Abstract
BACKGROUND AND PURPOSE:
Eye lenses are among the most sensitive organs to x-ray radiation and may be considered at risk during neurointerventional radiology procedures. The threshold dose to produce eye lens opacities has been recently reduced to 500 mGy by the International Commission on Radiologic Protection. In this article, the authors investigated the radiation doses delivered to patients' eyes during interventional neuroradiology procedures at a university hospital.
MATERIALS AND METHODS:
Small optically stimulated luminescence dosimeters were located over patients' eyes during 5 diagnostic and 31 therapeutic procedures performed in a biplane x-ray system. Phantom measurements were also made to determine the level of radiation to the eye during imaging runs with conebeam CT.
RESULTS:
The left eye (located toward the lateral C-arm x-ray source) received a 4.5 times greater dose than the right one. The average dose during embolization in the left eye was 300 mGy, with a maximum of 2000 mGy in a single procedure. The patient who received this maximum eye dose needed 6 embolization procedures to treat his high-volume AVM. If one took into account those 6 embolizations, the eye dose could be 2-fold. Sixteen percent of the embolizations resulted in eye doses of >500 mGy.
CONCLUSIONS:
A relevant fraction of patients received eye doses exceeding the threshold of 500 mGy. A careful optimization of the procedures and follow-up of these patients to evaluate potential lens opacities should be considered.
Interventional neuroradiology (INR) activity has increased in recent years, providing important benefits to patients, but the use of ionizing radiation adds risks that must be evaluated and minimized. Concerning eye lens irradiation during INR procedures particularly, little research has been conducted, yet it is important for physicians to know the level of risk for this organ in these kinds of procedures. The International Commission on Radiological Protection (ICRP) has recently published a report on the effects of radiation in tissues and organs, in which it recognizes that eye lenses may be more sensitive to ionizing radiation than previously thought.1 Until recently, the dose threshold suggested for the formation of lens opacities was 5 Gy in case of acute irradiation of the eye lens.2 However, as a result of new epidemiologic evidence,3,4 this threshold value has been reduced to 0.5 Gy,1 the legislation on radiation protection of workers has been amended, and the dose limits for the lens of the eye reduced in the International Basic Safety Standards5 and in the new European regulation.6 In the case of patients, to optimize procedures and reduce radiation doses, the new European legislation6 requires that the information relative to patient exposure be included in the medical report. In addition, radiation doses averaged from patient samples have to be compared with national diagnostic reference levels, and if relevant deviations are detected, optimization actions should be taken. As in the case of the authors' country, there may be no formal national diagnostic reference levels available. In such a case, other regional or local diagnostic reference levels could be used until the national diagnostic reference levels are established.
There is little information in the literature regarding the eye lens dose received by patients during INR procedures. Moritake et al7 reported average doses in patients' eyes of 380 mGy, with a maximum of 2079 mGy during cerebral embolizations, 4 times the threshold level of 500 mGy recommended by the ICRP. Sandborg et al8 reported mean and maximum doses in the eye of 71 and 515 mSv, respectively, also during cerebral embolizations. The variability of doses found then underlines the need for further investigations into the risks associated with these medical practices. Practitioners also need to know the order of magnitude of the radiation doses delivered to patients' eyes during INR procedures. These data will allow physicians to optimize radiation protection during clinical procedures, to better manage the information about the risks of radiation-induced lens opacities, and to give patients the appropriate counseling on the follow-up.
This article presents the measurement of patient eye lens doses, by using optically stimulated luminescence dosimeters (OSLDs). Eye doses were measured in a sample of diagnostic and therapeutic procedures performed in an interventional neuroradiology laboratory at a university hospital. The contribution of conebeam CT (CBCT) to eye lens doses was also investigated.
Materials and Methods
Cases of cerebral angiography (n = 5) and therapeutic (n = 31) procedures were randomly selected for this study. The therapeutic procedures consisted of embolizations of AVMs (n = 13) mainly with grades IV and V (Spetzler-Martin9); fistulas (n = 2); and aneurysm coiling (n = 16). All procedures were performed in the neuroradiology room equipped with an Allura FD 10/20 (Philips Healthcare, Best, the Netherlands) biplane x-ray unit at the Hospital Clinico San Carlos in Madrid, Spain. The diagonals of the flat detectors were 40 cm for the frontal C-arm and 25 cm for the lateral one. The lateral C-arm has its x-ray focus at the patient's left side (supine). When the patient's head is located at isocenter with the image detectors 10 cm from the patient's head and no collimation, the frontal detector covers the patient's surface area of approximately 27 × 27 cm2, and the lateral detector, 14 × 14 cm2. Both C-arms have transmission ionization chambers installed at the x-ray tube exit to monitor the radiation dose delivered to patients; the dose is included in the patient's dose reports. In most procedures, the digital subtraction angiography series was obtained at 2 images per second in the first 10 seconds and at 1 image per second in the remaining time.
The system, by using the conebeam CT technique, has the ability to acquire volumetric images. Depending on the CBCT mode selected, low dose or high resolution, 313 or 622 images can be acquired over a 240° arc rotation for volumetric reconstruction. The CBCT is performed by rotating the arc around the posterior side of the patient (in a supine position), with a rotation angle from −120° to 120°, minimizing irradiation to the patient's face. During CBCT acquisitions, the generator settings are as follows: 120 kV, 250 mA, 5 ms, 0.4-mm copper (Cu) +1-mm aluminum (Al) of added filtration. The maximum field size of 27 × 27 cm2 is set at the isocenter. At least 1 CBCT series was acquired during the therapeutic procedures. The x-ray system was submitted to regular quality control and calibration programs by the medical physics service, as recommended by the national guidelines. The neuroradiologists in charge have received training in radiation protection as required by national regulation.
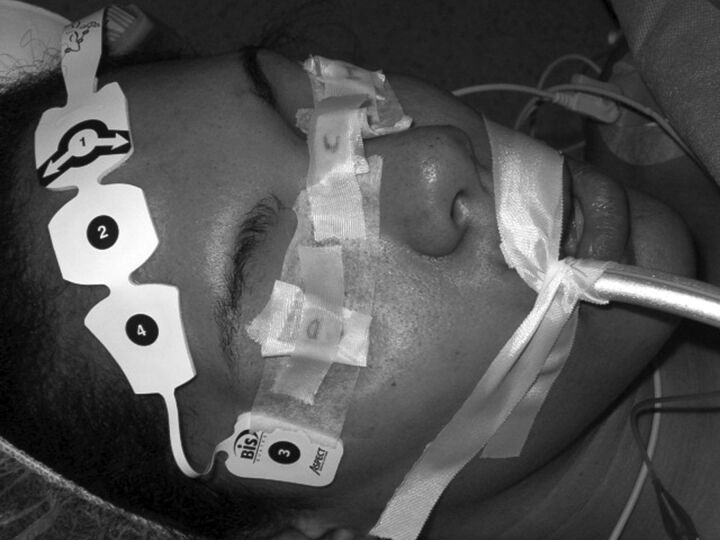
The radiation dose at the eye lens was estimated by measuring the entrance surface air kerma with small OSLDs. For simplicity, from now on, the word “dose” will refer to the entrance surface air kerma at the eye lens. For each patient, 2 OSLDs were located over the eyelids as shown in Fig 1. The OSLDs used were the nanoDot model (Landauer, Glenwood, Illinois). They are composed of a small disk of 4-mm diameter of optically stimulated luminescent material (Al2O3:C), which forms the active area, encased in a light-tight plastic protector of 10 × 10 × 2 mm3. Their small size makes them suitable for use near patients' eyes. OSLDs have been previously used to measure patient doses in different clinical situations,10–12 but when used with diagnostic energies, special attention must be paid to limitations such as energy and angular dependence.
Fig 1.
Position of the dosimeters on patient eyes.
In neuroradiology procedures, the x-ray beam quality may change with kilovolt settings and filtration. Kilovolt settings are adjusted by automatic dose control of the flat detector, depending on patient thickness. For the neuroradiology protocols programmed in Allura, the beam kilovolt is nearly constant around 70–80 kV. However, filtration may change depending on the operation mode selected by the user and may range from 0.1 mm of copper plus 1 mm of aluminum with the digital subtraction angiography mode to 0.9 mm of Cu plus 1 mm of Al with the fluoroscopic low-dose mode. The OSLDs have been calibrated “in house” by using typical clinical beam qualities from our interventional x-ray unit and verified by the Centro Nacional de Dosimetría in Valencia, Spain, a standard calibration laboratory. With these user x-ray beams, a difference of 6% in the calibration factor for 70 and 80 kV was observed for the same filtration, while a difference of 16% was measured for different filtrations. The uncertainty resulting from the response of OSLDs to kilovolt variation (6%) was assumed acceptable, but the effect of different filtration in the OSLD response had to be corrected. To reduce the influence of the different added filtration of the x-ray beams in the OSLD response, we used the information included in the patient dose report about the air kerma area product.13
The air kerma area product, commonly called dose-area product (DAP), is one of the standard magnitudes used to monitor patient doses in some x-ray modalities. Modern interventional x-ray equipment provides the DAP in both DICOM headers and patient dose reports and can be used as a patient dose indicator, provided it is duly calibrated. In this case, the DAP meter had a deviation of −10%, and all DAP values had been duly corrected. Our x-ray unit produces patient dose reports that provide the fraction of the DAP delivered with fluoroscopy (high added filtration) and with DSA (low added filtration). This information was used to calculate a corrected calibration factor for each procedure, by combining the calibration factors for fluoroscopic and DSA beams proportionally to the fraction of fluoroscopic and DSA DAP included in the dosimetric report. Once the calibration factor had been derived for each procedure, the OSLD reading was then translated into dose. Regarding angular dependence and for the beam qualities used in this study, angular dependence has been measured and resulted in a difference in response of −15% in the worst case (beam incidence of −90° in the lower energetic beam) and −3% for high-filtered beams. Besides the eye dose, other relevant parameters were recorded, such as the dose at the patient entrance reference point,14 the fluoroscopy time, the number of DSA and CBCT series, and the number of images.
To measure the dose contribution to the eyes during CBCT runs, we performed a phantom simulation. We laid down an anthropomorphic phantom model, Rando (The Phantom Laboratory, Salem, New York), over the examining couch, centering the phantom head at the isocenter. Optically stimulated luminescence dosimeters were attached over the phantom eyes (Fig 2). Doses at the phantom eyes were measured with the 2 modes of operation available in this x-ray system: low dose and high resolution. Both modes of operation have been described previously in this section. The specific calibration factor for the CBCT beam quality was measured for the OSLDs.
Fig 2.
Anthropomorphic phantom with OSLDs over the eyes.
Regression analysis between the eye dose and DAP was performed with the statistical package SPSS, Version 12 (IBM, Armonk, New York).
An independent local ethics committee approved this study under the title “Radiologic Risks in Fluoroscopy Guided Procedures” (code B-09/20). Patients agreed to allow anonymous dosimetric information to the investigation.
Results
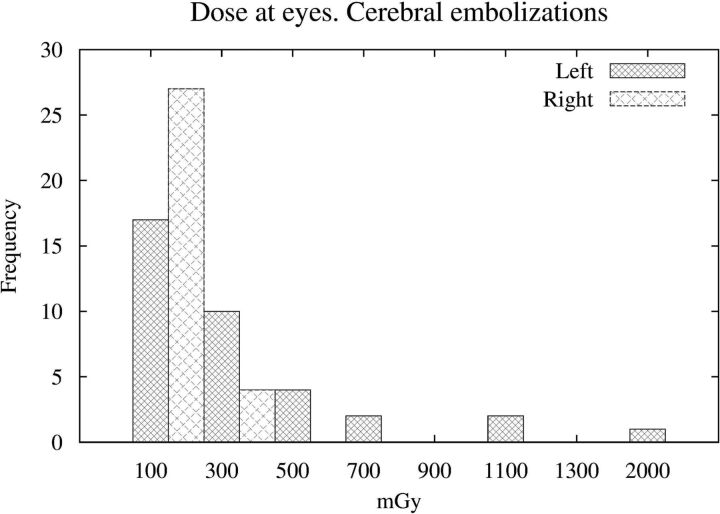
Of 36 procedures measured, 5 were diagnostic and 31 were therapeutic. Table 1 summarizes the main results of patient doses. The maximum doses delivered to the left eye, liable to receive direct radiation from the lateral C-arm, resulted in 81 mGy for diagnostic procedures and 2080 mGy for therapeutic ones. Five of the 31 embolizations (16%) resulted in doses in the left eye greater than the threshold of 500 mGy. Table 2 shows the main dosimetric parameters of those procedures. The linear regression between the DAP (in grays × square centimeters) and the dose at the left eye (in milligrays) resulted in an expression Dose at Eye = 2.1 × DAP, with a correlation coefficient of r2 = 0.63 (P < .001). Figure 3 shows the left and right eye dose histogram for cerebral embolizations.
Table 1:
Main statistics for parameters related to patient dose for diagnostic and therapeutic procedures
| DAP (Gy × cm2) | Fluoroscopy Time (sec) | No. of DSA Images | Right Eye K (mGy) | Left Eye K (mGy) | |
|---|---|---|---|---|---|
| Diagnostic (n = 5) | |||||
| Min | 36 | 169 | 87 | 17 | 24 |
| Max | 86 | 1581 | 958 | 28 | 81 |
| Mean ± SD | 56 ± 21 | 657 ± 560 | 484 ± 421 | 20 ± 11 | 67 ± 32 |
| Median | 44 | 407 | 293 | 23 | 67 |
| 1stQ | 44 | 362 | 165 | 21 | 52 |
| 3rdQ | 72 | 768 | 920 | 24 | 77 |
| Therapeutic (n = 31) | |||||
| Min | 63 | 680 | 112 | 9 | 32 |
| Max | 479 | 5250 | 2410 | 173 | 2084 |
| Mean ± SD | 203 ± 120 | 1680 ± 900 | 1030 ± 460 | 62 ± 37 | 303 ± 409 |
| Median | 164 | 1400 | 1000 | 57 | 172 |
| 1stQ | 115 | 1120 | 750 | 42 | 77 |
| 3rdQ | 248 | 2200 | 1180 | 76 | 315 |
Note:—1stQ indicates first quartile; 3rdQ, third quartile; K, air kerma; Min, minimum; Max, maximum.
Table 2:
The dosimetric data for the 5 therapeutic procedures with left eye doses >500 mGy
| Procedure | DAP (Gy × cm2) | Fluoroscopy Time (sec) | No. of Images | AKR Frontal (mGy) | AKR Lateral (mGy) | Right Eye Dose (mGy) | Left Eye Dose (mGy) |
|---|---|---|---|---|---|---|---|
| AVM | 227 | 1407 | 1283 | 2388 | 599 | – | 671 |
| Aneurysm | 271 | 2412 | 1020 | 1978 | 880 | 58 | 614 |
| Aneurysm | 214 | 1956 | 2412 | 2314 | 714 | 118 | 936 |
| AVM | 466 | 5254 | 979 | 3801 | 1711 | 129 | 2080 |
| AVM | 423 | 1801 | 1801 | 2220 | 724 | 173 | 911 |
Note:—AKR indicates the air kerma in the patient entrance reference point for the frontal and lateral C-arms.
Fig 3.
Left and right eye doses measured with OSLDs during cerebral embolizations.
During a CBCT irradiation, the doses at the patient entrance reference point were 32 and 64 mGy for the low dose and high resolution, respectively. On the anthropomorphic phantom's eyes, the doses were 10 mGy in the CBCT low-dose mode and 20 mGy for the high resolution.
Discussion
The dose values at the phantom's eyes of 10 and 20 mGy measured for this x-ray unit during the CBCT acquisitions correspond to 3%–6% of the average left eye dose for a therapeutic procedure and 15%–30% for a cerebral angiography. Compared with the dose at the patient entrance reference point reported by the x-ray system during a CBCT run, the eye dose resulted in a fraction of 30%. The values in our x-ray system are of the same order of magnitude as the ones reported by Koyama et al,15 who measured 20 mGy in eye lenses using diodes.
In diagnostic cases, patient DAPs were lower than those in therapeutic cases: 56 ± 21 Gy × cm2 versus 203 ± 120 Gy × cm2 (mean ± SD). These values of DAP are even lower than the ones reported by several authors16–18 who showed average DAPs from 68 to 158 Gy × cm2 for angiography and from 215 to 382 Gy × cm2 for embolization. Sandborg et al,8 who also included eye doses, reported 55 and 190 Gy × cm2 for angiographies and embolizations, respectively (ie, doses similar to those found in this investigation). The maximum eye dose recorded during a cerebral angiography was 81 mGy, much lower than the threshold of 500 mGy.
The OSLD located at the left eye (in front of the lateral C-arm x-ray tube) read an average dose 4.8 times greater than the one located at the right eye. The average dose of 300 mGy measured at the left eye can be considered important compared with the threshold recommended by ICRP (500 mGy). This mean value is of the same order of magnitude as the one reported by Moritake et al7 (380 mGy) but much higher than the one reported by Sandborg et al8 (71 mGy). The sample of Sandborg et al had a mean DAP similar to ours (190 versus 203 Gy × cm2) for embolizations, but in comparison, the eye doses were drastically lower (71 mGy versus our 300 mGy). In our sample, 5 cases (16%) of the 31 therapeutic procedures measured resulted in doses of >500 mGy at the left eye. With such a level of radiation, the possibility of producing opacities or cataracts in patients' eyes should be considered, especially in patients requiring several procedures to be treated properly. At the right eye, the dose measured was below 200 mGy, a value unlikely to produce opacities.
The maximum radiation dose measured at the left eye was 2080 mGy during an AVM located in the anterior fossa, with a DAP of 466 Gy × cm2 (88 fluoroscopy minutes and 979 images). In such a case, opacities in this eye are likely to occur; 4.4 mGy in the left eye per Gy × cm2 is an extreme case well above the average tendency of 2.1 mGy/(Gy × cm2). This uncommonly high dose may certainly result from the patient's pathology being located in the anterior fossa, close to the eye. This particular patient, with a high-volume AVM, needed 6 INR procedures within 18 months, with a total cumulative DAP of 800 Gy × cm2. It was not possible to measure the eye dose with OSLDs in the course of the 6 procedures, but if we assume that no additional measures could be taken to protect the eyes, this patient might have received almost 4000 mGy. This patient and his relatives were informed of the risks of developing cataracts and of how to proceed should this happen.
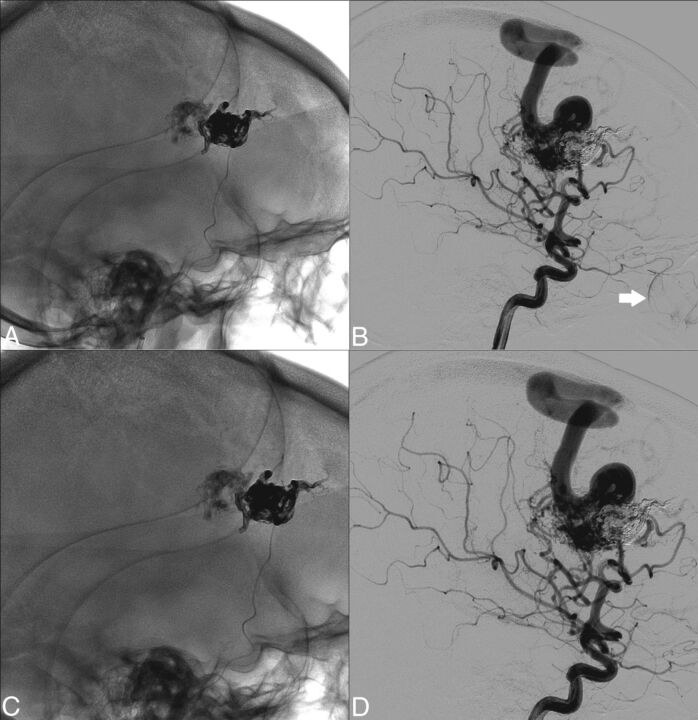
Another case of interest, with a high DAP of 480 mGy × cm2 but with a very low eye dose of 94 mGy, was an embolization located at the posterior side of the head, during which the neuroradiologist had taken precautions to protect the patient's eye lenses from the lateral beam in most DSA series. This example shows that even during complex procedures with a large DAP, it is still possible to reduce the eye dose when clinically compatible, provided proper collimation in the lateral beam is used to protect the eye. After further analysis of the sample of procedures, we concluded that in some cases, collimation could be optimized. Figure 4A, -B shows a nonoptimal lateral projection from an AVM embolization in which the left eye was irradiated. Figure 4C, -D shows the optimal proposal based on a retrospective analysis during a joint optimization session by neuroradiologists and medical physicists. This collimation provides eye protection while keeping enough FOV to monitor and prevent possible iatrogenic embolizations.
Fig 4.
A and B, Nonoptimal lateral projection without and with subtraction where the left eye is irradiated. C and D, The proposed collimation to avoid eye irradiation. The arrow in B indicates some contrast in colloids via the ophthalmic artery that may be chosen as the edge to collimate the lateral beam.
The correlation between DAP, probably the most frequently used dose indicator, and the dose at the left eye was small (r2 = 0.6), certainly limited by the influence of other factors like the collimation of the lateral beam and the lesion location (close or distant from the eyes). The combination of these 3 variables should, therefore, be taken into account to evaluate the risk of producing lens opacities.
So far the radiation dose has been analyzed during 1 single INR procedure, but it is, however, common for a patient to undergo >1 procedure. This hospital is a reference center for the treatment of AVMs of grades IV and V (Spetzler-Martin): 95% of the AVMs performed here are grades 4 and 5 and all of them require several procedures. In fact, only 6 (17%) of the 36 patients in this sample had undergone only 1 INR procedure at the time; 11 patients (30%) had undergone 3 or 4 procedures; 10 patients (28%), 5 or 6 procedures; and 9 patients (25%), ≥6 procedures. It was not possible to measure the eye doses in all these cases, but all the DAPs were recorded, giving an average of 566 Gy × cm2, with 17 patients (47%) with >300 Gy × cm2. This value of 300 Gy × cm2 is the DAP obtained from the linear regression equation that may produce eye lens doses over the threshold of 500 mGy. The average age of this patient sample was 59 years, with 9 patients (25%) younger than 50 years of age, therefore with a long life expectancy.
Finally, the difference of 10% in calibration factors used for the various procedures indicates that uncertainties due to the response of OSLDs to beam quality have been reduced. Nevertheless and despite the corrections made, other factors arose from the calibration process and the angular dependence of the dosimeters, which could increase the uncertainty to 20%.
Conclusions
During INR therapeutic procedures in a biplane x-ray system, it is possible to deliver relevant doses to the eye lens. For the sample presented in this article, 16% of the therapeutic procedures measured resulted in eye doses higher than the threshold of 500 mGy for lens opacities. The factors that could modify the eye doses are the DAP delivered, the lesion localization, and the possibility of collimating the lateral x-ray beam to protect the eye. Given that most patients in this sample had undergone several INR procedures, the fraction of patients with a DAP that potentially may result in lens doses over the recommended threshold (>300 Gy × cm2) was 47%. When optimizing the collimation in the lateral beam to prevent direct eye irradiation, the risk of eye lens opacities is reduced to negligible levels. A follow-up of patients receiving high doses in the eyes should be considered to evaluate potential lens opacities and to decide whether the possibility of producing induced opacities should be included in the informed consent. The most effective actions to minimize eye doses are to collimate to the necessary surgical field, especially in the lateral beam; to avoid unnecessary acquisition series; and to use, when possible, fluoroscopy runs instead of acquisitions.
ABBREVIATIONS:
- CBCT
conebeam CT
- DAP
dose-area product
- ICRP
International Commission on Radiological Protection
- INR
interventional neuroradiology
- OSLD
optically stimulated luminescence dosimeter
Footnotes
Disclosures: Roberto M. Sánchez—UNRELATED: Grants/Grants Pending: Consejo de Seguridad Nuclear,* Comments: research project about eye lens doses for medical professionals; Payment for Lectures (including service on Speakers Bureaus): International Atomic Energy Agency, Comments: participant as lecturer during a workshop on radiation protection in interventional practices. *Money paid to the institution.
References
- 1. Stewart FA, Akleyev AV, Hauer-Jensen M, et al. ; ICRP. ICRP Publication 118: ICRP statement on tissue reactions/early and late effects of radiation in normal tissues and organs: threshold doses for tissue reactions in a radiation protection context. Ann ICRP 2012;41:1–322 10.1016/j.icrp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 2. The 2007 recommendations of the International Commission on Radiological Protection: ICRP Publication 103. Ann ICRP 2007;37:1–332 10.1016/j.icrp.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 3. Nakashima E, Neriishi K, Minamoto A. A reanalysis of atomic-bomb cataract data, 2000–2002: a threshold analysis. Health Phys 2006;90:154–60 10.1097/01.HP.0000175442.03596.63 [DOI] [PubMed] [Google Scholar]
- 4. Neriishi K, Nakashima E, Minamoto A, et al. Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res 2007;168:404–08 10.1667/RR0928.1 [DOI] [PubMed] [Google Scholar]
- 5. International Atomic Energy Agency. Radiation protection and safety of radiation sources. International basic safety standards: IAEA Safety Standards Series. GSR part 3. Vienna: IAEA, July 21, 2014. http://www-pub.iaea.org/MTCD/publications/PDF/Pub1578_web-57265295.pdf. Accessed October 2, 2015. [Google Scholar]
- 6. Council Directive 2013/59/EURATOM of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation. Official journal of the European Union 17.1.2014, L13, chapter VIIAccessed October 2, 2015 pp. 25–28. http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013L0059&from=EN. Accessed October 2, 2015.
- 7. Moritake T, Matsumaru Y, Takigawa T, et al. Dose measurement on both patients and operators during neurointerventional procedures using photoluminiscence glass dosimeters. AJNR Am J Neuroradiol 2008;29:1910–17 10.3174/ajnr.A1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandborg M, Rossitti S, Pettersson H. Local skin and eye lens equivalent doses in interventional neuroradiology. Eur Radiol 2010;20:725–33 10.1007/s00330-009-1598-9 [DOI] [PubMed] [Google Scholar]
- 9. Spetzler R, Martin N. A proposed grading system for arteriovenous malformations: 1986. J Neurosurg 2008;108:186–93 10.3171/JNS/2008/108/01/0186 [DOI] [PubMed] [Google Scholar]
- 10. Al-Senan RM, Hatab MR. Characteristics of an OSLD in the diagnostic energy range. Med Phys 2011;38:4396 10.1118/1.3602456 [DOI] [PubMed] [Google Scholar]
- 11. Jursinic PA. Characterization of optically stimulated luminescents, OSLDs, for clinical dosimetric measurements. Med Phys 2007;34:4594–604 10.1118/1.2804555 [DOI] [PubMed] [Google Scholar]
- 12. Yukihara EG, McKeever SW. Optically stimulated luminescence (OSL) dosimetry in medicine. Phys Med Biol 2008;53:R351–79 10.1088/0031-9155/53/20/R01 [DOI] [PubMed] [Google Scholar]
- 13. International Commission on Radiation Units and Measurements. ICRU report 74: patient dosimetry for x rays used in medical imaging. J ICRU 2005;5(2, theme issue):29 [Google Scholar]
- 14. International Electrotechnical Commission. Medical electrical equipment. Part 2–43: particular requirements for the safety of x-ray equipment for interventional procedures. Report IEC 60601-2-43. Geneva, Switzerland: International Electrotechnical Commission; 2010:25–26 [Google Scholar]
- 15. Koyama S, Aoyama T, Oda N, et al. Radiation dose evaluation in tomosynthesis and C-arm cone-beam CT examinations with an anthropomorphic phantom. Med Phys 2010;37:4298–306 10.1118/1.3465045 [DOI] [PubMed] [Google Scholar]
- 16. D'Ercole L, Thyrion FZ, Bocchiola M, et al. Proposed local diagnostic reference levels in angiography and interventional neuroradiology and a preliminary analysis according to the complexity of the procedures. Phys Med 2012;28:61–70 10.1016/j.ejmp.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 17. Miller DL, Kwon D, Bonavia GH. Reference levels for patient radiation doses in interventional radiology: proposed initial values for U.S. practice. Radiology 2009;253:753–64 10.1148/radiol.2533090354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdun FR, Aroua A, Trueb R, et al. Diagnostic and interventional radiology: a strategy to introduce reference dose level taking into account the national practice. Radiat Prot Dosimetry 2005;114:188–91 10.1093/rpd/nch547 [DOI] [PubMed] [Google Scholar]