Abstract
Rationale:
Prior research suggests that the neural pathway from the lateral hypothalamic area (LHA) to the paraventricular nucleus of the thalamus (PVT) mediates the attribution of incentive salience to Pavlovian reward-cues. However, a causal role for the LHA and the neurotransmitters involved have not been demonstrated in this regard.
Objectives:
To examine: 1) the role of LHA in the acquisition of Pavlovian conditioned approach (PavCA) behaviors, and 2) the role of PVT orexin 1 receptors (OX1r) and orexin 2 receptors (OX2r) in the expression of PavCA behaviors and conditioned reinforcement.
Methods:
Rats received excitotoxic lesions of the LHA prior to Pavlovian training. A separate cohort of rats characterized as sign-trackers (STs) or goal-trackers (GTs) received the OX1r antagonist SB-334867, or the OX2r antagonist TCS-OX2-29, into the PVT, to assess their effects on the expression of PavCA behavior and on the conditioned reinforcing properties of a Pavlovian reward-cue.
Results:
LHA lesions attenuated the development of sign-tracking behavior. Administration of either the OX1r or OX2r antagonism into the PVT reduced sign-tracking behavior in STs. Further, OX2r antagonism reduced the conditioned reinforcing properties of a Pavlovian reward-cue in STs.
Conclusions:
The LHA is necessary for the development of sign-tracking behavior; and blockade of orexin signaling in the PVT attenuates the expression of sign-tracking behavior and the conditioned reinforcing properties of a Pavlovian reward-cue. Together, these data suggest that LHA orexin inputs to the PVT are a key component of the circuitry that encodes the incentive motivational value of reward cues.
Keywords: Incentive salience, paraventricular nucleus of the thalamus, lateral hypothalamus, sign-tracking, orexin/hypocretin
Introduction.
Forming stimulus-reward associations provides individuals with the fundamental capacity to identify stimuli in the environment that predict the availability of valuable resources. Such stimulus-reward associations result from Pavlovian conditioning, during which a previously neutral stimulus becomes a conditioned stimulus (CS) following repeated pairings with an unconditioned stimulus (US), such as food. In addition to acquiring predictive value, Pavlovian-conditioned stimuli (CSs) can also acquire incentive motivational value, thus becoming incentive stimuli (Bindra 1978; Robinson and Berridge 1993). While predictive stimuli indicate the future availability of the US, incentive stimuli evoke emotional and motivational states that render the CSs themselves ‘wanted’ (Berridge 2001; Robinson and Flagel 2009). Several psychiatric disorders, including substance use disorder, have been associated with an excessive attribution of incentive value to reward-cues (Berridge and Robinson 2003; Cofresi et al. 2019; Frank et al. 2019; Hellberg et al. 2019; Mahler and de Wit 2010; Versace et al. 2016).
Individuals differ in the extent to which they attribute incentive salience to reward-cues (Hearst and Jenkins 1974). Exploiting this individual variability in an animal model has allowed us to parse the predictive vs. incentive qualities of reward-cues (Flagel et al. 2009; Flagel et al. 2007). When a lever-cue is repeatedly paired with a food reward, some rats, referred to as sign-trackers (STs), attribute both predictive and incentive motivational value (i.e. incentive salience) to the reward-cue, and approach and interact with the cue itself upon its presentation (Flagel et al., 2009). In addition, STs will perform a novel instrumental action for presentation of the reward-cue, even in the absence of the reward with which it was initially paired (Hughson et al. 2019; Robinson and Flagel 2009). In contrast, other rats, referred to as goal-trackers (GTs), treat the reward-cue primarily as a predictive stimulus and approach the location of impending reward delivery upon presentation of the cue (Flagel et al. 2009; Flagel et al. 2007). Thus, while both STs and GTs attribute predictive value to the reward-cue, only STs attribute it with incentive salience (Robinson and Flagel 2009).
The ST-GT model has provided a unique platform to investigate the neurobiology that contributes to the attribution of predictive vs. incentive value to reward-cues. Research thus far suggests that GT rats rely primarily on “top-down” cortical mechanisms to guide their behavior (Campus et al. 2019; Flagel et al. 2010; Paolone et al. 2013; Sarter and Phillips 2018), while ST rats rely primarily on “bottom-up” subcortical circuitry (Flagel et al. 2011; Haight et al. 2017; Kuhn 2018; Sarter and Phillips 2018). Among the subcortical brain structures that have been shown to play a role, the paraventricular nucleus of the thalamus (PVT) has emerged as a critical mediator in regulating incentive salience attribution in STs (Campus et al. 2019; Flagel et al. 2011; Haight and Flagel 2014; Haight et al. 2015; Haight et al. 2017; Kuhn et al. 2018; Yager et al. 2015). The PVT is a midline thalamic nucleus that is connected with cortical, limbic and motor circuitries (Berendse and Groenewegen 1990; Canteras et al. 1995; Chen and Su 1990; Hsu and Price 2009; Kirouac et al. 2005; Kirouac et al. 2006; Lee et al. 2015; Li and Kirouac 2008; Li and Kirouac 2012; Li et al. 2014; Parsons et al. 2006; Parsons et al. 2007; Pinto et al. 2003; Su and Bentivoglio 1990; Van der Werf et al. 2002; Vertes 2004; Vertes and Hoover 2008; Vogt et al. 2008).
Another subcortical brain region that seems to play a role in sign-tracking behavior is the lateral hypothalamic area (LHA) (Haight et al. 2017), which, for these purposes, refers to the lateral hypothalamus and the adjacent perifornical area. We previously demonstrated that presentation of an incentive CS evokes greater neural activity in the LHA of STs, compared to GTs, and specifically in cells that project from the LHA to the PVT (Haight et al., 2017). These data support the hypothesis that the LHA-PVT circuit plays a role in the attribution of incentive salience to reward-associated stimuli (Haight and Flagel 2014), but the molecular identity of the cells or transmitter systems involved within this circuit remain unknown. While the inputs from the LHA to the PVT are heterogeneous, one potential candidate for mediating the incentive motivational value of reward-cues is the orexin/hypocretin system (Haight and Flagel 2014; Kelley et al. 2005).
The orexin/hypocretin system consists of two neuropeptides, orexin-A and orexin-B, that bind to two distinct G-protein coupled receptors, orexin receptor 1 (OX1r) and orexin receptor 2 (OX2r). Orexin positive neurons originate exclusively in the LHA (de Lecea et al. 1998; Sakurai et al. 1998), and project diffusely to multiple cortical and subcortical brain regions (Marcus and Elmquist 2006). While known primarily for its role in arousal, sleep and feeding behavior, orexinergic transmission has long been implicated in cue-motivated and addiction-related behaviors (Cason and Aston-Jones 2013; Cole et al. 2015; Keefer et al. 2016; Mahler et al. 2012; Petrovich et al. 2012; Sakurai 2014). Recent evidence suggests that the role of orexin in mediating such behaviors is, in part, localized to the PVT (Barson et al. 2015; James and Dayas 2013; Li et al. 2011; Martin-Fardon and Boutrel 2012; Matzeu et al. 2016; Matzeu and Martin-Fardon 2018; Matzeu et al. 2014), which receives dense orexinergic projections (Kirouac et al. 2005; Lee et al. 2015).
To better examine the role of the LHA and orexinergic signaling in the PVT on incentive motivational processes, we conducted two experiments. In Experiment 1, we performed bilateral excitotoxic lesions of the LHA in male Sprague-Dawley rats before training them in a Pavlovian conditioning paradigm. In Experiment 2, after rats had acquired Pavlovian conditioned approach (PavCA) behavior, we administered either the OX1r antagonist, SB-334867 (Experiment 2a), or the OX2r antagonist, TCS-OX2-29 (Experiment 2b), into the PVT and assessed the effects on the expression of sign- and goal-tracking behavior, and on the conditioned reinforcing properties of the Pavlovian-conditioned food-cue. We hypothesized that lesions of the LHA, and blockade of orexin signaling in the PVT, would attenuate the attribution of incentive salience to a Pavlovian conditioned food-cue and thereby the expression of sign-tracking behavior, as well as the conditioned reinforcing properties of the incentive stimulus.
Materials and methods.
All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee, and all experiments were conducted in accordance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals: Eighth Edition, revised in 2011.
Housing.
Male Sprague-Dawley rats (Charles River, Saint-Constant, Québec, Canada and Raleigh, NC, USA) were used. Rats were housed in a climate-controlled room (22±2 °C) with a 12-hour dark-light cycle (lights on at 06:00 or 07:00 depending on daylight savings time). All rats had ad-libitum access to food and water for the duration of the experiments. Behavioral testing took place during the light cycle between 11:00 and 17:00.
Surgeries.
Surgeries were performed under aseptic conditions. A surgical plane of anesthesia was induced with inhalation of 5% isoflurane, and anesthesia was maintained throughout the procedure with inhalation of 1-2% isoflurane. Prior to surgeries, while under anesthesia, rats received an injection of carprofen (5mg/kg, s.c.) for analgesia and were further prepared for surgeries by shaving the scalp and applying betadine (Purdue Products, Stamford, CT) followed by 70% alcohol as an antiseptic. Rats were then placed into a stereotaxic frame (David Kopf instruments, Tujunga, CA or Stoelting, Wood Dale, IL) and a small incision was made on the scalp to expose the skull. The skull was leveled within +/− 0.1 mm using bregma and lambda coordinates, and small holes were drilled above the regions of interest, as described below.
Pavlovian conditioned approach (PavCA) apparatus.
PavCA training occurred inside Med Associates chambers (St. Albans, VT, USA; 30.5 × 24.1 × 21 cm) located in sound-attenuating cabinets with a ventilation fan to create background noise. Each chamber contained a food magazine located in the center of one wall approximately 3 cm above the grid floor, connected to an automatic pellet dispenser. Each time the pellet dispenser was triggered, one 45-mg banana-flavored dustless pellet (Bio-Serve, Flemington, NJ) was delivered into the food cup. A retractable backlit metal lever was located either to the left or right of the food magazine, approximately 6 cm above the grid floor. A house light was located on the wall opposite to the food magazine and lever, approximately 1 cm from the top of the chamber. Magazine entries were recorded upon break of a photo-beam located inside the magazine and lever contacts were registered upon deflection of the lever, which required a minimum of 10 g of force. Behavioral data were collected using Med Associates’ Med PC software.
PavCA Procedure.
PavCA procedures were the same as described previously (Campus et al. 2019; Hughson et al. 2019). For two days prior to behavioral training rats were briefly handled by the experimenters in the housing room, and a small scoop (~25) of banana-flavored pellets were delivered in the home cage, to familiarize rats to the experimenters and to the novel food. Following these two days, all rats underwent one pretraining session, followed by 7 PavCA sessions. The pretraining session consisted of 5 minutes of acclimation time in the chamber, followed by illumination of the house light and 25 trials in which a food pellet was delivered into the food magazine on a variable time (VT) 30-s schedule (range 0-60 s). Prior to the start of the session, each food magazine was baited with 3 banana-flavored pellets, to direct the rats’ attention to the location of reward delivery. The lever remained retracted for the entirety of the session, which lasted an average of 12.5 minutes. Rats typically consumed all of the pellets delivered into the food cup during the pretraining session. After pre-training, rats underwent one daily session of PavCA training for 7 consecutive days. Each PavCA training session consisted of 1 minute of acclimation time, and then the house light was turned on and 25 trials under a VT-90 s schedule (range 30-150 s) were presented. During each trial, an illuminated lever was inserted into the chamber for 8 s, and was followed by the delivery of a banana-flavored food pellet into the food magazine upon lever retraction. The start of PavCA training was signaled by illumination of the house light and lasted an average of 40 minutes. For each PavCA session, the number of lever contacts and head entries into the food magazine, the probability of contacting the lever or entering the food magazine, and the latency to contact the lever or to enter the food magazine during each trial were recorded or calculated.
Statistical Analyses.
All statistical analyses were performed using IBM SPSS Statistics 26 (IBM, Armonk, NY, USA). Alpha was set at 0.05. When significant main effects or interactions were detected, Bonferroni post-hoc comparisons were performed. Graphic representations of the data were created with Prism 8 (Graphpad Software, San Diego, CA).
Detailed Methods.
Experiment 1- Effects of LHA lesion on the acquisition of Pavlovian conditioned approach behavior.
Subjects.
Sixteen adult male Sprague-Dawley rats (Charles River, Saint-Constant, Québec, Canada), weighing an average of 400 g (~11-12 weeks old) at the time of experimentation were used. These rats had previously undergone a brief, three-day fear conditioning pilot experiment. Rats were pair-housed for a two-week rest period following fear-conditioning, and then lesion and sham surgeries targeting the LH were performed. Following surgery, rats were single-housed for the remainder of the experiment, and allowed to rest for an additional two weeks prior to the start of PavCA training. The fear-conditioning pilot experiment was unlikely to affect the results of the current experiment, due to the substantial rest periods (4 weeks total) and the fact that all subjects were counter-balanced across treatment groups.
Drugs.
Excitotoxic lesions were performed using N-methyl-D-aspartate (NMDA; #M3262; Sigma-Aldrich, Inc.; St. Louis, MO). NMDA was dissolved in sterile saline and injected bilaterally in the LHA at a 90 mM concentration (pH = 7.34-7.36).
Lesion surgery and PaVCA training.
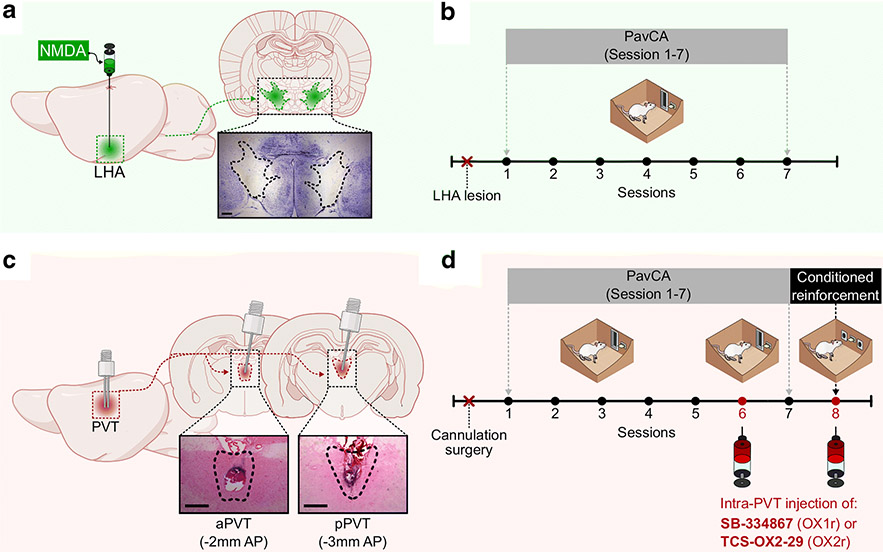
Excitotoxic lesions (Figure 1a) were performed by lowering one-barrel stainless steel guide-cannulas (26-gauge; Plastics One, Inc.; Roanoke, VA) bilaterally into the LHA at the following coordinates relative to bregma: AP: −2.2 mm, ML: +/−1.7 mm, DV: −8.1 mm. A stainless steel injector that projected 1mm beyond the guide cannula (33-gauge; Plastics One, Inc.; Roanoke, VA) was inserted into each cannula and connected with PE-20 tubing to a microsyringe (5 μL; Hamilton Company; Reno, NV) mounted in an infusion pump (Harvard Instruments; Holliston, MA). NMDA injections occurred over the course of 4 min at a rate of 0.15 μl/min, for a total delivered volume of 0.6 μl. The injector was left in place for two minutes following injection to allow for diffusion. Sham surgeries were done by performing the same incisions and by drilling holes over the LHA, but cannulas were not inserted and no injections were delivered. Following surgery, the incisions were sealed using surgical clips. After a 2-week recovery period rats went through seven sessions of PavCA training (for experimental timeline, see Figure 1b).
Figure 1. Experimental timelines.
Experiment 1: a) Schematic of the excitotoxic lesion of the LH and photomicrograph of a representative lesion, and b) experimental timeline. Rats received 0.6 μl of NMDA in the right and left LHA. Sham rats underwent the same surgery but no injection was performed. After recovery from surgery, rats were trained in a Pavlovian Conditioned Approach (PavCA) paradigm for 7 consecutive sessions. Experiment 2: c) Schematic of the cannulation surgery to target the aPVT and pPVT and representative images of aPVT and pPVT cannulation sites, and d) experimental timeline. After recovery from surgery, rats were trained in a Pavlovian Conditioned Approach (PavCA) paradigm for 5 consecutive sessions (Acquisition) and phenotyped as sign- (STs) or goal-trackers (GTs). On the following day rats went through a PavCA test session (session 6) in order to assess the effects of OX1 or OX2 receptor antagonism in the PVT on the expression of PavCA behavior. The next day, all rats went through an additional PavCA session (session 7) with no drug or vehicle infusions to assess any lasting effects of drug infusion. Following the completion of PavCA training, all subjects were tested in a conditioned reinforcement (CRT) paradigm, in order to assess the effects of OX1 or OX2 receptor antagonism in the PVT on the conditioned reinforcing properties of the lever-CS.
Tissue processing.
Rats were deeply anesthetized with 5% inhaled isoflurane. Following anesthetization, brains were extracted and flash frozen in isopentane cooled with dry ice. The frozen brains were subsequently sectioned in the coronal plane (40 μm) using a cryostat (Leica Biosystems Inc, Buffalo Grove, IL), mounted onto SuperFrost Plus microscope slides (Fisher Scientific), soaked in 4% formaldehyde for 30 minutes, and counterstained with cresyl violet. Sections containing the LHA were assessed for lesion accuracy by an experimenter blind to the experimental groups using a DM1000 light microscope coupled to an ICC50 HD camera (Leica-Microsystems, Wetzlar, GER).
Statistical analyses.
To assess differences in the acquisition of PavCA behavior, a linear mixed effects model with a restricted maximum likelihood estimation method was used. Session was used as the repeated variable and Group (Sham vs. Lesion) as the between-subject variable. Lever-directed behaviors (lever contacts, probability to contact the lever and latency to contact the lever), magazine-directed behaviors (magazine entries during the CS period, probability to enter the magazine during the CS period and latency to enter the magazine during the CS period), as well as magazine entries during the intertrial interval (ITI), were used as dependent variables. Before choosing the final model, several covariance structures were explored for each one of the dependent variables, and the best-fitting model was chosen by selecting the lowest Akaike Information Criterion (AIC) (Duricki et al. 2016; Verbeke and Molenberghs 2009).
Correlational analysis was performed to assess whether food magazine entry rate was associated with the PavCA Index Score. For this analysis, an average difference score for magazine entries was calculated by taking the CS magazine entry rate (total number of magazine entries during CS presentation divided by 8 [CS duration in seconds]) and subtracting the ITI magazine entry rate (total number of magazine entries during the ITI divided by 90 [average length of ITI in seconds]), from sessions 6 and 7. The magazine entry difference score was then correlated with PavCA Index scores (Meyer et al. 2012) calculated from the average of sessions 6 and 7. The PavCA Index is a composite score that is used to measure the degree to which an individual’s behavior is directed towards the lever-CS or food cup (location of US delivery) using three different metrics: response bias [(total lever contacts – total food cup contacts) / (sum of total contacts)], probability difference score [Prob(lever) – Prob(food cup)], and latency difference score [-(lever contact latency – food cup entry latency) / 8 seconds]. These three measures were averaged together and rounded to the nearest tenth of a decimal place to create the PavCA Index score, which ranges from −1.0 to 1.0, with −1.0 representing an individual whose behavior is directed solely towards the food cup (i.e. goal-tracker), and 1.0 representing an individual whose behavior is directed solely towards the lever-CS (i.e. sign-tracker).
Experiment 2- Effects of the pharmacological antagonism of orexin 1 or orexin 2 receptors on the expression of sign-tracking behavior and on the conditioned reinforcing properties of a Pavlovian reward-cue.
Subjects.
A total of 230 male Sprague Dawley rats (Charles River, Saint-Constant, Québec, Canada and Raleigh, NC, USA) were used for Experiment 2. Rats were 275-325 g (~7-9 weeks old) at the time of arrival and were initially pair-housed and allowed to acclimate to the housing room for at least 7 days prior to the surgery. Following cannula implantation surgery (described below), rats were single-housed for the remainder of the study to avoid damage to the implanted cannulas. As we were interested only in assessing STs (n=116) and GTs (n=62), rats with a PavCA index between −0.3 and +0.3 (intermediate rats, n = 52) were excluded from Experiment 2. Of the remaining 178 rats, 88 were excluded from the main statistical analysis due to missed cannula placement in either the aPVT or the pPVT (or both). In addition, 9 rats did not complete the experiment due to health or technical issues, resulting in a final n of 81 rats (52 STs, 29 GTs). Of the final 81 rats, 47 (29 STs, 18 GTs) were used for the OX1 r antagonism study (Experiment 2a) and 34 (23 STs, 11 GTs) were used for the OX2r antagonism study (Experiment 2b). To evaluate the selectivity of OX1r and OX2r antagonism in the PVT, STs with cannula placements outside of the aPVT and pPVT were allocated to a neuroanatomical control group (EXCLUDED group, see Supplemental Information). Data for Experiment 2a were collected across 3 rounds of testing, while data for Experiment 2b were collected across 2 rounds of testing.
Drugs.
To block orexin 1 receptors, the selective OX1r antagonist SB-334867 (SB; Lots 11B/185592, 11B/186281, Tocris Bioscience, Avonmouth, Bristol, UK) was dissolved in 100% dimethyl sulfoxide (DMSO) at a concentration of 15 μg per 300 nl. To block orexin 2 receptors, the selective OX2r antagonist TCS-OX2-29 (TCS; Lots 2A/179223, 2B191601, Tocris Bioscience) was dissolved in 0.9% sterile saline at a concentration of 15 μg per 300 nl. These drug doses were chosen from other studies in the literature investigating the role of orexin signaling in the PVT (for example, see Li et al., 2011; Matzeu et al., 2016).
Guide cannula implantation.
For both Experiment 2a and 2ba stainless steel double cannula aligned along the anterior-posterior axis (1 mm center to center gap, cut 6 mm below pedestal, 26 gauge, part # C235G-1.0-SP, Plastics One, Roanoke, VA) were implanted with the stereotaxic arm angled at 10° towards the midline to target the aPVT and pPVT (Figure 1c), at the following coordinates relative to bregma: AP −2.0 mm, ML −1.0 mm, DV −4.7 mm (aPVT) and AP −3.0 mm, ML −1.0 mm, DV −4.7 mm (pPVT). Four screws were then implanted in the skull, and the cannula was fixed in place using dental cement. Once the cement was dry, the incision was closed around the cement with stainless steel wound clips. In addition, the cannula was plugged with a dummy injector that was flush with the end of the cannula, and covered with a dust cap. Following cannulation surgeries rats were allowed to recover a minimum of 7 days prior to any behavioral testing.
PavCA training.
All subjects from Experiments 2a and 2b went through 1 session of pretraining, followed by 7 sessions of PavCA training as described above (for experimental timeline, see Figure 1d). Prior to pretraining and the first 5 PavCA sessions, rats were transported to a separate room and handled by the experimenters, increasing in time from approximately 30 seconds (pretraining) to 4 minutes (PavCA session 5). In addition, during the handling prior to sessions 4 and 5, all rats had their dust caps screwed on and off, in order to acclimate them to the infusion procedure.
Intra-PVT infusions and PavCA test.
Following session 5 of PavCA training, rats were classified as STs or GTs based on their average PavCA Index scores from sessions 4 and 5, as described above (Meyer et al. 2012). Rats were then split into experimental and control groups counterbalanced based on their PavCA Index score. On the following day rats went through a PavCA test session (session 6) in order to assess the effects of OX1r (Experiment 2a) or OX2r (Experiment 2b) antagonism in the aPVT and pPVT on the expression of PavCA behavior. Prior to the test session, each rat’s dust cap and dummy cannula was removed, and a double injector protruding 1 mm beyond the guide cannula was inserted (final infusion coordinates AP −2.0 mm, ML −1.0 mm, DV −5.7 mm and AP −3.0 mm, ML −1.0 mm, DV −5.7 mm). The injector was connected via P50 tubing to two 1 μl Hamilton syringes housed in a Harvard Apparatus double syringe pump. Rats from Experiment 2a were infused with either SB-334867 (SB) or 100% dimethyl sulfoxide (DMSO) as control vehicle. Rats from Experiment 2b were infused with either TCS-OX2-29 or 0.9% saline (SAL) as a control vehicle. Infusions occurred simultaneously in the aPVT and pPVT and lasted 2 minutes, at a flow rate of 150 nl per minute, for a total infusion volume of 300 nl per injection site. After the end of the infusion, the injector was left in place for 2 additional minutes to allow diffusion. An experimenter gently held each rat for the 4-minute duration of the infusion/diffusion period. After the injector was removed, the dummy cannula and dust cap were replaced, and the rat was placed back into its home cage and transported to the testing room, where it sat for 10 minutes under red light prior to being placed in the chambers for the PavCA test. The next day, all rats went through an additional PavCA session (session 7) with no drug or vehicle infusions to assess any lasting effects of drug infusion on PavCA behavior.
Conditioned Reinforcement Test (CRT).
Following the completion of PavCA training, all subjects were tested in a conditioned reinforcement (CRT) paradigm, in order to assess the effects of intra-PVT OX1r (Experiment 2a) or OX2r (Experiment 2b) antagonism on the conditioned reinforcing properties of the lever-CS. Injection procedures before CRT were the same as described above, and all treatment groups remained consistent from the PavCA experiment to the CRT experiment. For the CRT test, the chambers were rearranged so that the food cup and pellet dispenser were removed, and the lever-CS was moved to the center of the wall. Two nose poke ports were then installed to the right and left of the lever. The nose port installed opposite the previous position of the lever-CS was designated the “active” nose port, and pokes into this port resulted in the brief 2-second presentation of the illuminated lever-CS on a fixed-ratio 1 schedule. Pokes into the other nose port, designated the “inactive”, did not result in lever-CS presentation. Once the rats were placed into the test chambers, the house light remained off for 1 minute. After the 1-minute acclimation period, the house light was illuminated, and the CRT test session began. The session lasted 40 minutes, and the Med PC software program recorded the following measures for analysis: 1) the number of pokes into the active nose port, 2) the number of pokes into the inactive nose port, and 3) the number of lever contacts.
Tissue processing.
Rats were deeply anesthetized with an intraperitoneal injection of ketamine (90 mg/kg i.p.) and xylazine (10 mg/kg i.p.) and transcardially perfused with ~200 mL of room temperature 0.9% saline, followed by ~200 mL of room-temperature 4% formaldehyde (pH=7.30-7.4, diluted in 0.1M sodium phosphate buffer; Fisher Scientific, Hampton, NH). Brains were then extracted and post-fixed overnight in 4% formaldehyde at 4°C. Brains were cryoprotected over three nights in graduated sucrose solutions (10%, 20%, and 30%, dissolved in 0.1M sodium phosphate buffer with a pH=7.3-7.4) at 4°C. Following cryoprotection, brains were encased in Tissue-Plus O.C.T. (Fisher Healthcare, Houston, TX), frozen using dry ice and subsequently sectioned in the coronal plane (40 μm) using a cryostat (Leica Biosystems Inc, Buffalo Grove, IL). Brain slices containing the PVT were collected into well plates containing cryoprotectant and stored at −20 °C before being mounted onto SuperFrost Plus microscope slides (Fisher Scientific), and counterstained with Eosin-Y. Sections were assessed for accuracy of cannula placement using a DM1000 light microscope coupled to an ICC50 HD camera (Leica-Microsystems, Wetzlar, GER) by an experimenter blind to the experimental groups.
Statistical analyses.
Similar to Experiment 1, linear mixed effects models with a restricted maximum likelihood estimation method were used to assess differences in the acquisition and expression of PavCA behavior. For acquisition of PavCA behavior across all subjects, Session (1-5) was used as the repeated variable and Phenotype (ST vs. GT) was used as between-subjects variable. Lever-directed behaviors (lever contacts, probability to contact the lever and latency to contact the lever) and magazine-directed behaviors (magazine entries during the CS period, probability to enter the magazine during the CS period and latency to enter the magazine during the CS period) were used as dependent variables. Similarly, PavCA index scores during session 1-5 of PavCA training were analyzed across all subjects using a linear mixed effect model. For this analysis, session (1-5) was used as the repeated variable and Phenotype (ST vs. GT) as the between-subject variable. In addition, to ensure that subjects were counterbalanced between different experimental groups, linear mixed effects models with a restricted maximum likelihood estimation method were used to assess group differences in the acquisition PavCA behavior prior to treatment. Session (1-5) was used as the repeated variable and Phenotype (ST vs. GT) and Treatment (DMSO vs. SB vs. SAL vs. TCS) were used as between-subjects variables. Baseline differences in the expression of lever- and magazine-directed behaviors during session 5 (prior to treatment) and the average PavCA index scores from sessions 4-5 were assessed using a two-way ANOVA, with Phenotype (STs vs. GTs) and Treatment (DMSO vs. SB for Experiment 2a; SAL vs. TCS for Experiment 2b) as independent variables.
To assess the effects of OX1r or OX2r antagonism in the PVT on the expression of PavCA behavior, the effect of Treatment was assessed within each phenotype separately. Linear mixed effects models with a restricted maximum likelihood estimation method were used, with Session (5-7) as the repeated variable and Treatment (SB vs. DMSO for Experiment 2a; TCS vs. SAL for Experiment 2b) as the between-subject variable. Lever contacts and magazine entries were used as the dependent variables. For all linear mixed effects models, the covariance structure that was the best fit was chosen based on the lowest AIC.
To assess the effects of OX1r and OX2r antagonism in the PVT on the behaviors expressed during CRT, nose pokes, lever contacts and incentive value index (Campus et al. 2019; Hughson et al. 2019) were analyzed within each phenotype. Nose pokes were analyzed using a two-way ANOVA with nose port (Active vs. Inactive) and Treatment (DMSO vs. SB for Experiment 2a; SAL vs. TCS for Experiment 2b) as independent variables. Differences in lever contacts and in the incentive value index were analyzed using an unpaired t-test, with Treatment as the independent variable. The incentive value index was calculated as previously described (Hughson et al. 2019) by using the following formula: [(Active Nosepokes + Lever Contacts) – Inactive Nosepokes].
Results
Experiment 1- Effects of LHA lesion on the acquisition of Pavlovian conditioned approach behavior.
Lesion verification.
Sections containing the LHA were screened for accuracy of lesion placement using the Rat Brain Atlas (Paxinos & Watson, 2007). All subjects in the lesion group showed evidence of excitotoxic damage to the LHA, with lesions generally spanning from −2.12 to −2.80 AP, relative to bregma. A representative image of an LHA lesion can be found in Figure 1a, and the approximate spread of LHA lesions for all experimental rats can be found in Supplemental Figure 1.
Lesions of the LHA impair the acquisition of sign-tracking behavior.
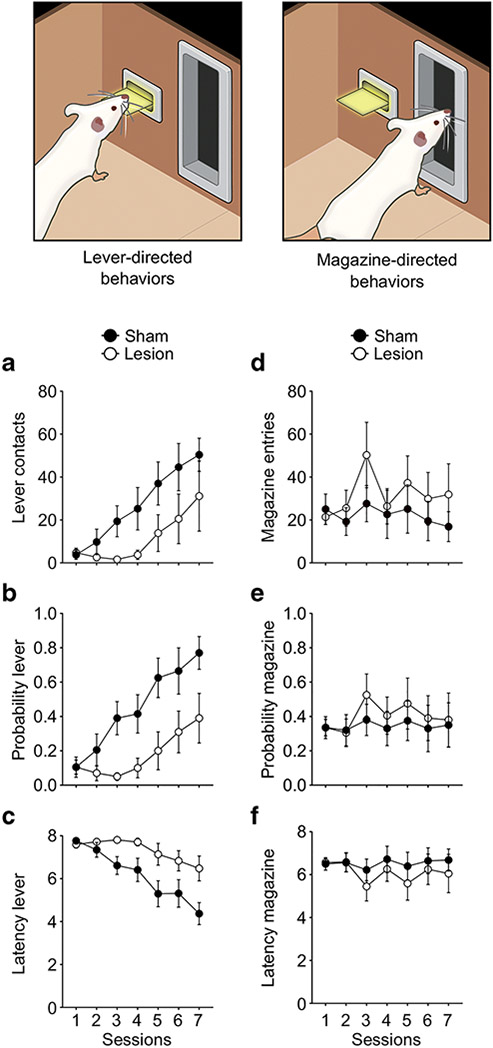
To assess the effects of LHA lesions on the acquisition of sign-and goal-tracking behavior, lever-directed behaviors (lever contacts, probability to contact the lever and latency to contact the lever) and magazine-directed behaviors (magazine entries during the CS period, probability to enter the magazine during the CS period and latency to enter the magazine during the CS period) were analyzed across 7 sessions of PavCA training. Relative to lesioned rats, control (sham) rats exhibited a greater number of lever contacts (F6,22.118 = 2.565, p = 0.049, Figure 2a), a greater probability to contact the lever (F6,36.592 = 3.000, p = 0.017, Figure 2b), and had a lower latency to approach the lever (F6,30.768 = 3.640, p = 0.008, Figure 2c). Post-hoc analyses revealed a significant difference between sham and lesioned rats from Session 3-7 (p < 0.044) for all lever-directed behaviors. There was not a significant effect of Group for magazine entries (F6,23.484 = 0.861, p = 0.537, Figure 2d), probability to enter the magazine (F6,78.090 = 0.501, p = 0.806, Figure 2e) or for latency to enter the magazine (F6,18.702 = 0.411, p = 0.862, Figure 2f).
Figure 2. Effects of LHA lesions on the acquisition of lever-directed (sign-tracking) and magazine-directed (goal-tracking) behaviors in Experiment 1.
Mean ± SEM for a) Number of lever contacts, b) probability to contact the lever, c) latency to contact the lever, d) number of magazine entries, e) probability to enter the magazine and f) latency to enter the magazine across 7 sessions. There was a significant effect of treatment (Sham vs. lesion) on sign-tracking (left column; P<0.05), but not goal-tracking behaviors (right column). N = 8/group.
It should be noted that, while there was a significant effect of Session for lever-directed behaviors (P<0.01 for all measures), there was only a trend towards significance for goal-directed behaviors (P≥0.06 for all measures). This could be due to the fact that the population of animals for this experiment was skewed towards sign-trackers, thus masking evidence of a learned goal-tracking response. Therefore, to determine whether animals were, in fact, learning the predictive value of the reward-cue over course of training, additional analyses were conducted. First, the number of magazine entries during the intertrial interval (ITI) were analyzed. There was a progressive reduction in the number of magazine entries across sessions (significant effect of Session, F6,70.288 = 7.677, p = 0.000), and this did not significantly differ between Treatment groups (Supplemental Figure 2a). That is, with Pavlovian training, both sham and lesion rats decreased their approach to the food cup in the absence of the CS (Flagel et al., 2007; Meyer et al., 2012). This reduction in magazine entries during the ITI has consistently been observed in both ST and GT rats, and is considered an index of learning (e.g. Flagel et al., 2007). Second, a correlational analysis was performed to assess whether magazine entry difference score (CS magazine entry rate – ITI magazine entry rate) was associated with the PavCA Index score at the end of training. There was a significant negative correlation between the magazine entry difference score and PavCA Index score (r(14) = −0.918, p < 0.001; Supplemental Figure 2b). Subjects with PavCA Index scores in the typical goal-tracking (negative) range tended to have a greater magazine entry difference score, indicating a higher rate of head entry into the food magazine during the CS period; while rats with PavCA Index scores in the typical sign-tracking (positive) range tended to have smaller magazine entry difference scores. Together, these data support the conclusion that lesions of the LHA impair the acquisition of lever-directed behaviors, without impeding predictive learning, and highlight a specific role for the LHA in the attribution of incentive salience to a reward-cue.
Experiment 2- Effects of the pharmacological antagonism of orexin 1 or orexin 2 receptors on the expression of Pavlovian conditioned approach behavior and on the conditioned reinforcing properties of a Pavlovian reward-cue.
Histological controls.
Cannula placements were verified for all subjects to ensure accuracy of drug infusions into the anterior and posterior PVT. Subjects with injector tracts abutting the dorsal border of the PVT, within the PVT, or immediately adjacent to the lateral or ventral borders of the PVT were considered accurate injections, and were included in the main analysis of the study (for an example of acceptable cannula placement, see Figure 1b). Subjects with at least one injection that did not touch the top of the PVT (e.g. in the ventricle, hippocampus, etc), or that was completely contained within the surrounding nuclei (e.g. the mediodorsal thalamic nucleus, habenula, etc), were excluded from the main analysis, and are not included below. Rats with cannula placements outside of both the aPVT and pPVT were analyzed separately to assess the selectivity of OX1 and OX2 receptor antagonism in the PVT (Supplemental Information).
Acquisition of PavCA behaviors.
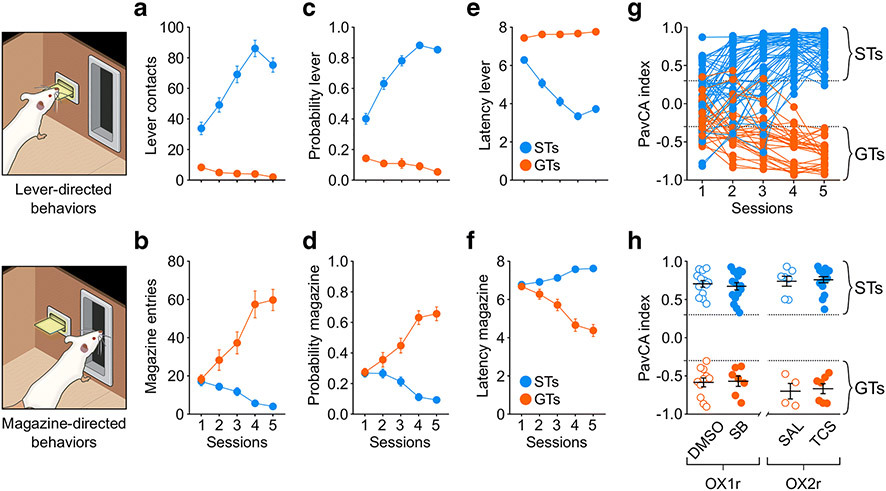
Similar to previous reports (Meyer et al. 2012; Robinson and Flagel 2009), there was variation in the conditioned responses acquired following 5 sessions of PavCA. Rats that directed their behavior towards the food magazine were classified as GTs, with average PavCA index scores from sessions 4 and 5 ranging between −0.3 and −1.0 (Figure 3g). Rats that displayed lever-directed behavior were classified as STs, with PavCA Index scores ranging from 0.3 to 1.0 (Figure 3g). Rats with PavCA index scores ranging from −0.3 to 0.3 (intermediate rats), were not included in the study
Figure 3. Acquisition of lever-directed (sign-tracking) and magazine-directed behaviors (goal-tracking) for Experiment 2.
a-f) Mean ± SEM for a) Number of lever contacts, b) number of magazine entries, c) probability to contact the lever, d) probability to enter the magazine, e) latency to contact the lever and f) latency to enter the magazine. g) Individual Pavlovian conditioned approach (PavCA) index scores during the 5 sessions of Pavlovian conditioning. PavCA scores from session 4 and 5 were averaged to determine the behavioral phenotype. Rats with a PavCA score <−0.3 were classified as goal-trackers (GTs), rats with a PavCA score >+0.3 were classified as sign-trackers (STs; n= 52 STs, 29 GTs). h) Allocation of experimental groups. Mean ± SEM for PavCA index. Rats with similar PavCA scores were assigned to receive different treatments. Rats assigned to the orexin 1 study received either vehicle (DMSO) or the orexin 1 antagonist SB-334867 (SB). Rats assigned to the orexin 2 study received either vehicle (SAL) or the orexin 2 antagonist TCS-OX2-29 (TCS). Baseline differences in PavCA index between experimental groups were assessed by using a 2-way ANOVA with Phenotype and Treatment as independent variables and PavCA index as dependent variable. A significant effect of phenotype was found (p < 0.001). There were no other significant differences between experimental groups. N = 4-11/group for GTs, 7-16/group for STs.
For all measures analyzed, there was a significant effect of Phenotype, Session and a Phenotype x Session (indicated below) interaction. Relative to GTs, ST rats exhibited a greater number of lever contacts (F4,118.507 = 18.793, p < 0.001, Figure 3a), a greater probability to contact the lever (F4,137.618 = 29.209, p < 0.001, Figure 3c) and had a lower latency to approach the lever (F4,155.316 = 32.657, p < 0.001, Figure 3e). Post-hoc analyses revealed a significant difference between phenotypes during all 5 sessions of PavCA training (p < 0.001). During the lever-CS presentation, subjects classified as GTs showed a greater number of magazine entries (F4,141.543 = 33.338, p < 0.001, Figure 3b), a greater probability to enter the magazine (F4,144.416 = 39.753, p < 0.001, Figure 3d), and a lower latency to enter the magazine (F4,138.611 = 40.725, p < 0.001, Figure 3f) compared to STs. Post-hoc analyses revealed a significant difference between phenotypes for all magazine-directed measures from session 2 through session 5 (p < 0.05).
Since the previous measures were calculated with all subjects from Experiments 2a and 2b collapsed, it was important to ensure that the experimental groups did not differ before drug administration. To assess this, Treatment was included as a between - group variable, and acquisition data was analyzed. There was no significant effect of Treatment, nor were there any significant interactions with this variable, as treatment did not occur during this phase of the study. To further explore baseline differences between experimental groups, the effect of Phenotype and Treatment was assessed for the average PavCA index from sessions 4 and 5 (Figure 3h) and for lever- and magazine-directed behavior during session 5 of PavCA training, prior to treatment (Supplemental Tables 1 and 2). As expected, there was a significant effect of Phenotype (F1,80 = 965.945, p< 0.001), for which STs had a greater PavCA index compared to GTs rats. There was not a significant effect of Treatment, nor a significant Phenotype x Treatment interaction, suggesting that all groups were counterbalanced prior to drug testing.
Experiment 2a- Effects of pharmacological antagonism of orexin 1 receptor on the expression of Pavlovian conditioned approach behavior and on the conditioned reinforcing properties of a Pavlovian reward-cue.
Lever contacts and magazine entries are presented as the primary dependent variable in the main text, but analyses of all other lever- and magazine-directed behaviors for STs and GTs are included in Supplemental Tables 3 and 4, respectively.
Antagonism of OX1r in the PVT attenuates subsequent sign-tracking behavior in STs.
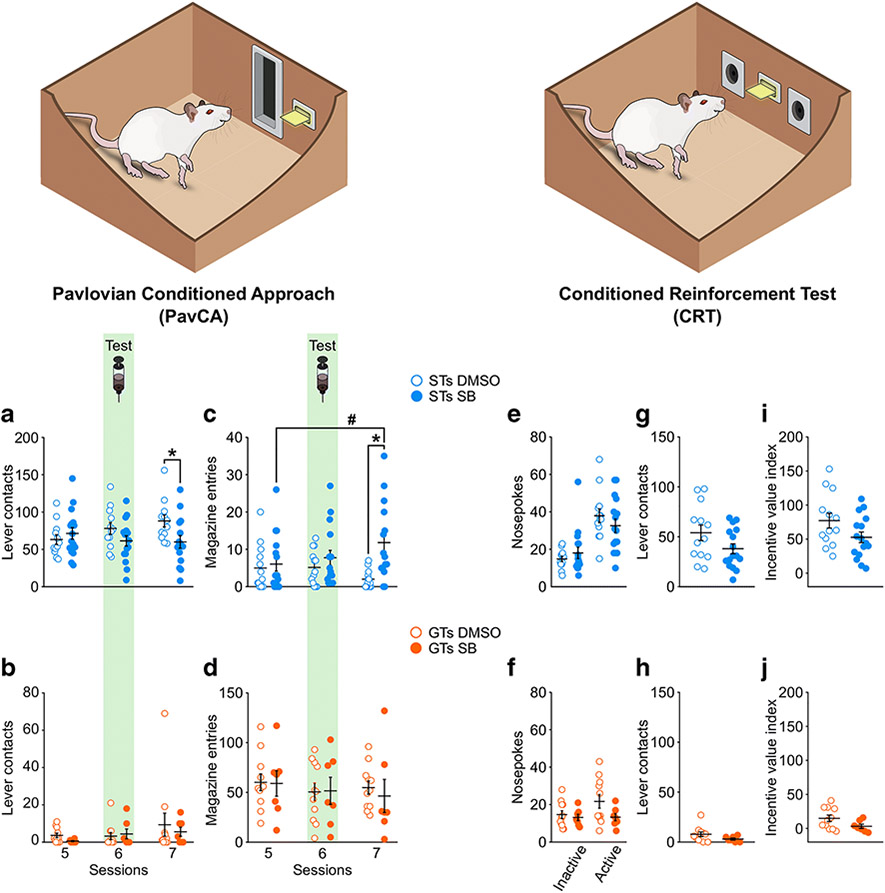
To assess the effects of OX1r antagonism in the PVT on PavCA behavior, PavCA data across sessions 5, 6, and 7 were analyzed for each Phenotype. The analysis of lever contacts (i.e. sign-tracking) in STs showed no significant effect of Treatment (F1,26.827 = 2.983, p = 0.101) nor Session (F2,52.951 = 0.568, p = 0.570). However, there was a significant Treatment x Session interaction (F2,52.951 = 3.735, p = 0.030), suggesting that the antagonism of OX1r in the PVT affected sign-tracking behavior in STs (Figure 4a). There was a trend towards a significant difference between vehicle- and drug-treated rats on session 6 (p = 0.067), when the administration of the OX1r antagonist occurred; but a more robust effect of treatment during session 7 (p = 0.023), which occurred 24 hours after drug administration. These data suggest that a single administration of the OX1r antagonist, SB-33658, may not immediately affect the attribution of incentive value to a reward-cue, but does disrupt the subsequent expression of sign-tracking behavior. There were no significant effects of Treatment (F1,16.391 = 0.318, p = 0.581), Session (F2,16.736 = 1.144, p = 0.342), nor a significant Treatment x Session interaction (F2,16.736 = 1.079, p = 0.363) for lever contacts in GT rats (Figure 4b), suggesting that the blockade of OX1r in the PVT has no effect on the expression of sign-tracking behavior in this phenotype.
Figure 4. Antagonism of OX1r in the PVT prevents the escalation of sign-tracking behaviors and increases goal-tracking behavior in STs, but has no effect on the conditioning reinforcing properties of a food-cue.
Left panel, mean ± SEM for a) number of lever contacts in sign-trackers during Session 5, 6 and 7 of PavCA training. There was a significant Treatment x Session interaction (p = 0.030). Post-hoc analyses revealed that, compared to control rats (DMSO), rats that received a single administration of the OX1r antagonist SB-334867 (SB) before Session 6 showed a decrease in lever contacts during session 7 (*p = 0.023 vs. DMSO), c) number of magazine entries in sign-trackers during Session 5, 6 and 7 of PavCA training. There was a significant Treatment x Session interaction (p = 0.008). Post-hoc analyses revealed that, compared to control rats (DMSO), rats that received a single administration of the OX1r antagonist SB-334867 (SB) before Session 6 showed an increase in magazine entries during session 7 (*p = 0.001 vs. DMSO; #p = 0.015 vs. Session 5). b) number of lever contacts and d) magazine entries in goal-trackers during Session 5, 6 and 7 of PavCA training. No effects were found in goal trackers. N = 13 STs-DMSO, 16 STs-SB, 11 GTs-DMSO, 7 GTs-SB. Right panel, mean ± SEM for e) nosepokes, g) lever contacts, and i) incentive value index in STs, and f) nosepokes, h) lever contacts, and j) incentive value index in GTs. There were no significant effects of OX1r antagonism in the PVT on any measures during the conditioned reinforcement test. N = 13 STs-DMSO, 16 STs-SB, 11 GTs-DMSO, 7 GTs-SB.
Antagonism of OX1r in the PVT increases goal-tracking behaviors in STs.
There was not a significant effect of Treatment (F1,26.909 = 4.187, p = 0.051) nor Session (F2,53.139 = 0.438, p = 0.648) on magazine entries for STs. There was, however, a significant Treatment x Session interaction (F2,53.139 = 5.357, p = 0.008), suggesting that the antagonism of OX1r in the PVT affects goal-tracking behavior in STs (Figure 4c). Similar to the sign-tracking results described above, post-hoc analyses revealed a significant difference between vehicle- and drug-treated rats 24 hours after drug administration (session 7, p = 0.001), but no significant difference during session 6, immediately following the administration of the OX1r antagonist (p = 0.333). Furthermore, post-hoc analyses revealed a significant difference between session 5 and 7 in drug-treated rats (p = 0.015). Thus, despite antagonism of OX1r in the PVT having no immediate effects on goal-tracking behavior, a single administration of the OX1r antagonist SB-33658 is sufficient to promote the expression of goal-tracking behavior in STs 24 hours following drug infusion. In contrast, in GTs, there was not a significant effect of Treatment (F1,16 = 0.042, p = 0.840), Session (F2,32 = 1.678, p = 0.203), nor a significant Treatment x Session interaction (F2,32 = 0.398, p = 0.675) for magazine entries (Figure 4d). These findings suggest that the blockade of PVT OX1r has no effect on the expression of goal-tracking behavior in rats with an inherent tendency for this behavior.
Antagonism of OX-1Rs in the PVT does not alter the conditioned reinforcing properties of the lever-CS for STs or GTs.
For STs, there was a significant effect of Port (F(1,27) = 52.409, p = 0.000), but no effect of Treatment (F(1,27) = 0.081, p = 0.778), nor a Treatment x Port interaction (F(1,27) = 2.682, p = 0.113) on the number of nosepokes during the conditioned reinforcement test (Figure 4e). An unpaired t-test showed a trend towards a significant effect of Treatment for the number of lever contacts (t(27) = 1.802, p = 0.083; Figure 4g) and incentive value index (t(27) = 1.876, p = 0.071; Figure 4i) for STs. For GTs, there was not a significant effect of Port (F(1,16) = 1.833, p = 0.195), Treatment (F(1,16) = 2.706, p = 0.119), nor a Treatment x Port interaction (F(1,16) = 1.691, p = 0.212; Figure 4f). In addition, GTs showed no effect of Treatment for lever contacts (t(16) = 1.614, p = 0.126; Figure 4h) and there was a trend towards significance for the incentive value index (t(16) = 1.818, p = 0.088; Figure 4j). These results suggest that OX1r antagonism in the PVT does not significantly alter the conditioned reinforcing properties of the lever-CS for either STs or GTs.
Experiment 2b- Effects of pharmacological antagonism of OX2r on the expression of Pavlovian conditioned approach behavior and the conditioned reinforcing properties of a Pavlovian reward-cue.
Lever contacts and magazine entries are presented as the primary dependent variable in the main text, but analyses of all other lever- and magazine-directed behaviors for STs and GTs are included in Supplemental Tables 5 and 6, respectively.
Antagonism of OX2r in the PVT prevents the escalation of sign-tracking behaviors in STs.
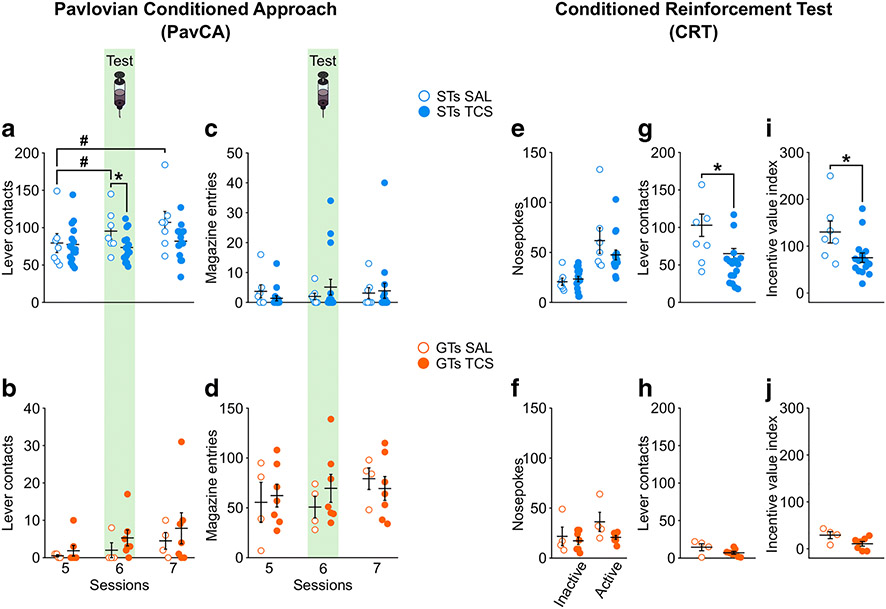
There was a significant effect of Session (F2,24.730 = 4.261, p = 0.026) and a significant Treatment x Session interaction (F2,24.730 = 6.937, p = 0.004) for the number of lever contacts in STs (Figure 5a), suggesting that antagonism of OX2r in the PVT affects sign-tracking behavior. Post-hoc analyses revealed a significant difference between vehicle- and drug-treated rats on session 6 (p = 0.039), during which antagonism of OX2r in the PVT reduced sign-tracking behavior compared to vehicle-treated controls of the same phenotype. In addition, post-hoc comparisons revealed a significant difference between session 5, 6 and 7 in vehicle-treated rats, but not in drug-treated rats. These data suggest that a single administration of the OX2r antagonist, TCS-OX2-29, is sufficient to prevent the increase in sign-tracking behavior shown by control rats. While there was a significant effect of Session (F2,9.740 = 4.837, p = 0.035; Figure 5b) in GTs, there was not a significant effect of Treatment (F1,9.016 = 0.704, p = 0.423), nor a significant Treatment x Session interaction (F2,9.740 = 0.370, p = 0.700). Together, these results indicate that pharmacological antagonism of OX2r in the PVT prevents the escalation of sign-tracking behavior in STs, but has no effect in GTs.
Figure 5. Antagonism of OX2r in the PVT prevents the escalation of sign-tracking behaviors and reduces the conditioned reinforcing properties of a reward-paired cue in sign-trackers, but has no effect in GTs.
Left panel, mean ± SEM for a) number of lever contacts in sign-trackers during Session 5, 6 and 7 of PavCA training. There was a significant Treatment x Session interaction (p = 0.004). Post-hoc analyses revealed that, compared to control rats (SAL), rats that received a single administration of the OX2r antagonist TCS-OX2-29 (TCS) before Session 6 showed a decrease in lever contacts (*p = 0.039 vs. SAL). c) Number of magazine entries in sign-trackers during Session 5, 6 and 7 of PavCA training. No effects were found for magazine entries in sign-trackers. b) number of lever contacts and d) magazine entries in goal-trackers during Session 5, 6 and 7 of PavCA training. There were no significant effects in goal trackers. N = 7 STs-SAL, 16 STs-TCS, 4 GTs-SAL, 7 GTs-TCS. Right panel, mean ± SEM for e) nosepokes, g) lever contacts, and i) incentive value index in STs. A significant reduction in lever contacts and incentive index was apparent in STs who received infusion of the OX2r antagonist TCS OX2 29 into the PVT (p<0.019 for both measures). For GTs, there was no significant effect of drug administration for f) nosepokes, h) lever contacts, or the j) incentive value index. N = 7 STs-SAL, 16 STs-TCS, 4 GTs-SAL, 7 GTs-TCS.
Antagonism of OX2r in the PVT does not affect goal-tracking behaviors.
There was not a significant effect of OX2r antagonism in the PVT on magazine entries in STs (Figure 5c), as there was no effect of Session (F2,21 = 0.283, p = 0.757), Treatment (F1,21 = 0.027, p = 0.871), nor a significant Treatment x Session interaction (F2,21 = 1.447, p = 0.258). Similarly, in GTs, there were no significant effects of Session (F2,17.943 = 2.803, p = 0.087) or Treatment (F1,9.392 = 0.084, p = 0.778), and just a trend towards a significant Treatment x Session interaction (F2,17.943 = 3.062, p = 0.072; Figure 5d). Thus, antagonism of OX2r in the PVT does not significantly affect goal-tracking behavior in either STs or GTs.
Antagonism of OX-2Rs in the PVT affects the conditioned reinforcing properties of the lever-CS in STs.
When assessing the effects of OX2r antagonism on nosepokes in the CRT paradigm, there was a significant effect of Port (F1,21 = 43.666, p = 0.000), but no effect of Treatment (F(1,21) = 0.695, p = 0.414), nor a Treatment x Port interaction (F(1,21) = 2.941, p = 0.101, Figure 5e) for STs. There was, however, a significant effect of OX2r antagonism on the number of lever contacts in STs (t(21) = 2.664, p = 0.015; Figure 5g). Even though pokes into the active port result in only a brief 2-s presentation of the lever-CS, vehicle-treated rats tended to engage with the lever-CS to a greater extent than drug-treated rats during the CRT session. In agreement, compared to vehicle-treated rats, antagonism of OX2r in the PVT reduced the incentive value index in STs (t(21) = 2.546, p = 0.019; Figure 5i). For GTs, there was a significant effect of Port (F1,9 = 11.078, p = 0.009), but no effect of Treatment (F1,9 = 1.817, p = 0.211), and only a trend towards significance for a Treatment x Port interaction (F1,9 = 4.225, p = 0.070; Figure 5f). There was not a significant effect of Treatment for lever contacts (t(9) = 1.710, p = 0.121; Figure 5h) nor the incentive value index (t(9) = 2.047, p = 0.089; Figure 5j) in GTs during CRT.
Discussion.
We performed two separate experiments to examine both the role of the LHA and orexin signaling within the PVT in the attribution of incentive salience to a Pavlovian-conditioned food cue. In Experiment 1, we tested a causal influence of the LHA on the acquisition of sign- and goal-tracking behavior. A bilateral excitotoxic lesion of the LHA before Pavlovian training resulted in an attenuation of lever-directed behaviors during PavCA training, without affecting magazine-directed behaviors (Figure 2, Supplemental Figure 2). These results indicate that an intact LHA is required for the acquisition of sign-tracking. Originally considered to be the feeding center of the brain (Anand and Brobeck 1951), the LHA has long been recognized to play a role in motivated and reward-related behaviors (Devenport and Balagura 1971; DiLeone et al. 2003; Margules and Olds 1962; Nieh et al. 2016; Stuber and Wise 2016; Tyree and de Lecea 2017). While the LHA has previously been shown to respond to or be activated by reward-paired and incentive-motivational cues (Haight et al. 2017; Nieh et al. 2015), this is the first study to causally link the LHA to the attribution of incentive salience to reward cues. Moreover, the fact that goal-tracking behavior was not affected, suggests that the LHA is not critical for the attribution of predictive value to reward cues. These findings are in agreement with our prior research, demonstrating that, relative to GTs, STs have greater c-fos counts in the LHA, and predominantly in neurons of the LHA that project to the PVT (Haight et al. 2017). Thus, the LHA appears to play a critical role in incentive motivational processes, and likely does so via the PVT.
The findings described above were expanded in Experiment 2, where we examined the role of orexinergic activity in the PVT on the attribution of incentive salience to a food-cue. To this end, we tested the effects of selective antagonism of either the OX1r (Experiment 2a) or OX2r (Experiment 2b) in the PVT on Pavlovian conditioned approach behavior and the conditioned reinforcing properties of the food-cue. Unlike Experiment 1, this study focused on the expression of Pavlovian conditioned approach behavior, after the conditioned responses had been acquired. We found that the OX2r antagonist immediately decreased the incentive-motivational value of the reward-cue when administered directly into the PVT of ST rats, evidenced by decreased lever contacts during the PavCA paradigm. Thus, orexin signaling at OX2r receptors in the PVT appears to be directly involved in mediating approach behavior directed towards an incentive-motivational stimulus. By blocking this signal, the incentive-motivational value of the cue is reduced, and a deficit in approach behavior can be readily observed. In addition, performance decrements in PavCA behavior were only observed in rats that developed a sign-tracking phenotype, and were not apparent in rats that developed a goal-tracking phenotype. Presumably, this is because GTs do not assign incentive motivational value to the reward cue (Robinson and Flagel 2009), further indicating that the decrease in behavior observed in STs was due to a loss in the incentive motivational value of the lever-CS, and not a general disruption of the stimulus-reward association.
Antagonism of OX1r receptors in the PVT did not immediately attenuate PavCA behavior. Instead, the observable effects of a single intra-PVT injection of the OX1r receptor antagonist were largely apparent 24 hours after drug administration (session 7). On session 7, there was both a decrease in lever-directed behavior (sign-tracking), and an increase in food-cup directed behavior (goal-tracking) in ST rats. Thus, blockade of OX1 receptors in the PVT appeared to disrupt the sign-tracking trajectory and permit STs to adapt a predictive learning strategy on subsequent sessions. In contrast, blockade of OX2r receptors in the PVT immediately inhibited lever-directed behaviors in STs, without affecting magazine-directed behaviors. Orexin, therefore, appears to play multiple roles in the PVT, with different receptor subtypes mediating distinct aspects of stimulus-reward processing. Speculatively, activity at OX2r may integrate incentive-salience signals from the LHA to the PVT in real time, while activity at OX1r may encode long-term incentive values associated with Pavlovian-conditioned cues. Thus, disruption of OX1r signaling does not manifest itself in behavior immediately, but only becomes apparent when the stimulus is presented the following day. This could also potentially explain why there was a concomitant increase in goal-tracking behavior. The cue-reward association remains intact, and it still able to elicit a conditioned response, but the long-term incentive value of the cue has been reduced, and the focus of the response shifts towards the location of reward delivery. Additional studies will be needed to further explore these diverging roles for orexin signaling.
In addition to PavCA behavior, the effects of orexin antagonism on behavior during a conditioned reinforcement test were assessed. The conditioned reinforcement test was performed as a second measure of the incentive value of the reward cue, since previous work has demonstrated that it can be quite difficult to interrupt an already-acquired sign-tracking phenotype, which is resistant to extinction (Ahrens et al. 2016; Fitzpatrick et al. 2019). There was no effect of administration of the OX1r antagonist on the conditioned reinforcing properties of the food cue. Similarly, administration of the OX2r antagonist did not affect instrumental responding for the food cue; but it did affect approach to the cue when it was presented and thereby the incentive value index. Our interpretation of these findings, which is in agreement with those described above, is that blockade of OX2r in the PVT attenuates cue-directed approach behaviors in real time, and this can be observed during both Pavlovian training and a conditioned reinforcement test. Thus, OX2r signaling in the PVT appears to encode the incentive motivational value of reward-cues, but may not be directly involved in the conditioned reinforcing properties. These findings are not necessarily discordant with one another, as different neural mechanisms underlie Pavlovian vs. instrumental responding for reward cues (Cardinal et al. 2002; Yin et al. 2008).
The data reported here add to a growing literature highlighting an important role for the PVT, as well as its extended circuitry, in motivated behaviors and cue-reward learning (Haight et al. 2017; James and Dayas 2013; Kirouac et al. 2005; Martin-Fardon and Boutrel 2012; Matzeu et al. 2014). Unlike previous studies, however, we were able to isolate the role of the LHA and orexin signaling in the PVT specifically in incentive motivational processes. Recently, Otis and colleagues found that projection cells from the prefrontal cortex (PFC) to the PVT send signals encoding cue-reward relationships, while projection cells from the LHA to the PVT (putatively GABAergic) send consummatory signals to the PVT (Otis et al. 2019). While 100% of the PVT neurons observed by Otis had an inhibitory response following LHA stimulation, 35% of them also showed excitatory responses. Orexin is an excitatory peptide that has been shown to increase activity of PVT neurons (Kolaj et al. 2014). Speculatively, a proportion of the LHA inputs targeted by Otis and colleagues were likely orexinergic, and, according to our data, send signals to the PVT encoding the incentive-motivational value of the reward-paired CS. This hypothesis is congruent with other data collected by Zhu and colleagues (2018), who demonstrated that PVT neurons are activated by a variety of salient stimuli, including appetitively-conditioned stimuli. While the source of activation was not investigated in the study by Zhu and colleagues, it was hypothesized that these salience signals are encoded by the PVT using information coming in part from the PFC or the hypothalamus, and, in turn, sent to the NAc to inform behavior (Zhu et al. 2018). Thus, the LHA-PVT pathway is likely not limited to consummatory signals. Rather, we speculate that the excitatory orexinergic activity that encodes the incentive motivational value of a reward cue is interwoven within the larger GABAergic input from the LHA to the PVT, and specifically leads to increased incentive salience signaling within the PVT. This incentive-salience signal is likely combined with other information about the cue-reward relationship coming to the PVT from the PFC (e.g. Campus et al. 2019) and other areas. Further, in agreement with Otis et al. (2019) and Zhu et al. (2018), we believe that output from the PVT to the nucleus accumbens is a critical component of reward processing. We propose, however, that this circuit is especially important for incentive motivational processes. In support, our prior findings show that neurons projecting from the PVT to the NAc are activated to a greater extent in sign-trackers than goal-trackers in response to a Pavlovian food cue (Haight et al., 2017). That is, these neurons are engaged by an incentive stimulus, but not a predictive stimulus. In addition, we have long-known that stimulation of PVT-NAc projections can elicit nucleus accumbens dopamine release, and can do so independent of the ventral tegmental area (Parsons et al., 2007). As we know that dopamine release in the NAc is critical for sign-tracking, but not goal-tracking, behavior, we propose that the PVT-NAc pathway is the final integrative component of a hypothalamic (LHA) – thalamic (PVT) – striatal (NAc) circuit mediating the incentive value of reward cues. To determine if this is indeed the case, future studies will need to combine elegant technical approaches, like that used by Otis et al. (2019) and Zhu et al. (2018), with behavioral outcome measures that permit the dissociation of complex psychological phenomenon, like those used here.
Given the wide-ranging effects of orexin signaling in the brain, there are a number of factors that need to be considered when interpreting the results of the current studies. First is the fact that a food stimulus was used, and both the LHA and PVT are considered critical components of the ingestive circuitry (Cheng et al. 2018; Stratford and Wirtshafter 2013). In terms of orexinergic involvement, previous work has demonstrated that administration of orexin-A into the posterior PVT leads to increased consumption of 2% sucrose solution (Barson et al. 2015), while knockdown of OX-1Rs in the PVT reduces hedonic feeding of high-fat chow in rats (Choi et al. 2012). Importantly, in the current study, subjects retrieved and consumed food pellets during PavCA regardless of treatment. Thus, the desire to consume the food reward was not affected by either LHA lesions or blockade of orexin signaling in the PVT.
Second, orexinergic inputs to the PVT have been recently identified as part of the circuity promoting wakefulness via signals to the NAc (Ren et al. 2018), demonstrating yet another role for this diverse and heterogeneous circuit. However, we do not believe this confounded the reported results. First, Ren and colleagues found that PVT manipulations had minimal effects on reducing wakefulness during the light cycle, when our animals were tested. In addition, if blockade of OX1r or OX2r in the PVT reduced wakefulness, then we would have expected to observe a decrease in PavCA behavior in both STs and GTs. Instead, we only observed selective behavioral deficits that were tied to incentive salience attribution. Thus, we believe we have identified an additional and novel role for orexinergic signaling in the PVT.
Third, orexin transmission in the PVT has been linked to negative affective states, including responses to stress (Heydendael et al. 2011) and anxiety-like behaviors (Li et al. 2010). While the PavCA training procedure used here was performed over multiple days, allowing the rats to acclimate to the procedure, the CRT test that followed involved placing the rats in a semi-novel context (addition of nose ports, removal of food magazine, location change for lever). It is possible that transitioning to this test context produced a state of anxiety prompted by a “conflict between curiosity (knowing more about it) and fear (how risky is it?)” (Steimer, 2011, p. 499). The PVT has recently been identified as a locus for selecting appropriate behavior during times of conflict (Choi et al. 2019). However, we do not believe that orexin’s role in these negative affective states influenced the findings presented here. First, stress responses appear to be mediated by OX1r in the posterior region of the PVT (Heydendael et al., 2011), and we observed no effect of OX1r blockade on CRT responses, likely ruling out stress as a factor in our results. Second, blockade of OX2r in the PVT with TCS OX2 29 has been shown to reduce anxiety-like behavior (Li et al., 2010). If OX2r antagonism produced an anxiolytic effect during CRT, one might expect a bias towards exploration of the semi-novel setting, potentially leading to an increase in nosepokes or contact with the lever-CS upon its insertion into the chamber. Instead, OX2r antagonism reduced lever-directed behavior, making it unlikely that a decrease in anxiety was influencing behavior. Nonetheless, further research exploring the role of orexin transmission in the PVT during times of conflict is warranted.
Last, we believe the effects reported here are due specifically to the blockade of orexin within the PVT, and not at another location within the thalamus or other parts of the brain accessed through the ventricular system. Previous work has shown dense orexinergic innervation of the PVT, while most of the surrounding thalamic nuclei tend to have sparser orexinergic input (Kirouac et al., 2005). This likely limits any potential effects that may have resulted from spread of the orexinergic antagonists outside of the PVT to the surrounding nuclei. Further, we analyzed data from STs with cannula placements outside of the PVT (Supplemental Information), and found no effect of orexin receptor antagonism on the behaviors of interest (Supplemental Figures 3 & 4). These data, combined with the neuroanatomical evidence above, support the notion that orexin transmission selectively in the PVT is involved in the attribution of incentive salience to reward-paired cues.
In conclusion, this work supports the notion that the attribution of incentive salience to reward-cues is mediated, predominantly, by bottom-up pathways. Specifically, we identify a novel role for orexinergic signaling in the PVT that appears to be originating from the lateral hypothalamic area. In addition, we have recognized potentially distinct roles for orexin activity at OX1r and OX2r within the PVT, but believe their convergent properties act to encode the incentive motivational value of reward-cues. While further work is needed to fully understand the complex role of orexin transmission in the PVT and the ability of the LHA to evoke these downstream effects, this study identifies a novel role for the LHA and orexin signaling within the PVT in incentive motivational processes.
Supplementary Material
Acknowledgements:
This work was supported by the National Institute on Drug Abuse branch of the National Institutes of Health (T32DA007268 [IRC], R01DA038599 [SBF], F31DA037680 [JLH], T32DA007821 [BNK], K08DA037912 & R01DA044960 [JDM],) and The National Science Foundation (GRFP [CMR]).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing interests: The authors report no biomedical financial interests or potential conflicts of interest.
References.
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE (2016) Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res 296:418–430 doi: 10.1016/j.bbr.2015.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR (1951) Localization of a "feeding center" in the hypothalamus of the rat. Proc Soc Exp Biol Med 77:323–324 doi: 10.3181/00379727-77-18766 [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF (2015) Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: Role of orexin receptor 2. Addiction Biology 20:469–481 doi: 10.1111/adb.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299:187–228 doi: 10.1002/cne.902990206 [DOI] [PubMed] [Google Scholar]
- Berridge KC (2001) Reward learning: Reinforcement, incentives, and expectations. Psychol Learn Motiv 40:223–278 [Google Scholar]
- Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513 doi: 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Bindra D (1978) How adaptive-behavior is produced - perceptual-motivational alternative to response-reinforcement. Behav Brain Sci 1:41–52 doi:Doi 10.1017/S0140525x00059380 [DOI] [Google Scholar]
- Campus P et al. (2019) The paraventricular thalamus is a critical mediator of top-down control of cue-motivated behavior in rats. Elife 8 doi: 10.7554/eLife.49041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW (1995) Organization of projections from the medial nucleus of the amygdala: A PHAL study in the rat. J Comp Neurol 360:213–245 doi: 10.1002/cne.903600203 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352 [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G (2013) Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 226:155–165 doi: 10.1007/s00213-012-2902-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Su H-S (1990) Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat — a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res 522:1–6 doi:http://dx.doi.orq/10.1016/0006-8993(90)91570-7 [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang J, Ma X, Ullah R, Shen Y, Zhou YD (2018) Anterior paraventricular thalamus to nucleus accumbens projection is involved in feeding behavior in a novel environment. Front Mol Neurosci 11:202 doi: 10.3389/fnmol.2018.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248 doi: 10.1016/j.neuroscience.2012.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EA, Jean-Richard-Dit-Bressel P, Clifford CWG, McNally GP (2019) Paraventricular thalamus controls behavior during motivational conflict. J Neurosci 39:4945–4958 doi: 10.1523/jneurosci.2480-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresi RU, Bartholow BD, Piasecki TM (2019) Evidence for incentive salience sensitization as a pathway to alcohol use disorder. Neurosci Biobehav Rev 107:897–926 doi: 10.1016/j.neubiorev.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD (2015) Orexin/hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 5:16143 doi: 10.1038/srep16143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L et al. (1998) The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327 doi: 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport LD, Balagura S (1971) Lateral hypothalamus: Reevaluation of function in motivated feeding behavior. Science 172:744–746 doi: 10.1126/science.172.3984.744 [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768 doi: 10.1016/s0024-3205(03)00408-9 [DOI] [PubMed] [Google Scholar]
- Duricki DA, Soleman S, Moon LD (2016) Analysis of longitudinal data from animals with missing values using SPSS. Nature Protocols 11:1112–1129 doi: 10.1038/nprot.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Geary T, Creeden JF, Morrow JD (2019) Sign-tracking behavior is difficult to extinguish and resistant to multiple cognitive enhancers. Neurobiol Learn Mem 163:107045 doi: 10.1016/j.nlm.2019.107045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology 56 Suppl 1:139–148 doi: 10.1016/j.neuropharm.2008.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE (2011) A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience 196:80–96 doi: 10.1016/j.neuroscience.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB et al. (2010) An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology 35:388–400 doi: 10.1038/npp.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H (2007) Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 191:599–607 doi: 10.1007/s00213-006-0535-8 [DOI] [PubMed] [Google Scholar]
- Frank DW et al. (2019) Toward precision medicine for smoking cessation: Developing a neuroimaging-based classification algorithm to identify smokers at higher risk for relapse. Nicotine Tob Res doi: 10.1093/ntr/ntz211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Flagel SB (2014) A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci 8:79 doi: 10.3389/fnbeh.2014.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fraser KM, Akil H, Flagel SB (2015) Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. Eur J Neurosci 42:2478–2488 doi: 10.1111/ejn.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fuller ZL, Fraser KM, Flagel SB (2017) A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus. Neuroscience 340:135–152 doi: 10.1016/j.neuroscience.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM (1974) Sign-tracking: The stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society. Austin, TX [Google Scholar]
- Hellberg SN, Russell TI, Robinson MJF (2019) Cued for risk: Evidence for an incentive sensitization framework to explain the interplay between stress and anxiety, substance abuse, and reward uncertainty in disordered gambling behavior. Cogn Affect Behav Neurosci 19:737–758 doi: 10.3758/s13415-018-00662-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S (2011) Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152:4738–4752 doi: 10.1210/en.2011-1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL (2009) Paraventricular thalamic nucleus: Subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol 512:825–848 doi: 10.1002/cne.21934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson AR, Horvath AP, Holl K, Palmer AA, Solberg Woods LC, Robinson TE, Flagel SB (2019) Incentive salience attribution, "sensation-seeking" and "novelty-seeking" are independent traits in a large sample of male and female heterogeneous stock rats. Sci Rep 9:2351 doi: 10.1038/s41598-019-39519-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Dayas CV (2013) What about me…? The PVT: A role for the paraventricular thalamus (PVT) in drug-seeking behavior. Front Behav Neurosci 7:18 doi: 10.3389/fnbeh.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer SE, Cole S, Petrovich GD (2016) Orexin/hypocretin receptor 1 signaling mediates Pavlovian cue-food conditioning and extinction. Physiol Behav 162:27–36 doi: 10.1016/j.physbeh.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE (2005) A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493:72–85 doi: 10.1002/cne.20769 [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S (2005) Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059:179–188 doi: 10.1016/j.brainres.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S (2006) Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol 497:155–165 doi: 10.1002/cne.20971 [DOI] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Hermes ML, Renaud LP (2014) Intrinsic properties and neuropharmacology of midline paraventricular thalamic nucleus neurons. Front Behav Neurosci 8:132 doi: 10.3389/fnbeh.2014.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BN, Campus P, Flagel SB (2018) Chapter 3: The neurobiological mechanisms underlying sign-tracking behavior. In: Morrow J, Tomie A (eds). Sign-tracking and drug addiction. Michigan Publishing, Ann Arbor, MI: doi: 10.3998/mpub.10215070 [DOI] [Google Scholar]
- Kuhn BN, Klumpner MS, Covelo IR, Campus P, Flagel SB (2018) Transient inactivation of the paraventricular nucleus of the thalamus enhances cue-induced reinstatement in goal-trackers, but not sign-trackers. Psychopharmacology (Berl) 235:999–1014 doi: 10.1007/s00213-017-4816-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Lee EY, Lee HS (2015) Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res 1598:97–113 doi: 10.1016/j.brainres.2014.12.029 [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506:263–287 doi: 10.1002/cne.21502 [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ (2012) Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 217:257–273 doi: 10.1007/s00429-011-0360-7 [DOI] [PubMed] [Google Scholar]
- Li S, Shi Y, Kirouac GJ (2014) The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front Neuroanat 8 doi: 10.3389/fnana.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ (2010) Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology 212:251–265 doi: 10.1007/s00213-010-1948-y [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ (2011) Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav 102:42–50 doi: 10.1016/j.physbeh.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H (2010) Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PloS one 5:e15475 doi: 10.1371/journal.pone.0015475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G (2012) Multiple roles for orexin/hypocretin in addiction. Prog Brain Res 198:79–121 doi: 10.1016/B978-0-444-59489-1.00007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Elmquist JK (2006) Orexin projections and localization of orexin receptors. In: Nishino S, Sakurai T (eds) The orexin/hypocretin system: Physiology and pathophysiology. vol Contemporary Clinical Neuroscience. Humana Press Inc., Totowa, NJ, pp 21–43 [Google Scholar]
- Margules DL, Olds J (1962) Identical "feeding" and "rewarding" systems in the lateral hypothalamus of rats. Science 135:374–375 doi: 10.1126/science.135.3501.374 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Boutrel B (2012) Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: Is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci 6:75 doi: 10.3389/fnbeh.2012.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F, Martin-Fardon R (2016) Orexin-A/hypocretin-1 mediates cocaine-seeking behavior in the posterior paraventricular nucleus of the thalamus via orexin/hypocretin receptor-2. J Pharmacol Exp Ther 359:273–279 doi: 10.1124/jpet.116.235945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Martin-Fardon R (2018) Drug seeking and relapse: New evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol 9:720 doi: 10.3389/fneur.2018.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R (2014) The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: Recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front Behav Neurosci 8:117 doi: 10.3389/fnbeh.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE (2012) Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one 7 doi: 10.1371/journal.pone.0038987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH et al. (2015) Decoding neural circuits that control compulsive sucrose seeking. Cell 160:528–541 doi: 10.1016/j.cell.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH et al. (2016) Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90:1286–1298 doi: 10.1016/j.neuron.2016.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM et al. (2019) Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron 103:423–431 e424 doi: 10.1016/j.neuron.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M (2013) Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci 33:8321–8335 doi: 10.1523/JNEUROSCI.0709-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ (2006) The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59:480–490 doi: 10.1002/syn.20264 [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ (2007) Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol 500:1050–1063 doi: 10.1002/cne.21224 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxin coordinates. 6th Ed. Academic Press, Burlingame, MA. [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ (2012) Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience 224:70–80 doi: 10.1016/j.neuroscience.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR (2003) Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol 459:142–155 doi: 10.1002/cne.10596 [DOI] [PubMed] [Google Scholar]
- Ren S et al. (2018) The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362:429–434 doi: 10.1126/science.aat2512 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65:869–873 doi: 10.1016/j.biopsych.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T (2014) The role of orexin in motivated behaviours. Nat Rev Neurosci 15:719–731 doi: 10.1038/nrn3837 [DOI] [PubMed] [Google Scholar]
- Sakurai T et al. (1998) Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585 doi: 10.1016/s0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Sarter M, Phillips KB (2018) The neuroscience of cognitive-motivational styles: Sign- and goal-trackers as animal models. Behav Neurosci 132:1–12 doi: 10.1037/bne0000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T (2011) Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialogues Clin Neurosci 13:495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D (2013) Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res 1490:128–133 doi: 10.1016/j.brainres.2012.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wise RA (2016) Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 19:198–205 doi: 10.1038/nn.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-S, Bentivoglio M (1990) Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297:582–593 doi: 10.1002/cne.902970410 [DOI] [PubMed] [Google Scholar]
- Tyree SM, de Lecea L (2017) Lateral hypothalamic control of the ventral tegmental area: Reward evaluation and the driving of motivated behavior. Front Syst Neurosci 11:50 doi: 10.3389/fnsys.2017.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39:107–140 [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G (2009) Linear mixed models for longitudinal data. Springer series in statistics. Springer, New York [Google Scholar]
- Versace F, Kypriotakis G, Basen-Engquist K, Schembre SM (2016) Heterogeneity in brain reactivity to pleasant and food cues: Evidence of sign-tracking in humans. Soc Cogn Affect Neurosci 11:604–611 doi: 10.1093/scan/nsv143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58 doi: 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237 doi: 10.1002/cne.21679 [DOI] [PubMed] [Google Scholar]
- Vogt B, Hof P, Friedman D, Sikes R, Vogt L (2008) Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Struct & Funct 212:465–479 doi: 10.1007/s00429-008-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology 40:1269–1277 doi: 10.1038/npp.2014.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW (2008) Reward-guided learning beyond dopamine in the nucleus accumbens: The integrative functions of cortico-basal ganglia networks. Eur J Neurosci 28:1437–1448 doi: 10.1111/j.1460-9568.2008.06422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nachtrab G, Keyes PC, Allen WE, Luo L, Chen X (2018) Dynamic salience processing in paraventricular thalamus gates associative learning. Science 362:423–429 doi: 10.1126/science.aat0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.