Twenty-one patients with previously stented recurrent aneurysms who later underwent Pipeline Embolization Device placement (group 1) were retrospectively identified and compared with 63 patients who had treatment with the Pipeline with no prior stent placement (group 2). Pipeline treatment resulted in complete aneurysm occlusion in 55.6% of patients in group 1 versus 80.4% of patients in group 2. The retreatment rate in group 1 was 11.1% versus 7.1% in group 2. The authors conclude that the Pipeline is less effective in the management of previously stented aneurysms than when used in nonstented aneurysms.
Abstract
BACKGROUND AND PURPOSE:
The use of the Pipeline Embolization Device in the management of recurrent previously stented cerebral aneurysms is controversial. The aim of this study was to evaluate the efficacy and safety of the Pipeline Embolization Device in the treatment of recurrent, previously stented aneurysms.
MATERIALS AND METHODS:
Twenty-one patients with previously stented recurrent aneurysms who later underwent Pipeline Embolization Device placement (group 1) were retrospectively identified and compared with 63 patients who had treatment with the Pipeline Embolization Device with no prior stent placement (group 2). Occlusion at the latest follow-up angiogram, recurrence and retreatment rates, clinical outcome, complications, and morbidity and mortality observed after treatment with the Pipeline Embolization Device were analyzed.
RESULTS:
Patient characteristics were similar between the 2 groups. The mean time from stent placement to recurrence was 25 months. Pipeline Embolization Device treatment resulted in complete aneurysm occlusion in 55.6% of patients in group 1 versus 80.4% of patients in group 2 (P = .036). The retreatment rate in group 1 was 11.1% versus 7.1% in group 2 (P = .62). The rate of good clinical outcome at the latest follow-up in group 1 was 81% versus 93.2% in group 2 (P = .1). Complications were observed in 14.3% of patients in group 1 and 9.5% of patients in group 2 (P = .684).
CONCLUSIONS:
The use of the Pipeline Embolization Device in the management of previously stented aneurysms is less effective than the use of this device in nonstented aneurysms. Prior stent placement can worsen the safety and efficacy profile of this device.
Since the introduction of detachable coils, cerebral aneurysm management has shifted considerably toward endovascular treatment. One of the main weaknesses of cerebral aneurysm coiling lies in the treatment of patients with large1,2 wide-neck or fusiform aneurysms.3 These aneurysms tend to have higher recurrence and retreatment rates after coiling. To make up for this deficiency, intracranial stents have emerged as an alternative in the management of this type of aneurysm. The most widely used stents include the Neuroform (Stryker Neurovascular, Kalamazoo, Michigan) and Enterprise self-expanding (Codman & Shurtleff, Raynham, Massachusetts) stents, which were approved for use as Humanitarian Use Devices in 2002 and 2007, respectively. Several techniques can be used with stents, including stent placement alone and stent-assisted coiling. Recanalization and retreatment rates are lower with stent placement and stent-assisted coiling than with coiling alone.4–6 However, the initial occlusion rates are suboptimal, particularly in large aneurysms.7,8
The management of recurrent previously stented aneurysms remains controversial. There are no recommendations to indicate the most appropriate management strategy, to our knowledge. In 2011, the Pipeline Embolization Device (PED; Covidien, Irvine, California) was FDA-approved for the treatment of large and giant wide-neck aneurysms in the internal carotid artery, from the petrous to the superior hypophyseal segments.9 The PED belongs to a family of devices known as flow diverters, which work by acting as a scaffold for endothelial overgrowth of the aneurysm neck.10 The main structural differences from previous stents are the higher metal surface area coverage compared with previous stents and the low porosity, which allows more flow reduction into the aneurysm neck.11,12 The overall use of the PED has gained popularity mainly because of its high success rate in achieving aneurysm occlusion and low aneurysm recurrence and retreatment rates, especially compared with other endovascular interventions.13,14 Early reports have questioned the efficacy and safety of the PED in treating previously stented aneurysms.15–17 The aim of this study was to evaluate the role of the PED, both its efficacy and safety, in the treatment of recurrent, previously stented cerebral aneurysms.
Materials and Methods
Patients
Patients with cerebral aneurysms treated with the PED between May 2011 and January 2015 at a single institution were reviewed. Twenty-one patients with previously stented aneurysms who later underwent PED placement for aneurysm recurrence were retrospectively identified (group 1). Blinded to outcome, we matched them with 63 patients who underwent treatment by using the PED without prior stent placement in a 3-to-1 ratio, on the basis of patient age and aneurysm size (group 2). PED treatment was offered for recurrent complex, wide-neck aneurysms, partially thrombosed aneurysms, and aneurysms with multiple recurrences treated unsuccessfully by other means. Baseline patient characteristics, aneurysm characteristics, and procedure characteristics were recorded. The study protocol was approved by the Thomas Jefferson University institutional review board.
PED Procedure
Patients were started on clopidogrel and aspirin for at least 10 days before the intervention. An angiographic evaluation was performed to assess aneurysm dimensions. Treatment with the PED was performed with the patient under general anesthesia and neurophysiologic monitoring. After embolization, another angiogram was obtained. Patients were evaluated in the hospital for any complications. After discharge, patients were scheduled for clinical and angiographic follow-up.18 Patients continued taking both aspirin and clopidogrel for 6 months after the procedure, at which time clopidogrel was to be discontinued.
Outcomes
The outcomes of interest in this study were the following: 1) the efficacy of the PED in the management of previously stented cerebral aneurysms evaluated for angiographic occlusion at the latest follow-up angiogram, recurrence and retreatment rates after PED placement, and clinical outcome as measured by the modified Rankin Scale at the latest available follow-up; and 2) the safety of the PED in the treatment of recurrent cerebral aneurysms with a stent in situ assessed by complications, morbidity, and mortality observed after treatment. Modified Rankin Scale scores of 0–2 were considered favorable outcomes, and mRS scores of 3–6 were considered poor outcomes. Modified Rankin Scale scores were assessed by the treating neurosurgeon on the basis of the last follow-up visit. Angiographic evaluations were obtained to monitor any recanalization or residual filling after PED treatment. Aneurysm occlusion at follow-up was evaluated by the treating neurosurgeon and was categorized as complete or incomplete.
Statistical Analysis
Data are presented as mean and range for continuous variables and as frequency for categoric variables. Analysis was performed by using the Wilcoxon matched signed rank test and the McNemar test as appropriate. P values ≤ .05 were considered statistically significant. Statistical analysis was performed with STATA 10.0 (StataCorp, College Station, Texas).
Results
Patient and Aneurysm Characteristics
In the 21 cases, the mean patient age was 51 years. Seven patients presented initially (before the first endovascular intervention) with a subarachnoid hemorrhage (33.3%) (Table 1). Aneurysm locations are detailed in Table 2. Fifteen aneurysms were saccular (71.4%); 4, fusiform (19%); and 2, pseudoaneurysms (9.5%). The average initial aneurysm size before any attempted treatment was 12.5 mm. Before PED treatment, 12 patients had a recurrence on follow-up angiography, whereas 9 patients had aneurysm remnants that eventually grew.
Table 1:
Baseline characteristics in patients with previous stent placement
| Patient | Age (yr) | Sex | Presented with SAH | Location | Aneurysm Form | Initial Size (mm) |
|---|---|---|---|---|---|---|
| 1 | 45 | Female | No | Cavernous ICA | Saccular | 7 |
| 2 | 73 | Female | No | VB | Saccular | 11 |
| 3 | 80 | Female | No | Cavernous ICA | Saccular | 18 |
| 4 | 48 | Female | No | VB | Fusiform | 15 |
| 5 | 60 | Female | No | CO | Saccular | 5 |
| 6 | 54 | Female | No | CO | Saccular | 6 |
| 7 | 33 | Male | No | VB | Saccular | 27 |
| 8 | 44 | Female | No | VB | Fusiform | 22 |
| 9 | 48 | Female | No | PcomA | Saccular | 9 |
| 10 | 46 | Male | Yes | VB | Pseudoaneurysm | 2.5 |
| 11 | 61 | Female | No | Cavernous ICA | Saccular | 5 |
| 12 | 55 | Male | No | VB | Fusiform | 23 |
| 13 | 56 | Female | No | Superior hypophyseal | Saccular | 10 |
| 14 | 80 | Female | Yes | Superior cerebellar | Saccular | 9 |
| 15 | 64 | Male | No | PcomA | Saccular | 15 |
| 16 | 31 | Female | Yes | CO | Saccular | 11 |
| 17 | 51 | Female | Yes | PcomA | Fusiform | 10 |
| 18 | 56 | Female | Yes | CO | Saccular | 18 |
| 19 | 16 | Female | No | Cavernous ICA | Pseudoaneurysm | 6 |
| 20 | 24 | Male | Yes | PcomA | Saccular | 24 |
| 21 | 50 | Female | Yes | Posterior wall ICA | Saccular | 10 |
Note:—VB indicates vertebrobasilar artery; CO, carotid ophthalmic artery; PcomA, posterior communicating artery.
Table 2:
Aneurysm location
| Patients with Prior Stent (%) | Patients without Prior Stent (%) | |
|---|---|---|
| ICA | 23.8 | 35 |
| Vertebrobasilar artery | 28.6 | 9.5 |
| Posterior communicating artery | 19 | 4.8 |
| Carotid ophthalmic artery | 19 | 38 |
| Superior hypophyseal artery | 4.8 | 6.3 |
| Superior cerebellar artery | 4.8 | 0 |
| Middle cerebral artery | 0 | 4.8 |
| Anterior choroidal artery | 0 | 1.6 |
Although patients with no stents were matched on the basis of age and aneurysm size, there was no statistical difference in other baseline characteristics between the 2 groups except for a higher percentage of patients who presented initially with SAH in group 1 (P = .004) (Table 3).
Table 3:
Comparison between patients with and without a prior stent
| Patients with Prior Stent Placement | Patients without Prior Stent | P Value | |
|---|---|---|---|
| Mean age (yr) | 51.2 | 53.6 | .523 |
| Initial aneurysm size (mm) | 12.5 | 12.2 | .862 |
| Sex (male/female) | 76.2:23.8 | 85.7:14.3 | .310 |
| Smoking (%) | 57.1 | 50 | .571 |
| Initial presentation with SAH (%) | 33.3 | 8.06 | .004 |
| Aneurysm form (saccular/fusiform/pseudoaneurysm) (%) | 71.4/19.05/9.5 | 80.95/15.87/3.17 | .447 |
| Average No. of PEDs used | 1.33 | 1.39 | .742 |
| Retreatment post-PED (%) | 11.11 | 7.14 | .629 |
| Complete aneurysm occlusion (%) | 55.6 | 80.4 | .036 |
| Time of last follow-up angiogram (mo) | 10.38 | 10.58 | .816 |
| Complications (%) | 14.29 | 9.52 | .684 |
| Good clinical outcome (%) | 80.95 | 93.22 | .1 |
| Time of last follow-up (months) | 15.67 | 18.71 | .278 |
| Average preoperative PRU | 128.1 | 127.6 | .981 |
Note:—PRU indicates P2Y12 reaction units.
Treatment
In patients who had prior stent placement, the mean number of interventions before PED placement was 2.3, ranging from 1 prior intervention to 4 previous attempts (Table 4). Previous stent interventions included stent-assisted coiling in 57.1% and stent placement alone in 42.9% of patients. Sixteen aneurysms were initially coiled before stents were deployed (76%). Stents that were initially used included the Enterprise stent in 52% of patients and the Neuroform stent in 48% of patients.
Table 4:
Treatment and outcomes in patients with previous stents
| Patient No. | No. of Interventions before PED | Time from Stenting to Pipeline (mo) | No. of PEDs Used | Occlusion on Follow-Up Angiogram | Complications | mRS at Latest Follow-Up | Retreatment Post-PED |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 3 | 1 | Incomplete | No | 0 | No |
| 2 | 1 | 38 | 2 | Complete | No | 0 | No |
| 3 | 3 | 54 | 2 | Complete | No | 0 | No |
| 4 | 3 | 74 | 1 | Incomplete | No | 0 | No |
| 5 | 3 | 25 | 1 | Complete | Retinal emboli causing visual changes | 1 | No |
| 6 | 2 | 22 | 1 | Incomplete | No | 1 | No |
| 7 | 1 | 13 | 4 | Incomplete | No | 4 | Yes |
| 8 | 3 | 52 | 1 | Complete | No | 0 | No |
| 9 | 4 | 49 | 1 | Complete | No | 0 | No |
| 10 | 2 | 3 | 1 | Complete | No | 0 | No |
| 11 | 3 | 75 | 1 | Incomplete | No | 0 | No |
| 12 | 2 | 44 | 3 | Incomplete | PCA infarct | 5 | No |
| 13 | 1 | 44 | 1 | Complete | No | 0 | No |
| 14 | 2 | 39 | 1 | Incomplete | No | 1 | Yes |
| 15 | 2 | 76 | 1 | Complete | No | 0 | No |
| 16 | 2 | 39 | 1 | Incomplete | No | 0 | No |
| 17 | 2 | 48 | 1 | NA | No | 0 | NA |
| 18 | 2 | 91 | 1 | Complete | No | 0 | No |
| 19 | 2 | 1 | 1 | Complete | No | 0 | No |
| 20 | 2 | 17 | 1 | NA | No | 3 | NA |
| 21 | 6 | 41 | 1 | NA | ICH | 6 | NA |
Note:—PCA indicates posterior cerebral artery; NA, not applicable; ICH, intracerebral hemorrhage.
The mean time from stent placement to recurrence was 23 months. The mean time from the initial treatment to PED placement was 40.4 months. The mean number of PEDs placed was 1.3, ranging from 1 to 4. The average procedure length for PED treatment of previously stented aneurysms was 66 minutes versus 58 minutes for nonstented aneurysms (P = .6). Technical difficulties were observed in 5 patients with prior stent placement (23.8%). Technical difficulties were reported by the treating neurosurgeon and identified by reviewing the operative reports. In 1 patient, because of the prior stent placement, several catheter advancements and several readjustments of the microcatheter and wire were required to achieve a good position for PED deployment. In another patient, the distal two-thirds of the PED was deployed with good wall apposition; however, because of prior stent placement, the proximal third did not open and good wall apposition was not achieved. In 2 patients, there were difficulties in placing the PED across the neck of the aneurysm and achieving appropriate overlap with the previous stent and covering the proximal tines of the stent. In another patient, 4 PEDs were required to span the proximal portion of the aneurysm and extend proximally to the pre-existing stent. On the other hand, technical difficulties were encountered in 3 patients with no prior stent placement (4.8%). There were difficulties in opening the proximal part of the PED during deployment in 2 patients who had a severe tortuosity of the inflow of the aneurysm, and a balloon was used to expand the device.
Preoperative P2Y12 reaction unit values were available in 19 patients in group 1 (mean, 128.1) and 51 patients in group 2 (mean, 127.6) (P = .98). In-stent stenosis was observed in 2 of the 21 patients with previous stent placement (9.5%), one patient with 50% stenosis and the other with <50% stenosis.
Efficacy of PED Treatment of Previously Stented Aneurysms
Angiographic Occlusion.
In group 1, 3 patients did not have a follow-up angiographic evaluation because of loss to follow-up. Of the 18 patients who had follow-up angiograms, PED treatment resulted in complete aneurysm occlusion in 10 patients (55.6%) (Fig 1). In group 2, 7 patients did not have available angiograms, 3 patients because of death following the procedure and 4 patients who were lost to follow-up. Of the 56 patients with follow-up angiograms, 45 patients had complete aneurysm occlusion (80.4%). In group 1, 8 aneurysms (44.4%) were found to have incomplete occlusion with residual filling or recurrence after PED placement (Figs 2 and 3), whereas in group 2, 11 aneurysms were found to have incomplete occlusion (19.6%). This difference of 24.8% was statistically significant (P = .036). The mean angiographic follow-up time from PED placement to the last angiogram was 10.4 months in group 1 and 10.6 months in group 2 (P = .816).
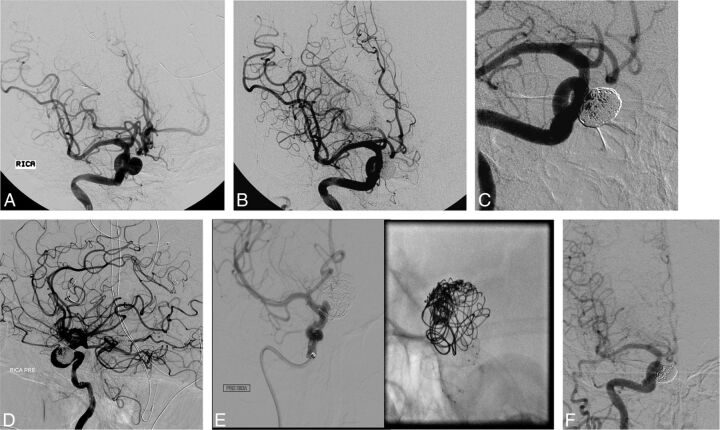
Fig 1.
A, A 53-year-old woman who presented with headache and was found to have a 10-mm right superior hypophyseal artery aneurysm. B, Stent-assisted coiling was performed, and the control angiogram shows complete occlusion. C, A follow-up angiogram at 6 months shows complete occlusion of the aneurysm dome and minimal filling of the interstices. D, A follow-up angiogram after 2 years shows recurrence of the aneurysm. E, At that point, embolization by using the PED was performed. F, A 6-month follow-up angiogram shows complete occlusion of the aneurysm.
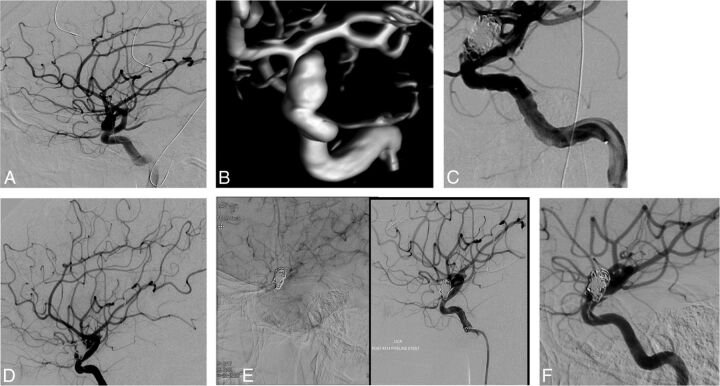
Fig 2.
A and B, A 28-year-old woman who presented with subarachnoid hemorrhage resulting from a ruptured 11-mm left carotid ophthalmic artery aneurysm. C, Stent-assisted coiling was performed. This resulted in incomplete occlusion of the aneurysm. D, Three-year follow-up angiogram shows growth of the residual. E, Treatment with the PED was performed, with incomplete wall apposition. F, Six-month follow-up angiogram shows incomplete occlusion.
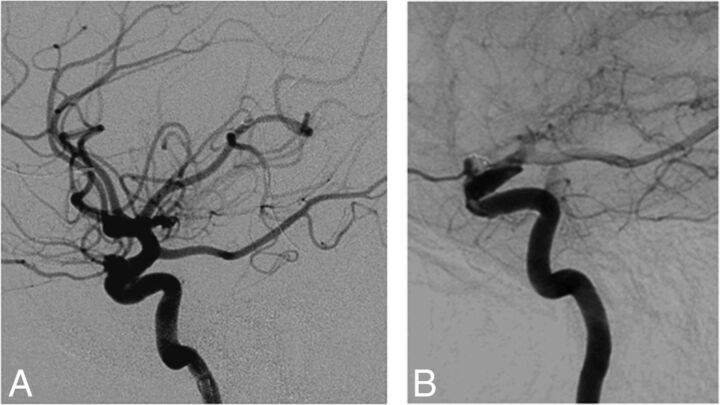
Fig 3.
A, A 54-year-old woman with a 6-mm left carotid ophthalmic artery aneurysm, which was initially treated with stent-assisted coiling but recurred. B, Treatment with the PED was performed. A 12-month follow-up angiogram shows incomplete occlusion.
The 8 aneurysms with incomplete occlusion in patients with prior stent placement had different characteristics. In general, 3 aneurysms were larger than the average size (12.5 mm) and 5 aneurysms were smaller. More specifically, incompletely occluded aneurysms included 3 vertebrobasilar aneurysms of >15 mm, 2 carotid ophthalmic aneurysms (6 and 9 mm), 2 aneurysms in the cavernous internal carotid artery (5 and 7 mm), and a 9-mm superior cerebellar artery aneurysm. Five aneurysms were previously coiled, and 3 were not.
The 11 incompletely occluded aneurysms in patients without prior stent placement included 3 cavernous ICA aneurysms (10, 20, and 21 mm), 2 paraclinoid ICA aneurysms (9 and 16 mm), 3 carotid ophthalmic aneurysms (5, 10, and 13 mm), one 12-mm middle cerebral artery aneurysm, one 6-mm superior hypophyseal aneurysm, and one 7-mm posterior carotid wall aneurysm.
Retreatment.
Two previously stented aneurysms managed with the PED required another retreatment, with placement of another PED (11.1%). No aneurysms required additional coiling or clipping. In patients with no prior stents, 4 patients required retreatment post-PED placement (7.1%). The rate of retreatment was nonsignificantly higher in patients with a prior stent (P = .62).
Clinical Outcome.
Mean follow-up was 15.7 months in group 1 and 18.7 months in group 2 (P = .278). In patients with previous stent treatment, 17 patients had a good clinical outcome (mRS 0–2) at the latest follow-up (81%), with 14 patients having an mRS score of zero (66.7%) and 3 patients having an mRS score of 1 (14.3%). Four patients had an unfavorable outcome (19%), with 1 patient having an mRS score of 3, 1 patient with an mRS score of 4, 1 patient with an mRS of 5, and 1 patient with an mRS of 6. In patients with no prior stent placement, 93.2% had a good clinical outcome versus 6.8% with a poor clinical outcome. There was a trend toward worse clinical outcome in patients with prior stent placement (19%) as opposed to patients without prior stent treatment (6.8%) (P = .1).
Safety of PED Treatment of Previously Stented Aneurysms
Complications within 30 days after treatment were observed in 3 patients (14.3%) in group 1 and 6 patients in group 2 (9.5%) (P = .684). In group 1, 1 patient with a giant vertebrobasilar artery aneurysm had a posterior cerebral artery territory infarct and later developed hydrocephalus requiring ventriculoperitoneal shunting and then developed multiple shunt infections (mRS = 5). Another patient had retinal emboli causing visual changes (mRS = 1) after PED treatment of a carotid ophthalmic aneurysm, and another patient had a right intracerebral hemorrhage after PED treatment of a right ICA aneurysm and died (mRS = 6). Mortality was similar in both groups (4.76%). In group 2, 5/6 patients with complications had giant aneurysms. Complications in this group included 2 intracerebral hemorrhages, 3 MCA infarcts, and 1 basal ganglia stroke.
There were no statistically significant differences in the rate of complete occlusion (62% versus 50%, P = .7), complications (10% versus 18%, P = .9), or good clinical outcome (82% versus 80%, P = 1) among patients who had prior Neuroform or Enterprise stent placement, respectively, though patients with prior Neuroform stents had better overall outcomes after PED deployment.
Discussion
Our results show that stent placement negatively affects the efficacy of the PED in the management of recurrent aneurysms. The rate of complete aneurysm occlusion was significantly low (55.6%), especially compared with the high success rate of PED treatment reported in this study for subjects without prior stent placement (80.4%) and in other studies, with most reporting a high occlusion rate of >80%.12–14,19 In addition, the rate of retreatment (11.1%) was considerably higher than that reported with PED treatment of nonstented aneurysms. Zanaty et al13 reported a 5% rate of retreatment after PED placement.
The complication rate seen with PED treatment of recurrent previously stented aneurysms (14.3%) falls within the higher spectrum reported in the literature (0%–12%). Kallmes et al20 reported a morbidity and mortality rate of 8.4% with PED treatment. Brinjikji et al19 reported a procedure-related morbidity rate of 5%. In patients with prior stent treatment, 1 patient had a posterior cerebral artery infarct after PED treatment and then developed hydrocephalus requiring ventriculoperitoneal shunt placement and another patient developed emboli to the ophthalmic artery causing visual disturbances. Thromboembolic events are well-known complications of PED treatment.12,19,20 Because this device consists of a bare metal stent that aids in neointimal tissue formation, it can lead to platelet activation causing local thrombosis and cerebral infarction or distal embolization. The large metal surface area coverage of the Pipeline Embolization Device and frequent use of multiple devices also contribute to the occurrence of thromboembolic events.
Treatment with the PED poses a risk for hemorrhagic complications as well. This was observed in 1 patient who had an intracerebral hemorrhage and died. The occurrence of intracerebral hemorrhage in patients treated with the PED can be due to multiple factors, including the dual-antiplatelet therapy that patients receive, hemorrhagic transformation of ischemic stroke, and hemodynamic changes related to placement of the PED. Furthermore, the presence of a prior Neuroform or Enterprise stent may increase the risk of both thromboembolic and hemorrhagic complications associated with PED placement because these stents act as foci of thrombus formation and patients are started on aspirin and clopidogrel (Plavix) before stent placement. Hemorrhagic and thromboembolic complications occurred at a nonsignificantly higher rate in previously stented aneurysms.
Since the initial reports about treatment with the PED, the use of this device has been controversial in the management of recurrent previously stented aneurysms. Fischer et al15 criticized both the safety and efficacy of the PED in the treatment of aneurysms with prior stent placement in a series that included 30 lesions treated previously with conventional stents. They reported that adverse events occurred in only 1 of 54 patients (2%) without a previous stent placement after PED treatment and in 4 of 30 patients (13%) in whom a stent was previously placed before PED treatment. They added that the observed successful occlusion rate was lower for aneurysms with a previous stent placement (65%). The complication rate of 13% and the occlusion rate of 65% reported in their study are very much comparable with the rates obtained in our study (14.3% and 66.7%, respectively). They concluded that the presence of a previous stent may reduce the hemodynamic effect of the PED. This negative interaction was attributed to a disruption of the process of wall apposition of the PED to the parent vessel, preventing the process of appropriate device endothelialization.
Nelson et al17 reported that 1 of 2 aneurysms (n = 31) not initially occluded with the PED was previously treated with another stent. They concluded as well that prior stent placement impairs effective apposition of the PED to the parent artery, thus slowing or preventing the neointimal formation and endothelialization processes and potentially inhibiting complete aneurysm occlusion. This outcome was indeed observed in our series in several patients with prior stent placement, in whom appropriate wall apposition was impeded by the previously placed stent.
Similarly, Lylyk et al16 observed that of 63 aneurysms treated with the PED, the only aneurysm that remained patent at 12-month follow-up had been treated previously with stent-assisted coiling. They deduced that the presence of a pre-existing stent may have limited the efficacy of the PED by impairing the apposition of the PED to the wall of the parent artery, which led to endoleaks that maintained patency of the aneurysm and disrupted the endothelialization process over the surface of the PED. In addition, the presence of these devices can complicate the navigation of the delivery catheter into position and the actual deployment of the PED, increasing the technical difficulty of the procedure. Technical difficulties involving catheter advancements were encountered in our study as well. These increased the complexity of the procedure and the ability to properly deploy the PED.
Chalouhi et al21 highlighted the technical difficulties observed with PED placement in aneurysms with a previous stent in situ. Because the PED should be deployed distal to the stent, the distal end of the PED may “catch” on the previously placed stent, which may cause anchoring and stretching of the device, leading to less effective results. They suggested that performing simultaneous balloon angioplasty may be useful in optimizing PED apposition to the vessel wall.
Given the negative association between stent placement and later PED treatment, physicians should be careful when selecting the primary intervention to treat large complex wide-neck cerebral aneurysms that are amenable to PED treatment and less likely to be cured with conventional endovascular procedures. If a stent was placed initially, recurrence would be less eligible for PED treatment and might require surgical clipping to achieve aneurysm occlusion.
The main limitations of this study include the single-center experience with a small number of aneurysms. Another important limitation is the retrospective nature of this study. Furthermore, the mean time of angiographic follow-up was limited (10 months).
Conclusions
The use of the PED in the management of previously stented cerebral aneurysms is less effective than the use of this device in nonstented aneurysms. Prior stent placement may decrease the rate of aneurysm occlusion following treatment with the PED, may increase the rate of complications associated with the procedure, and may increase the chances of encountering technical difficulties during PED deployment. Initial management of large wide-neck complex cerebral aneurysms amenable to PED treatment with conventional stents should be carefully planned because recurrence of stented aneurysms is less likely to respond to PED placement. More studies are required to determine the best treatment for recurrent previously stented aneurysms.
ABBREVIATION:
- PED
Pipeline Embolization Device
Footnotes
Disclosures: Stravropoula Tjoumakaris—UNRELATED: Consulting Fee or Honorarium: Covidien. Pascal Jabbour—UNRELATED: Consultancy: Covidien.
References
- 1. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 10.1161/01.STR.0000073841.88563.E9 [DOI] [PubMed] [Google Scholar]
- 2. Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 2009;40:e523–29 10.1161/STROKEAHA.109.553099 [DOI] [PubMed] [Google Scholar]
- 3. Ries T, Siemonsen S, Thomalla G, et al. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol 2007;28:1755–61 10.3174/ajnr.A0649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010;41:110–15 10.1161/STROKEAHA.109.558114 [DOI] [PubMed] [Google Scholar]
- 5. Nishido H, Piotin M, Bartolini B, et al. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol 2014;35:339–44 10.3174/ajnr.A3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. King B, Vaziri S, Singla A, et al. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with Enterprise and Neuroform stents: a comparative analysis of the literature. J Neurointerv Surg October 28 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Santillan A, Greenberg E, Patsalides A, et al. Long-term clinical and angiographic results of Neuroform stent-assisted coil embolization in wide-necked intracranial aneurysms. Neurosurgery 2012;70:1232–37; discussion 1237 10.1227/NEU.0b013e3182422a68 [DOI] [PubMed] [Google Scholar]
- 8. Hong Y, Wang YJ, Deng Z, et al. Stent-assisted coiling versus coiling in treatment of intracranial aneurysm: a systematic review and meta-analysis. PLoS One 2014;9:e82311 10.1371/journal.pone.0082311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 10. Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007;38:2346–52 10.1161/STROKEAHA.106.479576 [DOI] [PubMed] [Google Scholar]
- 11. Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the Pipeline embolization device. Neurosurgery 2008;62:1115–20; discussion 1120–21 10.1227/01.neu.0000325873.44881.6e [DOI] [PubMed] [Google Scholar]
- 12. D'Urso PI, Lanzino G, Cloft HJ, et al. Flow diversion for intracranial aneurysms: a review. Stroke 2011;42:2363–68 10.1161/STROKEAHA.111.620328 [DOI] [PubMed] [Google Scholar]
- 13. Zanaty M, Chalouhi N, Starke RM, et al. Flow diversion versus conventional treatment for carotid cavernous aneurysms. Stroke 2014;45:2656–61 10.1161/STROKEAHA.114.006247 [DOI] [PubMed] [Google Scholar]
- 14. Chalouhi N, Tjoumakaris S, Starke RM, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013;44:2150–54 10.1161/STROKEAHA.113.001785 [DOI] [PubMed] [Google Scholar]
- 15. Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012;54:369–82 10.1007/s00234-011-0948-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009;64:632–42; discussion 642–43; quiz N6 10.1227/01.NEU.0000339109.98070.65 [DOI] [PubMed] [Google Scholar]
- 17. Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34–40 10.3174/ajnr.A2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chitale R, Gonzalez LF, Randazzo C, et al. Single center experience with Pipeline stent: feasibility, technique, and complications. Neurosurgery 2012;71:679–91; discussion 691 10.1227/NEU.0b013e318260fe86 [DOI] [PubMed] [Google Scholar]
- 19. Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013;44:442–47 10.1161/STROKEAHA.112.678151 [DOI] [PubMed] [Google Scholar]
- 20. Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the Pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015;36:108–15 10.3174/ajnr.A4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chalouhi N, Chitale R, Starke RM, et al. Treatment of recurrent intracranial aneurysms with the Pipeline embolization device. J Neurointerv Surg 2014;6:19–23 10.1136/neurintsurg-2012-010612 [DOI] [PubMed] [Google Scholar]