Goal:
The goal of this study was to assess the clinical performance of an investigational in vitro fecal calprotectin immunoassay for differentiating inflammatory bowel disease (IBD) from irritable bowel syndrome (IBS).
Background:
Fecal calprotectin is a stool biomarker that can assist in the detection of intestinal inflammation and is utilized to identify individuals who have a higher chance of having IBD and who require further invasive tests. Current assays exhibit variable performance.
Materials and Methods:
This study was a multicenter, cross-sectional analysis of prospectively collected stool samples from patients 4 years of age or older who presented with gastrointestinal (GI) symptoms and underwent colonoscopy for diagnostic confirmation. IBD was diagnosed based on clinical, endoscopic, and histologic findings. IBS was diagnosed based on Rome III Criteria and negative colonoscopy. Stool samples were extracted and tested on the DiaSorin LIAISON XL using the LIAISON Calprotectin Assay.
Results:
A total of 240 patients (67% female) were included in the study. In total, 102 patients had IBD (54% ulcerative colitis), 67 had IBS, and 71 had other GI disorders. Median fecal calprotectin levels were significantly higher in patients with IBD [522 μg/g; 95% confidence interval (CI): 354-970 μg/g] compared with IBS (34.5 μg/g; 95% CI: 19.7-44.2 μg/g, P<0.001) and other GI disorders (28.6 μg/g; 95% CI: 18.7-40.3 μg/g, P<0.001). Receiver operating characteristic curve analysis indicated a fecal calprotectin cutoff of 94 μg/g for distinguishing IBD from other GI disorders with an area under the curve of 0.964 (sensitivity=92.2%, specificity=88.4%).
Conclusion:
The automated LIAISON Calprotectin assay brings efficient calprotectin testing to the laboratory with a time to the first result of 35 minutes and is a sensitive marker for distinguishing IBD from IBS with a cutoff of ∼100 μg/g.
Key Words: fecal calprotectin, inflammatory bowel disease, irritable bowel syndrome, Crohn’s disease, ulcerative colitis, stool
Fecal calprotectin provides a noninvasive approach for objectively measuring intestinal inflammation, and is increasingly utilized in clinical practice to differentiate organic from functional gastrointestinal (GI) diseases. The calprotectin protein is a heterocomplex composed of the calcium and zinc-binding proteins S100A8 and S100A9.1 It constitutes >60% of total protein in the cytosol of neutrophils, which infiltrate the intestinal mucosa as part of the inflammatory response in intestinal inflammation, such as in inflammatory bowel disease (IBD).2 As a consequence of inflamed, hyperpermeable gut mucosal leakage of activated neutrophils into the feces, measurement of stool samples for calprotectin has shown promise for discriminating patients with IBD from those with irritable bowel syndrome (IBS).3–5
Although the currently used serological markers C-reactive protein and erythrocyte sedimentation rate are reasonable indicators of the presence and severity of systemic inflammation, they are not specific for intestinal inflammatory disease and in many instances do not reflect intestinal mucosal inflammation.6 Other methods for assessing inflammation, such as radiology and endoscopy, are invasive, expensive and time-intensive. Clinical disease (activity) scores are hindered as a result of compiling a number of subjective component inaccuracies and do not discriminate between symptoms of IBS.7,8 Thus, noninvasive assessments, such as fecal calprotectin, are needed to evaluate for bowel inflammation. S100 proteins are remarkably resistant to degradation by fecal bacteria, making them suitable markers for gut wall inflammation.9
The main forms of IBD are ulcerative colitis (UC) and Crohn’s disease (CD). Both are chronic inflammatory disorders characterized by a relapsing-remitting clinical behavior, and they are grouped based on the location of the inflammation: in CD, the inflammation can be in any part of the intestine, while in UC, the inflammation is limited to the colon.10 The course of IBD is unpredictable and is associated with decreased quality of life, especially during relapses when treatment must be intensified. However, IBS is common in IBD, and the chronic nature of the disease requires a continuous reassessment of symptom activity to distinguish noninflammatory symptoms from true disease to adapt a therapeutic strategy enabling the maintenance of remission.11,12 Noninvasive, reliable tools are needed to support this important, yet erstwhile onerous, evaluation of disease activity.
Currently, available calprotectin assays are not standardized, with different manufacturers’ tests giving markedly different values and cutoffs with each assay, as well as variability in time to the first result.13,14 In this study, the LIAISON Fecal Calprotectin Assay was evaluated for its ability to distinguish IBD from IBS and other GI disorders.
MATERIALS AND METHODS
Design, Setting, and Participants
This study comprised a multicenter, cross-sectional analysis of stool samples collected between October 2017 and August 2018 from subjects enrolled from 12 sites across the United States. The study population (4 y or older, of either gender) presented with GI symptoms and underwent diagnostic, colonoscopic evaluation. The following conditions excluded subjects from the study: (1) pregnancy or lactation, (2) ingestion of nonsteroidal anti-inflammatory drugs within 7 days of colonoscopy and stool sample collection, (3) use of immunomodulators or biological therapies within 6 months preceding colonoscopy, and (4) having had a prior surgical resection. Patients with IBD were instructed to collect a stool sample either within 3 days before starting colonoscopy bowel preparation or after normal bowel movements had resumed, but not >7 days following a colonoscopy. Subjects diagnosed with IBS or other GI disorders within 1 year were instructed to collect a stool sample within 7 days of clinical evaluation. Stool samples were frozen upon collection at each enrollment site and shipped to the testing sites. The study was approved by the respective local IRB committees.
Diagnosis
IBD was diagnosed based on clinical, endoscopic and histologic findings. Physicians were asked to rate the severity of IBD: for UC, physicians were asked to ascribe a local endoscopic Mayo score (0 to 3), while for CD local physicians ascribed remission, mild, moderate, or severe. IBS was diagnosed based on Rome III Criteria and negative colonoscopy within the past 1 year. Other GI disorders included diverticular disease, celiac disease, and chronic diarrhea or recurrent abdominal pain (not meeting criteria for another more specific diagnosis), and were diagnosed based on clinical, laboratory, imaging, and endoscopic assessment as deemed appropriate by the local site physician.
Stool Testing
Calprotectin was measured using the automated LIAISON Calprotectin assay (DiaSorin Inc., Stillwater, MN), a quantitative, chemiluminescent, sandwich immunoassay that uses a monoclonal antibody on paramagnetic beads for the capture of calprotectin from stool samples, followed by detection with another monoclonal antibody of different specificity conjugated to an isoluminol derivative. The assay is run on an automated platform, as opposed to a manual enzyme-linked immunosorbent assay, allows for fast time to first result (35 min) with high sample throughput (170 samples/h). Testing was performed following the manufacturer’s instructions on the DiaSorin LIAISON XL (DiaSorin Inc.). Extraction was performed using the weigh method. This assay was evaluated against the Genova Diagnostics PhiCal test, and showed good correlation (R=0.98) and a Passing-Bablock regression of y=0.97×+1.50, with a positive percent agreement of 97.8% and a negative percent agreement of 94.4%.15
Statistical Analyses
MedCalc 18.11.6 was utilized for all analyses presented. The nonparametric Mann-Whitney rank test was used to assess significant differences between groups. R was used to explore log-linear models using the general linear model procedure, and for follow-up type II analyses of deviance using the car package.
RESULTS
Patient Characteristics
Clinical assessment of the LIAISON Calprotectin assay was performed in a multicenter, clinical trial. While 410 total patients were enrolled, 170 were excluded due to withdrawal due to lack of stool collection, failure to meet inclusion/exclusion criteria, stool samples being outside of the stability allowance, or inconclusive or incomplete data due to lack of diagnosis or confirmation of diagnosis (Fig. 1). Of the 240 patients included in the study, 67% were female: 102 patients had IBD (54% UC), 67 were categorized with IBS and 71 had other GI disorders such as diverticular disease, chronic diarrhea, or recurrent abdominal pain. The basic characteristics of enrolled subjects are shown in Table 1. Significantly more females were enrolled in the non-IBD group, and there were significant differences with regard to age, but not racial distribution.
FIGURE 1.

Flow diagram of enrolled patients, with exclusion classification.
TABLE 1.
Basic and Clinical Characteristics of the Subjects According to IBD Status
| n (%) | |||
|---|---|---|---|
| Variable | IBD (N=102) | Non-IBD (N=138) | P |
| Sex | <0.001 | ||
| Male | 46 (45) | 34 (25) | |
| Female | 56 (55) | 104 (75) | |
| Age (y) | <0.0001 | ||
| 4-21 | 17 (17) | 2 (1) | |
| 22-35 | 30 (29) | 21 (15) | |
| 36-45 | 13 (13) | 19 (14) | |
| 46-55 | 11 (11) | 27 (20) | |
| 56-65 | 19 (19) | 36 (26) | |
| >65 | 12 (12) | 33 (24) | |
| Race | 0.43 | ||
| White | 90 (88) | 126 (91) | |
| Nonwhite | 12 (12) | 12 (9) | |
| Diagnosis | |||
| Crohn’s disease | 36 (15) | — | |
| Ulcerative colitis | 58 (24) | — | |
| IBDU | 8 (3.3) | — | |
| IBS | — | 67 (28) | |
| Diverticular disease | — | 35 (15) | |
| Chronic diarrhea | — | 16 (6.7) | |
| Recurrent abdominal pain | — | 11 (4.6) | |
| Other | — | 9 (3.7) | |
IBD indicates inflammatory bowel disease; IBDU, inflammatory bowel disease unclassified; IBS, irritable bowel syndrome.
Test Characteristics
Using the GLM procedure and analyses of deviance we explored the contribution of collection site and age: while a small part of the association between fecal calprotectin and IBD was due to confounding by site and age, it remained highly significant.
Clinical Performance
Mean fecal calprotectin levels were significantly higher in patients with IBD with median levels of 522 µg/g [95% confidence interval (CI): 354-970 µg/g] compared with median levels of 34.5 µg/g (95% CI: 19.7-44.2 µg/g; P<0.001) for the IBS group and 28.6 µg/g (95% CI: 18.7-40.3 µg/g; P<0.001) for those with other GI disorders (Fig. 2).
FIGURE 2.

Box-and-whisker plots comparing fecal calprotectin levels by clinical diagnosis of IBD versus IBS or other GI disorders (diverticular disease, chronic diarrhea, or recurrent abdominal pain). Fecal calprotectin levels are significantly higher in IBD compared with non-IBD (P<0.0001). GI indicates gastrointestinal; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
A receiver operating characteristic analysis was performed to locate a cutpoint suitable for dichotomizing the subjects into IBD and non-IBD subjects based on diagnosis and to assess the clinical performance of the test (Fig. 3). The Youden Index associated criterion was calculated at 94 μg/g (95% CI: >56 to >146 μg/g) for distinguishing IBD from IBS and other GI disorders with an area under the curve of 0.964 (95% CI: 0.932-0.984, Fig. 3). The associated clinical performance showed sound agreement (κ=0.903), with good sensitivity (92.2%) and specificity (88.4%) (Table 2).
FIGURE 3.

Receiver operating curve for distinguishing IBD from IBS and other gastrointestinal disorders using the LIAISON Calprotectin test in a group of 240 (N=102 for IBD) subjects undergoing colonoscopy. IBD diagnosis was established based on clinical, endoscopic, and histologic findings. AUC=0.964 (95% CI: 0.932-0.984). The Youden Index associated cutoff is 94 μg/g (95% CI >56 to >146 μg/g). AUC indicates area under the curve; CI, confidence interval; IBD, inflammatory bowel disease.
TABLE 2.
LIAISON Calprotectin Assay Clinical Performance in Relation to Inflammatory Bowel Disease Diagnosis Based on Clinical, Endoscopic, and Histologic Findings
| LIAISON Calprotectin | ||
|---|---|---|
| Test | %/κ | 95% Confidence Interval |
| Sensitivity (%) | 92.2 | 85.1-96.5 |
| Specificity (%) | 88.4 | 81.8-93.2 |
| Positive predictive value (%) | 85.4 | 78.7-90.3 |
| Negative predictive value (%) | 93.8 | 88.7-96.7 |
| Interrater agreement (κ) | 0.903 | 0.858-0.937 |
Severity Classification
Fecal calprotectin levels significantly correlated with overall physician rank of disease severity in CD patients (N=94, Fig. 4) with medians of 56.8 μg/g (95% CI: 35.3-65.0 μg/g), 185 μg/g (95% CI: 128-374 μg/g), 1230 μg/g (95% CI: 369-2190 μg/g), and 3435 μg/g (95% CI: 2245-5645 μg/g) for the remission, mild, moderate, and severe groups, respectively. Similarly, in UC patients (N=50), the Mayo Endoscopic Severity Score correlated with fecal calprotectin levels with medians of 56.8 μg/g, 188 μg/g (95% CI: 135-377 μg/g), 1580 μg/g (95% CI: 1034-2328 μg/g), and 4620 μg/g (95% CI: 2031-7963 μg/g) for Mayo scores of 0 to 3, respectively (Fig. 5).
FIGURE 4.
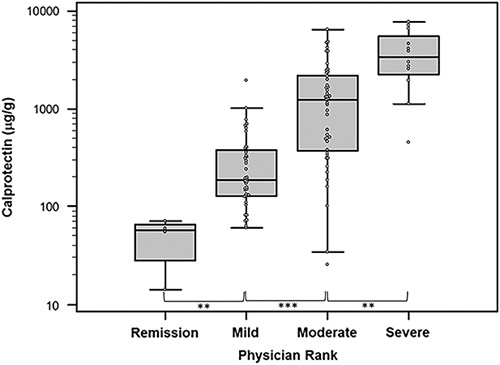
Relationship of fecal calprotectin levels in Crohn’s disease patients based on overall physician rank of disease severity. Significantly different levels between severity groups are marked by asterisks (**P<0.005, ***P<0.0001).
FIGURE 5.

Relationship of fecal calprotectin in ulcerative colitis patients based on Mayo Endoscopic Score. Significantly different levels between score groups are marked by asterisks (*P<0.05, **P<0.005, ***P<0.0001).
DISCUSSION
Fecal calprotectin is increasingly used to differentiate IBD from IBS. However, existing assays have variable cutoffs, ranges, and time to results. The automated LIAISON Calprotectin assay provides advantages including an extended assay range (up to 8000 µg/g), enabled autodilution and a time to the first result of 35 minutes. The data in this study support the measurement of fecal calprotectin as a sensitive marker for distinguishing IBD from IBS, with an optimal cutoff of ∼100 µg/g (92.1% sensitivity and 88.4% specificity). Using the cutoff of 50 μg/g suggested by the manufacturer increases sensitivity to 98.0% (95% CI: 93.1%-99.7%) at the expense of decreasing specificity to 69.6% (95% CI: 61.2%-77.1%). Currently, standardization of calprotectin assays is nonexistent which results in different manufacturer’s tests giving markedly different values.14 A number of cutoffs have been proposed that are assay dependent, but generally, fecal calprotectin measurement can reliably distinguish between functional disease and IBD with sensitivities ranging from 60.4% to 100% and specificities from 44% to 100% depending on the test of choice and its respective cutoff.1
The strengths of this study are the multicenter nature and broad inclusion criteria for non-IBD diagnoses, ideally representing clinical practice variation in calprotectin testing and providing more generalizable results. There are a few notable limitations of this study. First, the lack of additional demographic, clinical, and disease variables, especially for those participants with IBD, make it difficult to accurately assess the performance of calprotectin across all patients with IBD. For example, we do not have complete data on disease activity or the number of patients in remission at the time of testing, disease history, or complete endoscopic findings. However, we assume patients likely had active disease given the need to have had GI symptoms and a diagnostic (not surveillance) colonoscopy to be enrolled in the study. There may have been less of a difference between IBD and non-IBD disorders if patients with quiescent or treated IBD had been admitted. In addition, we did not specify what time of day samples needed to be collected, thus there may have been variability in results based on the time of collection.16,17 In this study, stool samples were weighed for a preanalytical workup to provide consistent amounts of stool irrespective of the stool’s Bristol score.18 Further studies are needed to compare analytical and clinical accuracy of the weigh method to the LIAISON Calprotectin Stool Extraction Device, as well as understanding the variability of fecal calprotectin levels measured from samples taken from stools collected at different times.19,20 Last, patient selection was nonrandomized, and thus bias may have been introduced into the results.
Existing studies have shown the effectiveness of fecal calprotectin measurement for clinical diagnosis of IBD versus IBS. Moving forward, there is potential for this test to be used as a monitoring tool for IBD disease activity,21 relapse detection22–24 and the monitoring and adapting of biological drug therapy to patient care.25
In conclusion, the LIAISON Calprotectin assay is an accurate and efficient method for differentiating IBD from IBS. Future studies will assess the stability of calprotectin levels day-to-day in patients with IBD using this assay, and the performance of this assay in IBD disease monitoring.
ACKNOWLEDGMENTS
The authors thank all of the patients and principal investigators (Brian P. Garvin, MD; Magued Beshay, MD; William J. Barlow, MD; Bhaktasharan Patel, MD; Alex Sherman, MD; Javier Sobrado, MD; Suzy L. Kim, MD; Benjamin Gold, MD; Keith A. Friedenberg, MD; Daniel F. Jackson III, MD; Marc Couturier, PhD; Salika M. Shakir, PhD) for participating in the study. They also thank all the coordinators for data and sample collection, as well as LIAISON Calprotectin testing personnel.
Footnotes
Supported by DiaSorin to support this clinical trial and all reagents for laboratory testing.
C.Z., A.M.R., and F.A.B. are employees of DiaSorin the manufacturer of the LIAISON Calprotectin test. B.P.V. has received honoraria from Abbvie and Janssen and research support from Roche, Takeda, Celgene, DiaSorin, and Crestovo within the past 5 years. J.P.C. declares that there is nothing to disclose.
REFERENCES
- 1.Mumolo MG, Bertani L, Ceccarelli L, et al. From bench to bedside: fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24:3681–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oyaert M, Boel A, Jacobs J, et al. Analytical performance and diagnostic accuracy of six different faecal calprotectin assays in inflammatory bowel disease. Clin Chem Lab Med. 2017;55:1564–1573. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee A, Srinivas M, Eyre R, et al. Faecal calprotectin for differentiating between irritable bowel syndrome and inflammatory bowel disease: a useful screen in daily gastroenterology practice. Frontline Gastroenterol. 2015;6:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 5.Caviglia GP, Pantaleoni S, Touscoz GA, et al. Fecal calprotectin is an effective diagnostic tool that differentiates inflammatory from functional intestinal disorders. Scand J Gastroenterol. 2014;49:1419–1424. [DOI] [PubMed] [Google Scholar]
- 6.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Däbritz J, Musci J, Foell D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahiff C, Safaie P, Awais A, et al. The Crohn’s disease activity index (CDAI) is similarly elevated in patients with Crohn’s disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37:786–794. [DOI] [PubMed] [Google Scholar]
- 9.Acevedo D, Salvador MP, Girbes J, et al. Fecal calprotectin: a comparison of two commercial enzymoimmunoassays and study of fecal extract stability at room temperature. J Clin Med Res. 2018;10:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller R, Major G. IBS and IBD—separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol. 2016;13:613–621. [DOI] [PubMed] [Google Scholar]
- 12.Zhulina Y, Cao Y, Amcoff K, et al. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016;44:495–504. [DOI] [PubMed] [Google Scholar]
- 13.Whitehead SJ, Ford C, Gama RM, et al. Effect of faecal calprotectin assay variability on the management of inflammatory bowel disease and potential role of faecal S100A12. J Clin Pathol. 2017;70:1049–1056. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead SJ, French J, Brookes MJ, et al. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem. 2013;50:53–61. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration (FDA). Medical Devices 510(k) Premarket Notification. 510(k) substantial equivalence determination: decision memorandum; 2018.
- 16.Calafat M, Cabre E, Manosa M, et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21:1072–1076. [DOI] [PubMed] [Google Scholar]
- 17.Du L, Foshaug R, Huang VW, et al. Within-stool and within-day sample variability of fecal calprotectin in patients with inflammatory bowel disease: a prospective observational study. J Clin Gastroenterol. 2018;52:235–240. [DOI] [PubMed] [Google Scholar]
- 18.Amarenco G. Bristol Stool Chart: prospective and monocentric study of “stools introspection” in healthy subjects. Prog Urol. 2014;24:708–713. [DOI] [PubMed] [Google Scholar]
- 19.Husebye E, Tøn H, Johne B. Biological variability of fecal calprotectin in patients referred for colonoscopy without colonic inflammation or neoplasm. Am J Gastroenterol. 2001;96:2683–2687. [DOI] [PubMed] [Google Scholar]
- 20.Moum B, Jahnsen J, Bernklev T. Fecal calprotectin variability in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1091–1092. [DOI] [PubMed] [Google Scholar]
- 21.Puolanne A-M, Kolho K-L, Alfthan H, et al. Rapid fecal calprotectin test and symptom index in monitoring the disease activity in colonic inflammatory bowel disease. Dig Dis Sci. 2017;62:3123–3130. [DOI] [PubMed] [Google Scholar]
- 22.Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, et al. Accuracy of consecutive fecal calprotectin measurements to predict relapse in inflammatory bowel disease patients under maintenance with anti-TNF therapy. J Clin Gastroenterol. 2018;52:229–234. [DOI] [PubMed] [Google Scholar]
- 23.Theede K, Holck S, Ibsen P, et al. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2016;22:1042–1048. [DOI] [PubMed] [Google Scholar]
- 24.Heida A, Park K, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. [DOI] [PubMed] [Google Scholar]


