Abstract
BACKGROUND AND PURPOSE:
The development of resistance to antiepileptic drugs is explained well by the transporter hypothesis, which suggests that drug resistance is caused by inadequate penetration of drugs into the brain barrier as a result of increased levels of efflux transporter such as p-glycoprotein. To evaluate the brain expression of p-glycoprotein in patients with drug-resistant epilepsy, including neocortical epilepsy, we developed a noninvsive quantitative analysis including asymmetry indices based on (R)-[11C]-verapamil PET/MR imaging with cyclosporin A, a p-glycoprotein inhibitor.
MATERIALS AND METHODS:
Six patients with drug-resistant epilepsy, 5 patients with drug-sensitive epilepsy, and 8 healthy controls underwent dynamic (R)-[11C]-verapamil PET/MR imaging with an intravenous infusion of cyclosporin A. Asymmetry indices [(Right Region − Left Region)/(Right Region + Left Region) × 200%] of the standard uptake values in each of the paired lobes were calculated.
RESULTS:
All patients with drug-resistant epilepsy had significantly different asymmetry from the healthy controls, whereas all patients with drug-sensitive epilepsy had asymmetry similar to that in healthy controls. In the temporal lobe, the asymmetry indices of patients with left temporal lobe drug-resistant epilepsy were more positive than those of healthy controls (healthy controls: 4.0413 ± 1.7452; patients: 7.2184 ± 1.8237; P = .048), and those of patients with right temporal drug-resistant epilepsy were more negative (patients: −1.6496 ± 3.4136; P = .044). In addition, specific regions that had significant asymmetry were different between the lateral and medial temporal lobe epilepsy groups. In the frontal lobe, the asymmetry index of patients with right frontal lobe drug-resistant epilepsy was more negative than that in healthy controls.
CONCLUSIONS:
We confirmed that statistical parametric mapping analysis by using asymmetry indices of (R)-[11C]-verapamil PET/MR imaging with cyclosporin A could be used as a surrogate marker for drug-resistant epilepsy, and this approach might be helpful for localizing or lateralizing the epileptic zone.
Epilepsy, which is characterized by recurrent spontaneous seizures, is one of the most common neurologic disorders.1 Despite the development of third-generation antiepileptic drugs during the past 3 decades, 20%–30% of patients with epilepsy remain resistant to drug treatment.2,3 The mechanisms of drug resistance in epilepsy remain unclear. Among the mechanisms of drug resistance in epilepsy, increasing experimental and clinical evidence supports the transporter hypothesis.4 Several studies have suggested that the increased expression of efflux transporters, such as p-glycoprotein (Pgp), at the blood-brain barrier in the focal tissue limits the penetration of antiepileptic drugs into the focus.5,6 In the human brain, Pgp exhibited a highly localized overexpression on the vascular endothelium and the end-feet of the vascular glia in cases of drug-resistant epilepsy (DRE).7
Consequently, the problem of drug resistance in epilepsy might be resolved by the development of a new treatment strategy targeting the transporter mechanism. In developing such a strategy, surrogate markers should objectively pinpoint the expression of transporters such as Pgp. More important, surrogate markers should be used noninvasively to render the technique readily applicable in clinical practice. Several recent clinical trials have evaluated Pgp expression in humans via positron-emission tomography by using the Pgp substrate (R)-[11C]-verapamil and Pgp inhibitors, such as tariquidar or cyclosporin A (CS).8,9 Several investigators have performed (R)-[11C]-verapamil PET in patients with epilepsy to compare the expression of Pgp between patients with DRE and healthy controls, by using the percentage changes in verapamil influx or the efflux rate before and after Pgp inhibitor injection. However, to our knowledge, there has been no study for MRI-negative neocortical epilepsies, and previous methods were applied only to medial temporal lobe epilepsy (TLE). Furthermore, they confirmed that the change in the verapamil influx rate was smaller in patients with DRE compared with healthy controls; this difference necessitated the use of complex analytic methods, including invasive serial arterial sampling, which has limited applicability in clinical practice.
Another problem is that in (R)-[11C]-verapamil PET, it is impossible to find focal hypo- or hyper-radio uptake regions because when one uses the Pgp inhibitor, individually distinct Pgp functions of the whole brain and regional differences in Pgp expression may result in interindividual variability of radiotracer uptake in the whole brain and specific brain regions.10,11
Therefore, to develop a more feasible surrogate marker that can be used in clinical practice, we performed (R)-[11C]-verapamil PET and MR imaging with intravenous infusion of cyclosporin A without serial arterial sampling (VPM-PET/MR-CS). The subject population comprised patients who were drug-resistant and seizure-free with various types of epilepsy, MRI-negative neocortical epilepsy. We analyzed the data by using statistical probabilistic anatomical mapping (SPAM) with the asymmetry index (AI) of the standard uptake value (SUV). We hypothesized that Pgp activity is higher in the epileptic foci compared with the contralateral normal area in patients with drug-resistance, and the asymmetry will be larger than that in healthy individuals or patients who are seizure-free.
Materials and Methods
Subjects
Patients with epilepsy (age range, 18–53 years) who were either drug-resistant or drug-sensitive were recruited between January 2013 and March 2014 from epilepsy clinics led by an epileptologist (S.K.L.) at Seoul National University Hospital. All patients had been examined for 24 hours by using video electroencephalography monitoring, and patients with DRE had previously undergone presurgical evaluation. “DRE” was defined as the failure of adequate trials of 2 tolerated and appropriately chosen and used antiepileptic drugs schedules (whether as monotherapies or in combination) to achieve sustained freedom from seizures.12 “Seizure-free drug-sensitive epilepsy” (DSE) was defined as a well-controlled state, free of all seizures, in a patient receiving antiepileptic drugs for at least 1 year before PET scanning. We investigated the baseline characteristics and the results of the previous video electroencephalography monitoring, brain MR imaging, single-photon emission CT, and [18F]-fluorodeoxyglucose-PET examinations in each patient (On-line Table 1).
Healthy control subjects (age range, 20–40 years) without other disorders, including neurologic or psychiatric disorders, who were not taking any drugs were recruited through a notice on the bulletin board of the Biomedical Research Institute of the Seoul National University Hospital. Before inclusion, all subjects underwent a screening interview, neurologic and general medical examinations, complete blood count, and routine biochemical (liver and kidney function) testing. This study was approved by the institutional review board at Seoul National University Hospital and was performed in accordance with the Investigational New Drug application of the Korea Food and Drug Administration and was registered at www.ClinicalTrials.gov (NCT02144792).
PET/MR Imaging and Experimental Procedures
The PET imaging procedures in our study were performed as described previously.8 Participants underwent a 60-minute PET scan after an intravenous injection of 370 MBq of (R)-[11C]-verapamil radiotracer (range, 333–407 MBq). Simultaneous acquisitions of 3D dynamic PET images and T1-weighted MR images were performed by using a Biograph mMR (Siemens, Erlangen, Germany). During the scans, subjects were instructed to lie in a supine position with their heads affixed to a device designed to minimize movement. After routine corrections such as normalization, ultrashort echo time MR imaging–based attenuation, scatter, and decay corrections, the PET imaging data acquired in list mode were reconstructed by using a filtered back-projection. The dynamic volumetric images were sequenced by using the following framing: 8 × 2.5, 16 × 5, 10 × 60, and 10 × 240 seconds. To evaluate Pgp expression, we initiated intravenous CS infusion (2.5 mg/kg/h and 50 mg/mL; Sandimmune; Novartis Pharmaceuticals, Basel, Switzerland) 1 hour before the acquisition of the PET/MR imaging scans. CS infusion was continued during the PET scan to a maximum of 2 hours from the start of the infusion. Blood samples were taken before and after the PET scan to determine CS concentrations by high-performance liquid chromatography.
PET Data Analysis and Acquisition of Asymmetry Indices
PET images were coregistered to the subject's T1 MR images and spatially normalized to a T1 template provided by statistical parametric mapping by using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) (Fig 1). Voxelwise calculations of SUV, [Voxel Intensity (Bq/mL)/Injected Dose (Bq)/Body Weight (g)], was performed in each dynamic PET frame. Dynamic SUVs in 98 brain ROIs were obtained by using the population-based SPAM, which was constructed by incorporating anatomic and functional variabilities among 152 healthy volunteers and automatically labeling brain structures in functional data in the Montreal Neurological Institute space.13,14 Because the uptake of (R)-[11C]-verapamil in the choroid plexus results in a spillover of radioactivity into the neighboring medial temporal structures, including the hippocampus, parahippocampal gyrus, and amygdala, our investigator (S.A.S.), who was unaware of the participants' clinical statuses, masked out the choroid plexus in the individual normalized PET image; volumes for the hippocampus of the individually modified SPAM maps were calculated (On-line Table 2). Static SUVs were then obtained as the weighted mean of the dynamic SUVs during the 2.5- to 40-minute scans with the frame duration as weight. On the basis of the static SUVs in each cortex, AIs were calculated by using the equation [(Right Region − Left Region)/(Right Region + Left Region) × 200%] for all the respective participants.
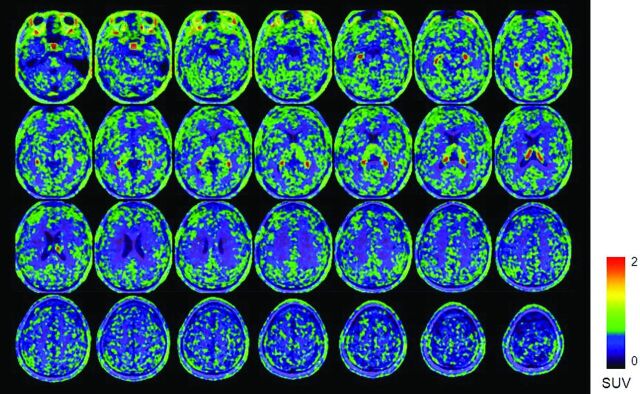
Fig 1.
(R)-[11C]-verapamil PET/MR uptake images after 1 hour of CS infusion in a patient who had drug-resistant left neocortical temporal lobe epilepsy. The color scale reflects the SUV as shown by the heat map.
If Pgp is overexpressed in the right region, the SUV of (R)-[11C]-verapamil in the right region is smaller than that in the left region, resulting in a negative AI. If Pgp is overexpressed in the left region, the SUV in the left region is smaller than that in the right region, resulting in a positive AI. We compared asymmetry in healthy controls and patients with epilepsy by using the AIs. To compare the AIs between patients with lateral TLE and the medial TLE group, AIs were assessed in 6 ROI pairs of the temporal lobe. The medial temporal structures included the hippocampus, amygdala, and parahippocampal gyrus. The lateral temporal structures included the superior, middle, and inferior temporal lobes (Fig 2).
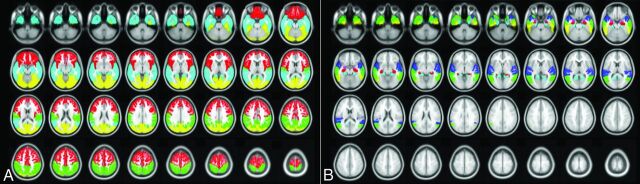
Fig 2.
T1-weighted MR images (axial view, A) marking volumes of interest including the frontal, parietal, temporal, occipital cortices, and temporal ROIs (B), including the hippocampus, amygdala, parahippocampus, superior temporal gyrus, middle temporal gyrus, and inferior temporal gyrus by the population-based SPAM. A, Red indicates the frontal cortex; cyan, the temporal cortex; green, the parietal cortex; yellow, the occipital cortex. B, Red indicates the hippocampus; violet, the amygdala; cyan, the parahippocampus; blue, the superior temporal gyrus; green, the middle temporal gyrus; yellow, the inferior temporal gyrus.
Genotyping Methods
We used the genotyping method described by Kim et al.15 Genotyping of the ABCB1 2677G>T, 1236C>T, and 3435C>T single-nucleotide polymorphisms was performed by using validated TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, California).
Statistical Analyses
Statistical analyses were performed by using SPSS for Windows (Version 21.0; IBM, Armonk, New York). A Mann-Whitney U test and Fisher exact test were used to compare the basal characteristics between the DRE and DSE groups, including age, sex, duration of epilepsy, and the number of antiepileptic drugs taken. In addition, AIs of healthy controls, patients with DRE, and those with DSE were compared by using a Mann-Whitney U test. Associations between ABCB1 polymorphisms and whole-brain SUVs and associations between serum concentration of CS and whole-brain SUVs were determined by using Spearman correlations. A P value < .05 was considered significant.
Results
Ten healthy men (median age, 27 years; range, 22–36 years), 6 patients with DRE (median age, 37 years; range, 26–52 years), and 5 patients with DSE (median age, 25 years; range, 18–53 years) underwent VPM-PET/MR–CS. Data from 2 healthy controls and 1 patient with DSE were excluded due to technical errors. In 2 healthy controls, the PET/MR imaging stopped working during the scan. One patient with DSE did not receive the full dose of cyclosporin A due to the infusion pump stopping during the PET/MR imaging. No difference in basal characteristics was found between the patients with DRE and those with DSE, except for the duration of epilepsy (DRE: median, 20.5 years; range, 5–42 years; DSE: median, 2.6 years; range, 1–4 years; P < .001), number of antiepileptic drugs (DRE: median 3.5; range, 2–4; DSE: median, 1.1; range 1–3; P = .009), and seizure frequency (DRE: median, 10.3 per month; range, 0.2–30 per month; DSE, no seizure event per month; P < .001).
There was interindividual variability in the radioactivity of the whole brain in healthy participants, and regional distributions of radioactivity were different in each individual (On-line Fig 1A). Moreover, radioactivity of the whole brain was independent of the serum concentration of CS (r = .100, P = .701) and ABCB1 up-regulation (On-line Fig 1B and On-line Table 3).
Five healthy controls reported hot flushes in the body during the infusion of CS, and 1 healthy control had mild nausea. However, no other side effects were observed during the experimental period.
Different Values of AIs in Patients with DRE and Healthy Subjects
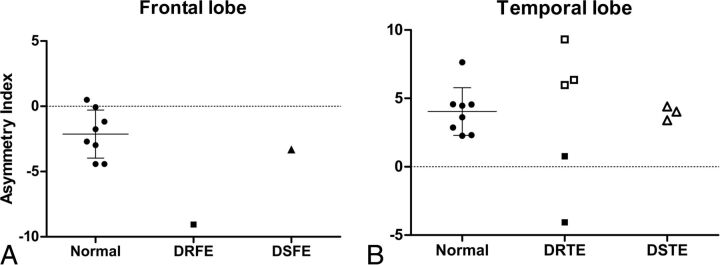
All patients with DRE had more asymmetry between ipsilateral ROIs and contralateral ROIs than healthy subjects, while there were no significant differences in AIs between patients with DSE and healthy controls (Figs 3 and 4). In TLE, PET data from 3 patients with left temporal lobe DRE showed significantly more positive AIs than those from healthy controls (healthy controls: 4.0413 ± 1.7452; patients: 7.2184 ± 1.8237; P = .048), suggesting that Pgp overexpression was in the left temporal area. PET data from 2 patients who had right temporal lobe DRE showed significantly more negative AIs than those from healthy controls (patients, −1.6496 ± 3.4136; P = .044), suggesting Pgp overexpression in the right temporal area (Fig 3B).
Fig 3.
A, Asymmetry indices of the frontal lobes in healthy controls and in patients with drug-resistant or drug-sensitive right frontal lobe epilepsy. B, AIs of temporal lobes in healthy controls and patients with DRE or DSE temporal lobe epilepsy. Filled squares are diagnosed right temporal lobe epilepsy, and blank squares and triangles are diagnosed left temporal lobe epilepsy. When the AI was positive, the standard uptake value of (R)-[11C]-verapamil in the left region was lower than that in the right region in each paired lobe. The bars represent mean ± SD. DRFE indicates drug-resistant frontal lobe epilepsy; DSFE, drug-sensitive frontal lobe epilepsy; DRTE, drug-resistant temporal lobe epilepsy; DSTE, drug-sensitive temporal lobe epilepsy.
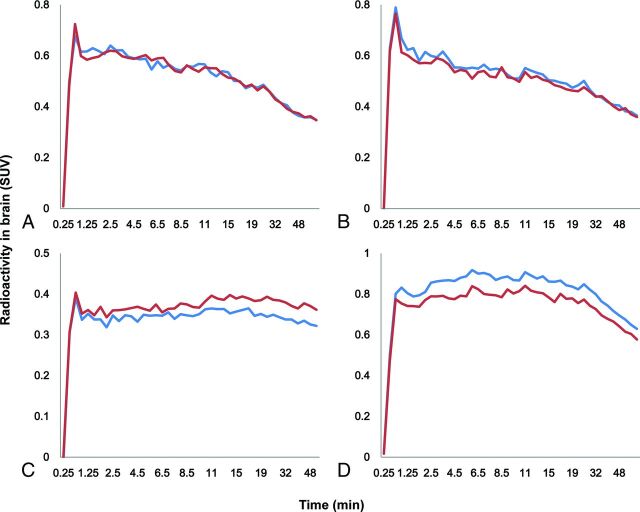
Fig 4.
The time-activity curves of (R)-[11C]-verapamil in the frontal lobe of a healthy subject (A); the temporal lobe of a healthy subject (B); the frontal lobe of patient 6, who had drug-resistant right frontal lobe epilepsy (C); and the temporal lobe of patient 2, who had drug-resistant left temporal lobe epilepsy (D). The blue line indicates right lobe; the red line, left lobe.
PET data from a patient who had a right frontal lobe DRE showed the largest negative AI. This means that the SUV of the right frontal lobe in this patient was much lower than that of the left frontal lobe and that the AI of this patient was different from AIs of healthy controls (healthy controls: −1.9963 ± 1.6329; patient, −9.0502) (Fig 3A).
In addition, we compared AIs in the temporal lobe between patients with drug-resistant and drug-sensitive left TLE. AIs in patients with DRE were larger than those in patients with DSE, though this result was not statistically significant (DRE: 7.2183 ± 1.8237; DSE: 3.9473 ± 0.5101, P = .100).
Possibility of Localization by Using VPM-PET/MR–CS
We investigated whether localization of the epileptic focus is possible by using our method. We compared the characteristics of AIs between the medial TLE and lateral TLE groups. The lateral TLE group had significantly different AIs from those of healthy subjects in the superior (healthy subjects: 3.7592 ± 2.7825; patients: 15.0673 ± 10.2462; P = .024) and middle temporal gyri (healthy subjects: 0.4077 ± 3.0872; patients: 7.3093 ± 1.9225; P = .012), but not in the medial temporal structures. Moreover, significant differences in AIs were observed between patients with medial TLE and healthy subjects in the hippocampus but not in the lateral structures (healthy subjects: 10.4622 ± 4.9800; patients: −4.2948 ± 2.7194; P = .044) (On-line Table 4).
Discussion
The primary objective of this study was to confirm the usefulness of noninvasive quantitative PET methods including SPAM, with AIs of the SUVs as a surrogate marker in DRE. We also wanted to determine whether this method could help us localize the epileptic zone. In our study, significant asymmetry of Pgp expression was confirmed in all patients with DRE compared with healthy controls, and the absolute values of AIs in patients with drug-resistant left TLE also were larger than those in healthy subjects and patients with drug-sensitive left TLE. There was no difference between the DSE group and healthy controls. In addition, we were able to find different asymmetry patterns between patients with lateral TLE and those with medial TLE.
Two previous studies examined patients with DRE by using (R)-[11C]-verapamil PET. In a pilot study, (R)-[11C]-verapamil PET was performed in 7 patients with drug-resistant TLE who had hippocampal atrophy.16 In this study, the increased efflux of (R)-[11C]-verapamil in the ipsilateral region was more pronounced than in the contralateral region, though this difference was not statistically significant. In a recent case-control study, 14 patients with drug-resistant TLE caused by unilateral hippocampal sclerosis underwent (R)-[11C]-verapamil PET with tariquidar; when compared with patients with DSE, the influx of (R)-[11C]-verapamil was less significantly increased in the patients with drug-resistant TLE.17 In our study, AIs of the left TLE group were more positive than those in healthy controls, suggesting an overexpression of Pgp in the left temporal lobe and a low uptake of (R)-[11C]-verapamil. The AIs of the right TLE were also more negative than those in healthy controls. In addition, asymmetries in patients with drug-resistant left TLE were larger than those in patients with drug-sensitive left TLE. Considering the coherence of the results of several studies, including our results, (R)-[11C]-verapamil PET might serve as an important and useful noninvasive surrogate marker for DRE.
To our knowledge, this is the first report to confirm that (R)-[11C]-verapamil PET could facilitate evaluation of a surrogate marker in patients with DRE including neocortical epilepsy. In addition to lateral temporal lobe epilepsy, a patient who had drug-resistant MRI-negative right frontal lobe epilepsy had different asymmetry in the frontal lobe than healthy subjects. This patient had a much larger negative AI than the healthy controls, suggesting an overexpression of Pgp in the right frontal lobe. In addition, there also was no difference in drug-sensitive frontal lobe epilepsy.
For localization, we tried to compare the asymmetry patterns between patients with DRE with medial TLE and lateral TLE. All patients with lateral TLE had a significant asymmetry in the superior and middle temporal gyrus, but not in the medial temporal structures. Moreover, 2 patients with medial TLE had significantly different asymmetry in the hippocampus, but not in the lateral temporal structures. Given the different patterns between the medial and lateral TLE groups, this method could be helpful in localizing the epileptic zone.
Among the cases, a second patient (patient 2) had the longest standing drug-resistant left TLE, and decreased SUV in all of the left lobes indicated broad Pgp overexpression in the left hemisphere (data not shown). Considering that the DRE group, including this patient, had a longer duration of disease than the DSE group, seizure duration might be an important factor in the overexpression of Pgp. We also analyzed the relationship between the duration of epilepsy and the absolute value of the AIs in patients with left DRE and DSE TLE (n = 6) by using the Spearman nonparametric correlation coefficient and found a significant correlation (r = 0.928, P = .008). Indeed, a recent study investigated the correlation between Pgp overexpression and clinical features (age, age at the onset of habitual seizures, duration of seizure history, seizure frequency per year, and number of Pgp inducers or substrates taken). There was a positive correlation among seizure frequency, number of medications that induce Pgp, and overexpression of Pgp, with no other significant relationships observed.7 Given the various results from many studies, there is ongoing debate regarding which clinical features affect the overexpression of Pgp, and additional studies are required to address this issue.
The use of (R)-[11C]-verapamil PET with a Pgp inhibitor in humans presents several difficulties. First, high dosages of Pgp that cannot be used in (R)-[11C]-verapamil PET could be used with a medium dosage of a Pgp inhibitor to increase the baseline signal and thus create larger differences in the brain concentrations of the tracer between the healthy and diseased brain.9 At an acceptable dose, 17%–58% of Pgp inhibition with tariquidar (2–8 mg/kg) and 38%–50% of Pgp inhibition with CS (2.5 mg/kg/h) were observed by evaluating Pgp inhibition on (R)-[11C]-verapamil PET imaging.18 However, interindividual variability regarding affinity and response to the substrate or inhibitor may also complicate the outcome when using a moderate dose of a Pgp inhibitor.19 Our data also showed individual variability among all the participants, similar to that observed in previous studies.10 In addition, the interindividual variability was unrelated to polymorphisms of the ABCB1 gene, including 3435T/T carriers, which had influenced the influx rate of the Pgp substrate drug in a previous study.20
Given the small sample size in our study, we could not determine the influence of these polymorphisms on regional Pgp activity statistically. Also, several studies have shown that the effect of Pgp inhibitors is rapid, and the Pgp function is promptly restored on the elimination of the inhibitors.21,22 Our study showed a rapid decrease in the radioactivity of the whole brain soon after the infusion of Pgp inhibitor was terminated, despite the maintenance of the serum concentration of CS (data not shown). Therefore, a novel Pgp inhibitor with enhanced affinity and a longer half-life for binding Pgp will be required. This study has several limitations. First, due to the small sample size in each group, we were unable to detect statistically significant differences in patients with frontal lobe epilepsy. However, despite the small sample size, we showed robust differences in the asymmetry patterns of patients with DRE and healthy controls and patients with DSE. Second, our study did not consider cerebral blood flow measurements. The regional uptake of (R)-[11C]-verapamil can become dependent on regional cerebral blood flow when Pgp is partially inhibited.23 However, because (R)-[11C]-verapamil is a low-extraction radiotracer, its uptake is considered insensitive to changes in cerebral blood flow.10 In addition, Muzi et al8 reported that the CS modulation of Pgp increased the blood-brain transfer of (R)-[11C]-verapamil into the brain by 73%, and this increase was significantly greater than changes in blood flow by 13%.
Despite some problems with the use of VPM-PET/MR-CS, considering the different asymmetry in the DRE group, but not in the DSE group, from that of healthy subjects, the AI could be a useful surrogate marker of DRE in clinical practice. If one used SPAM with the AI of VPM-PET/MR-CS, it might be possible to predict the drug response in each patient and consider the appropriate surgical treatment more proactively. In addition, patients with DRE exhibiting Pgp overexpression could be treated with a Pgp inhibitor. A recent pilot study reported that the adjunctive use of verapamil (120 mg/day), which was used as a Pgp inhibitor in patients with DRE, improved the response rate.24 In the future, our method should be applied to more variable neocortical epilepsy cases.
Conclusions
On the basis of the results obtained to date, we confirmed the importance of Pgp expression in DRE by using a noninvasive method including SPAM analysis with AIs. Therefore, an AI obtained by using VPM-PET/MR-CS might be used as a surrogate marker of Pgp expression in patients with epilepsy and might serve as an important prognostic factor for individualized drug therapy. Moreover, the results showing that ipsilateral lesions had low SUVs and the difference in asymmetry patterns between medial and lateral temporal epilepsy groups showed that our method may be a useful tool for localization of the epileptic zone. In the future, prospective studies by using VPM-PET/MR-CS in patients with recent-onset epilepsy are necessary for assessing predictive value.
Supplementary Material
ABBREVIATIONS:
- AI
asymmetry index
- CS
cyclosporin A
- DRE
drug-resistant epilepsy
- DSE
seizure-free drug-sensitive epilepsy
- Pgp
p-glycoprotein
- SPAM
statistical probabilistic anatomic mapping
- SUV
standard uptake value
- TLE
temporal lobe epilepsy
- VPM-PET/MR-CS
(R)-[11C]-verapamil PET and MR imaging with intravenous infusion of cyclosporin A without serial arterial sampling
Footnotes
This study was supported by the Ministry of Health and Welfare (A0700001-1232-1280100), Republic of Korea.
REFERENCES
- 1. Browne TR, Holmes GL. Epilepsy. N Engl J Med 2001;344:1145–51 10.1056/NEJM200104123441507 [DOI] [PubMed] [Google Scholar]
- 2. Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657–78 10.1111/j.1528-1167.2011.03024.x [DOI] [PubMed] [Google Scholar]
- 3. Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain 2006;129:617–24 10.1093/brain/awh726 [DOI] [PubMed] [Google Scholar]
- 4. Löscher W, Klitgaard H, Twyman RE, et al. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 2013;12:757–76 10.1038/nrd4126 [DOI] [PubMed] [Google Scholar]
- 5. Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 2005;6:591–602 10.1038/nrn1728 [DOI] [PubMed] [Google Scholar]
- 6. Potschka H. Role of CNS efflux drug transporters in antiepileptic drug delivery: overcoming CNS efflux drug transport. Adv Drug Deliv Rev 2012;64:943–52 10.1016/j.addr.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 7. Liu JY, Thom M, Catarino CB, et al. Neuropathology of the blood-brain barrier and pharmaco-resistance in human epilepsy. Brain 2012;135:3115–33 10.1093/brain/aws147 [DOI] [PubMed] [Google Scholar]
- 8. Muzi M, Mankoff DA, Link JM, et al. Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J Nucl Med 2009;50:1267–75 10.2967/jnumed.108.059162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner CC, Bauer M, Karch R, et al. A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J Nucl Med 2009;50:1954–61 10.2967/jnumed.109.063289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer M, Karch R, Neumann F, et al. Assessment of regional differences in tariquidar-induced P-glycoprotein modulation at the human blood-brain barrier. Cereb Blood Flow Metab 2010;30:510–15 10.1038/jcbfm.2009.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dukart J, Mueller K, Horstmann A, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage 2010;49:1490–95 10.1016/j.neuroimage.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 12. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–77 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 13. Kang KW, Lee DS, Cho JH, et al. Quantification of F-18 FDG PET images in temporal lobe epilepsy patients using probabilistic brain atlas. Neuroimage 2001;14:1–6 10.1006/nimg.2001.0783 [DOI] [PubMed] [Google Scholar]
- 14. Lee JS, Lee DS. Analysis of functional brain images using population-based probabilistic atlas. Current Medical Imaging Reviews 2005;1:81–87 10.2174/1573405052953056 [DOI] [Google Scholar]
- 15. Kim DW, Lee SK, Chu K, et al. Lack of association between ABCB1, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res 2009;84:86–90 10.1016/j.eplepsyres.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 16. Langer O, Bauer M, Hammers A, et al. Pharmacoresistance in epilepsy: a pilot PET study with the P-glycoprotein substrate R-[(11)C]verapamil. Epilepsia 2007;48:1774–84 10.1111/j.1528-1167.2007.01116.x [DOI] [PubMed] [Google Scholar]
- 17. Feldmann M, Asselin MC, Liu J, et al. P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol 2013;12:777–85 10.1016/S1474-4422(13)70109-1 [DOI] [PubMed] [Google Scholar]
- 18. Kalvass JC, Polli JW, Bourdet DL, et al. ; International Transporter Consortium. Why clinical modulation of efflux transport at the human blood-brain barrier is unlikely: the ITC evidence-based position. Clin Pharmacol Ther 2013;94:80–94 10.1038/clpt.2013.34 [DOI] [PubMed] [Google Scholar]
- 19. Syvänen S, Eriksson J. Advances in PET imaging of P-glycoprotein function at the blood-brain barrier. ACS Chem Neurosci 2013;4:225–37 10.1021/cn3001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taubert D, von Beckerath N, Grimberg G, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther 2006;80:486–501 10.1016/j.clpt.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 21. Syvänen S, Blomquist G, Sprycha M, et al. Duration and degree of cyclosporin induced P-glycoprotein inhibition in the rat blood-brain barrier can be studied with PET. Neuroimage 2006;32:1134–41 10.1016/j.neuroimage.2006.05.047 [DOI] [PubMed] [Google Scholar]
- 22. Syvänen S, Hooker A, Rahman O, et al. Pharmacokinetics of P-glycoprotein inhibition in the rat blood-brain barrier. J Pharm Sci 2008;97:5386–400 10.1002/jps.21359 [DOI] [PubMed] [Google Scholar]
- 23. Deo AK, Borson S, Link JM, et al. Activity of P-glycoprotein, a β-amyloid transporter at the blood-brain barrier, is compromised in patients with mild Alzheimer disease. J Nucl Med 2014;55:1106–11 10.2967/jnumed.113.130161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asadi-Pooya AA, Razavizadegan SM, Abdi-Ardekani A, et al. Adjunctive use of verapamil in patients with refractory temporal lobe epilepsy: a pilot study. Epilepsy Behav 2013;29:150–54 10.1016/j.yebeh.2013.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.