Table 2. Excipient-target activities determined in vitro.
The targets are drawn from safety profiling assays generally used for determining off-target effects of drug candidates and marketed drugs. Functions are drawn from the Handbook of Pharmaceutical Excipients (64) except as noted. Examples of marketed drugs containing the excipient, and maximal excipient dosages occurring in any single formulation, are shown. Biochemical and radioligand binding data (IC50) are unformatted; functional data (antagonism/inhibition, IC50; agonism, half maximal effective concentration EC50) are in bold in the Target column. Activity values are accurate to ±20%.
| Excipient | Function | Structure | Example marketed drug | Highest excipient amount/dose | Target | Ki/IC50 Or EC50 (μM) |
|---|---|---|---|---|---|---|
| Aspartame | Sweetener | 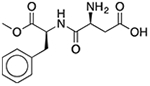 |
Purixan | 450 mg† | NR1I2 | 13 |
| Benzethonium chloride | Antimicrobial preservative; antiseptic; disinfectant | 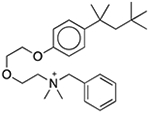 |
Ketalar/ketamine hydrochloride§ | 0.1%‡ | ADORA3 ADRA1A DRD1 DRD3 ESR1 HRH3 PRG OPRM PDE4D PTGS2 ABCB11 SLC18A2 KCNH2 |
5.3 4.7 3.8 1.6 11 27 6.2 6.0 14 24 29 0.18 1.9 |
| Benzoic acid | Antimicrobial preservative; therapeutic agent | 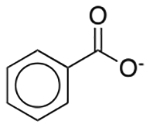 |
Excedrin Migraine | 25 mg/5 ml† |
ABCB11 SLC18A2 |
260 18 |
| Butylparaben | Antimicrobial preservative | 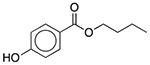 |
Fexofenadine (children’s allergy) | 8 mg/5 ml† |
SLCO2B1 ADRA1A SLC6A2 SLC6A3 TBXA2R SLC18A2 SCN5A |
39 16 20 18 19 6.6 33 |
| Cetylpyridinium chloride | Antimicrobial preservative; antiseptic; surfactant; disinfectant; solubilizer; wetting agent | Dyazide | 1.5 mg† |
ADRA1A DRD1 DRD3 ESR1 HRH3 PRG OPRM1 PDE4D PTGS1 PTGS2 TBXA2R SLC18A2 KCNH2 SCN5A |
6.4 4.8 0.55 18 13 4.5 5.1 13 7.5 2.5 3.4 0.73 1.3 34 |
|
| Chloroxylenol | Antimicrobial preservative; antiseptic; disinfectant | 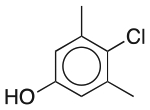 |
Miconazole nitrate | 0.1%‡ |
CHRM1 ADRA1A SLC18A2 |
9.9 14 0.79 |
| Cysteine | Antioxidant* | 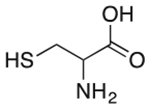 |
Zyban | 16.2 mg† | PTGS2 | 4.7 |
| D&C Red No. 28 | Colorant | 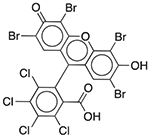 |
Nexium 24HR | 2.5 mg/5 ml† |
SLCO2B1 ADRA1A DRD1 ADORA3 CHRM2 OPRM1 PDE3A PDE4D |
0.60 0.48 0.59 0.48 0.68 0.82 0.13 0.94 |
| Ethylparaben | Antimicrobial preservative | 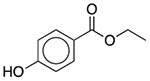 |
Clindamycin (pediatric) | 2 mg/5 ml† | SLC18A2 | 22 |
| FD&C Red No. 3 | Colorant | 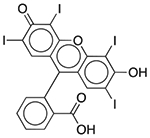 |
Esomeprazole | 2 mg/5 ml† | ACHE PDE3A PDE4D |
0.42 0.092 0.32 |
| FD&C Yellow No. 5 (Tartrazine) | Colorant | 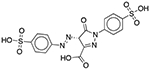 |
Pepcid Complete (tropical flavor) | 652 mg† | DRD1 OPRM1 GABRA1 |
23 16 13 |
| Methylene Blue | Colorant | 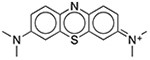 |
Diurex Water Pills† | 1%§ | ACHE CHRM2 HRH3 KCNH2 |
0.090 0.19 0.88 27.5 |
| Oleic acid | Emulsifying agent; skin penetrant | 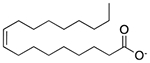 |
Verapamil | 598.6 mg† |
PTGS1 PTGS2 SLC18A2 |
6.7 3.5 4.7 |
| Palmitic acid | Emulsifying agent; skin penetrant; tablet and capsule lubricant |  |
Captopril | 6 mg† |
PDE3A PDE4D SLC18A2 |
4.1 6.6 4.4 |
| Phenylmercuric acetate | Antimicrobial preservative; antiseptic |  |
- | 0.01%¶ | DRD1 DRD3 SLC6A2 SLC6A3 CHRM1** SLC18A2 KCNH2 |
2.2 0.024 1.7 1.8 8.1 0.36 6.2 |
| Propyl gallate | Antioxidant |  |
Janumet XR | 2 mg† |
ALOX5 PTGS2 TY3H KDM4C FTO |
0.43 7.2 1.9 39 3.0 |
| Propylparaben | Antimicrobial preservative |  |
Keppra | 200 mg/5 ml† |
SLC6A2 KDR LTA4H |
30 24 260 |
| Sodium lauryl sulfate | Anionic surfactant; detergent; emulsifying agent; skin penetrant; lubricant; wetting agent | 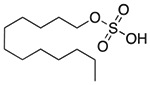 |
Cetirizine | 96 mg† | HTR1A ADORA3 CHRM1 ADRA1A DRD3 PRG PDE4D |
15 15 29 15 18 15 23 |
| Stearic acid | Emulsifying agent; solubilizing agent; tablet and capsule lubricant | 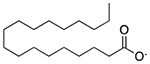 |
Kariva | 1203 mg/5 ml† |
PDE3A PDE4D |
3.1 6.7 |
| Thimerosal | Antimicrobial preservative; antiseptic | 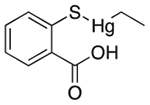 |
Influenza vaccines multidose packaging | 0.03%§ | ADORA3 ADRA2A SLC18A2 DRD1 DRD3 5HT2b** |
1.6 3.8 0.97 2.4 0.32 15 |
| Ethyl mercury | Thimerosal major metabolite | 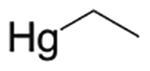 |
NA | NA | DRD3 DRD5 |
0.21 0.15 |
| Thymol | Antioxidant; antiseptic; cooling agent; disinfectant; flavoring agent; skin penetrant |  |
Listerine† | 0.01%# |
CHRM1 ADRA1A SLC6A2 SLC18A2 |
14 26 28 25 |
From (65).
Oral.
Auricular.
Intravenous.
Topical.
Inhalation.
An agonist effect was observed.
