SUMMARY:
Initially used in the treatment of prostate cancer and uterine fibroids, the role of focused ultrasound has expanded as transcranial acoustic wave distortion and other limitations have been overcome. Its utility relies on focal energy deposition via acoustic wave propagation. The duty cycle and intensity of focused ultrasound influence the rate of energy deposition and result in unique physiologic and biomechanical effects. Thermal ablation via high-intensity continuous exposure generates coagulative necrosis of tissues. High-intensity, pulsed application reduces temporally averaged energy deposition, resulting in mechanical effects, including reversible, localized BBB disruption, which enhances neurotherapeutic agent delivery. While the precise mechanisms remain unclear, low-intensity, pulsed exposures can influence neuronal activity with preservation of cytoarchitecture. Its noninvasive nature, high-resolution, radiation-free features allow focused ultrasound to compare favorably with other modalities. We discuss the physical characteristics of focused ultrasound devices, the biophysical mechanisms at the tissue level, and current and emerging applications.
The use of therapeutic ultrasound predates its role in diagnostic imaging. Early applications of therapeutic ultrasound were primarily in physical therapy for the treatment of musculoskeletal injuries, albeit in a low-energy, nonfocused manner.1 More recently, higher energy focused ultrasound (FUS) has demonstrated enormous therapeutic and research potential via newly discovered, unique bioeffects. Therapeutic FUS relies on acoustic wave propagation directed at a specific focus, generating high-resolution focal energy deposition while sparing intervening and adjacent tissues. Acoustic waves can be generated by single-element transducers. Newer generation devices offer multielement phased array transducers, allowing electronic steering of the focal zone.2
Experimentation with neurologic FUS applications began in earnest more than 60 years ago with partial ablation of the basal ganglia in cats and monkeys in 1955 by Fry et al3 and intracranial tumor therapy in humans performed by Heimburger4 3 decades later. Initially, impedance mismatch and nonuniformity at the soft-tissue-calvarial interface necessitated surgical craniotomy for intracranial FUS application. Once reliable transcranial propagation was achieved, the morbidity associated with craniotomy was no longer an obstacle in neurologic FUS application and the technique gained more widespread acceptance as a viable noninvasive alternative to current therapeutic options. Clinical and preclinical investigations into potential applications of the controlled deposition of mechanical energy, in the form of ultrasound, have since accelerated and yielded a variety of bioeffects based on the level of energy deposition. At high levels of deposition, tissue heating generates irreversible necrosis of the target area. As the temporally averaged rate of energy deposition is decreased, via decreasing the ultrasound intensity and duty cycle (ie, ratio of ON and OFF), mechanical effects can increase the permeability of the blood-brain barrier and influence neuronal activity, via both reversible suppression and stimulation.
Advances in Transcranial FUS
In 2002, the biggest obstacle in the translation of FUS to neurologic applications was overcome when Clement and Hynynen5 achieved reproducible, high-resolution focal energy deposition via transcranial acoustic wave propagation, obviating craniotomy. This is accomplished via registration of data from CT interrogation of the calvaria, specifically to assess its attenuation, contour, and thickness. These data are registered with MR images and serve as input to the MR imaging–guided FUS (MRgFUS) apparatus, which can individually steer up to 1024 ultrasound elements to compensate for predicted acoustic wave distortions at the soft-tissue–calvarial interface. Focused transcranial acoustic wave propagation can then be achieved with a resolution of approximately 1 mm.5 The phased array transducer also offers the versatility of creating numerous focal points and hence treating larger volumes. This is currently being evaluated for generating regional hyperthermia, which may enhance the efficacy of chemotherapy and radiation therapy (Fig 1).6
Fig 1.
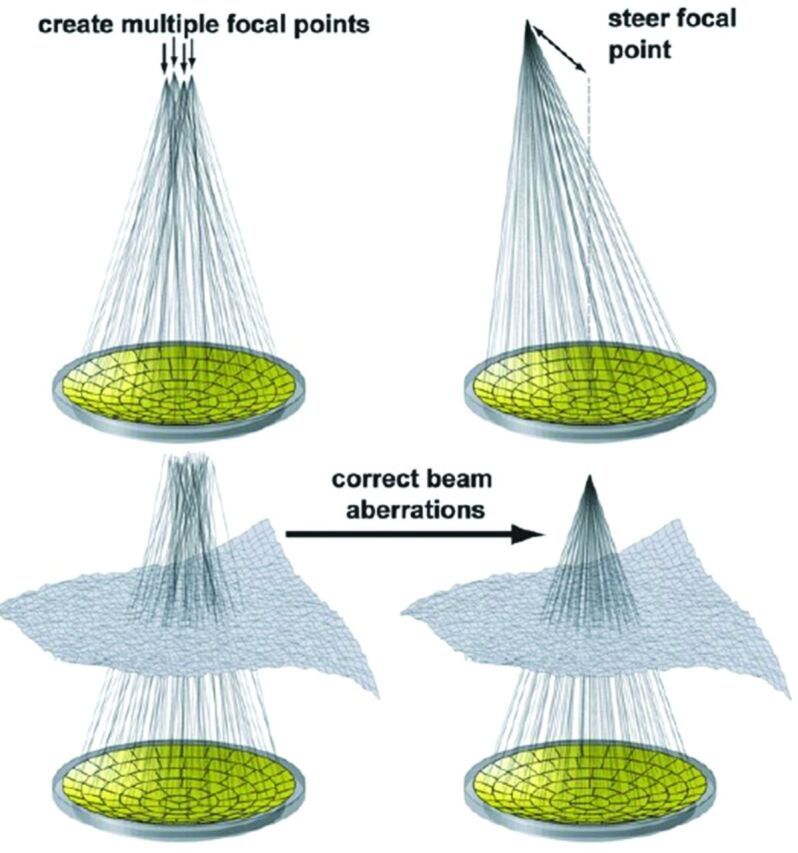
With a multielement, hemispheric phased array transducer, a single focus can be electronically steered (upper right), multiple focal points can be generated (upper left), and corrections can be achieved for aberrations in the beam path. Reprinted with permission from Tempany et al.37
Developments in real-time image guidance and monitoring have expanded the scope of FUS therapies, namely the integration of MR imaging–guided therapeutic systems. Early image guidance of FUS relied entirely on diagnostic ultrasound images for treatment planning and monitoring tissue-level effects, such as in the treatment of prostate cancer.7 Current MR imaging–guided therapeutic systems offer superior soft-tissue detail, allowing preservation of nontarget tissues and accurate identification of tumor margins and other potential ROIs. Temperature maps via noninvasive, near-real-time MR thermometry enable validation of effective treatment for thermal therapies (ie, ablation, hyperthermia) and determination whether the nontargeted tissue in adjacent regions has been spared.
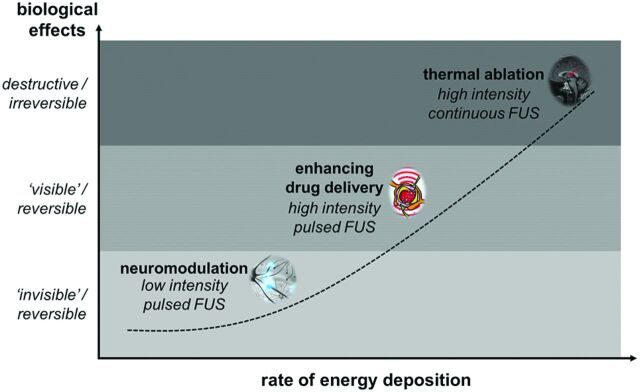
The versatility of the MRgFUS apparatus is a product of its ability to manipulate the volume and degree of energy deposition. The magnitude of local energy deposition generated by focused acoustic wave propagation is influenced by the intensity and duty cycle of the ultrasound application. High-intensity, continuous FUS application can generate marked focal temperature elevation, reaching up to 65°C or greater (with tissue devitalization generally achieved at temperatures exceeding 55°C)8 within a matter of seconds. When FUS is applied in a pulsed, high-intensity fashion, cooling can occur between the pulses and the temporally averaged intensity can be lowered substantially. These factors lower the temperature elevations to just a few degrees Celsius. As a result, mechanical effects predominate on local cytoarchitecture, without irreversible thermal injury.9 Further reduction in energy deposition is achieved with a pulsed low-intensity application, which may generate unique neuromodulatory effects with negligible temperature elevations (Fig 2).
Fig 2.
Unique biologic effects can be achieved over a range of energy-deposition rates by manipulating the intensity and duty cycle of the ultrasound application. These include neuromodulation, localized reversible enhancement of blood-brain barrier permeability, and thermal ablation.
High-Intensity, Continuous FUS
The therapeutic benefit of high-intensity continuous FUS application is related to the degree of localized temperature elevation, resulting in irreversible coagulative necrosis at the tissue level, and is the basis of ablative therapies. The treatment of benign prostatic hyperplasia and prostate cancer was the earliest clinical application of this form of FUS and used a transrectal transducer and a collinear ultrasound imaging transducer for both treatment planning and monitoring. Newer generation devices use MR imaging guidance and were first used for the treatment of uterine fibroids.7 The use of MRgFUS in the treatment of uterine fibroids has since acquired FDA approval and is reimbursed by insurance providers on an individual basis. The advent of transcranial devices has provided the impetus for investigation into a variety of neurologic applications.
One of the more promising applications of FUS for thermal ablation has been in the treatment of medication-refractory essential tremor. While the exact etiology of the disorder is not entirely understood, disruption of the ventral intermediate nucleus of the thalamus, via stereotactic radiosurgery or deep brain stimulation, has been shown to be effective in the management of symptoms.10 In 2013, Elias et al11 demonstrated reliable improvement in tremor-related disability up to 1 year after MRgFUS-induced unilateral thalamotomy of the ventral intermediate nucleus. Other institutions have since successfully replicated these findings, and the procedure was recently FDA approved. Recent evidence also suggests a role for FUS-induced thermal ablation in cases of Parkinson disease refractory to pharmacologic intervention. In a small trial (n = 13), FUS-induced thermal ablation of the pallidothalamic tract resulted in significant reduction in symptoms as measured by the Unified Parkinson Disease Rating Scale and patient assessment of global symptom relief. Patients undergoing MRgFUS-induced thermocoagulation of the pallidothalamic tract experienced a reduction in symptomatology that was comparable with that in stereotactic radiosurgery when effective parameters were applied.12
Similarly, MRgFUS-induced central lateral thalamotomy in patients with medication-refractory neuropathic pain syndrome has also been shown to result in symptomatic relief. In a small study (n = 11) performed in 2012, immediate pain relief was achieved during sonication in more than half the subjects. All except 1 patient experienced substantial symptomatic relief, which persisted 1 year after therapy (57% mean pain relief). The study included patients with both peripheral and central etiologies of neuropathic pain syndrome, and symptomatic benefit was similar in both groups.13
An effective and safe protocol for MRgFUS in the treatment of primary solid intracranial neoplasms remains more elusive. The infiltrative nature of primary gliomas makes ablation uniquely challenging. In a small clinical trial assessing the role of MRgFUS in the ablation of high-grade gliomas, coagulative necrosis and complete ablation of the tumors were not achieved in 3 patients undergoing MRgFUS treatment. After modification of the protocol, a fourth patient who underwent treatment had successful ablation of the tumor but with fatal intracranial hemorrhage a few days after treatment, possibly related to an underlying coagulopathy. Current understanding is that lower frequency application, as attempted in the fourth patient, seems to be associated with an increased risk of bleeding.14 A universal protocol has not yet been established in the ablation of primary gliomas, and continued investigations are necessary.
High-Intensity, Pulsed FUS
By applying FUS in a pulsed mode rather than a continuous application, the temporally averaged rate of energy deposition is reduced. As a result, lower temperature elevations are achieved without irreversible thermal injury. Rather, mechanical effects will predominate, which may be used to enhance drug and gene delivery9 via reversible localized enhanced permeability of the BBB (Fig 3).15 Early investigations into FUS-induced BBB permeability enhancement were inconsistent and required acoustic energy levels that resulted in localized tissue damage in the region of treatment. Later development demonstrated that when these exposures were used in conjunction with ultrasound contrast agents in the form of “microbubbles,” lower acoustic energies were required and BBB permeability was more reproducible. The underlying mechanism is related to the controlled oscillation of the bubbles by the varying pressure field of the ultrasound wave. It is theorized that bubble interactions with endothelial cells lead to the compromised integrity of the tight junctions and subsequent “leakiness” of the endothelial membrane.16
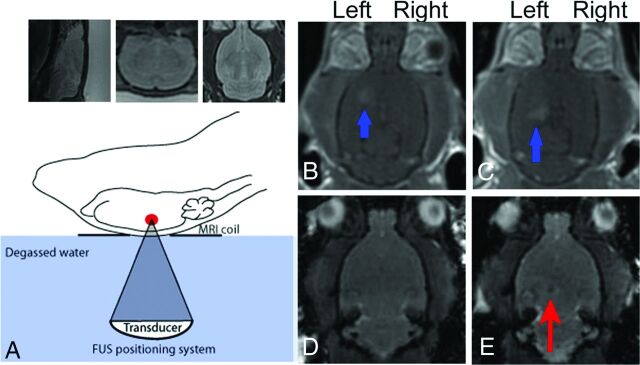
Fig 3.
Application of MRgFUS for the delivery of iron-labeled neural stem cells. Schematic of the FUS apparatus (lower left). Sagittal, coronal, and axial T2-weighted images (A) are used to identify the intended targets of neural stem cell delivery in the left hippocampus and left striatum. T1-weighted, postcontrast images after local FUS sonication (B and C) demonstrate enhancement in the striatum and hippocampus (blue arrows) compatible with enhanced BBB permeability. Fast gradient-echo sequences are obtained before (D) and after (E) sonication, localizing a focus of hypointense signal (red arrow) confirming delivery of iron-labeled neural stem cells. Reprinted with permission from Burgess et al.40
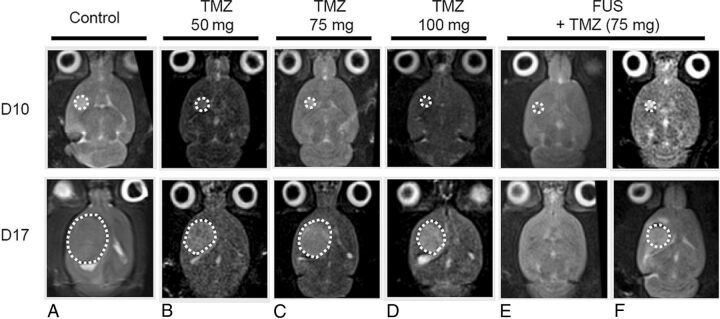
Enhanced drug delivery by using FUS is actively being investigated in several studies to determine a role in the treatment of CNS neoplasms.17,18 Glioblastoma multiforme, the most common primary brain tumor in adults, is among the most lethal CNS neoplasms and has limited chemotherapeutic options. Temozolomide (TMZ) is one of the few agents that has shown a survival benefit in multiple large phase III trials, though posttreatment, 5-year mortality remains abysmal.19 In a small preclinical study with a noninvasive glioma model, Wei et al18 compared small-, medium-, and high-dose TMZ groups with a medium-dose TMZ + FUS group in regard to CSF/plasma TMZ concentrations, volume of tumor progression, and survival time in a rat model. The TMZ + FUS group demonstrated the highest TMZ CSF/plasma ratio, and MR imaging confirmed that the TMZ + FUS group showed the lowest rate of tumor progression compared with TMZ alone, regardless of concentration (Fig 4). Most important, the TMZ + FUS group was the only group to demonstrate a survival benefit compared with the control group (15% median survival time benefit).18
Fig 4.
Comparison of TMZ versus TMZ + FUS. At day 10, tumors in all groups are similar in size, designating the start of treatments. At day 17, the FUS + TMZ group demonstrate the slowest rate of tumor growth as evidenced by the degree of T2-weighted signal. The FUS + TMZ group also shows the longest survival of any of the TMZ-only groups (not shown). Reprinted with permission from Wei et al.18
Enhanced CNS delivery via FUS has also been investigated by using different classes of therapeutic agents, including gene-carrying vectors, stem cells, and immunotherapies such as targeted antibodies.20 Specifically in the case of Alzheimer disease, immunotherapy in the form of anti-amyloid-β plaque antibodies has yielded promising results.21 Independent investigations by Raymond et al22 and Jordão et al23 have shown that FUS can be used to significantly increase CNS concentrations of anti-amyloid-β antibodies in a mouse model (approximately 3-fold in the Raymond et al group). By comparing the amyloid-β plaque burden between antibody and FUS + antibody cerebral hemispheres, the Jordão et al group further demonstrated a significant reduction in amyloid-β plaque burden in the antibody + FUS treatment hemisphere relative to the contralateral FUS-naive cerebral hemisphere. More recently, a novel modified FUS protocol developed by Leinenga and Götz24 applied in a mouse model demonstrated that diffuse BBB permeability could be achieved via multiple sonication sites throughout the forebrain, reducing amyloid-β plaque burden without pharmacologic intervention. The etiology of amyloid-β plaque reduction is proposed to be related to enhancement of intrinsic microglial phagocytosis.24
Low-Intensity, Pulsed FUS
Neuromodulation, or the ability to reversibly influence neuronal activity, either via excitation or reversible suppression, has enormous therapeutic potential. Other neuromodulatory modalities have already been used extensively in the treatment of a variety of diseases. For instance, deep brain stimulation has gained widespread acceptance in the treatment of movement disorders, including Parkinson disease and essential tremor.25 Electroconvulsive therapy26 and, more recently, transcranial magnetic stimulation27 have also demonstrated efficacy in the treatment of major depression. When used in a low-intensity manner, FUS can also produce local neuromodulatory effects creating negligible temperature elevations and complete preservation of target cytoarchitecture.28
The ability of ultrasound to influence neuronal activity was first established by Fry et al29 in 1958 via ultrasound-induced reversible inhibition of visual-evoked potentials. As the role of FUS has expanded, so has the number of trials into the mechanisms and efficacy of ultrasound-induced neuromodulation. Mechanistic investigation into the effects of low-intensity ultrasound on neuronal electrophysiology by Tyler et al30 in 2008 showed that ultrasound application triggered opening of sodium (Na+) voltage-dependent channels, free calcium (Ca2+) channels, and ultimately an increase in soluble N-ethylmaleimide sensitive fusion proteins attachment receptor–mediated synaptic vesicle exocytosis and synaptic transmission in excised hippocampal sections of mouse brain.30 In vivo neurostimulation through an intact skull in mice has also been shown, without an increase in apoptosis or loss of BBB integrity. This was measured indirectly by using antibodies targeting apoptotic mediators and via a lack of intra-axial fluorescein isothiocyanate-dextran (10 kDa), which does not cross the BBB under normal conditions.31
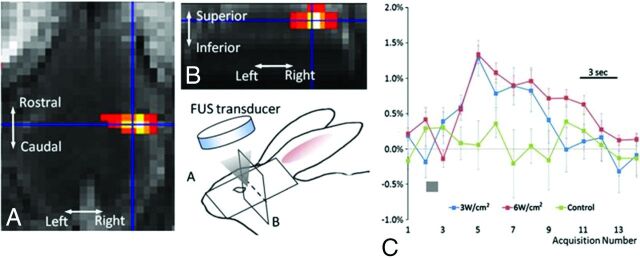
More recently, Yoo et al28 achieved reliable neurostimulation and reversible suppression by using remote low-intensity FUS in a rabbit model, as confirmed by fMRI and electroencephalographic recordings (Fig 5). Concurrent MR thermometry measured temperature elevations of approximately 0.7°C, well below levels required to cause tissue devitalization.8 Most important, the FUS exposures did not result in damage to the BBB as demonstrated by the absence of enhancement on postcontrast MR imaging or cytoarchitectural distortion as confirmed by histologic evaluation.28 Low-intensity FUS for neuromodulation is one of the newest applications of FUS currently being investigated. Preliminary studies have been promising, and research is ongoing.
Fig 5.
Neuromodulation of the rabbit motor cortex. An fMRI activation map shows increased blood oxygen level–dependent–weighted signal in the right motor cortex (A and B). The blue crosshairs on the fMRI images correspond to the sonication focus. Cartoon schematic illustrates the experimental setup and spatial orientation. The graph (C) demonstrates the percentage of blood oxygen level–dependent signal change as a function of the time/acquisition number at 2 different FUS intensities: 6 W/cm2 (red curve) and 3 W/cm2 (blue curve). The green dataset represents the control group. The gray bars indicate the sonication time. Reprinted with permission from Yoo et al.28
Advantages and Obstacles of FUS
High-intensity MRgFUS shares a great deal of therapeutic overlap with surgical resection and stereotactic radiosurgery, albeit with a few important distinctions. Both MRgFUS and radiosurgery compare favorably with surgical intervention in that both do not require surgical craniotomy. While radiosurgery is a mainstay in the treatment of intracranial neoplasms, its role in nonneoplastic pathologies is somewhat limited due to ionizing radiation exposure. In contradistinction, MRgFUS offers reliable, high-resolution energy deposition without radiation exposure or thermal injury to nontarget intracranial tissues.
As a tool for therapeutic agent delivery, FUS is the only available method of generating localized reversible BBB permeability in a noninvasive manner. Selective transport across the BBB is a challenge in ensuring CNS delivery.32 A common obstacle in the development of CNS immunotherapy is the requirement of molecular modification to allow prohibitively large agents to traverse the BBB. While other noninvasive methods have been developed,33 customized modification of each agent is required to take advantage of endogenous transport mechanisms, imposing a costly and time-intensive burden on translation to clinical trials. Use of an FUS-induced BBB opening would substantially decrease the size restrictions dictating molecular permeability and expedite assessment of clinical efficacy.34
FUS offers unique benefits as a therapeutic technique in influencing neuronal activity. FUS-induced neuromodulation does not require surgical craniotomy as is required for deep brain stimulation or subdural and epidural cortical stimulation. Other noninvasive systems, such as transcranial magnetic stimulation and electroconvulsive therapy can be effective; however, they offer inferior spatial resolution.35 The superior spatial resolution offered by FUS could complement functional imaging modalities such as fMRI, to potentially serve as a powerful tool in functional connectivity studies.
Important obstacles remain in the development of FUS as a clinical tool. Further investigations into safe and effective protocols in the ablation of gliomas are needed because its role has lagged behind that of thalamotomy in the treatment of movement disorders and neuropathic pain. Achieving generalizability has also been challenging, most notably due to the limitations of acoustic accessibility in the ablation of superficial lesions or treatment envelope and in patient selection based on heat generation at the calvaria. Overcoming these limitations is actively being investigated.
Conclusions
The discovery of a noninvasive method of focusing mechanical energy, in the form of acoustic waves, within the parenchyma has heralded a new age of investigations into clinical and research applications. In the brief time since transcranial acoustic wave propagation was achieved, FUS has empirically demonstrated efficacy in the ablation of tissues, therapeutic agent delivery, and neuromodulation. The ability to focus acoustic wave propagation noninvasively on the scale of a few millimeters while manipulating the magnitude of energy deposition to create unique bioeffects offers versatility that is unparalleled in neurotherapeutics and research (Table). The role of FUS appears destined to expand as investigations into its utility continue at a rigorous pace.
Clinical applications of focused ultrasound and the proposed mechanisms of action
| FUS Exposures and Effects on Biologic Tissues |
||
|---|---|---|
| Mechanism of Action | Applications | |
| High-intensity continuous application | Thermal → coagulative necrosis | Thalamotomy: essential tremor,11 chronic |
| neuropathic pain,13 obsessive- | ||
| compulsive disorder36 | ||
| Pallidotomy: Parkinson disease12 | ||
| Solid tumor ablation14 | ||
| High-intensity pulsed application | Oscillation of ultrasound “microbubble” contrast agents | Enhanced agent CNS delivery22 |
| Thermal → regional hyperthermia | Enhanced chemotherapy/radiotherapy37 | |
| Mechanical → radiation force–induced displacements | Induction of microglial activation27 | |
| Sonothrombolysis in ischemic stroke38 | ||
| Intracranial hematoma evacuation39 | ||
| Low-intensity pulsed application | Mechanical → activation/inhibition of Na+ and Ca2+-gated ion channels | Noninvasive neuromodulation33,34 |
ABBREVIATIONS:
- FUS
focused ultrasound
- MRgFUS
MR imaging–guided focused ultrasound
- TMZ
temozolomide
Footnotes
Disclosures: Dheeraj Gandhi—UNRELATED: Expert Testimony: Occasionally (<1 year) provided expert testimony on medicolegal cases related to cerebrovascular disease; Royalties: Cambridge Press. RELATED: Grants/Grants Pending: Insightec, Medtronic, National Institutes of Health, Stryker. Victor Frenkel—UNRELATED: Grants/Grants Pending: Focused Ultrasound Foundation,* Comments: This was a 1-year grant of $100,000 to Dr Paul Fishman (Principal Investigator) of the VA Hospital affiliated with the University of Maryland School of Medicine. From this grant, I received 5% effort for my salary for 1 year, during which our article was conceived and written. The funding was, however, for my work on a preclinical study on using focused ultrasound to enhance stem cell delivery to the brain. It was not in any way related to working on this article. The Foundation is a nonprofit organization whose mandate is to increase awareness of focused ultrasound and its implementation in the clinic. This funding has ended, and we recently received notification from the VA System that our grant, which follows this work, is also to be funded. *Money paid to the institution.
Paper previously presented in part at the Annual Meeting of the American Society of Neuroradiology and the Foundation of the ASNR Symposium, April 25–30, 2015; Chicago, Illinois.
References
- 1. Lehmann JF. The biophysical basis of biologic ultrasonic reactions with special reference to ultrasonic therapy. Arch Phys Med Rehabil 1953;34:139–52 [PubMed] [Google Scholar]
- 2. Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007;23:89–104 10.1080/02656730601186138 [DOI] [PubMed] [Google Scholar]
- 3. Fry WJ, Barnard JW, Fry EJ, et al. Ultrasonic lesions in the mammalian central nervous system. Science 1955;122:517–18 10.1126/science.122.3168.517 [DOI] [PubMed] [Google Scholar]
- 4. Heimburger R. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med 1985;78:469–76 [PubMed] [Google Scholar]
- 5. Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol 2002;47:1219–36 10.1088/0031-9155/47/8/301 [DOI] [PubMed] [Google Scholar]
- 6. Madersbacher S, Pedevilla M, Vingers L, et al. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res 1995;55:3346–51 [PubMed] [Google Scholar]
- 7. Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005;5:321–27 10.1038/nrc1591 [DOI] [PubMed] [Google Scholar]
- 8. Meshorer A, Prionas SD, Fajardo LF, et al. The effects of hyperthermia on normal mesenchymal tissues: application of a histologic grading system. Arch Pathol Lab Med 1983;107:328–34 [PubMed] [Google Scholar]
- 9. Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev 2008;60:1193–208 10.1016/j.addr.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pahwa R, Lyons KE, Wilkinson SB, et al. Comparison of thalamotomy to deep brain stimulation of the thalamus in essential tremor. Mov Disord 2001;16:140–43 [DOI] [PubMed] [Google Scholar]
- 11. Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640–48 10.1056/NEJMoa1300962 [DOI] [PubMed] [Google Scholar]
- 12. Magara A, Bühler R, Moser D, et al. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound 2014;2:11 10.1186/2050-5736-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus 2012;32:E1 10.3171/2011.10.FOCUS11248 [DOI] [PubMed] [Google Scholar]
- 14. McDannold N, Clement G, Black P, et al. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 2010;66:323–32; discussion 332 10.1227/01.NEU.0000360379.95800.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hynynen K, McDannold N, Vykhodtseva N, et al. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir Suppl 2003;86:555–58 [DOI] [PubMed] [Google Scholar]
- 16. Sheikov N, McDannold N, Vykhodtseva N, et al. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004;30:979–89 10.1016/j.ultrasmedbio.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 17. Kinoshita M, McDannold N, Jolesz FA, et al. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A 2006;103:11719–23 10.1073/pnas.0604318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei KC, Chu PC, Wang HY, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One 2013;8:e58995 10.1371/journal.pone.0058995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 20. Kinoshita M, McDannold N, Jolesz FA, et al. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006;340:1085–90 10.1016/j.bbrc.2005.12.112 [DOI] [PubMed] [Google Scholar]
- 21. Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000;6:916–19 10.1038/78682 [DOI] [PubMed] [Google Scholar]
- 22. Raymond SB, Treat LH, Dewey JD, et al. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS One 2008;3:e2175 10.1371/journal.pone.0002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One 2010;5:e10549 10.1371/journal.pone.0010549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leinenga G, Götz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci Transl Med 2015;7:278ra233 10.1126/scitranslmed.aaa2512 [DOI] [PubMed] [Google Scholar]
- 25. Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337:403–06 10.1016/0140-6736(91)91175-T [DOI] [PubMed] [Google Scholar]
- 26. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003;361:799–808 10.1016/S0140-6736(03)12705-5 [DOI] [PubMed] [Google Scholar]
- 27. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med 2009;39:65–75 10.1017/S0033291708003462 [DOI] [PubMed] [Google Scholar]
- 28. Yoo SS, Bystritsky A, Lee JH, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage 2011;56:1267–75 10.1016/j.neuroimage.2011.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science 1958;127:83–84 10.1126/science.127.3289.83 [DOI] [PubMed] [Google Scholar]
- 30. Tyler WJ, Tufail Y, Finsterwald M, et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One 2008;3:e3511 10.1371/journal.pone.0003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tufail Y, Matyushov A, Baldwin N, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010;66:681–94 10.1016/j.neuron.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 32. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2005;2:3–14 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci U S A 2000;97:7567–72 10.1073/pnas.130187497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi JJ, Wang S, Tung YS, et al. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol 2010;36:58–67 10.1016/j.ultrasmedbio.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng 2007;9:527–65 10.1146/annurev.bioeng.9.061206.133100 [DOI] [PubMed] [Google Scholar]
- 36. Jung HH, Kim SJ, Roh D, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study. Mol Psychiatry 2015;20:1205–11 10.1038/mp.2014.154 [DOI] [PubMed] [Google Scholar]
- 37. Tempany CM, McDannold NJ, Hynynen K, et al. Focused ultrasound surgery in oncology: overview and principles. Radiology 2011;259:39–56 10.1148/radiol.11100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burgess A, Huang Y, Waspe AC, et al. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS One 2012;7:e42311 10.1371/journal.pone.0042311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monteith SJ, Harnof S, Medel R, et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J Neurosurg 2013;118:1035–45 10.3171/2012.12.JNS121095 [DOI] [PubMed] [Google Scholar]
- 40. Burgess A, Ayala-Grosso CA, Ganguly M, et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 2011;6:e27877 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]