SUMMARY:
Previous studies using diffusion tensor imaging to examine white matter in Niemann-Pick disease type C have produced mixed results. However, diffusion tensor imaging does not directly measure myelin and may be affected by other structural changes. We used myelin water imaging to more directly examine demyelination in 2 patients with Niemann-Pick disease type C. The results suggest that this technique may be useful for identifying regional changes in myelination in this condition.
Niemann-Pick disease type C (NPC) is a progressive lysosomal storage disease caused by a mutation in the NPC1 or NPC2 gene leading to intracellular accumulation of cholesterol systemically and glycosphingolipids in the nervous system. Previous studies have shown reduced gray matter in a variety of cerebral regions, including the thalamus and hippocampus,1 while others have correlated reductions in cerebellum gray matter with clinical severity scores.2 Measures of white matter may also be useful, though studies using diffusion tensor imaging have provided mixed results, with some noting widespread changes in white matter tracts,1 while others found localized reductions to specific structures.3,4 Nevertheless, callosal fractional anisotropy values and callosal volume correlated with clinical severity scores,4 suggesting that indices of the integrity of white matter tracts could also be an objective gauge of disease status. Such findings are consistent with reduced myelination being a noticeable neuropathologic finding in NPC.5
However, inferences about the status of myelin from measures of fractional anisotropy on diffusion tensor imaging can be problematic because fractional anisotropy can also be altered by a reduction in the number of axons or changes in axonal structure caused by swelling or abnormal branching. This is particularly relevant because animal NPC models have shown abnormal branching and swelling of axons.6 Hence, a more direct in vivo measure of myelin may be clinically desirable. Here, we evaluated the use of myelin water imaging,7 a technique that measures the amount of water present within the myelin of white matter tracts and has been used previously to measure abnormalities in multiple sclerosis,8 to examine the distribution and extent of demyelination in 2 patients with NPC.
Materials and Methods
Subjects
Patient 1 (P01) is a man who presented at 29 years of age. He developed a mild hand-action tremor at 16 years of age and slurring of speech at 17 years of age. In his early twenties, he developed progressive imbalance and incoordination of his hands. His examination showed a score of 29/30 on the Mini-Mental State Examination. He had slurred dysarthria, intention tremor, and limb dysmetria as well as truncal and gait ataxia. MR imaging showed mild cortical atrophy. Genetic testing showed 2 mutations in the NPC1 gene: allele 1 c.C3019G (p.P1007A) and allele 2 c.C2780T (p.A927V). He started N-butyl-deoxynojirimycin (Miglustat) in September 2008 (29 years of age) and was scanned at 33 and 34 years of age.
Patient 2 (P02) is a woman who presented at 26 years of age. A psychological assessment showed problems with visuomotor sequencing, complex verbal reasoning, and written expression. Otherwise, she had only noted some mild balance problems. She scored 29/30 on the Mini-Mental State Examination. She had mildly impaired tandem gait. MR imaging showed patchy confluent hyperintense white matter changes, most prominent in the posterior periventricular regions. Genetic testing showed 2 mutations associated with Niemann-Pick disease type C: exon 18 c.2621A>T (p.Asp874Val) and exon 23 c.3508C>G (p.His1170Asp). She began treatment with Miglustat in April 2011 (27 years of age) and was scanned at 28 and 29 years of age.
Fifteen healthy control subjects took part in this study. Eight men (mean age, 31.9 years) acted as control subjects for P01, while 7 women (mean age, 30.3 years) acted as control subjects for P02.
Imaging Parameters
A T1-weighted high-resolution structural scan (3D-T1-turbo field echo with sensitivity encoding: TR, 10 ms; turbo field echo, 3000 ms; TE, 6 ms; TI, 845.88 ms; 170 sections; FOV, 240 mm; voxel size, 1 × 1 mm; section thickness, 1 mm) was collected as well as a multiecho axial gradient and spin-echo scan7 for T2 measurement (TR, 1000 ms; TE, 10, 20, 30…320 ms; 20 sections acquired at 5-mm thickness reconstructed at 2.5-mm section thickness; in-plane voxel size, 1 × 1 mm; sensitivity encoding, 2232 × 192 matrix).
Myelin Water Fraction Analysis
The signal decay curve obtained by the T2 relaxation sequence was modeled by multiple exponential components, and the T2 distribution was estimated by using non-negative least squares with the extended phase graph algorithm.7 The myelin water fraction (MWF) in each image voxel was computed as the ratio of the area under the T2 distribution with times of 10–40 ms to the total area under the distribution. The MWF images were then registered to the T1-weighted anatomic scan.
To compare the patients' myelin water fraction maps with those of the healthy population, we matched a normative 3D atlas representing the mean and SD for myelin water fraction from each group of age-matched healthy controls. The healthy myelin water fraction maps were calculated, nonlinearly aligned to Montreal Neurological Institute standard space, and averaged. MWF maps for patients were also nonlinearly aligned to Montreal Neurological Institute space. For each voxel, a z score was calculated comparing the patient MWF values with the corresponding control group distribution.
The Juelich histologic white matter atlas in FSL (http://neuro.debian.net/pkgs/fsl-juelich-histological-atlas.html) was used to create tracts of interest. Average MWF values from each fiber tract were then extracted and averaged across the hemisphere. MWF values from the patients were compared with those from aged-matched controls by using Crawford t-tests.9
Results
Paired sample tests of the tract-of-interest analysis showed that the results were stable across the 2 visits for P01 [t(60) = −1.62, P = .11] and P02 [t(60) = 1.44, P = .15]; therefore, because our analysis of the results across visits showed high reproducibility and stability of data, we averaged data across the 2 visits.
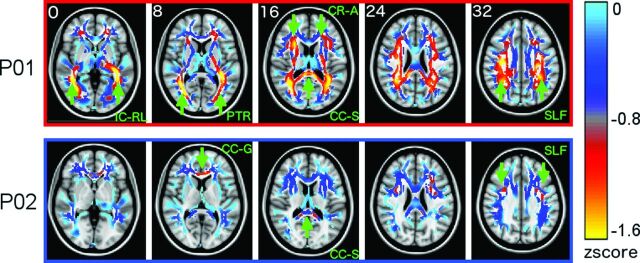
Figure 1 shows the average MWF z score maps for both patients, indicating the location of regions with reduced myelin compared with those of the controls. The patient with the less clinically severe condition (P02) showed focal, patchy reductions in MWF, while the patient with the more clinically severe condition (P01) showed extensive, widespread reductions across entire fiber tracts.
Fig 1.
Whole-brain myelin water z score maps in the 2 patients. Statistical z score maps show the difference between patients and their respective control subjects, where negative z scores indicate reduced myelin water. For clarity, maps were spatially smoothed with a Gaussian 6-mm filter and are shown on a diluted fractional anisotropy skeleton. Statistical maps (minimum thresholded at z = − 1.6 for clarity) are overlaid on the average Montreal Neurological Institute brain. Green arrows highlight areas where the myelin is substantially reduced in the patients compared with their controls. IC-RL indicates retrolenticular portion of internal capsule; PTR, posterior thalamic radiation including the optic radiation; CR-A, anterior corona radiata; SLF, superior longitudinal fasciculus; CC-G, genu of corpus callosum; CC-S, splenium of the corpus callosum.
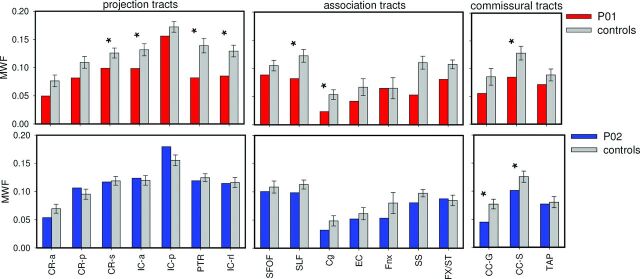
A global analysis for each of the 3 classes revealed that P01 had a reduction in MWF for projection [t(7) = −2.46, P < .05, η = −2.61], association [t(7) = −2.49, P < .05, η = −2.64], and commissural fibers [t(7) = −2.41, P < .05, η = −2.56]. For P02, MWF values were not reduced in this global analysis, for association [t(6) = −0.18, P = .43, η = −0.2], projection [t(6) = −1.09, P = .16, η = −1.17], or commissural fibers [t(6) = −1.33, P = .12, η = −1.42]. Regional analysis of different tracts (Fig 2) revealed significant MWF reductions for P01 in 4 projection tracts, 4 association tracts, and 1 commissural tract. In P02, the less affected patient, only 2 commissural tracts showed significantly reduced MWF.
Fig 2.
Tract analysis for patients (red and blue bars) and their age-matched control subjects (gray bars). CR-a indicates anterior corona radiata, CR-p, posterior corona radiata; CR-s, superior corona radiata; IC-a, anterior limb of internal capsule; IC-p, posterior limb of internal capsule; PTR, posterior thalamic radiation including optic radiation; IC-rl, retrolenticular portion of internal capsule; SFOF, superior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; Cg, cingulum including the hippocampus; EC, external capsule; Fnx, column and body of fornix; SS, sagittal stratum including inferior longitudinal fasciculus and inferior fronto-occipital fasciculus; Fx/ST, fornix and stria terminalis; CC-g, genu of the corpus callosum; CC-s, splenium of corpus callosum; TAP, tapetum. Asterisks indicate P < .05 as determined by Crawford t-tests.9 Error bars show 1 standard error of the mean.
A paired-samples t test, collapsed across all fibers, showed significantly lower MWF for the patient with the more clinically severe condition, P01, compared with P02 [t(16) = −4.488, P < .001].
Discussion
We conducted a detailed examination of the distribution of reduced myelination in the cerebral cortex of 2 patients with NPC. In the clinically worse patient, there was reduced MWF in a large number of association fibers. We also observed large reductions of MWF in the corpus callosum of both patients, paralleling prior reports of reduced callosal fractional anisotropy4 and cortical thickness of the corpus callosum10 in this disorder. However, reductions in the MWF of projection tracts were only observed in the more clinically affected patient.
The physiologic basis for regional variation in NPC remains unclear1; nevertheless, this is a well-documented phenomenon in animal and human models.11,12 For example, in humans, some studies show regions with selectively reduced fractional anisotropy, such as in the corpus callosum4 and the superior cerebellar peduncle,3 while others have shown widespread variations in the reduction of fractional anisotropy.1
Our results also contain 3 important observations relevant to the possible use of MWF to monitor disease status. First, the data for MWF were consistent during a year, indicating that they are reproducible and reliable. Second, the contrast between our patients indicates that MWF may correlate with clinical severity. However, further studies are required to show whether the measure is sensitive to changes in clinical severity with time, which could be either deterioration through natural progression or improvement with therapy. Third, our whole-brain analysis showed reduced MWF in some but not all areas of a fiber tract, suggesting that the effects of this disorder are focal and patchy in milder stages of the disease. This effect of severity may explain discrepancies in prior studies, with some showing focal and others diffuse effects.
Because other neuronal abnormalities are present in NPC, combined use of both MWF and diffusion tensor imaging in future assessment of this disease may help elucidate the contributing pathologic factors affecting white matter tracts in NPC.
ABBREVIATIONS:
- NPC
Niemann-Pick disease type C
- MWF
myelin water fraction
- P01
Patient 1
- P02
Patient 2
Footnotes
Disclosures: Jodie Davies-Thompson—UNRELATED: Grants/Grants Pending: Canadian Institutes of Health Research.* Irene Vavasour—UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Hoffman-La Roche, Comments: reimbursement for conference expenses (International Society of Magnetic Resonance in Medicine, 2014). Jason J.S. Barton—RELATED: serves on the Scientific Advisory Board for Vycor Medical Corporation and is funded by the Canadian Institutes of Health Research operating grants MOP-102567, MOP-106511, and MOP-130566. *Money paid to the institution.
References
- 1. Walterfang M, Fahey M, Desmond P, et al. White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology 2010;75:49–56 10.1212/WNL.0b013e3181e6210e [DOI] [PubMed] [Google Scholar]
- 2. Walterfang M, Abel LA, Desmond P, et al. Cerebellar volume correlates with saccadic gain and ataxia in adult Niemann-Pick type C. Mol Genet Metabol 2013;108:85–89 10.1016/j.ymgme.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 3. Scheel M, Abegg M, Lanyon LJ, et al. Eye movement and diffusion tensor imaging analysis of treatment effects in a Niemann-Pick type C patient. Mol Genet Metabol 2010;99:291–95 10.1016/j.ymgme.2009.10.180 [DOI] [PubMed] [Google Scholar]
- 4. Lee R, Apkarian K, Jung ES, et al. Corpus callosum diffusion tensor imaging and volume measures are associated with disease severity in pediatric Niemann-Pick disease type C1. Pediatr Neurol 2014;51:669–674.e5 10.1016/j.pediatrneurol.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elleder M, Jirásek A, Smíd F, et al. Niemann-Pick disease type C: study on the nature of the cerebral storage process. Acta Neuropathol 1985;66:325–36 10.1007/BF00690966 [DOI] [PubMed] [Google Scholar]
- 6. Zervas M, Somers KL, Thrall MA, et al. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol 2001;11:1283–87 10.1016/S0960-9822(01)00396-7 [DOI] [PubMed] [Google Scholar]
- 7. Prasloski T, Rauscher A, MacKay AL, et al. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage 2012;63:533–39 10.1016/j.neuroimage.2012.06.064 [DOI] [PubMed] [Google Scholar]
- 8. Vavasour IM, Whittall KP, MacKay AL, et al. A comparison between magnetization transfer ratios and myelin water percentages in normals and multiple sclerosis patients. Magn Reson Med 1998;40:763–68 10.1002/mrm.1910400518 [DOI] [PubMed] [Google Scholar]
- 9. Crawford JR, Garthwaite PH. Methods of testing for a deficit in single-case studies: evaluation of statistical power by Monte Carlo simulation. Cogn Neuropsychol 2006;23:877–904 10.1080/02643290500538372 [DOI] [PubMed] [Google Scholar]
- 10. Walterfang M, Fahey M, Abel L, et al. Size and shape of the corpus callosum in adult Niemann-Pick type C reflects state and trait illness variables. AJNR Am J Neuroradiol 2011;32:1340–46 10.3174/ajnr.A2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta 2004;1685:48–62 10.1016/j.bbalip.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 12. March PA, Thrall MA, Brown DE, et al. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol 1997;94:164–72 10.1007/s004010050689 [DOI] [PubMed] [Google Scholar]