SUMMARY:
In current practice, gadolinium-based contrast agents have been considered safe when used at clinically recommended doses in patients without severe renal insufficiency. The causal relationship between gadolinium-based contrast agents and nephrogenic systemic fibrosis in patients with renal insufficiency resulted in new policies regarding the administration of these agents. After an effective screening of patients with renal disease by performing either unenhanced or reduced-dose-enhanced studies in these patients and by using the most stable contrast agents, nephrogenic systemic fibrosis has been largely eliminated since 2009. Evidence of in vivo gadolinium deposition in bone tissue in patients with normal renal function is well-established, but recent literature showing that gadolinium might also deposit in the brain in patients with intact blood-brain barriers caught many individuals in the imaging community by surprise. The purpose of this review was to summarize the literature on gadolinium-based contrast agents, tying together information on agent stability and animal and human studies, and to emphasize that low-stability agents are the ones most often associated with brain deposition.
Gadolinium-based contrast agents (GBCAs) have been widely used in clinical MR imaging studies since the initial FDA approval of gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) in 1988. To date, 9 GBCAs are available for clinical use in 1 or more regions of the world (Table 1), and it is estimated that >200 million doses have been administered worldwide.1
Table 1:
Gadolinium-based contrast agents currently approved for clinical use: biochemical properties
| Chemical Structure | Trade Name | Thermodynamic Stability Constant | Conditional Stability Constant | Elimination Pathway |
|---|---|---|---|---|
| Linear | ||||
| Nonionic | ||||
| Gadodiamide | Omniscan, 0.5 mmol/mL | 16.8 | 14.9 | Renal |
| Gadoversetamide | OptiMARK, 0.5 mmol/mL | 16.6 | 15 | Renal |
| Ionic | ||||
| Gadopentetate dimeglumine | Magnevist, 0.5 mmol/mL | 22.1 | 17.7 | Renal |
| Gadobenate dimeglumine | MultiHance, 0.5 mmol/mL | 22.6 | 18.4 | 93% Renal 3% Biliary |
| Gadoxetic acid disodium | Primovist, 0.25 mmol/mL | 23.5 | NA | 50% Renal 50% Biliary |
| Gadofosveset trisodium | Vasovist, 0.25 mmol/mLa | 22 | NA | 91% Renal 9% Biliary |
| Macrocyclic | ||||
| Nonionic | ||||
| Gadoteridol | ProHance, 0.5 mmol/mL | 22.8 | 17.1 | Renal |
| Gadobutrol | Gadavist, 0.5 mmol/mL | 21.8 | NA | Renal |
| Ionic | ||||
| Gadoterate meglumine | Dotarem, 0.5 mmol/mL | 25.4 | 19 | Renal |
Note:—NA indicates not applicable.
Bayer Schering Pharma.
All GBCAs approved for clinical use have been considered to have a wide safety margin when used at relatively low doses (0.1–0.3 mmol/kg) in patients with normal renal function. The accumulated safety record is excellent, with serious adverse reactions occurring in roughly 0.03% of all administrations.2,3 These adverse reactions are more common in patients with history of asthma, allergies, and renal insufficiency and in patients injected at faster rates.1,4,5
GBCAs had an exceptional safety reputation from 1988 to 2006, to the point that in 2004 and 2005 GBCAs were recommended as a substitute for iodine-based contrast media in patients with renal failure for CT and in interventional studies.6–9
In 2006, the association between the administration of GBCAs and the development of nephrogenic systemic fibrosis (NSF) in patients with renal insufficiency was described.10,11 NSF is a debilitating and potentially life-threatening disease characterized by widespread progressive tissue fibrosis that results from the deposition of fibroblasts and collagen. It predominantly involves the skin but may also affect other organs such as the lungs, liver, heart, and muscles.
The exact pathophysiology of NSF remains unknown, but the dissociation of gadolinium ions from their chelating ligands has been accepted as the primary etiology, which is more likely to occur in patients with renal failure than in those with normal renal function because the excretion rate is reduced in the former, allowing time for the chelates to disassociate in vivo. Most cases of NSF reported in the literature have been associated with administration of nonionic, linear gadodiamide (Omniscan; GE Healthcare, Piscataway, New Jersey),12 though reports also described substantial incidents with another nonionic linear agent, gadoversetamide (OptiMARK; Covidien, Irvine, California), and with an ionic linear agent, gadopentetate dimeglumine (Magnevist).13–17
Since mid-2009, no new cases of NSF have been reported. This finding reflects the use of more stable GBCAs and limiting the use of GBCAs in patients with renal failure. As a result, from 2009 to 2014, confidence in the safety profile of GBCAs has been largely restored. However, in the past 2 years, numerous studies regarding gadolinium deposition in neural tissues in patients with normal renal function have been published.
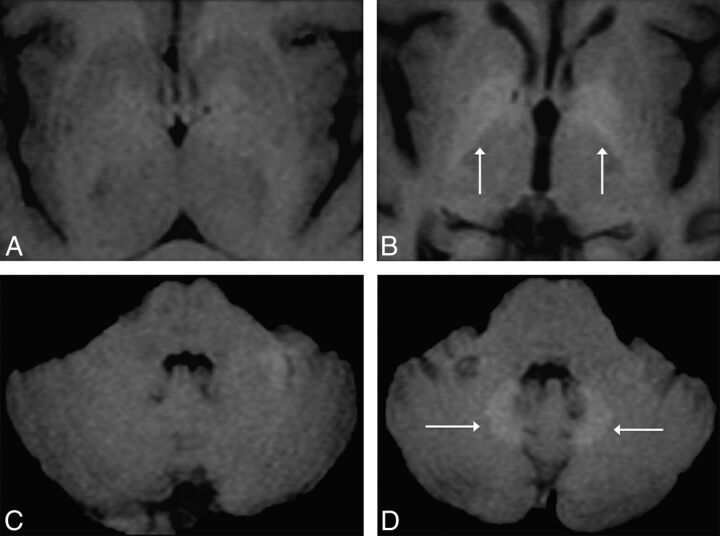
This deposition was first postulated by MR imaging studies in which progressively increased signal intensity in the globi pallidi and/or dentate nuclei (DN) on unenhanced T1-weighted images in patients with normal renal function was related to multiple administrations of GBCAs. As with NSF, the agent most associated with this finding was gadodiamide (Omniscan) (Fig 1),18–21 but it has also been shown with gadopentetate dimeglumine (Magnevist).18,22
Fig 1.
Axial MR images in a 51-year-old woman with parkinsonism. Unenhanced T1-weighted MR imagings of the first (A and C) and fifth (3 years later; B and D) gadolinium-enhanced MR imagings performed with a nonionic linear GBCA (Omniscan) at the level of the basal ganglia (A and B) and the level of the dentate nuclei of the cerebellum (C and D). The images show progressively increased T1 signal of the globi pallidi and dentate nuclei (white arrows, B and D), undetectable on the first MR imaging.
T1 hyperintensity in the DN was previously described in the progressive subtype of multiple sclerosis and was associated with increased clinical disability, lesion load, and brain atrophy.23 Similar findings were also reported with brain irradiation.24 From the perspective of our current understanding, none of these studies considered the number of contrast-enhanced MR imaging studies performed in their analyses, raising the question of whether these findings reflect gadolinium deposition rather than a primary disease manifestation, as demonstrated recently by Adin et al25 in a study with 184 subjects who were treated with brain irradiation.
A study by McDonald et al26 was the first to document that the high signal in the neural tissues reflected deposited gadolinium. In brain specimens from postmortem examinations of 13 subjects who underwent at least 4 MR imaging examinations with gadodiamide (Omniscan), the presence of gadolinium was histologically confirmed by using inductively coupled plasma mass spectroscopy. They also showed a dose-dependent relationship between intravenous gadodiamide administrations and subsequent neural tissue deposition that was independent of renal function. Kanda et al27 confirmed neural tissue deposition in 5 patients with normal renal function who had received gadopentetate dimeglumine (Magnevist), gadodiamide (Omniscan), or gadoteridol (ProHance; Bracco Diagnostics, Princeton, New Jersey) in varying combinations.
Confirming human studies, an animal study28 also demonstrated that repeated administrations of linear gadodiamide (Omniscan) to healthy rats was associated with progressive and persistent T1 signal hyperintensity in the DN and with histologic gadolinium deposits in the cerebellum, in contrast to those who received the macrocyclic agent gadoterate meglumine (Dotarem; Guerbet, Aulnay-sous-Bois, France), in whom no effects were observed.
The more stable macrocyclic GBCAs, such as gadoteridol (ProHance),22 and gadoterate meglumine (Dotarem),28,29 were not associated with substantial MR imaging changes or even brain deposition in the case of gadoterate meglumine (Dotarem),28 supporting the concept that gadolinium accumulation varies depending on the stability of the agent used. Gadobenate dimeglumine (MultiHance; Bracco Diagnostics), an agent of intermediate stability, was associated with fewer MR imaging changes compared with the linear gadodiamide (Omniscan) and was only appreciated in the DN.21 Recently, Weberling et al30 suggested that this agent releases less gadolinium than gadopentetate dimeglumine (Magnevist) but more than gadoterate meglumine (Dotarem). Most surprising, a more stable macrocyclic agent, gadobuterol (Gadavist; Bayer Schering Pharma, Berlin, Germany), has also been shown to result in brain deposition.31 These findings suggest that all GBCAs should be evaluated individually, despite their molecular structures.
Gadolinium-Based Contrast Agents: In Vitro Stability, Pharmacokinetics, and Biodistribution
GBCAs are used as MR imaging contrast agents because of their excellent paramagnetic properties. Gadolinium is a rare earth element and one of the 15 metallic atoms in the lanthanide series. On the periodic table, its symbol is Gd, and its atomic number is 64. Free gadolinium (Gd3+) is toxic in humans, and to be used in vivo, it must be chelated to organic ligands.32
Depending on the ligand structure, GBCAs can be classified in 2 major groups: macrocyclic molecules, in which the Gd3+ is caged in the preorganized cavity of the ligand; and linear or open chain molecules, in which the ligand is not fully closed. From a chemical structure perspective, each category may be further subclassified, according to their charges, into ionic and nonionic.33,34
Frenzel et al33 reported that under physiologic conditions (human serum, at 37°C), GBCAs can be divided into 3 distinct stability classes: nonionic linear, ionic linear, and macrocyclic. Macrocyclic chelates are more stable than linear chelates, and ionic linear chelates are more stable than the nonionic linear ones.
The dissociation of Gd3+ from its ligand is an equilibrium process defined by 2 distinct and independent parameters: kinetic and thermodynamic stabilities.
Kinetic stability of a gadolinium complex is characterized by its dissociation rate, which describes how fast a resting equilibrium is reached and thus how fast Gd3+ is released from a gadolinium complex.33 If the kinetic stability is high, the dissociation rate is considerably slower than the elimination rate from the body, and the release of Gd3+ becomes negligible during the in vivo residence time of the gadolinium complex. A simple way to understand kinetic stability is the speed at which the chelated gadolinium agent dissociates. At present, kinetic stability of GBCAs is reported for a pH of 1 (hence, we prefer to designate these values as “pH 1, kinetic stability”), in large part, because the kinetic stability of some of the macrocyclic agents would need to be expressed in terms of months or years at a pH of 7.4.
Thermodynamic stability reflects the energy required for the metalloligand to release the Gd3+ ion. When thermodynamic stability is high, the chelate less readily releases the free Gd3+ ion. A simple way to understand thermodynamic stability is that it represents the final equilibrium state between chelated and unchelated gadolinium. Thermodynamic stability is also determined at a pH of 1, but a more appropriate measure when considering an in vivo environment is to calculate it at the physiologic pH of 7.4,4,33 which is termed “conditional stability” (we prefer the term “pH 7, thermodynamic stability”).
Other factors, including the concentration of competing ions or ligands and the interaction times between the gadolinium chelates and the competitors,35,36 contribute to the stability of GBCAs.4
In vivo, the gadolinium complex is surrounded by a variety of competitors, which have the potential to interact with either the Gd3+ or the ligand. Different endogenous cations (eg, Fe3+, Mg2+, Cu2+, Zn2+, or Ca2+) compete with Gd3+ ions for the ligand, and endogenous anions (eg, phosphate, carbonate, hydroxide) compete for the Gd3+ ions. This competition may destabilize the gadolinium complex in biologic fluids and shift the dissociation equilibrium toward its free components. The components do not exist as free ions but bind to other agents rapidly. This exchange process is termed “transmetallation.”1,33,37–39 Most often, if the ligand releases Gd3+ ions, they quickly rebind. On the basis of the availability of other cations and the affinity of the ligand to them, the ligand may bind to another cation.4,33 The same phenomenon is experienced by the anionic component.
GBCAs are also classified according to their biodistribution as extracellular, combined extracellular-intracellular, and blood-pool agents. An intravenously administered chelate rapidly equilibrates in the intravascular and interstitial fluid compartments (extracellular compartment). Depending on its structure, the complex may also be distributed in the intracellular compartment (including the liver and kidneys) by passive diffusion or specific uptake processes.40 Most GBCAs in clinical use are nonspecific extracellular contrast agents, which, like iodine-based contrast agents, are cleared almost exclusively by the kidneys. Combined extracellular-intracellular agents are distributed into the extracellular and intracellular compartments of hepatocytes; therefore, they are also described as “hepatocyte-specific agents.” These agents (gadobenate dimeglumine [MultiHance] and gadoxetic acid/gadoxetate disodium [Primovist/Eovist; Bayer Schering Pharma]) when taken up by hepatocytes are excreted into the bile ducts, thus exhibiting dual-elimination routes (renal and biliary). The biliary route is an important pathway of elimination of contrast if the kidneys are functioning poorly.32,41 With normally functioning kidneys, most of the administered dose of GBCAs, regardless of which agent was given, should be eliminated in <2 hours after injection and >95% by 24 hours. However, patients with renal impairment have reduced GBCA elimination, and the Gd complex remains inside the body for extended periods, allowing dissociation to occur.4,33 In this setting, GBCAs with dual elimination (biliary and renal) have an alternative elimination pathway, which helps decrease the gadolinium burden in the body.
Gadolinium Toxicity
Most of the known toxicity of the free Gd3+ ion is related to 2 properties: its insolubility at physiologic pH, resulting in very slow systemic excretion; and an ionic radius close to that of Ca2+ (Gd3+ = 107.8 pm and Ca2+ = 114 pm) that allows Gd3+ to compete biologically with Ca2+.3,34
Gadolinium is a well-known blocker of many types of voltage-gated calcium channels at very low concentrations, and consequently, it can inhibit physiologic processes such as contraction of smooth, skeletal, and cardiac muscles; transmission of nerve impulses; and blood coagulation. It also inhibits the activity of certain enzymes such as Ca2+-activated-Mg2+-adenosine triphosphatase, some dehydrogenases and kinases, and glutathione S-transferases. It also acts as an agonist on the calcium-sensing receptors.42 Gadolinium may also increase the expression of some cytokines,43 inhibit mitochondrial function, and induce oxidative stress.44,45
Major lesions related to single-dose administration of gadolinium chloride (0.07–0.35 mmol/kg) in rats consist of mineral deposition in capillary beds, phagocytosis of minerals by macrophage-like cells, hepatocellular and splenic necrosis followed by dystrophic mineralization, decreased platelet numbers, and increased coagulation times.46 Gadolinium is also a potent inhibitor of the reticuloendothelial system.34,47 All GBCAs and gadolinium chloride have been found to stimulate fibroblast proliferation in tissues taken from healthy subjects.48–51 This last process may be a major factor responsible for NSF because proliferation of CD34+ fibroblasts is the hallmark histologic feature of this disease.52,53
Gadolinium Retention and Tissue Deposition
Several studies describe a complex pharmacokinetic behavior after intravenous administration of GBCA. Even in patients with normal renal function, in vivo clinical exposure to gadolinium chelates results in gadolinium incorporation into body tissues such as bone matrix54–56 or brain tissues.26,27 As early as 1991, Rocklage et al57 stated, “Minute amounts of chelated or unchelated metals are likely to remain in the body for an extended period and could possibly result in a toxic effect.”
Gibby et al54 used inductivity coupled plasma atomic emission spectroscopy to quantify gadolinium deposition in the bones of patients who underwent total hip arthroplasty after an injection of 0.1 mmol/kg of gadodiamide (Omniscan) or gadoteridol (ProHance) no less than 3 days and not more than 8 days before the operation. The authors found that Omniscan resulted in 2.5 times the amount of gadolinium deposition as ProHance. In a follow-up study, White et al55 confirmed these findings by using a more sensitive analytic method and reported that Omniscan deposited 4 times more than did ProHance.
Later, Darrah et al56 also analyzed bone tissue. The authors confirmed that gadolinium incorporates into bone and is retained for >8 years. However, no differences were observed in bone gadolinium concentration between patients dosed with Omniscan (n = 6) and ProHance (n = 5). It is difficult to explain the different findings between these 2 groups, and perhaps the small number of patients may have affected the results of Darrah et al. Other researchers have previously estimated that approximately 1% of the injected gadolinium from each dose of the evaluated GBCAs could be released from the contrast agent and deposited in the bones, including in patients with normal kidney function.58
The methods of gadolinium sequestration and deposition remain poorly understood. Little is known about the levels of gadolinium required to induce tissue structural changes and to achieve clinical significance in humans. Recently, Christensen et al52 analyzed the skin of 13 patients with NSF and found significant differences in the amounts of gadolinium in affected-versus-nonaffected regions. Gadolinium was also present in unaffected skin. The authors also found elevated gadolinium concentration in the skin of 2 healthy individuals months after the GBCA exposure. These findings suggest that there may be a threshold level for gadolinium required for the development of disease.52
Regarding brain tissue deposition, Xia et al59 used scanning electron microscopy with energy dispersive x-ray spectroscopy to evaluate gadolinium deposition within brain tumor tissues that had blood-brain barrier disruption and found that gadolinium deposition occurred in patients without severe renal disease. Deposition of gadolinium in the cerebellum was also reported in a patient who developed NSF after several administrations of Omniscan.60 Gadolinium deposition in neural tissues in patients with intact blood-brain barrier and normal renal function was only recently established by McDonald et al,26 followed by Kanda et al.27 Postmortem brain specimens from the 2 studies showed no obvious gadolinium-mediated histologic changes26 or macroscopic changes27 in areas of gadolinium deposition.
Another intriguing finding is the nonuniform gadolinium deposition in neural structures. Among all sampled neuroanatomic locations (globi pallidi, thalami, DN, and pons), McDonald et al26 found that the DN contained the highest median concentrations of elemental gadolinium, followed by the globi pallidi. Confirming this finding, Kanda et al27 found that the DN and globi pallidi showed significantly higher gadolinium concentrations than the other evaluated brain regions (ie, cerebellar white matter, frontal lobe cortex, and frontal lobe white matter).
Similar MR imaging signal-intensity changes in the dentate and/or deep gray nuclei are seen in patients with multiple sclerosis, neurofibromatosis, hypoparathyroidism, manganism, inherited metabolic disorders, and Fahr disease, suggesting that these areas are particularly susceptible to metal deposition18,19,26,61; but these anatomic preferences remain poorly understood.
In bone and other tissues, gadolinium deposition can be explained, in part, by the presence of fenestrated capillary systems, in combination with the analogous nature of Gd and Ca. However, neural tissue deposition with an otherwise intact blood-brain barrier as reported by McDonald et al26 and Kanda et al27 is not clearly understood. Kanda et al27 found that gadolinium was prominently clustered in large foci within the endothelial wall but 18%–42% of gadolinium appeared to have crossed the blood-brain barrier and was deposited into the neural tissue interstitium.
It also remains unclear whether the gadolinium present in tissues, including neuronal tissues, is present in a chelated or unchelated state. Dissociated gadolinium often binds to phosphates or carbonates in vivo, but it may also bind to proteins or other macromolecules and may also be taken up by macrophages or similar immune cells. Phosphate- and carbonate-bound gadolinium is considered insoluble and thought to not generate increased T1 signal.62 Tissue gadolinium deposits have often been associated with the presence of calcium, phosphorus, and sometimes iron or zinc.58,63,64 It is likely that chelated, protein-bound, insoluble, and intramacrophage gadolinium may all be present in different proportions depending on the type of GBCA. Future studies are needed to investigate in detail the behavior of GBCA molecules taken up in brain areas associated with T1 high signal intensity.28
Clinical Significance of Gadolinium Deposition
The retention of gadolinium is important clinically. Gadolinium is not a naturally occurring biologic constituent, and once within the tissues of animals, it persists for long periods.54 Additionally, heavy metals are known to be toxic.
The risks associated with the administration of weaker chelate GBCAs to patients with severely impaired kidney function are well-documented, and NSF is the result. As described in this review, the published literature, most of which is recent, indicates that some gadolinium from each dose given may remain in the body of all patients regardless of their renal function. The long-term and cumulative effects of retained gadolinium are, at present, unknown in patients with normal renal function.
Preclinical safety studies performed on animals failed to reveal any neurologic effects of chelated gadolinium when given intravenously.60 There is, however, proof of gadolinium toxicity in the brain when administered by the intraventricular route in rats65 and also by the intravenous route after blood-brain barrier disruption.66
It is conceivable that patients may be adversely affected by retained gadolinium, especially in the brain. Despite being a difficult-to-prove cause-effect relationship, an MR imaging gadolinium-toxicity support group has been created. This group reported symptoms that they considered consistent with what is known about the toxic effects of gadolinium. In a recent survey performed in 17 patients, an association between chronic effects and GBCA exposure was suggested.67 Although no specific conclusions can be drawn from the survey, the results indicated that the symptoms appeared within 1 month after the last contrast-enhanced MR imaging and chronic pain was present in all 17 subjects (Table 2).
Table 2:
Summary of the results of the “Survey of Chronic Effects of Retained Gadolinium from Contrast MRIs”67,a
| Symptoms | Percentage (%)b |
|---|---|
| Pain: ache (dull, continuous pain), burning, numbness, tingling, or prickling sensations (paraesthesia), deep bone pain, and electric-like feelings | 100%c |
| Pain location: extremities (feet, legs, hands, arms), hips, joints, and ribs | |
| Muscle symptoms: twitching and weakness | 88% |
| Ocular symptoms: worsening vision, dry and bloodshot eyes | 76% |
| Dermal changes: discoloration, rash, skin lesions (ulcers, papules, macules, nodules, or other lesions), tight skin, thickened tissue | 71% |
| Cognitive symptoms: brain fog, difficulty concentrating | 65% |
| ENT symptoms: ringing in ears, swallowing and voice problems | 65% |
| Low body temperature, hair loss, and itchy skin | 59% |
| Balance problems | 53% |
| Swelling of extremities | 53% |
Note:—ENT indicates ear, nose, and throat.
Data obtained directly from the survey.
Percentage of patients who reported the symptoms.
Highest priority chronic symptom in 59%.
We recommend future investigations to evaluate a possible relation between gadolinium retention and clinical symptoms in subjects with normal renal function.
Conclusions
All GBCAs probably deposit in vivo in humans to some degree. At present, it is unclear why only the weaker chelates appear to result in meaningful clinical disease such as NSF, despite the fact that more stable GBCAs also show deposition. This presumably reflects the concentration of gadolinium deposited in tissues, though it is likely that the molecular state of the administered and deposited gadolinium strongly influences both deposition and clinical manifestations.
Recent literature confirms that gadolinium deposition occurs in the human brain after multiple gadolinium contrast administrations, despite an intact blood-brain barrier and normal renal function. On MR imaging, this accumulation is seen as increased signal intensity within the DN and globi pallidi on T1-weighted images. Gadolinium-associated findings gleaned from in vitro, animal, and human studies suggest that the greatest deposition and most deleterious effects are associated with GBCAs with the lowest stability. The ultimate significance of this deposition in subjects with normal renal function, in their brain and elsewhere, remains to be determined. Careful evaluation, especially in children, is recommended when administering GBCAs.
ABBREVIATIONS:
- DN
dentate nuclei
- GBCA
gadolinium-based contrast agent
- NSF
nephrogenic systemic fibrosis
REFERENCES
- 1. Hao D, Ai T, Goerner F, et al. MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 2012;36:1060–71 10.1002/jmri.23725 [DOI] [PubMed] [Google Scholar]
- 2. Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol 2008;191:W307–11 10.2214/AJR.07.3951 [DOI] [PubMed] [Google Scholar]
- 3. Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 2015;275:630–34 10.1148/radiol.2015150805 [DOI] [PubMed] [Google Scholar]
- 4. Ersoy H, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 2007;26:1190–97 10.1002/jmri.21135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging 2007;25:884–99 10.1002/jmri.20955 [DOI] [PubMed] [Google Scholar]
- 6. Gierada DS, Bae KT. Gadolinium as a CT contrast agent: assessment in a porcine model. Radiology 1999;210:829–34 10.1148/radiology.210.3.r99mr06829 [DOI] [PubMed] [Google Scholar]
- 7. Quinn AD, O'Hare NJ, Wallis FJ, et al. Gd-DTPA: an alternative contrast medium for CT. J Comput Assist Tomogr 1994;18:634–36 10.1097/00004728-199407000-00022 [DOI] [PubMed] [Google Scholar]
- 8. Henson JW, Nogueira RG, Covarrubias DJ, et al. Gadolinium-enhanced CT angiography of the circle of Willis and neck. AJNR Am J Neuroradiol 2004;25:969–72 [PMC free article] [PubMed] [Google Scholar]
- 9. Bonvento MJ, Moore WH, Button TM, et al. CT angiography with gadolinium-based contrast media. Acad Radiol 2006;13:979–85 10.1016/j.acra.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 10. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2005;21:1104–08 10.1093/ndt/gfk062 [DOI] [PubMed] [Google Scholar]
- 11. Marckmann P. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17:2359–62 10.1681/ASN.2006060601 [DOI] [PubMed] [Google Scholar]
- 12. Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am 2009;47:827–31, vi 10.1016/j.rcl.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 13. Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol 2008;66:230–34 10.1016/j.ejrad.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 14. Abujudeh HH, Kaewlai R, Kagan A, et al. Nephrogenic systemic fibrosis after gadopentetate dimeglumine exposure: case series of 36 patients. Radiology 2009;253:81–89 10.1148/radiol.2531082160 [DOI] [PubMed] [Google Scholar]
- 15. Wertman R, Altun E, Martin DR, et al. Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four American universities. Radiology 2008;248:799–806 10.1148/radiol.2483072093 [DOI] [PubMed] [Google Scholar]
- 16. Hope TA, Herfkens RJ, Denianke KS, et al. Nephrogenic systemic fibrosis in patients with chronic kidney disease who received gadopentetate dimeglumine. Invest Radiol 2009;44:135–39 10.1097/RLI.0b013e31819343ba [DOI] [PubMed] [Google Scholar]
- 17. Fretellier N, Idée JM, Guerret S, et al. Clinical, biological, and skin histopathologic effects of ionic macrocyclic and nonionic linear gadolinium chelates in a rat model of nephrogenic systemic fibrosis. Invest Radiol 2011;46:85–93 10.1097/RLI.0b013e3181f54044 [DOI] [PubMed] [Google Scholar]
- 18. Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 19. Errante Y, Cirimele V, Mallio CA, et al. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014;49:685–90 10.1097/RLI.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 20. Quattrocchi CC, Mallio CA, Errante Y, et al. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 2015;50:470–72 10.1097/RLI.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 21. Ramalho J, Castillo M, AlObaidy M, et al. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 2015;276:836–44 10.1148/radiol.2015150872 [DOI] [PubMed] [Google Scholar]
- 22. Kanda T, Osawa M, Oba H, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015;275:803–09 10.1148/radiol.14140364 [DOI] [PubMed] [Google Scholar]
- 23. Roccatagliata L, Vuolo L, Bonzano L, et al. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology 2009;251:503–10 10.1148/radiol.2511081269 [DOI] [PubMed] [Google Scholar]
- 24. Kasahara S, Miki Y, Kanagaki M, et al. Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology 2011;258:222–28 10.1148/radiol.10100508 [DOI] [PubMed] [Google Scholar]
- 25. Adin ME, Kleinberg L, Vaidya D, et al. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 2015;36:1859–65 10.3174/ajnr.A4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–82 10.1148/radiol.15150025 [DOI] [PubMed] [Google Scholar]
- 27. Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015;276:228–32 10.1148/radiol.2015142690 [DOI] [PubMed] [Google Scholar]
- 28. Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 2015;50:473–80 10.1097/RLI.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015;275:783–91 10.1148/radiol.2015150337 [DOI] [PubMed] [Google Scholar]
- 30. Weberling LD, Kieslich PJ, Kickingereder P, et al. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 2015:50:743–48 10.1097/RLI.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 31. Stojanov DA, Aracki-Trenkic A, Vojinovic S, et al. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 2015. June 25. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32. de Campos RO, Herédia V, Ramalho M, et al. Application of gadolinium based contrast agents in abdominal magnetic resonance imaging: important considerations. In: Thompson CC, ed. Gadolinium: Compounds, Production and Applications. New York: Nova Science Publishers; 2009:chap 5 [Google Scholar]
- 33. Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol 2008;43:817–28 10.1097/RLI.0b013e3181852171 [DOI] [PubMed] [Google Scholar]
- 34. Idée JM, Port M, Robic C, et al. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 2009;30:1249–58 10.1002/jmri.21967 [DOI] [PubMed] [Google Scholar]
- 35. Prince MR, Erel HE, Lent RW, et al. Gadodiamide administration causes spurious hypocalcemia. Radiology 2003;227:639–46 10.1148/radiol.2273012007 [DOI] [PubMed] [Google Scholar]
- 36. Laurent S, Elst LV, Copoix F, et al. Stability of MRI paramagnetic contrast media: a proton relaxometric protocol for transmetallation assessment. Invest Radiol 2001;36:115–22 10.1097/00004424-200102000-00008 [DOI] [PubMed] [Google Scholar]
- 37. Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol 1995;30:372–80 10.1097/00004424-199506000-00008 [DOI] [PubMed] [Google Scholar]
- 38. Tweedle MF, Hagan JJ, Kumar K, et al. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging 1991;9:409–15 10.1016/0730-725X(91)90429-P [DOI] [PubMed] [Google Scholar]
- 39. Corot C, Idée JM, Hentsch AM, et al. Structure-activity relationship of macrocyclic and linear gadolinium chelates: investigation of transmetallation effect on the zinc-dependent metallopeptidase angiotensin-converting enzyme. J Magn Reson Imaging 1998;8:695–702 10.1002/jmri.1880080328 [DOI] [PubMed] [Google Scholar]
- 40. Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 2009;30:1259–67 10.1002/jmri.21969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Altum E, Semelka RC, Cakit C. Nephrogenic systemic fibrosis and management of high-risk patients. Acad Radiol 2009;16:897–905 10.1016/j.acra.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 42. Quarles LD, Hartle JE 2nd, Middleton JP, et al. Aluminum-induced DNA synthesis in osteoblasts: mediation by a G-protein coupled cation sensing mechanism. J Cell Biochem 1994;56:106–17 10.1002/jcb.240560115 [DOI] [PubMed] [Google Scholar]
- 43. Pałasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol 2000;47:1107–14 [PubMed] [Google Scholar]
- 44. Feng X, Xia Q, Yuan L, et al. Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity. Neurotoxicology 2010;31:391–98 10.1016/j.neuro.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 45. Xia Q, Feng X, Huang H, et al. Gadolinium-induced oxidative stress triggers endoplasmic reticulum stress in rat cortical neurons. J Neurochem 2011;117:38–47 10.1111/j.1471-4159.2010.07162.x [DOI] [PubMed] [Google Scholar]
- 46. Spencer AJ, Wilson SA, Batchelor J, et al. Gadolinium chloride toxicity in the rat. Toxicol Pathol 1997;25:245–55 10.1177/019262339702500301 [DOI] [PubMed] [Google Scholar]
- 47. Idée JM, Port M, Medina C, et al. Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: a critical review. Toxicology 2008;248:77–88 10.1016/j.tox.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 48. Piera-Velazquez S, Louneva N, Fertala J, et al. Persistent activation of dermal fibroblasts from patients with gadolinium-associated nephrogenic systemic fibrosis. Ann Rheum Dis 2010;69:2017–23 10.1136/ard.2009.127761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhagavathula N, Dame MK, DaSilva M, et al. Fibroblast response to gadolinium: role for platelet-derived growth factor receptor. Invest Radiol 2010;45:769–77 10.1097/RLI.0b013e3181e943d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edward M, Quinn JA, Burden AD, et al. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology 2010;256:735–43 10.1148/radiol.10091131 [DOI] [PubMed] [Google Scholar]
- 51. Varani J, DaSilva M, Warner RL, et al. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol 2009;44:74–81 10.1097/RLI.0b013e31818f76b5 [DOI] [PubMed] [Google Scholar]
- 52. Christensen KN, Lee CU, Hanley MM, et al. Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Dermatol 2011;64:91–96 10.1016/j.jaad.2009.12.044 [DOI] [PubMed] [Google Scholar]
- 53. Cowper SE, Bucala R, Leboit PE. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: setting the record straight. Semin Arthritis Rheum 2006;35:208–10 10.1016/j.semarthrit.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 54. Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol 2004;39:138–42 10.1097/01.rli.0000112789.57341.01 [DOI] [PubMed] [Google Scholar]
- 55. White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol 2006;41:272–78 10.1097/01.rli.0000186569.32408.95 [DOI] [PubMed] [Google Scholar]
- 56. Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, et al. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 2009;1:479–88 10.1039/b905145g [DOI] [PubMed] [Google Scholar]
- 57. Rocklage SM, Worah D, Kim SH. Metal ion release from paramagnetic chelates: what is tolerable? Magn Reson Med 1991;22:216–21; discussion 229–32 10.1002/mrm.1910220211 [DOI] [PubMed] [Google Scholar]
- 58. Abraham JL, Thakral C, Skov L, et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol 2008;158:273–80 [DOI] [PubMed] [Google Scholar]
- 59. Xia D, Davis RL, Crawford JA, et al. Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 2010;51:1126–36 10.3109/02841851.2010.515614 [DOI] [PubMed] [Google Scholar]
- 60. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis: an autopsy-based review. Nephrol Dial Transplant 2011;26:3616–26 10.1093/ndt/gfr085 [DOI] [PubMed] [Google Scholar]
- 61. Ogi S, Fukumitsu N, Tsuchida D, et al. Imaging of bilateral striopallidodentate calcinosis. Clin Nucl Med 2002;27:721–24 10.1097/00003072-200210000-00008 [DOI] [PubMed] [Google Scholar]
- 62. Fretellier N, Idée JM, Dencausse A, et al. Comparative in vivo dissociation of gadolinium chelates in renally impaired rats: a relaxometry study. Invest Radiol 2011;46:292–300 10.1097/RLI.0b013e3182056ccf [DOI] [PubMed] [Google Scholar]
- 63. Schroeder JA, Weingart C, Coras B, et al. Ultrastructural evidence of dermal gadolinium deposits in a patient with nephrogenic systemic fibrosis and end-stage renal disease. Clin J Am Soc Nephrol 2008;3:968–75 10.2215/CJN.00100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thakral C, Abraham JL. Gadolinium-induced nephrogenic systemic fibrosis is associated with insoluble Gd deposits in tissues: in vivo transmetallation confirmed by microanalysis. J Cutan Pathol 2009;36:1244–54 10.1111/j.1600-0560.2009.01283.x [DOI] [PubMed] [Google Scholar]
- 65. Ray DE, Holton JL, Nolan CC, et al. Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. AJNR Am J Neuroradiol 1998;19:1455–62 [PMC free article] [PubMed] [Google Scholar]
- 66. Roman-Goldstein SM, Barnett PA, McCormick CI, et al. Effects of gadopentetate dimeglumine administration after osmotic blood-brain barrier disruption: toxicity and MR imaging findings. AJNR Am J Neuroradiol 1991;12:885–90 [PMC free article] [PubMed] [Google Scholar]
- 67. Gadolinium Toxicity: A Survey of the Chronic Effects of Retained Gadolinium from Contrast MRIs. https://gdtoxicity.files.wordpress.com/2014/09/gd-symptom-survey.pdf. Accessed September 1, 2015.