Dear Editor,
Neurological complications of COVID-19 are reported with an increasing rate from the beginning of COVID-19 pandemic in December 2019. Some of these complications occur following immune response to SARS-CoV-2 [1]. There are reported cases of acute disseminated encephalomyelitis (ADEM) and Guillain-Barre Syndrome following developing COVID-19 [2]. In addition, one case of multiple sclerosis (MS) was also reported following developing COVID-19 [3].
This study reported a patient who simultaneously had developed COVID-19 and MS.
The patient was a 31-years-old woman with developed blurred vision of the left eye in July 2020. Blurred vision was gradually initiated and reached its peak over three days. Eye movements of the patient were painful, and visual acuity was about 20/200 in the examination.
Notably, other neurologic examinations were normal. The patient had no history of previous medical disease. Moreover, history of the patient's family on autoimmune diseases was negative.
Concomitant with the initiation of blurred vision, the patient lost her smell and taste senses. However, she had no complain about fever, cough or short breathing. Although routine laboratory tests and chest computed tomography (CT) scan were normal, nasopharyngeal swab real-time polymerase chain reaction (rt-PCR) test was positive regarding SARS-CoV-2.
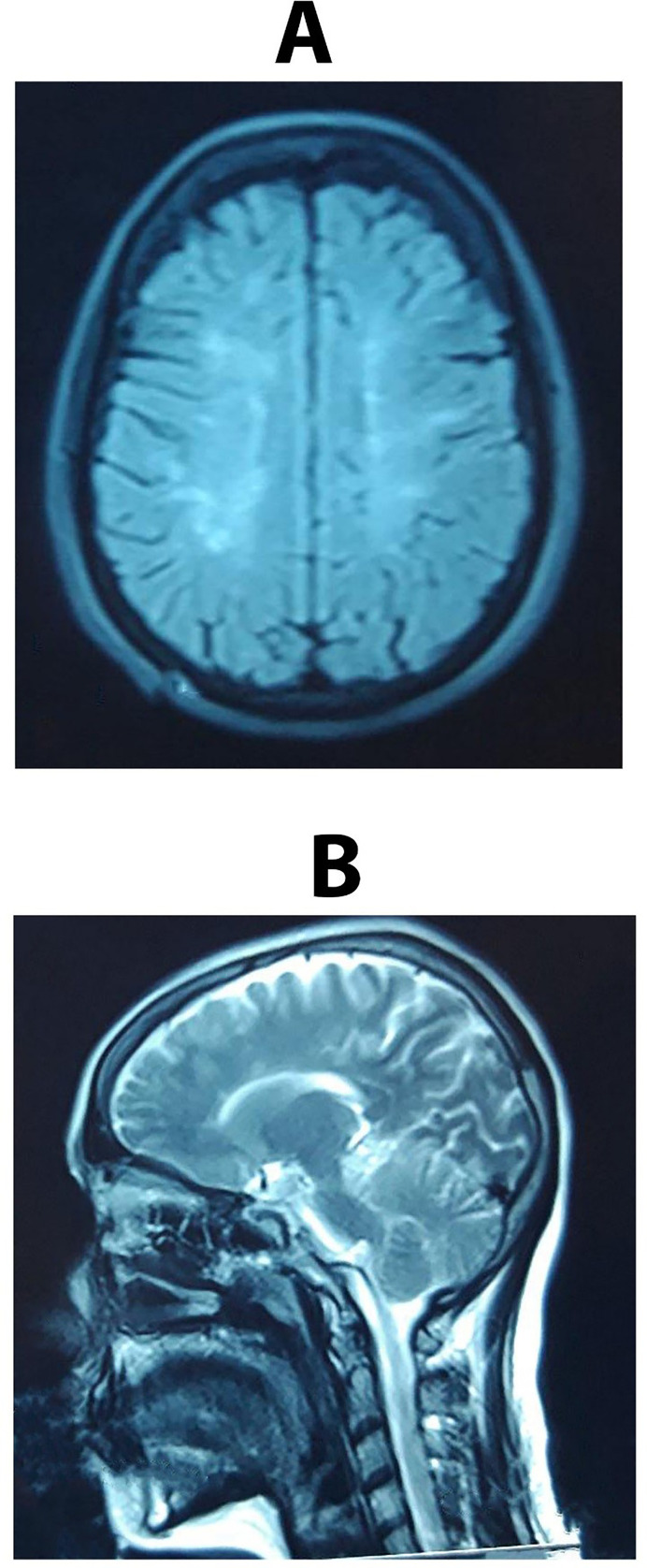
Brain magnetic resonance imaging (MRI) revealed numerous periventricular and pericallosal lesions that did not enhance with gadolinium injection (Fig. 1 A & B). Also, cervical MRI was normal.
Fig. 1.
A & B - Brain MRI revealed multiple periventricular and pericallosal lesions.
Vasculitis tests, anti-aquaporin antibody, and anti- myelin oligodendrocyte glycoprotein (MOG) antibody were negative.
Simple analysis of cerebrospinal fluid (CSF) was normal. Notably, PCR of SARS-CoV-2 was negative in CSF. However, the oligoclonal bands (OCB) result was positive. So, the patient underwent treatment with intravenous methylprednisolone and the vision of the patient was thoroughly recovered.
Smell and taste signs of the patient have improved over this period. The patient diagnosed with MS underwent treatment with glatiramer acetate.
Co-occurring of MS and COVID-19 in this patient can be justified through several ways.
Firstly, none of the plaques of MRI was enhanced. Accordingly, this lack of enhancement can indicate that the lesions are old, meaning that in fact the infection by COVID-19 acts as a predisposing factor for the clinical manifestations. Unfortunately, Orbit MRI was not done regarding the involvement of optic nerve in this patient.
However, the symptoms of the disease and examinations confirmed the optic neuritis. Therefore, symptoms observed in the patient were also related to acute involvement of optic nerve, and the plaques observed in the brain MRI were asymptomatic.
Besides, various studies pointed out the role of viruses in the pathogenesis of MS [4].
However, we still do not know whether SARS-CoV-2 can also be involved in the pathogenesis of MS or not.
This issue will be clarified through further investigations and the number of reported cases.
But, the role of SARS-CoV-2 in affecting immune system increases this probability. There are several evidences indicating the possible role of SARS-CoV-2 in the pathogenesis of different autoimmune diseases. Different autoantibodies are detected in patients with COVID-19. In addition, increasing reports of different autoimmune diseases secondary to COVID-19 enhance this possibility. Strong relationship between human leukocyte antigen (HLA) genetic polymorphism and susceptibility to COVID-19 is another important evidence of the role of SARS-CoV-2 in the pathogenesis of autoimmune disorders [5,6]. Also, molecular mimicry of human proteomes and virus spike glycoprotein should be considered as an important factor in the pathogenesis of autoimmune diseases following COVID-19 [5,7].
Currently, we know that one of the important factors in critical patients with developed COVID-19 is severe reaction of immune system to this virus followed by some destruction in the organs such as lung [5,8,9]. This cytokine storm can explain high mortality rate in severe cases of COVID-19 [8]. This reaction is also involved in developing brain manifestations.
However, as mentioned earlier, our patient developed mild covid-19 and the only symptom was loss of taste and smell senses.
Therefore, the question is raised about whether milder responses of the immune system to the coronavirus such as developing and producing neutralizing antiviral T cell and antibody immunity are involved in establishing autoimmune diseases such as MS or not?
Answering this question needs more investigations on the behavior of the virus and the immune response to it, as well as the effect of immune response on other organs such as central nervous system.
Funding
Not funded.
Declaration of competing interest
The author declares there is no conflict of interest.
References
- 1.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020:1–4. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrodan M., Alessandro L., Farez M.F., Correale J. The role of infections in multiple sclerosis. Mult Scler. 2019;25(7):891–901. doi: 10.1177/1352458518823940. [DOI] [PubMed] [Google Scholar]
- 5.Halpert G., Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19(12):102695. doi: 10.1016/j.autrev.2020.102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68(5):310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreakos E., Tsiodras S. COVID-19: lambda interferon against viral load and hyperinflammation. EMBO Mol Med. 2020;12(6) doi: 10.15252/emmm.202012465. [DOI] [PMC free article] [PubMed] [Google Scholar]