Abstract
BACKGROUND AND PURPOSE:
Few studies have examined the prevalence of intracranial aneurysms in connective tissue diseases such as Marfan syndrome, Ehlers-Danlos syndrome, neurofibromatosis type 1, and Loeys-Dietz syndrome. We studied the prevalence of intracranial aneurysms and other intracranial neurovascular pathologies such as arteriovenous malformations and intracranial dissections, in these 4 patient populations.
MATERIALS AND METHODS:
We retrospectively reviewed all patients who had a clinical diagnosis of Marfan syndrome, Ehlers-Danlos syndrome, neurofibromatosis type 1, or Loeys-Dietz syndrome who underwent MRA, CTA, and/or DSA imaging of the intracranial circulation between January 1, 2005, and January 31, 2015. The presence, location, and maximum dimensions of intracranial aneurysms were catalogued. Other neurovascular findings studied included intracranial dissections and arteriovenous fistulas and shunts. Baseline data collected included demographic characteristics (sex, age, smoking history), imaging modality, and cardiovascular comorbidities.
RESULTS:
The prevalence of intracranial saccular and fusiform aneurysms was as follows: 14% (8/59) among patients with Marfan syndrome, 12% (12/99) among patients with Ehlers-Danlos syndrome, 11% (5/47) among patients with neurofibromatosis type 1, and 28% (7/25) among patients with Loeys-Dietz syndrome. Intracranial dissections were found in 2 patients (3%) with Marfan syndrome and 1 patient (1%) with Ehlers-Danlos syndrome. No intracranial dissections were found in patients with neurofibromatosis type 1 or Loeys-Dietz syndrome.
CONCLUSIONS:
Patients with connective tissue disorders, including Marfan syndrome, Ehlers-Danlos syndrome, neurofibromatosis type 1, and Loeys-Dietz syndrome, have a high prevalence of intracranial aneurysms.
The association between neurovascular lesions such as intracranial aneurysms and connective tissue diseases has long been a topic of debate. Early studies suggesting an association between Marfan syndrome and intracranial aneurysms were limited to small case series and case reports.1 However, larger studies have since suggested that there is no association between aneurysms and Marfan syndrome.1–3 Nonetheless, the association is still widely cited in the literature.1–3 In addition to Marfan syndrome, associations between connective tissue diseases, such as neurofibromatosis type 1 (NF1), Ehlers-Danlos syndrome (EDS), and Loeys-Dietz syndrome (LDS), and neurovascular lesions such as intracranial aneurysms have also been suggested. However, relatively few series have been published on the prevalence of aneurysms in these populations.
In this study, we sought to retrospectively characterize neurovascular findings in patients with connective tissue diseases, including Marfan, EDS, NF1, and LDS. Our primary outcome of interest was the prevalence of intracranial aneurysms. Secondary outcomes included the prevalence of intracranial dissections and arteriovenous malformations. We hypothesized that patients with connective tissue diseases would have a higher prevalence of intracranial aneurysms than the general population (ie, >3%).
Materials and Methods
Patient Population
Following institutional review board approval, we conducted a retrospective chart review of all patients diagnosed with Marfan syndrome, EDS, NF1, and LDS who underwent MRA, CTA, or DSA of the intracranial circulation from January 2005 through January 2015. All patients had clinically confirmed Marfan, EDS, NF1, or LDS as determined by medical geneticists at our institution. Patients who had other forms or unconfirmed connective tissue diseases were excluded. All of the above information pertinent to the inclusion and exclusion criteria was obtained through the electronic medical record.
Data Collection
Once the patients were selected, we collected the following data: demographic characteristics (sex, age, smoking history), imaging used (CT angiography, MR angiography, or digital subtraction angiography), and medical history (diabetes, hypertension, stroke, coronary artery disease, dyslipidemia).
The primary outcome of this study was the prevalence of intracranial aneurysms in these disease populations. The presence, location, and maximum dimensions of aneurysms were evaluated by reviewing CTA, MRA, or DSA images and reports. All images and reports were reviewed by a radiologist with 4 years of experience. In addition, we studied the prevalence of intracranial arteriovenous malformations, arteriovenous fistulas, and intracranial dissections. The symptomatic status of the intracranial aneurysms and dissections was collected in addition to the presence of aneurysm rupture.
Statistical Analysis
Continuous variables are presented as mean ± SD, and categoric variables, as frequency in percentages. To determine variables independently associated with intracranial aneurysm presence, we performed a multivariate logistic regression analysis adjusting for age, sex, smoking, hypertension, diabetes mellitus, abdominal/thoracic aortic aneurysms, coronary artery disease, and history of stroke. A separate analysis was performed for each connective tissue disease. All statistical analysis was performed by using JMP, Version 12.0.0 (SAS Institute, Cary, North Carolina).
Results
A total of 230 patients fit our inclusion criteria: Fifty-nine patients had Marfan syndrome, 99 patients had EDS, 47 patients had NF1, and 25 patients had LDS. The data presented below are organized in Table 1, along with additional demographic characteristics and imaging findings. The symptom status of each patient with a confirmed intracranial aneurysm is presented in Table 2. A brief description of each connective tissue disease is summarized in Table 3. The presentation and reason for neuroimaging referral for each patient who qualified for our study are summarized in the On-line Table.
Table 1:
Summary of all data collected for patients with connective tissue diseases who underwent MRA, CTA, or DSA imaging of the head
| Marfan | Ehlers-Danlos | NF1 | Loeys-Dietz | |
|---|---|---|---|---|
| No. of patients | 59 | 99 | 47 | 25 |
| Mean age (yr) | 49.4 | 41.7 | 38.3 | 36.5 |
| No. (%) female | 29 (49%) | 81 (82%) | 23 (49%) | 13 (52%) |
| Cerebral imaging modality | ||||
| CTA, No. (%) | 16 (27%) | 24 (24%) | 4 (9%) | 9 (36%) |
| MRA, No. (%) | 53 (90%) | 85 (86%) | 44 (94%) | 22 (88%) |
| DSA, No. (%) | 1 (2%) | 2 (2%) | 9 (19%) | 0 (0%) |
| No. (%) intracranial aneurysms | 8 (14%) | 12 (12%) | 5 (11%) | 7 (28%) |
| No. (%) multiple intracranial aneurysms | 2 (3%) | 2 (2%) | 1 (2%) | 1 (4%) |
| Total No. of aneurysms | 12 | 14 | 7 | 8 |
| Location, No. (%): | ||||
| ICA | 9 (15%) | 12 (12%) | 3 (6%) | 5 (20%) |
| MCA | 0 (0%) | 1 (1%) | 2 (4%) | 0 (0%) |
| ACA | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) |
| AcomA | 1 (2%) | 1 (1%) | 1 (2%) | 0 (0%) |
| PCA | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Basilar | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vertebral | 0 (0%) | 0 (0%) | 1 (2%) | 2 (8%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mean (SD) aneurysm size (mm) | 4.4 (7.4) | 6.9 (6.5) | 11.2 (9.8) | 4.8 (4.5) |
| Other intracranial vascular findings, No. (%) | ||||
| AVM | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| AVF | 1 (2%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Dissection | 2 (3%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Intracranial hemorrhage, No. (%) | 0 (0%) | 2 (2%) | 2 (4%) | 1 (4%) |
| Comorbidities, No. (%) | ||||
| Hypertension | 21 (36%) | 24 (24%) | 15 (32%) | 7 (28%) |
| Hyperlipidemia | 24 (41%) | 15 (15%) | 9 (19%) | 5 (20%) |
| Diabetes mellitus | 4 (7%) | 3 (3%) | 2 (4%) | 1 (4%) |
| Smoking | 23 (39%) | 32 (32%) | 14 (30%) | 11 (44%) |
| Stroke | 10 (17%) | 7 (7%) | 9 (19%) | 2 (8%) |
| Coronary artery disease | 8 (14%) | 5 (5%) | 3 (6%) | 2 (8%) |
Note:—ACA indicates anterior cerebral artery; AcomA, anterior communicating artery; PCA, posterior cerebral artery.
Table 2:
Symptomatic status at presentation of patients with confirmed intracranial aneurysms
| Marfan | Ehlers-Danlos | NF1 | Loeys-Dietz | |
|---|---|---|---|---|
| No. with intracranial aneurysms | 8 | 12 | 5 | 7 |
| Symptomatic status, No. (%) | ||||
| Asymptomatic/incidental | 3 (38%) | 7 (58%) | 2 (40%) | 7 (100%) |
| Headache | 3 (38%) | 4 (33%) | 2 (40%) | 0 (0%) |
| Aneurysm rupture/SAH | 0 (0%) | 0 (0%) | 3 (60%) | 0 (0%) |
| Cranial nerve palsy | 4 (50%) | 3 (25%) | 0 (0%) | 0 (0%) |
Table 3:
Most common features of connective tissue diseases
| Clinical Manifestations | Associated Genes | Gene Product |
|---|---|---|
| Marfan | ||
| Marfanoid habitus | FBN1 | Fibrillin-1 |
| Aortic root diseases | ||
| Ectopic lentis | ||
| Dural ectasia | ||
| Ehlers-Danlos | ||
| Joint hypermobility | COL5A1 | Type V collagen |
| Skin hyperextensibility | COL5A2 | Type III collagen |
| Abnormal wound healing | COL3A1 | |
| Mitral valve prolapse | ||
| NF1 | ||
| Café-au-lait macules | NF1 | Neurofibromin |
| Lisch nodules | ||
| Neurofibromas | ||
| Osteoporosis | ||
| Loeys-Dietz | ||
| Aortic aneurysms | TGFBR1 | Transforming growth factor β receptor I |
| Generalized arterial tortuosity | TGFBR2 | Transforming growth factor β receptor II |
| Hypertelorism | TGFB2 | Transforming growth factor β |
| Bifid/broad uvula or cleft palate |
Marfan Syndrome
Of the 59 patients who underwent angiographic imaging of the head, 8 individuals (14%) had a total of 12 intracranial aneurysms: 9 saccular aneurysms and 3 fusiform aneurysms. The mean size of the intracranial aneurysms was 5.5 ± 7.4 mm. Among patients with aneurysms, 2 of 8 (33%) were males and 2 patients were younger than 50 years of age. Other intracranial findings were a direct cavernous carotid fistula (n = 1) and dissection (n = 2). One patient with dissection presented with acute ischemic stroke, and another presented with transient ischemic attacks. There were no cases of subarachnoid hemorrhage. Increasing age was the only variable independently associated with the presence of an intracranial aneurysm (OR, 1.09; 95% CI, 1.01–1.22; P = .03). Representative images are provided in Fig 1.
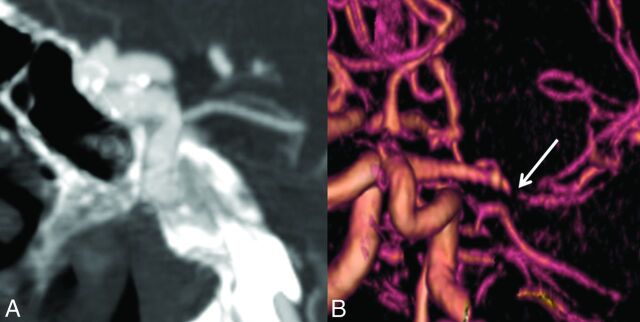
Fig 1.
Imaging findings in Marfan syndrome. A, A 25-year-old man with Marfan syndrome with a 4-mm periophthalmic aneurysm of the right ICA. B, 3D reconstruction of a CTA performed for evaluation of acute ischemic stroke and headache in a patient with Marfan syndrome demonstrates smooth tapering of the right MCA, consistent with an acute dissection (arrow).
Ehlers-Danlos Syndrome
Of the 99 patients who underwent angiographic imaging of the head, 12 individuals (12%) had a total of 14 intracranial aneurysms: 9 saccular aneurysms and 5 fusiform aneurysms. The mean size of the intracranial aneurysms was 6.9 ± 6.5 mm. Of the 12 patients with aneurysms and EDS, 1 had type I EDS, 3 had type III EDS, and 7 had type IV EDS. In 1, the type of EDS was unknown. One patient had both a vertebral AVF on the right and a contralateral dissection of the intradural vertebral artery, which presented as acute ischemic stroke. Intracranial hemorrhage occurred in 2 patients, with 1 patient dying from massive hemorrhage secondary to cerebral venous thrombosis and another with repeat incidences of subarachnoid hemorrhages with unknown etiology despite multiple cerebral angiograms. Increasing age was the only variable independently associated with the presence of an intracranial aneurysm (OR, 1.12; 95% CI, 1.02–1.26; P = .04). Representative images are provided in Fig 2.
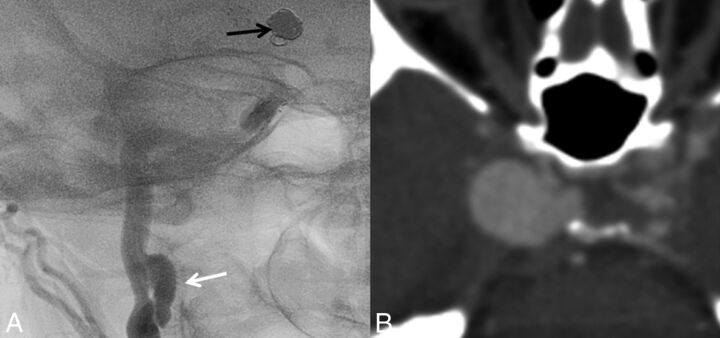
Fig 2.
Imaging findings in Ehlers-Danlos syndrome. A, DSA of a cervical carotid dissecting pseudoaneurysm (white arrow) in a patient with Ehlers-Danlos syndrome. Note the coil mass in the supraclinoid ICA from previous coiling of an ICA aneurysm. B, A giant cavernous carotid artery aneurysm in a patient with Ehlers-Danlos syndrome.
Neurofibromatosis Type 1
Of the 47 patients who underwent angiographic imaging of the head, 5 individuals (11%) had a total of 7 intracranial aneurysms: 6 saccular aneurysms and 1 fusiform aneurysm. The mean size of the intracranial aneurysms was 11.2 ± 9.8 mm. Among patients with aneurysms, 3 of 5 were male (60%), and 3 were younger than 50 years of age. Subarachnoid hemorrhage occurred in 1 patient secondary to intracranial aneurysm rupture and in another secondary to trauma. No patients had intracranial dissections. Hypertension was the only variable independently associated with aneurysm presence (OR, 27.4; 95% CI, 1.69–177.72; P = .02). Representative images are provided in Fig 3.
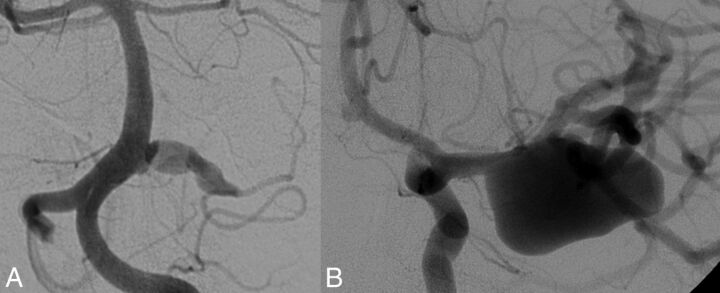
Fig 3.
Imaging findings in neurofibromatosis type 1. A, Fusiform aneurysm of the right anterior inferior cerebellar artery in a 22-year-old patient with NF1. B, The same patient had a giant right MCA aneurysm and a third fusiform aneurysm of an M2 branch.
Loeys-Dietz Syndrome
Of the 25 patients who underwent angiographic imaging of the head, 7 (28%) individuals had a total of 8 intracranial aneurysms: 7 saccular aneurysms and 1 fusiform aneurysm. The mean size of the intracranial aneurysms was 4.8 ± 4.5 mm. Among patients with aneurysms, 4 of 7 (57%) were male, and 4 were younger than 50 years of age. Subarachnoid hemorrhage of unknown etiology in a patient without an intracranial aneurysm occurred in 1 individual. No variables were independently associated with aneurysm presence. Representative images are provided in Fig 4.
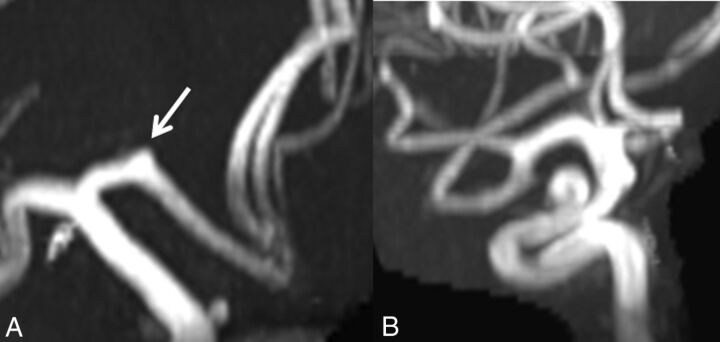
Fig 4.
Aneurysms in Loeys-Dietz syndrome. A, A 3-mm A1 aneurysm (arrow). B, A 6-mm supraclinoid ICA aneurysm in a patient with Loeys-Dietz syndrome.
Discussion
Our study demonstrates that patients with Marfan syndrome, EDS, NF1, and LDS have a high prevalence of intracranial aneurysms. Compared with the 3.2% of the general population who have intracranial aneurysms, the patients in our study had prevalences ranging from 9% to 28%.4 Most of these intracranial aneurysms were found in the anterior circulation, particularly the ICA. On our logistic regression analysis, increasing age was the only variable independently associated with intracranial aneurysm in patients with Marfan syndrome and EDS, and hypertension was the only variable associated with intracranial aneurysm in patients with NF1. The high prevalence of intracranial aneurysms in the population with connective tissue disease has implications for screening these patients, especially because most aneurysms were asymptomatic.
While it is still an ongoing area of debate, relatively large clinical series have called into doubt the association between both Marfan syndrome and EDS and intracranial aneurysms. In a postmortem series of 25 patients with Marfan syndrome, Conway et al1 found an aneurysm prevalence of just 4%. In addition, in a series of 129 patients with Marfan syndrome, van den Berg et al2 found no patients who presented with a symptomatic intracranial aneurysm. In a study of 419 patients with EDS or a family history of the disease, Pepin et al5 found 6 (1.4%) patients who had intracranial aneurysms. However, the exact prevalence of aneurysms in the Pepin et al series is not known because the number of patients who underwent angiographic imaging was not reported.
To our knowledge, our study represents the largest imaging study to date examining the prevalence of intracranial aneurysms in Marfan syndrome and EDS. Distinct from prior studies, all patients in our series underwent intracranial imaging with CTA, DSA, or MRA, thus allowing a more accurate representation of aneurysm prevalence. More than half of the patients in the Marfan and EDS cohorts were asymptomatic at the time of diagnosis. There is no clear consensus in the literature regarding the prevalence of intracranial aneurysms in patients with NF1. In a large case-control study including 39 patients with NF1 and 526 controls, Maya et al6 found a 9% aneurysm prevalence in the NF1 population versus the 0% in controls. However, in a separate study of children with NF1, Rosser et al7 found that only 1 of the 316 patients with NF1 undergoing brain MR imaging had an intracranial aneurysm. However, only 8 of these 316 patients had an MRA performed in addition to the MR imaging.7
To date, only 2 previously published studies have examined the prevalence of cerebral aneurysms in a consecutive series of patients with LDS. In the landmark article describing Loeys-Dietz syndrome including 90 patients, Loeys et al8 found that 10% of patients with LDS had intracranial aneurysms. Two patients died from cerebral bleeding. In a study of 25 patients with LDS, Rodrigues et al9 found that 7 patients (28%) had intracranial aneurysms; most were in the anterior circulation. These findings are similar to those in our study, which also found that 7 of 25 patients (28%) had intracranial aneurysms. All 7 of the patients with intracranial aneurysms in our LDS cohort were asymptomatic and had incidental findings.
Limitations
Our study has limitations. First, it is retrospective in nature. The multiple imaging modalities (CTA, MRA, and DSA) used to identify intracranial aneurysms can cause differences in the accuracy of aneurysm detection among patients. Although DSA is the most sensitive imaging technique for aneurysm detection, most of our patients underwent only CTA or MRA. In addition, the nongenetic clinical diagnosis used to identify patients with Marfan, EDS, NF1, and LDS can result in misdiagnosis. There is also the possibility that our study overestimates the prevalence of intracranial aneurysms in all of these patients because our center is a tertiary referral site for patients with complicated forms of these diseases, and it is a high-volume intracranial aneurysm treatment center. If one were to prospectively study a random group of patients with these connective tissue diseases and obtain imaging, the prevalence estimates may be substantially different from those presented here.
Conclusions
Our study suggests that patients with Marfan syndrome, Ehlers-Danlos syndrome, neurofibromatosis type 1, and Loeys-Dietz syndrome have a high prevalence of intracranial aneurysms. However, it is of key importance to recognize the inevitable yet substantial selection bias of this prevalence study. Nonetheless, these findings further support the need for future prospective studies to understand the risks and benefits of preventative screening in patients with these connective tissue disorders.
Supplementary Material
ABBREVIATIONS:
- EDS
Ehlers-Danlos syndrome
- LDS
Loeys-Dietz syndrome
- NF1
neurofibromatosis type 1
Footnotes
Disclosures: David F. Kallmes—UNRELATED: Board Membership: GE Healthcare (Cost-Effectiveness Board)*; Consultancy: ev3/Covidien/Medtronic,* Comments: planning and implementing clinical trials; Grants/Grants Pending: MicroVention,* Sequent Medical,* SurModics,* Codman,* ev3/Covidien/Medtronic,* NeuroSigma*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: ev3/Covidien/Medtronic,* Comments: presentation at an FDA panel meeting. *Money paid to the institution.
References
- 1. Conway JE, Hutchins GM, Tamargo RJ. Marfan syndrome is not associated with intracranial aneurysms. Stroke 1999;30:1632–36 10.1161/01.STR.30.8.1632 [DOI] [PubMed] [Google Scholar]
- 2. van den Berg JS, Limburg M, Hennekam RC. Is Marfan syndrome associated with symptomatic intracranial aneurysms? Stroke 1996;27:10–12 10.1161/01.STR.27.1.10 [DOI] [PubMed] [Google Scholar]
- 3. Pfohman M, Criddle LM. Epidemiology of intracranial aneurysm and subarachnoid hemorrhage. J Neurosci Nurs 2001;33:39–41 10.1097/01376517-200102000-00005 [DOI] [PubMed] [Google Scholar]
- 4. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 2014;13:393–404 10.1016/S1474-4422(14)70015-8 [DOI] [PubMed] [Google Scholar]
- 5. Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000;342:673–80 10.1056/NEJM200003093421001 [DOI] [PubMed] [Google Scholar]
- 6. Schievink WI, Riedinger M, Maya MM. Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. Am J Med Genet A 2005;134A:45–48 10.1002/ajmg.a.30475 [DOI] [PubMed] [Google Scholar]
- 7. Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology 2005;64:553–55 10.1212/01.WNL.0000150544.00016.69 [DOI] [PubMed] [Google Scholar]
- 8. Loeys BL, Schwarze U., Holm T., et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788–98 10.1056/NEJMoa055695 [DOI] [PubMed] [Google Scholar]
- 9. Rodrigues VJ, Elsayed S, Loeys BL, et al. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol 2009;30:1614–19 10.3174/ajnr.A1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.