions, with penicillin alternatives accounting for 22%. Our study could not distinguish whether amoxicillin alternatives were prescribed owing to a history of penicillin allergy or for other reasons. However, of the penicillin alternative prescriptions dispensed, 20 (51%) were for patients who were prescribed amoxicillin AP at other times. Only on 2 occasions was the penicillin alternative prescribed in the 3 weeks after an amoxicillin prescription. This suggests that penicillin alternatives are prescribed frequently, even when a patient does not have a history of penicillin allergy. Given the comparative safety of AP prophylaxis with amoxicillin,22 and the greater risk of developing adverse drug reactions with alternatives,22,23 this raises antibiotic stewardship concerns.
We also found that although some dentists issue a separate prescription for each AP course, others prescribe multiday courses, multiples of the normal AP dose, or permit prescription refills to provide patients with supplies they need to cover multiple dental visits. There are several potential concerns with this. First, from a study perspective, this makes it more difficult to determine whether a dental visit was covered by means of AP. This is particularly the case with longer-duration prescriptions, for example, 5 days, when there is also uncertainty whether the prescribed course of antibiotics was intended to treat an infection or to provide AP cover for multiple dental visits. Examining dental treatment codes for each visit and timing of prescriptions, however, often made it possible to establish whether a prescription was intended for treatment or AP purposes.
Although multiday prescriptions can reduce cost for patients, they raise the following antibiotic stewardship questions: Will patients remember to take AP for a future dental visit up to 1 year later? Will they take the required single dose? Will the antibiotic be used before its use-by date? Will patients take the antibiotic for non-AP purposes? Some patients in our cohort were clearly issued antibiotics more frequently than needed (for example, 3- to 5-day courses prescribed each time a patient had a red procedure dental visit). Another possibility is that some dentists are not following the single-dose recommendation for AP cover, as identified in some questionnaire surveys.18
Our investigation was used to validate 3 algorithms to identify when a dental visit or procedure was covered by means of AP in large data studies and to determine their sensitivity and specificity.
Study limitations
The data described here are based on the case histories of patients selected randomly from populations at high risk, moderate risk, or low or unknown risk of developing IE. Because they were selected randomly, it is assumed that that they represent all such patients, but that might not be the case. As we described, the different strategies that dentists use to prescribe AP, particularly the practice of covering multiple courses of AP with a single prescription, made it difficult to verify when or whether a particular dental visit was covered. Even when a single dose of AP was prescribed immediately before an invasive dental procedure visit, we could not be certain that the patient took the AP. Similarly, even when there was no evidence that an antibiotic prescription was issued, we cannot be certain that the patient was not provided AP through some other means, for example, via the dentist dispensing the antibiotic from their office without a prescription record. For these reasons, we chose to use the terms “likely,” “possible,” and “unlikely” to describe the probability that AP was prescribed rather than using more definitive categorical terms.
Although our study results suggest substantial levels of underprescribing of AP to those at high risk of developing IE complications after undergoing invasive dental procedures, we have to caution that it is possible we have underestimated AP prescribing owing to nonrecorded direct dispensing of AP via dentists. The level of this practice would have to be high, however, to account for there being no AP records in 40% of high-risk patients or for AP cover to have been likely for only 26.7% of high-risk red dental visits.
Ultimately, the best way to capture dental antibiotic prescribing practices is likely via a prospective process that incorporates patient comorbidities, antibiotic prescriptions data (including both pharmacy and in office), dental procedure, and indication for prescription. These data would help provide baseline information to drive future antibiotic stewardship interventions.
CONCLUSIONS
This study identified substantial underprescribing of AP in patients at high risk of developing IE complications undergoing invasive dental procedures. Of the invasive dental procedures performed, scaling accounted for most (80%–90%). The study also identified a number of different prescribing strategies to provide AP, particularly for repeat dental visits, some of which might not be consistent with modern antibiotic stewardship recommendations. In addition, this study validates, for the first time to our knowledge, the sensitivity and specificity of algorithms to identify AP prescribing in big data studies.
Supplementary Material
Disclosures.
Drs. Thornhill, Gibson, Lockhart, and O’Gara received support from the Delta Dental Research and Data Institute for the submitted work. Dr. O’Gara received support from Medtronic, Edwards Scientific, and the National Heart Lung Blood Institute, National Institutes of Health, which was unconnected to the submitted work. Dr. Dayer received reports from Biotronik, which was unconnected to the submitted work. None of the other authors reported financial relationships with companies that might have an interest in the submitted work. Drs. Thornhill, Gibson, Durkin, and O’Gara have no nonfinancial interests that might be relevant to the submitted work.
Dr. Lockhart is a member of the Writing Committee for the next American Heart Association’s guidelines on antibiotic prophylaxis to prevent infective endocarditis. Drs. Baddour and Lockhart were members of the American Heart Association’s Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease and were involved in producing the 2007 American Heart Association guideline on prevention of infective endocarditis. Dr. Dayer was a consultant to the review committee that produced the 2015 update to National Institute for Health and Care Excellence (UK) clinical guideline 64 on prophylaxis against infective endocarditis.
This study was funded by a research grant from Delta Dental of Michigan and its Research and Data Institute. The funding source had no role in the study design, collection, analysis or interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
The authors acknowledge the indispensable free advice, comments, and assistance of several colleagues in general and specialty dental practice in regard to matters of dental practice and coding. In particular, they wish to acknowledge the assistance of Dr. Richard Potter and colleagues of the Texas Dental Association 20th District, San Antonio District Dental Society, Dr. Julianne K. Ruppel (Ruppel Orthodontics, St. Louis, MO), Dr. Thomas Paumier (general dentist, Ohio), Dr. Jeffery Johnston (Delta Dental of Michigan, Ohio, and Indiana), and Dr. Jed Jacobson (Ann Arbor, MI). Although they provided invaluable advice to the research team, their views might not reflect any views expressed in this article.
ABBREVIATION KEY
- AHA
American Heart Association
- AP
Antibiotic prophylaxis
- CDT
American Dental Association Code on Dental Procedures and Nomenclature
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IE
Infective endocarditis
- NA
Not applicable
Footnotes
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
SUPPLEMENTAL DATA
Supplemental data related to this article can be found at: https://doi.org/10.1016/j.adaj.2020.07.021.
Supplemental material is available online.
Contributor Information
Martin H. Thornhill, Dr. Thornhill is a professor, Translational Research in Dentistry, University of Sheffield, Sheffield, UK; and an adjunct professor, Department of Oral Medicine, Carolinas Medical Center, Atrium Health, Charlotte, NC..
Teresa B. Gibson, Dr. Gibson is a senior director, Health Outcomes Research, IBM Watson Health, Ann Arbor, MI..
Michael J. Durkin, Dr. Durkin is an assistant professor of medicine, Division of Infectious Diseases, Department of Internal Medicine, Washington University in Saint Louis School of Medicine, St Louis, MO..
Mark J. Dayer, Dr. Dayer is a consultant cardiologist, Department of Cardiology, Taunton and Somerset National Health Service Trust, Taunton, Somerset, UK..
Peter B. Lockhart, Dr. Lockhart is a research professor, Department of Oral Medicine, Carolinas Medical Center, Atrium Health, Charlotte, NC..
Patrick T. O’Gara, Dr. O’Gara is the director of strategic planning, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA; and the Watkins Family Distinguished Chair in cardiology and a professor, Harvard Medical School, Boston, MA..
Larry M. Baddour, Dr. Baddour is an emeritus professor of medicine, Division of Infectious Diseases, Departments of Medicine and Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN..
References
- 1.American Heart Association. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation. 1960;21(1):151–155. [Google Scholar]
- 2.Cahill TJ, Harrison JL, Jewell P, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart. 2017;103(12):937–944. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117(24):3118–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson W, Taubert KA, Gewitz M, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736–1754. [DOI] [PubMed] [Google Scholar]
- 5.DeSimone DC, El Rafei A, Challener DW, et al. Effect of the American Heart Association 2007 Guidelines on the Practice of Dental Prophylaxis for the Prevention of Infective Endocarditis in Olmsted County, Minnesota. Mayo Clin Proc. 2017;92(6):881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornhill MH, Gibson TB, Cutler E, et al. Antibiotic prophylaxis and incidence of endocarditis before and after the 2007 AHA recommendations. J Am Coll Cardiol. 2018;72(20):2443–2454. [DOI] [PubMed] [Google Scholar]
- 7.Suda KJ, Calip GS, Zhou J, et al. Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw Open. 2019;2(5):e193909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IBM Watson Health. The IBM MarketScan Research Databases for Life Sciences Researchers: Data Brochure. Somers, NY: IBM Watson Health; 2019. [Google Scholar]
- 9.IBM Watson Health. The Truven Health MarketScan Databases for Health Services Researchers: White Paper. Somers, NY: IBM Watson Health; 2019. [Google Scholar]
- 10.The Health Insurance Portability and Accountability Act of 1996. Pub. L. 104–191. Stat. 1936. Web. 11 Aug. 2014.
- 11.Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available at: https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed September 8, 2019.
- 12.World Health Organization. International Classification of Diseases. 10th Revision. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 13.American Medical Association. CPT Professional 2020. Chicago, IL: American Medical Association; 2019. [Google Scholar]
- 14.Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. Circulation. 1997;96(1):358–366. [DOI] [PubMed] [Google Scholar]
- 15.Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. 2011;342:d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Dental Association (ADA) Code on Dental Procedures and Nomenclature (CDT Code). Available at: https://www.ada.org/en/publications/cdt. Accessed September 8, 2019.
- 17.Cummins J, McCarthy M, Esterman A, Karve A, Lee A. Knowledge and compliance of dentists’ and dental students’ with respect to relevant guidelines for prescribing antibiotic prophylaxis for the prevention of infective endocarditis: a systematic review. J Evid Based Dent Pract. 2020;20(1):101311. [DOI] [PubMed] [Google Scholar]
- 18.Lockhart PB, Thornhill MH, Zhao J, et al. Prophylactic antibiotic prescribing in dental practice: findings from a national dental PBRN questionnaire. JADA. 2020; 151(10):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tubiana S, Blotiere PO, Hoen B, et al. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: nationwide population based cohort and a case crossover study. BMJ. 2017; 358:j3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart PB, Hanson NB, Ristic H, Menezes AR, Baddour L. Acceptance among and impact on dental practitioners and patients of American Heart Association recommendations for antibiotic prophylaxis. JADA. 2013; 144(9):1030–1035. [DOI] [PubMed] [Google Scholar]
- 21.Reis LC, Rôças IN, Siqueira JF Jr., et al. Bacteremia after supragingival scaling and dental extraction: culture and molecular analyses. Oral Dis. 2018;24(4):657–663. [DOI] [PubMed] [Google Scholar]
- 22.Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother. 2015;70(8):2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornhill MH, Dayer MJ, Durkin MJ, Lockhart PB, Baddour LM. Risk of adverse reactions to oral antibiotics prescribed by dentists. J Dent Res. 2019;98(10):1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


