Abstract
BACKGROUND AND PURPOSE:
Preterm neonates are at risk for neurodevelopmental impairment, but reliable, bedside-available markers to monitor preterm brain growth during hospital stay are still lacking. The aim of this study was to assess the feasibility of corpus callosum–fastigium length as a new cranial sonography marker for monitoring of preterm brain growth.
MATERIALS AND METHODS:
In this longitudinal prospective cohort study, cranial ultrasound was planned on the day of birth, days 1, 2, 3, and 7 of life; and then weekly until discharge in preterm infants born before 29 weeks of gestational age. Reproducibility and associations between clinical variables and corpus callosum–fastigium growth trajectories were studied.
RESULTS:
A series of 1–8 cranial ultrasounds was performed in 140 infants (median gestational age at birth, 27+2 weeks (interquartile range, 26+1 to 28+1; 57.9% male infants). Corpus callosum–fastigium measurements showed good-to-excellent agreement for inter- and intraobserver reproducibility (intraclass correlation coefficient >0.89). Growth charts for preterm infants between 24 and 32 weeks of gestation were developed. Male sex and birth weight SD score were positively associated with corpus callosum–fastigium growth rate.
CONCLUSIONS:
Corpus callosum–fastigium length measurement is a new reproducible marker applicable for bedside monitoring of preterm brain growth during neonatal intensive care stay.
Brain growth is an important predictor of neurodevelopmental outcome in preterm infants.1–4 In neonatal intensive care units (NICUs), brain growth is usually monitored by manual measurement of head circumference. However, head circumference measurement has a low interrater agreement and does not correspond well with actual brain development.5,6 Therefore, there is a need for a new reliable bedside marker for monitoring preterm brain growth in clinical practice.
Brain structures measured by cranial ultrasound (CUS) could provide clinically applicable markers for brain growth. A few sonographic markers of brain growth have been used in the past, mainly measuring the corpus callosum (CC) or cerebellum, thereby reflecting growth of a small part of the brain only.7–11 In addition to currently available markers of preterm brain development, we propose that the length between the genu of the CC and the fastigium (roof of the fourth ventricle) could serve as a new marker for brain growth.
The aim of this study was to evaluate the usefulness of corpus callosum–fastigium (CCF) length and CC length, an existing marker, as markers for monitoring brain growth in preterm infants during the NICU stay. We assessed the reproducibility of CC and CCF length measurements, developed growth charts for preterm infants between 24 and 32 weeks of gestation, and evaluated prenatal and postnatal characteristics possibly associated with CC and CCF growth trajectories. We hypothesized that both measurements are highly reproducible. Furthermore, we hypothesized that CCF and CC growth trajectories are associated with prenatal and postnatal determinants of neurodevelopmental outcome in preterm infants.
Materials and Methods
This prospective observational cohort study was performed at the level III NICU of the Sophia Children's Hospital, Erasmus MC, Rotterdam, the Netherlands. The local medical ethics review board approved this study. Written parental consent was obtained before participation. Between 2010 and 2012, all newly admitted singleton, preterm infants born before 29 weeks' gestational age (GA) were eligible for enrollment. We applied the following exclusion criteria: 1) unknown GA at birth; 2) major congenital abnormalities, and 3) extensive brain injury (including intraventricular hemorrhage grade III, posthemorrhagic ventricular dilation, and venous infarction). The latter complications are expected to influence the validity of the measurements due to possible midline shift and expected altered brain growth. GA at birth was dated by using the first day of the last menstrual period and was confirmed by first trimester crown rump length measurement on sonography. Postnatal age was expressed by postmenstrual age, calculated as GA at birth + weeks and days of postnatal age. Pregnancy and neonatal characteristics were collected prospectively. Maternal characteristics were collected retrospectively from medical records. Pregnancy complications, including intrauterine growth retardation and pre-eclampsia and hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome were obtained from obstetric records and were defined on the basis of clinical definitions according to national guidelines.12
Cranial Sonography and Measurements
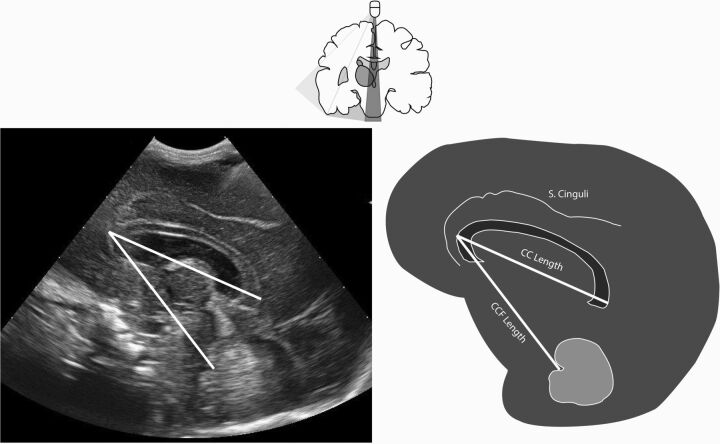
CUS was performed according to the standard local protocol on the day of birth; on days 1, 2, 3, and 7 of life; and then weekly until discharge. The protocol was only disregarded on clinical grounds (eg, hemodynamic instability). One researcher (M.M.A.R.) performed all CUS by using a MyLab 70 scanner (Esaote, Genoa, Italy), with a convex neonatal probe (7.5 MHz). Measurements were performed off-line by using the Mylab software (Esaote). Measurements of CC and CCF length were performed on a standard sagittal plane. In this plane, a complete corpus callosum (genu to the splenium) and distinct vermis of the cerebellum, including the fastigium, had to be visualized. CCF length was measured from the genu of the corpus callosum (outer border) to the fastigium. CC length was measured from outer to outer border (genu to the splenium, Fig 1). All measurements were performed by 1 investigator (M.M.A.R.). To establish the reliability, a second investigator (J.A.R), blinded to the previous results, measured 30 randomly selected scans of varying quality and of neonates with different GAs.
Fig 1.
In the upper part, we show the coronal view of the brain and the position of the sonography probe for assessment of the corresponding correct sagittal plane below. Measurements of the corpus callosum–fastigium and corpus callosum length are displayed in the sagittal sonography view (left) and schematically (right). S. Cinguli indicates sulcus cinguli.
Statistical Methods
Data were analyzed by using SPSS (Release 21 for Windows; IBM, Armonk, New York) and R statistical and computing software (http://www.r-project.org/). P values < .05 were statistically significant. Median value and interquartile range and means and SDs were used as appropriate.
Intraobserver and interobserver agreements for CC and CCF lengths were evaluated by using the intraclass correlation coefficient and Bland-Altman plots.13 The intraclass correlation coefficient was analyzed by using a 2-way mixed model. Cutoff values were in accordance with Landis and Koch.14 Growth charts were developed for CCF and CC growth as a function of postmenstrual age (weeks) and weight (grams). To model the relation between the measured CCF and CC lengths and a predefined list of covariates, we estimated linear mixed models by using lme (in the R nlme package; http://www.inside-r.org/r-doc/nlme/lme).15 To account for the within-subject correlation, we used a random intercept and random coefficient of GA and a power variance function to model the residual covariance. The predefined covariates were GA at birth, birth weight (BW) SD score, sex, intrauterine growth retardation (defined as estimated fetal weight below 10th percentile), pre-eclampsia/HELLP, chorioamnionitis, death, sepsis, and days on mechanical ventilation. In all models, both GA and GA2 (square of GA) were used as covariates. The additional predictors were added to this basic model separately (termed “univariable models” below) and all at once (the multivariable model).
Results
Of 336 neonates admitted to our NICU during the study period, 152 were eligible for inclusion. Twelve neonates were excluded because they met the exclusion criterion of extensive brain injury, resulting in a sample size of 140 neonates. Baseline maternal and neonatal characteristics are listed in Table 1. The median gestational age at birth was 27+2 weeks (interquartile range, 26+1–28+1); the median birth weight was 955 g (interquartile range, 780–1125 g). The number of sonography scans per neonate ranged from 1 to 8.
Table 1:
Baseline characteristicsa
| N = 140 | Missingb | |
|---|---|---|
| Maternal characteristics | ||
| Age (yr) (mean) (SD) | 30 (5.6) | 0 |
| Ethnicityc | 0 | |
| Dutch | 74 (52.9%) | |
| Other Western | 9 (6.4%) | |
| Non-Western | 57 (40.7%) | |
| Maternal smoking during pregnancy | 26 (18.6%) | 17 |
| IVF/ICSI | 9 (6.4%) | 0 |
| IUGR | 42 (30%) | 4 |
| PE/HELLP syndrome | 37 (26.4%) | 0 |
| Chorioamnionitis | 37 (26.4%) | 0 |
| PPROM | 32 (22.9%) | 0 |
| Neonatal characteristics | ||
| GA at birth (wk+ days) | 27+2 (26+1–28+1) | 0 |
| Male sex | 81 (57.9%) | 0 |
| BW (g) | 955 (780–1125) | 0 |
| Use of antenatal steroids | 127 (90.7%) | 2 |
| Apgar score at fifth minute | 8 (7–9) | 0 |
| CRIB score | 3 (1–6) | 1 |
| Death | 17 (12.1%) | 0 |
| Days on mechanical ventilation | 5 (1–14) | 3 |
| Days to regain birth weight | 9 (7–12) | 14 |
| Sepsis | 67 (47.9%) | 0 |
| IVH grade I or II | 32 (22.9%) | 0 |
| Severe BPD | 15 (10.7%) | 33 |
Note:—IVF/ICSI indicates in vitro fertilization with or without intracytoplasmic sperm injection; IUGR, intrauterine growth retardation; PE, pre-eclampsia; PPROM, prolonged premature rupture of membranes; CRIB, clinical risk index for babies; IVH, intraventricular hemorrhage; BPD, bronchopulmonary disease.
Baseline data of maternal and neonatal characteristics are presented as median (interquartile range) or No. (%) unless otherwise specified.
Missing data were mainly due to early transfer to a secondary hospital.
Ethnicity was reported to provide insight in the generalizablity of the study population.
Reproducibility
The mean interobserver difference was −0.3207 ± 1.4527 mm for CCF (P = .244) and 0.4600 ± 1.8463 mm for CC length (P = .183).
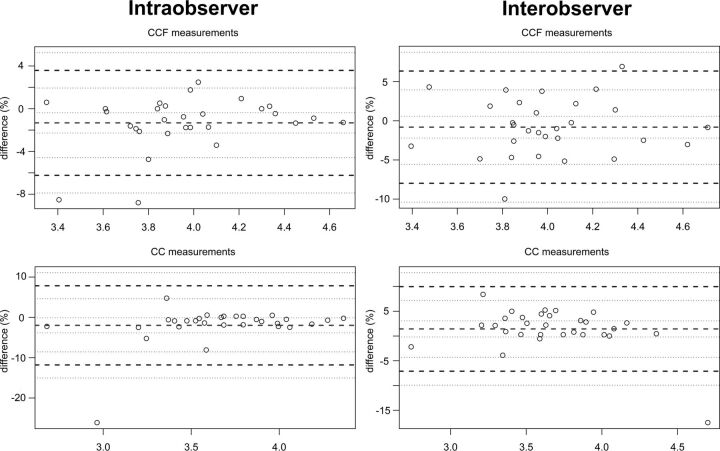
The ICCs for interobserver and intraobserver analysis showed excellent agreement for both CCF and CC length (respectively, intraobserver: 0.958; 95% CI, 0.912–0.980; interobserver: 0.885; 95% CI, 0.770–0.944; and intraobserver: 0.922; 95% CI, 0.844–0.962; and interobserver: 0.893; 95% CI, 0.783–0.948). Figure 2 shows Bland-Altman plots of interobserver and intraobserver agreement for both measurements.
Fig 2.
Reproducibility of corpus callosum–fastigium and corpus callosum lengths by using Bland-Altman plots. The middle dashed lines depict the average measurement bias in percentage differences. The bold dashed horizontal lines represent the 95% limits of agreement for these percentage differences.
CC and CCF Length
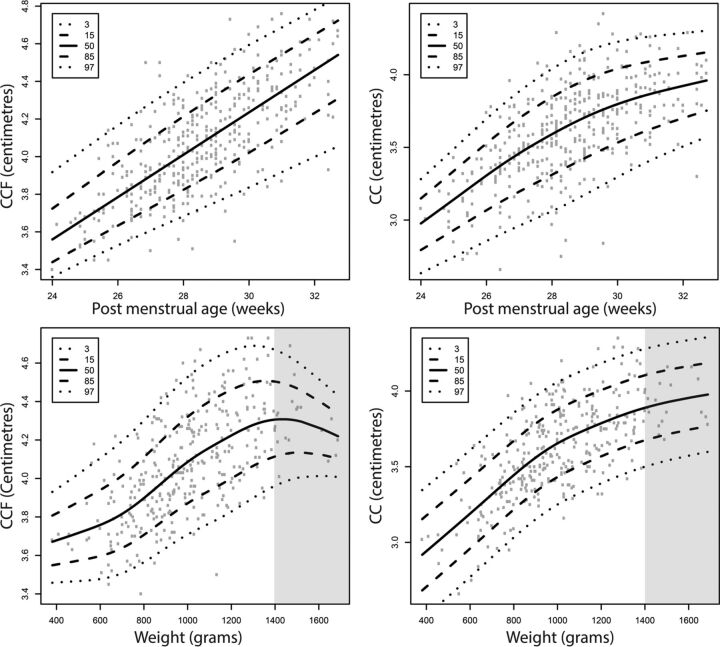
The mean CCF length was 40.9 ± 2.97 mm, with a range from 34.0 to 54.3 mm. The mean CC length was 36.3 ± 3.33 mm, with a range from 26.6 to 48.8 mm. Growth charts of CCF and CC lengths by postmenstrual age and by weight are shown in Fig 3.
Fig 3.
Growth charts of corpus callosum–fastigium (left) and corpus callosum (right) length for preterm neonates as a function of postmenstrual age (in days) and weight (in grams). On the y-axis, CCF (left) and CC (right) lengths are presented in centimeters. The gray areas indicate the parts of the weight charts that should not be used as reference curves.
Linear Mixed Models
Results of univariable analyses are shown in Table 2 for CC and CCF growth. The multivariable analysis confirmed a positive association between the BW SD score and the CCF growth rate and a negative association between female sex and the CCF growth rate. For the CC growth rate, a positive association was found with the BW SD score by using multivariable analysis.
Table 2:
Linear mixed modelsa
| CCF Growth |
CC Growth |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||||||
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| GA at birth | 0.029 | 0.012 | .022b | 0.011 | 0.017 | .518 | 0.024 | 0.017 | .146 | 0.004 | 0.021 | .857 |
| BW SDS | 0.053 | 0.009 | <.0001b | 0.050 | 0.014 | <.001b | 0.094 | 0.011 | <.001b | 0.075 | 0.017 | <.001b |
| Sex (female) | −0.109 | 0.030 | <.001b | −0.070 | 0.029 | .018b | −0.066 | 0.043 | .124 | −0.003 | 0.035 | .938 |
| IUGR (no) | 0.094 | 0.033 | .005b | −0.034 | 0.045 | .451 | 0.267 | 0.041 | <.001b | 0.046 | 0.054 | .390 |
| PE/HELLP (yes) | −0.064 | 0.035 | .068 | 0.000 | 0.038 | .992 | −0.200 | 0.045 | <.001b | −0.052 | 0.046 | .260 |
| Chorioamnionitis (yes) | 0.030 | 0.035 | .397 | 0.031 | 0.035 | .370 | 0.136 | 0.047 | .004b | 0.069 | 0.042 | .106 |
| Death (yes) | −0.103 | 0.048 | .033b | −0.061 | 0.046 | .186 | −0.200 | 0.064 | .002b | −0.105 | 0.054 | .057 |
| Sepsis (yes) | −0.034 | 0.031 | .272 | −0.021 | 0.029 | .477 | −0.050 | 0.042 | .239 | −0.043 | 0.035 | .218 |
| Days on mechanical ventilation | −0.001 | 0.002 | .432 | 0.002 | 0.002 | .340 | −0.003 | 0.002 | .160 | 0.002 | 0.002 | .397 |
Note:—SDS indicates SD score; SE, standard error; IUGR, intrauterine growth retardation; PE, pre-eclampsia.
The effect estimates of maternal and neonatal characteristics on CCF and CC growth in both univariable and multivariable linear mixed models are shown. The effect estimates (β), standard errors, and P values are given.
Significant.
Discussion
In this report, we demonstrated that CCF length, measured by using CUS, is a reproducible and feasible marker that could serve as a new bedside tool to monitor preterm infant brain growth during the NICU stay. We provided growth charts of CCF and CC length for preterm infants from 24 to 32 weeks' postmenstrual age. We found that a higher BW SD score results in an increased CCF and CC growth rate during the hospital stay, while female infants have a slower CCF growth compared with male infants.
Previous sonography studies have evaluated only a limited number of brain structures as potential markers for brain growth or predictors for neurodevelopmental outcome in preterm infants.7–10 One explanation for this is that the brain has few easily recognizable and consistent landmarks for reliable measurements on CUS. The CC, a flat bundle of white matter that connects the left and right hemispheres, is one of the brain structures that is easily visualized and recognizable on CUS.16 Prematurity is known to affect CC development, by the early transition from intrauterine to extrauterine life and by postnatal stress and injury,17 leading to both structural and functional impairment.18,19 Associations have been found between the length and thickness of the CC and brain volumes and neurodevelopmental outcome.11,20,21 Further studies should elucidate whether CC length can be considered a proxy of telencephalon development, creating an impression of white matter development and brain maturation.
The advantages of using CCF length in the monitoring of brain growth rely on anatomic and practical issues. CCF length may be considered a marker of diencephalon and mesencephalon development and vermis growth. The diencephalon includes the thalamus, a neural relay center crucial for adequate cognitive function.22 Altered development of the thalamus, and thus of the diencephalon, may lead to adverse neurodevelopmental outcome. Several studies showed impaired thalamus volume and extreme vulnerability of the thalamus to be neonatal risk factors after preterm birth.23,24 Whether thalamic injury or growth impairment directly influences CCF length needs to be further studied.
One of the other advantages of CCF length measurement is the use of CUS instead of MR imaging or head circumference measurements. In Table 3, the pros and cons of every method are depicted. Although volumetric MR imaging is increasingly used for growth assessment of the preterm brain, its use for serial assessment is still very limited.2 Head circumference measurement has a low interrater agreement and limited association with long-term outcome and does not measure actual brain growth, but growth of the skull and the subarachnoid spaces, which are frequently enlarged in preterm infants.5,6,25 Measurement of CCF length is not considered a burden compared with head circumference measurement because it can be performed on routine CUS, which is often recommended weekly in preterm infants.26 Both CCF length and CC length can already be measured prenatally because the CC and the fastigium are visible on sonography at around 18 weeks of gestation; this feature allows the use of the same marker prenatally and postnatally for monitoring of brain growth.27
Table 3:
Pros and cons of different methods for assessment of brain growth
| HC | CUS | MRI | |
|---|---|---|---|
| Patient friendly | ++ | ++ | − |
| Bedside available | ++ | ++ | − |
| Serial measurement possible | ++ | ++ | − |
| Fast measurement | ++ | + | − |
| Reproducible | ± | + | ++ |
| Reflecting actual brain growth | − | + | ++ |
| Low costs | ++ | + | − |
| Dimension | 1D | 2D | 3D |
Note:—++ indicates very good; +, acceptable; −, bad agreement with the corresponding item; ±, mediocre; HC, head circumference.
In accordance with previous studies, we showed satisfactory reproducibility for CC length.7 CCF reproducibility was excellent too; this finding suggests that both measurements are feasible for longitudinal evaluation of brain growth. Increasing lengths with increasing ages and weights, as shown in the growth charts, support the use of these markers in clinical practice.
We observed a nonlinear growth pattern for CC and CCF length. Previous studies found an intrauterine constant growth rate of 0.20–0.22 mm/day of the CC.28,29 Also in preterm infants, a constant-though-slower growth rate was observed.7 In contrast to previous studies, we performed longitudinal measurements (1–8 scans per infant), allowing a more reliable estimation of CC growth. Other brain structures, such as the vermis of the cerebellum, show a nonlinear growth pattern as well.8 Because we are the first to evaluate the use of CCF length, no literature is available for comparison, to our knowledge. We did expect a nonlinear growth pattern based on current literature.
In Fig 3, parts of the weight charts are gray because we advise not using these parts as a reference curve. We chose to analyze and present the complete original data of infants with a postmenstrual age between 24 and 32 weeks and not to select ideal reference cases. The drawback is seen in the upper part of the weight charts; the curves appear to go down above 1400 g and, despite the very small numbers, the confidence interval narrows. This finding, of course, does not reflect an incline of brain size, but rather selection and censoring. These data are not “first measurements” (reflecting intrauterine accomplished growth) but are follow-up data of patients with prolonged NICU admission, representing the most complex cases (eg, with severe chronic lung disease) not stable enough to be discharged early. In conclusion, the last part of this curve depicts valid data that you would expect in a NICU population, but we consider these not representative of normal growth in preterm infants.
The decreased growth rate of the CCF length in female infants is in accordance with previous studies, which identified sex differences in brain structures and neurodevelopmental outcome.30,31 The positive association between BW SD score and CCF and CC growth rate is also in accordance with current literature.32
One investigator who was trained in visualizing a standard sagittal plane performed all the scans. This likely improved the quality of the scans and may have enhanced the reproducibility. We realize, therefore, that the clinical applicability is probably overestimated in our cohort. Reliable measurements and a correct sagittal plane by using CUS depend on the experience of the observer but are easy to learn. Recently developed software to identify the sagittal plane automatically may further increase the reproducibility and clinical applicability.33
This study has some limitations. First, in the Netherlands, preterm neonates are transferred to a secondary hospital relatively early, accounting for very little data in our cohort of infants born at 29 weeks' gestation and limited data of infants after 30 weeks' gestation. Although white matter injury is already visible on scans after a few days, brain atrophy is often only noticeable after weeks to months.34 Our short follow-up time could explain why we did not find an association between expected clinical variables, such as sepsis and days on mechanical ventilation, and CCF or CC growth rate. Second, including all scans between 24 and 32 weeks' postmenstrual age may have influenced the reliability of the growth charts; that preterm infants lose weight after birth and start to grow days later is a common finding. Brain growth may be limited before regaining birth weight (usually after 10 days). This limitation may have increased variation in CC and CCF lengths. Extremely preterm and clinically unstable infants have longer NICU stays and are likely to undergo more CUS. This feature might have biased our growth charts. On the other hand, our data reflect clinical practice in a neonatal intensive care setting.
In future studies, it would be interesting to compare fetal and preterm CCF growth. Currently, we are scanning fetuses in the second and third trimesters of pregnancy to develop reference curves for fetal brain growth, which could also serve as an ideal growth curve for preterm neonates. We were not yet able to assess the association between feeding regimens and growth during the NICU stay and CCF growth trajectories. This is of interest because it may have clinical implications for nutritional practices. Moreover, CCF length can possibly be used as an outcome measure in nutritional and other intervention studies. It would be of main interest to assess whether CCF length, possibly combined with other available markers of brain growth such as CC length, could serve as a predictor of neurodevelopmental outcome. The clinical applicability may extend beyond the NICU stay into the outpatient follow-up period because the anterior fontanelle can be used as an acoustic window until approximately 6 months in most infants.
Conclusions
There is a lack of bedside markers for brain growth in preterm infants during the NICU stay. We propose a feasible, new sonography measurement called “corpus callosum–fastigium length” with high reproducibility for monitoring brain growth in preterm infants during the hospital stay. This marker may help clinicians determine whether preterm infants show adequate postnatal brain growth and may eventually be used as an outcome measure in nutritional and other intervention studies. Further research is warranted to assess whether this marker could also serve as an early predictor for short-term and long-term neurodevelopmental outcome.
ABBREVIATIONS:
- BW
birth weight
- CC
corpus callosum
- CCF
corpus callosum–fastigium
- CUS
cranial ultrasound
- GA
gestational age
- HELLP
hemolysis, elevated liver enzymes, low platelet count
- NICU
neonatal intensive care unit
Footnotes
The authors report no conflict of interest.
Paper previously presented in part at: First Congress of the Joint European Neonatal Societies, September 16–20, 2015; Budapest, Hungary.
References
- 1. Young JM, Powell TL, Morgan BR, et al. Deep grey matter growth predicts neurodevelopmental outcomes in very preterm children. Neuroimage 2015;111:360–68 10.1016/j.neuroimage.2015.02.030 [DOI] [PubMed] [Google Scholar]
- 2. Lee W, Al-Dossary H, Raybaud C, et al. Longitudinal cerebellar growth following very preterm birth. J Magn Reson Imaging 2015. November 23. [Epub ahead of print] 10.1002/jmri.25098 [DOI] [PubMed] [Google Scholar]
- 3. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev 2010;20:327–48 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park HW, Yoon HK, Han SB, et al. Brain MRI measurements at a term-equivalent age and their relationship to neurodevelopmental outcomes. AJNR Am J Neuroradiol 2014;35:599–603 10.3174/ajnr.A3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kan E, Roberts G, Anderson PJ, et al. ; Victorian Infant Collaborative Study Group. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev 2008;84:409–16 10.1016/j.earlhumdev.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 6. Sutter K, Engstrom JL, Johnson TS, et al. Reliability of head circumference measurements in preterm infants. Pediatr Nurs 1997;23:485–90 [PubMed] [Google Scholar]
- 7. Anderson NG, Laurent I, Cook N, et al. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol 2005;26:2685–90 [PMC free article] [PubMed] [Google Scholar]
- 8. Imamoglu EY, Gursoy T, Ovali F, et al. Nomograms of cerebellar vermis height and transverse cerebellar diameter in appropriate-for-gestational-age neonates. Early Hum Dev 2013;89:919–23 10.1016/j.earlhumdev.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 9. Hagmann CF, Robertson NJ, Acolet D, et al. Cerebral measurements made using cranial ultrasound in term Ugandan newborns. Early Hum Dev 2011;87:341–47 10.1016/j.earlhumdev.2011.01.044 [DOI] [PubMed] [Google Scholar]
- 10. Maunu J, Parkkola R, Rikalainen H, et al. ; PIPARI Group. Brain and ventricles in very low birth weight infants at term: a comparison among head circumference, ultrasound, and magnetic resonance imaging. Pediatrics 2009;123:617–26 10.1542/peds.2007-3264 [DOI] [PubMed] [Google Scholar]
- 11. Rademaker KJ, Lam JN, Van Haastert IC, et al. Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol 2004;28:279–87 10.1053/j.semperi.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 12. NVOG (Dutch Society of Obstetrics and Gynaecology). www.nvog.nl. Accessed September 7, 2015.
- 13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 15. Pinheiro JC, Bates DM. Mixed Effect Models in S and S-Plus. New York: Springer-Verlag; 2000 [Google Scholar]
- 16. Aboitiz F, Scheibel AB, Fisher RS, et al. Fiber composition of the human corpus callosum. Brain Res 1992;598:143–53 10.1016/0006-8993(92)90178-C [DOI] [PubMed] [Google Scholar]
- 17. Shim SY, Jeong HJ, Son DW, et al. Altered microstructure of white matter except the corpus callosum is independent of prematurity. Neonatology 2012;102:309–15 10.1159/000341867 [DOI] [PubMed] [Google Scholar]
- 18. Grunewaldt KH, Fjortoft T, Bjuland KJ, et al. Follow-up at age 10 years in ELBW children: functional outcome, brain morphology and results from motor assessments in infancy. Early Hum Dev 2014;90:571–78 10.1016/j.earlhumdev.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 19. Shim SY, Jeong HJ, Son DW, et al. Serial diffusion tensor images during infancy and their relationship to neuromotor outcomes in preterm infants. Neonatology 2014;106:348–54 10.1159/000363218 [DOI] [PubMed] [Google Scholar]
- 20. Andronikou S, Ackermann C, Laughton B, et al. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: a pilot study in children with HIV-related brain disease and controls. Pediatr Radiol 2015;45:1016–25 10.1007/s00247-014-3255-y [DOI] [PubMed] [Google Scholar]
- 21. Liu F, Cao S, Liu J, et al. Ultrasound measurement of the corpus callosum and neural development of premature infants. Neural Regen Res 2013;8:2432–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eaton-Rosen Z, Melbourne A, Orasanu E, et al. Longitudinal measurement of the developing thalamus in the preterm brain using multi-modal MRI. Med Image Comput Comput Assist Interv 2014;17(pt 2):276–83 [DOI] [PubMed] [Google Scholar]
- 23. Keunen K, Kersbergen KJ, Groenendaal F, et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J Matern Fetal Neonatal Med 2012;25(suppl 1):89–100 10.3109/14767058.2012.664343 [DOI] [PubMed] [Google Scholar]
- 24. Rose J, Vassar R, Cahill-Rowley K, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. Neuroimage Clin 2014;5:169–77 10.1016/j.nicl.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armstrong DL, Bagnall C, Harding JE, et al. Measurement of the subarachnoid space by ultrasound in preterm infants. Arch Dis Child Fetal Neonatal Ed 2002;86:F124–26 10.1136/fn.86.2.F124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Govaert P, De Vries LS. An Atlas of Neonatal Brain Sonography. 2nd ed. London: Mac Keith; 2010 [Google Scholar]
- 27. Tepper R, Kidron D, Hershkovitz R. Sonographic measurements of the fetal fastigium between 20 and 40 weeks' gestation. J Ultrasound Med 2009;28:1657–61 [DOI] [PubMed] [Google Scholar]
- 28. Achiron R, Achiron A. Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol 2001;18:343–47 10.1046/j.0960-7692.2001.00512.x [DOI] [PubMed] [Google Scholar]
- 29. Malinger G, Zakut H. The corpus callosum: normal fetal development as shown by transvaginal sonography. AJR Am J Roentgenol 1993;161:1041–43 10.2214/ajr.161.5.8273605 [DOI] [PubMed] [Google Scholar]
- 30. Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 2008;121:306–16 10.1542/peds.2007-0414 [DOI] [PubMed] [Google Scholar]
- 31. Skiöld B, Alexandrou G, Padilla N, et al. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr 2014;164:1012–18 10.1016/j.jpeds.2013.12.051 [DOI] [PubMed] [Google Scholar]
- 32. Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009;123:e101–09 10.1542/peds.2008-1352 [DOI] [PubMed] [Google Scholar]
- 33. Yaqub M, Rueda S, Kopuri A, et al. Plane localization in 3D fetal neurosonography for longitudinal analysis of the developing brain. IEEE J Biomed Health Inform 2015. May 20. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34. Dudink J, Mercuri E, Al-Nakib L, et al. Evolution of unilateral perinatal arterial ischemic stroke on conventional and diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2009;30:998–1004 10.3174/ajnr.A1480 [DOI] [PMC free article] [PubMed] [Google Scholar]