Abstract
BACKGROUND AND PURPOSE:
The selection of patients for endovascular therapy is an important issue in stroke imaging. The aim of this study was to determine the predictive value of 3 different dynamic CT angiography parameters, occlusion length, collateralization extent, and time delay to maximum enhancement, for latest generation of stent retriever thrombectomy recanalization outcomes in patients with acute ischemic stroke.
MATERIALS AND METHODS:
In this study, subjects were selected from an initial cohort of 2059 consecutive patients who had undergone multiparametric CT, including whole-brain CT perfusion. We included all patients with a complete occlusion of the M1 segment of the MCA or the carotid T and subsequent intra-arterial stent retriever thrombectomy. Dynamic CT angiography was reconstructed from whole-brain CT perfusion raw datasets. Angiographic outcome was scored by using the modified TICI scale; and clinical outcome, by using the modified Rankin Scale. Logistic regression analyses were performed to determine independent predictors of a favorable angiographic (mTICI = 3) and clinical outcome (mRS ≤2).
RESULTS:
Sixty-nine patients (mean age, 68 ± 14 years; 46% men) were included for statistical analysis. In the regression analysis, a short occlusion length was an independent predictor of favorable angiographic outcome (OR, 0.41; P < .05). Both collateralization grade (OR, 1.00; P > .05) and time delay to peak enhancement (OR, 0.90; P > .05) failed to predict a favorable angiographic outcome. None of the dynamic CT angiography predictors were significantly associated with clinical outcome on discharge (OR, 0.664–1.011; P = .330–.953) or at 90 days (OR, 0.779–1.016; P = .130–.845).
CONCLUSIONS:
A short occlusion length as determined by dynamic CT angiography is an independent predictor of a favorable angiographic outcome of stent retriever thrombectomy in patients with ischemic stroke.
Endovascular treatment in patients with acute stroke is known to be effective for emergency vascularization. Recently published data of randomized clinical trials focusing on the latest generation stent retriever devices (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands [MR CLEAN],1 Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke [ESCAPE],2 Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment [SWIFT-PRIME],3 and Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial [EXTEND-IA]4) have demonstrated a benefit of intra-arterial treatment over intravenous thrombolysis with respect to reperfusion, functional outcome, and mortality.
While the stent retriever trial results are an important milestone in the therapy of ischemic stroke, patient selection for this treatment option should be an important focus for future studies. To date, the decision to perform endovascular treatment is primarily based on the presence or absence of the demarcation of infarction on nonenhanced CT and the site of the vessel occlusion. In addition, a recent study suggests that more robust leptomeningeal collaterals as assessed by conventional angiography are associated with better recanalization results.5 However, invasive catheter-based angiography is not available as a screening technique for all patients with stroke.
Occlusion length is another potential predictor of endovascular therapy success because it has been shown to predict intravenous thrombolysis (IVT) outcome.6,7 Animal model studies have shown that clots with a length of >10 mm are associated with decreased success of endovascular procedures and increased rates of complications such as distal embolization during mechanical thrombectomy.8 In contrast, previous studies using nonenhanced CT and single-phase CTA have failed to find an association between occlusion length and the success of mechanical recanalization.9–11 Clarification of these conflicting results is important because endovascular recanalization procedures are especially considered as a treatment option in patients with large-vessel occlusions in whom IVT would be expected to have a low success rate.
Dynamic CTA is a promising new technique that has been shown to noninvasively predict the success of IVT.12 Dynamic CTA images can be reconstructed from time-resolved CT perfusion data and provide a broad temporal coverage from nonenhanced through arterial-to-venous phases,13 thus allowing a noninvasive assessment of the extent of leptomeningeal collateralization.12,14,15 Moreover, dynamic CTA is more sensitive to delayed contrast arrival and therefore more closely defines thrombus burden than single-phase CTA.16 It has been shown that the time delay of maximum enhancement predicts the final infarction size.17 These advantages of dynamic CTA qualify the technique as a candidate for the prediction of vascular recanalization, which is a major prerequisite for a favorable clinical outcome and reduced mortality in acute ischemic stroke.18
The aim of this study was to determine the predictive value of 3 different dynamic CTA parameters, occlusion length, collateralization extent, and time delay to maximum enhancement, for the latest generation stent retriever thrombectomy success in patients with acute ischemic stroke.
Materials and Methods
Study Design and Population
Institutional review board approval was obtained for this retrospective study, and the requirement for informed consent was waived. Our initial cohort consisted of 2059 consecutive patients who had undergone whole-brain CT perfusion (WB-CTP) for suspected stroke between April 2009 and June 2014.
Of this cohort, we included all subjects with the following:
Complete occlusion of the middle cerebral artery in the M1 segment or carotid T occlusion as demonstrated by single-phase CT angiography (spCTA)
Subsequent intra-arterial stent retriever recanalization therapy.
We excluded patients with the following:
Incomplete WB-CTP raw datasets
Nondiagnostic quality of dynamic CTA
Failure of visualization of thrombus length in dynamic CTA
Cancelled stent retriever thrombectomy.
CT Examination Protocol and Image Processing
All patients underwent a standardized multiparametric CT protocol. It consisted of nonenhanced CT, spCTA, and WB-CTP. All CT examinations were performed by using 1 of the following 3 CT scanners: Somatom Definition Flash, a 128-section dual-source CT scanner, and Somatom Definition Edge and Somatom Definition AS+, both 128-section CT scanners (all by Siemens, Erlangen, Germany).
WB-CTP was performed with 0.6-mm collimation and 100-mm scan coverage in the z-axis by using an adaptive spiral scanning technique. The datasets were acquired continuously for 48 seconds (32 cycles, 1 sweep every 1.5 seconds). Tube voltage and current were 80 kV and 200 mAs, respectively. The CT dose index was 276 mGy. A total of 35 mL of iodinated contrast agent (400 mg/mL) was administered at a flow rate of 5 mL/s, followed by a saline flush of 40 mL at 5 mL/s.
CT perfusion raw datasets were reconstructed as dynamic angiographies by using the syngo.CT Dynamic angio module (Syngo.via VA 20; Siemens). The image processing included motion correction and automated bone removal. Computation of temporal MIPs included a 4D noise reduction.19
Defining the Predictors: Dynamic CTA Image Analysis
Dynamic CTA information was used to define 3 predictors: length of occlusion, collateralization grade, and collateral blood flow time delay. All measurements were performed by a reader (S.E.B.) with >2 years of experience in stroke imaging. For quantitative assessment of the collateralization status and in unclear cases, a board-certified neuroradiologist (H.J.) with >12 years of experience in neuroimaging was consulted. Both readers were blinded to clinical data and spCTA.
Length of Occlusion
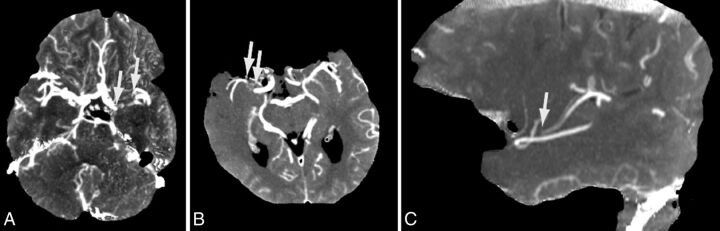
The length of the filling defect (length of occlusion) was measured on temporal maximum intensity projections that combined all scans of the WB-CTP examination through temporal fusion (temporal MIP).19 Temporal MIP images depict the maximum enhancement during the entire scan time for every voxel, thereby fusing contrast opacification from early arterial-to-late venous phases of the WB-CTP examination into 1 CT dataset. The length of the filling defect within the occluded artery was measured by connecting straight lines in axial, sagittal, or coronal planes in all cases in which the reader could define a proximal and distal end of the filling defect (Fig 1).16 We then approximated the occlusion length, taking into account the vessel course over >1 section by using the Pythagorean theorem. If the distal end of the filling defect extended to >1 M2 segment, the average of the filling defects of the single M2 branches was calculated and used as a predictor.
Fig 1.
Occlusion-length measurement. Temporal MIP images show the occluding thrombus as a filling defect. A, Axial temporal MIP image of a left-sided carotid T occlusion. B and C, Axial and sagittal images of a right-sided M1 occlusion.
Collateralization Grade
Leptomeningeal collateral vessels of the affected hemisphere were graded by using a score described by Menon et al12 and were used in different dynamic CTA studies.15 Briefly, this approach uses a 20-point score that is obtained by summing up 10 single scores for 10 MCA territory regions that were defined by the Alberta Stroke Program Early CT Score.20 For each of the 10 regions, the collateral vessels of the affected hemisphere are compared with those on the contralateral hemisphere and are assigned a score from 0 to 2: 0, no collateral filling; 1, less than the contralateral hemisphere; 2, equal or greater than the contralateral hemisphere.
Collateral Blood Flow Time Delay
The time delay distal to the occlusion was measured as described previously by Beyer et al.17 Briefly, the time delay was defined as the mean difference between time-to-peak contrast enhancement of the M2 segment distal to the occlusion and the corresponding M2 segment of the contralateral side. We used the delay of enhancement of the M2 segment as a surrogate marker for the collateral blood flow. The time-attenuation curves of all ROIs were interpolated by using cubic spline interpolation to improve temporal resolution as previously practiced with CT perfusion time-attenuation curves.21 Delay was defined as the difference of the mean of all M2 TTP measurements and the averaged TTP measurements of the contralateral M2 segment.
Endovascular Recanalization Procedure
Endovascular recanalization procedures were performed either with the patient under general anesthesia or, whenever deemed appropriate by the interventional neuroradiologist and the anesthesiologist, with the patient under conscious sedation. All procedures were performed in a triaxial fashion by using a distal-access catheter and a microcatheter to deploy a stent retriever device. All stent retrievers used in this cohort were the latest generation devices (Solitaire; Covidien, Irvine, California; pREset; phenox, Bochum, Germany; Trevo; Stryker, Kalamazoo, Michigan). Because these devices are almost identically constructed, we did not include the device type as a separate predictor in our statistical analysis. After affirmation of mainstem recanalization, we removed the catheter material.
Evaluation of Angiographic Data to Determine Technical Therapy Success
Two readers (1 board-certified neuroradiologist with >12 years [H.J.] and 1 radiologist [K.M.T.] with >3 years of experience in stroke imaging) independently evaluated the angiographic images with respect to recanalization and reperfusion. In case of disagreement, a consensus was reached in a separate session. Reperfusion of the corresponding arterial territory was scored by using the modified Thrombolysis in Cerebral Infarction (mTICI) scale,22 by using 50% as the threshold for achieving reperfusion grade 2b or higher: 0, no perfusion; 1, minimal flow past the occlusion with little to no perfusion; 2a, antegrade partial perfusion of <50% of the downstream ischemic territory; 2b, antegrade partial perfusion of ≥50% of the downstream ischemic territory; and 3, antegrade complete perfusion of the downstream ischemic territory. An mTICI of 3 was defined as a favorable recanalization outcome.
Statistical Analysis
All statistical analyses were performed with SPSS Statistics 20 (IBM, Armonk, New York). Normal distribution was evaluated by using the Kolmogorov-Smirnov test. In case of normal distribution, we used a 2-tailed Student t test. In case of non-normal distribution, a Wilcoxon test was performed.
Univariate logistic regression analysis was used to test the association between predictors and outcome variable (angiographic and clinical outcome). Predictors were the following: age, sex, additional internal carotid artery occlusion, additional IVT, time from symptom onset to initial imaging, length of occlusion, collateral blood flow delay, and morphologic collateral extent. Variables significantly associated with a favorable angiographic/clinical outcome (P < .2) in the univariate analysis were included in multivariate models.
All metric and normally distributed variables are reported as mean ± SD; non-normally distributed variables are presented as medians (interquartile range). Categoric variables are presented as frequency and percentage. P values below .05 indicate statistical significance.
Results
Study Population
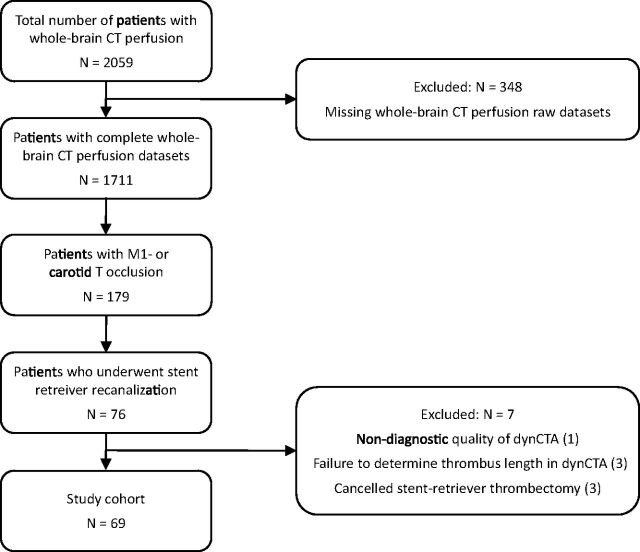
From our initial cohort of 2059 patients, 348 patients were excluded due to missing WB-CTP raw datasets. Among the remaining 1711 patients, 179 were found to have an M1 or carotid T occlusion on spCTA. In 76 of these patients, stent retriever recanalization was performed. From these patients, we excluded 3 patients in whom stent retriever thrombectomy was eventually not performed (no evidence of occlusion in the diagnostic angiographic series [1 patient], impassable proximal ICA occlusion [2 patients]). Furthermore, 1 patient was excluded due to nondiagnostic quality of dynamic CTA; and 3 patients, due to failure to determine the distal thrombus end in dynamic CTA. The remaining 69 patients formed the final study cohort. The selection process of the patients is illustrated in Fig 2.
Fig 2.
Flow chart of patient selection. dynCTA indicates dynamic CT angiography.
Patient Characteristics
The mean age of the 69 included patients was 68.4 ± 14.4 years (range, 28–97 years). Thirty-two (46%) patients were male. Fifty patients had an occlusion of the MCA, and 19 had an occlusion of the carotid T. Fifty-four (78%) were treated with IVT before stent retriever thrombectomy. The mean time from symptom onset to CT was 161 ± 88 minutes (n = 46). The mean time from CT to recanalization was 119 ± 82 minutes (n = 69).
On admission, the mean National Institutes of Health Stroke Scale score was 15.6 ± 4.7 (n = 57). Baseline NIHSS was significantly associated with better angiographic outcome (OR, 0.809; P = .005), a better clinical outcome on discharge (OR, 0.787; P = .008), and a better clinical outcome at 90 days (OR, 0.774; P = .015). The mean baseline Glasgow Coma Scale score on admission was 12.9 ± 2.4 (n = 69).
With respect to angiographic outcome, mTICI scores after recanalization were as follows: mTICI = 0, 5 patients; mTICI = 1, 3 patients; mTICI = 2a, 6 patients; mTICI = 2b, 24 patients; mTICI = 3, 31 patients. The results of univariate logistic regression analyses for mTICI scores after recanalization are shown in Table 1.
Table 1:
Characteristics of patients with favorable (mTICI = 3) and nonfavorable (mTICI < 3) recanalization outcomea
| Overall (N = 69) | mTICI = 3 (n = 31) | mTICI < 3 (n = 38) | Odds Ratio | P Value | |
|---|---|---|---|---|---|
| Age (yr) | 68.4 ± 14.4 | 66.7 ± 14.1 | 69.8 ± 14.5 | 1.000 | .981 |
| Male sex (No.) | 32 (46%) | 15 (48%) | 17 (45%) | 1.152 | .775 |
| Additional ICA occlusion (No.) | 22 (32%) | 9 (29%) | 13 (34%) | 0.708 | .485 |
| Carotid T occlusion (No.) | 19 (28%) | 5 (16%) | 14 (37%) | 1.768 | .274 |
| Time to recanalization (min) | 119 ± 82 | 108 ± 69 | 127 ± 92 | 0.859 | .389 |
| IVT (No.) | 54 (78%) | 24 (77%) | 30 (79%) | 1.094 | .878 |
| Occlusion length (mm) | 15.6 ± 7.9 | 12.9 ± 6.8 | 17.7 ± 8.1 | 0.409 | .018b |
| Collateralization grade (0–20) | 13.8 ± 4.3 | 13.7 ± 4.5 | 13.7 ± 4.1 | 1.003 | .958 |
| Time delay (sec) | 6.5 ± 2.8 | 6.2 ± 2.1 | 6.7 ± 3.2 | 0.896 | .251 |
The last 2 columns show the results of the univariate logistic regression analysis to determine the effect of predictors on recanalization success (N = 69).
Statistically significant.
Regarding clinical patient outcome, modified Rankin Scale scores on discharge were as follows: mRS = 0, 6 patients; mRS = 1, 4 patients; mRS = 2, 7 patients; mRS = 3, 7 patients; mRS = 4, 10 patients; mRS = 5, 27 patients; mRS = 6, 8 patients. The mean mRS score on discharge was 3.8 ± 1.7. The results of univariate logistic regression analyses for the modified Rankin Scale score on discharge are presented in Table 2.
Table 2:
Characteristics of patients with favorable (mRSdisc ≤ 2) and nonfavorable (mRSdisc > 2) clinical outcomea
| Overall (N = 69) | mRSdisc ≤ 2 (n = 17) | mRSdisc > 2 (n = 52) | Odds Ratio | P Value | |
|---|---|---|---|---|---|
| Age (yr) | 68.4 ± 14.4 | 65.4 ± 16.5 | 69.4 ± 13.8 | 0.982 | .380 |
| Male sex (No.) | 32 (46%) | 7 (41%) | 25 (48%) | 0.877 | .839 |
| Additional ICA occlusion (No.) | 22 (32%) | 4 (23%) | 18 (35%) | 0.960 | .949 |
| Carotid T occlusion (No.) | 19 (28%) | 6 (35%) | 13 (25%) | 1.023 | .974 |
| Time to recanalization (min) | 119 ± 82 | 101 ± 77 | 126 ± 86 | 0.694 | .181b |
| IVT (No.) | 54 (78%) | 15 (88%) | 39 (75%) | 0.202 | .142b |
| Occlusion length (mm) | 15.6 ± 7.9 | 13.6 ± 7.4 | 16.1 ± 8.2 | 0.664 | .330 |
| Collateralization grade (0–20) | 13.8 ± 4.3 | 13.9 ± 4.5 | 13.8 ± 4.2 | 0.996 | .953 |
| Time delay (sec) | 6.5 ± 2.8 | 6.7 ± 3.2 | 6.4 ± 2.8 | 1.011 | .917 |
Note:—mRSdisc indicates mRS at discharge.
The last 2 columns show the results of the univariate logistic regression analysis to determine the effect of predictors on clinical outcome (N = 69).
Below < .2 and therefore included in multivariate analysis.
mRS after 90 days (mRS90) was available in only 42 patients (61%). Scores of mRS after 90 days in this subgroup were the following: mRS = 0, 4 patients; mRS = 1, 9 patients; mRS = 2, 2 patients; mRS = 3, 6 patients; mRS = 4, 10 patients; mRS = 5, 2 patients; mRS = 6, 9 patients. The mean mRS score after 90 days was 3.1 ± 2.1.
There were 5 cases of postinterventional hemorrhage, all of which were not space-occupying. The mean values for the dynamic CTA predictors in the cases of hemorrhage were 14.7 ± 5.0 mm for occlusion length, 6.6 ± 2.6 for collateralization grade, and 8.6 ± 2.5 seconds for time delay. With respect to angiographic outcome, 2 of these cases were mTICI 3, and 3 were mTICI 2b. Four of these cases had a nonfavorable functional outcome after 90 days (mRS after 90 days of >2). In 1 case with hemorrhage, the mRS score after 90 days was unknown (mRS score on discharge was favorable in this case).
Association of Predictors with Thrombectomy Outcome
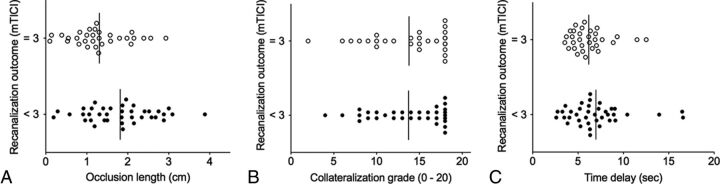
In the univariate analysis for angiographic outcome, occlusion length was significantly associated with recanalization outcome: The mean occlusion length of patients with an mTICI score of 3 (n = 31, 12.9 ± 6.8 mm) was significantly shorter than the mean occlusion length in patients with an mTICI score of <3 (n = 38, 17.7 ± 8.1 mm; OR, 0.41, P > .05). Both collateralization grade (OR, 1.00; P > .05) and time delay to peak enhancement (OR, 0.90; P > .05) failed to predict a favorable angiographic outcome (Table 1). The correlation between dynamic CTA predictors and favorable angiographic outcome is shown in Fig 3. Age, sex, the presence of an additional ICA occlusion, and additional IV thrombolysis did not show a significant correlation with thrombectomy outcome. Because occlusion length was the only predictor to show an association with angiographic thrombectomy outcome, multivariate analysis was not performed.
Fig 3.
Correlation between dynamic CTA predictors and angiographic outcome. Shorter occlusion length (A) is associated with favorable technical thrombectomy outcome. Both collateralization grade (B) and time delay (C) do not show a significant correlation with angiographic thrombectomy outcome.
In the univariate analyses for clinical outcome on discharge and after 90 days, none of the predictors reached statistical significance. The results for the mRS score on discharge, which was available in all patients, are presented in Table 2. Time to recanalization (P = .181) and IV thrombolysis (P = .142) were below the P threshold of .2 and were included in a multivariate analysis. In this analysis, neither of these 2 predictors reached statistical significance (P = .143 and P = .108, respectively).
mRS after 90 days was available in only 42 (61%) patients. In a separate univariate analysis of these 42 patients, none of the predictors were significantly associated with mRS at 90 days. Odds ratios and P values for the 3 imaging parameters were as follows: occlusion length: OR, 0.918, P = .845; collateralization grade: OR, 1.016, P = .833; time delay: OR, 0.779, P = .130.
Discussion
The present study investigated the predictive value of time-resolved dynamic CT angiography on endovascular treatment success. In patients with acute large-vessel occlusion who underwent stent retriever thrombectomy, our study demonstrated a shorter thrombus length as assessed by dynamic CTA associated with a better recanalization success, while collateralization grade and time delay of maximum enhancement distal to the occlusion failed to predict angiographic thrombectomy outcome. None of the imaging parameters were significantly associated with a better clinical outcome.
Occlusion length plays an important role in intravenous thrombolysis and may therefore be considered a potential predictor of endovascular recanalization success.23 Our observed association between occlusion length and recanalization success is in accordance with animal model studies that have shown longer occlusions to be associated with a lower success rate of endovascular procedures and higher rates of complications.8
Previous studies in humans, however, have failed to find an association between thrombus length and success of mechanical recanalization.9,11,24 These studies used thin-section nonenhanced CT or spCTA for clot depiction. It has been shown that the nonenhanced CT appearance of clot depends on the thrombus composition, suggesting that some (ie, low-density) portions of clot could be less well-discerned with this technique.25,26 In addition, nonenhanced CT is more challenging when vascular calcifications or a high hematocrit cause intra-arterial hyperdensities. On spCTA, visibility of thrombus extent depends on the strength of collateral flow, particularly with state-of-the-art fast-acquisition protocols.27 Dynamic CTA, on the other hand, is more sensitive to delayed contrast arrival and therefore more closely defines thrombus burden than spCTA.16 It might be reasonably assumed that dynamic CTA is the superior method for clot-length measurement, indicating that, in fact, there is an association between clot length and technical recanalization success.
In contrast to occlusion length, the grading of collateralization did not predict a favorable angiographic outcome of stent retriever recanalization. This finding is not consistent with recently published results of the Interventional Management of Stroke (IMS) III Trial.5 This trial showed an association between a more robust collateral grade and both recanalization of the occluded arterial segment and downstream reperfusion. Unlike our study, the IMS III trial used catheter-based angiography to determine the collateralization grade. Possible reasons for the discordant findings could be the imaging technique itself, the method of assessment of collateralization, and the different patient samples.
With respect to thrombectomy recanalization success, an mTICI score of 3, antegrade complete perfusion of the downstream ischemic territory, is, by nature, the most desirable goal for endovascular therapy. Therefore, unlike previous studies that mostly defined a favorable recanalization outcome as an mTICI score of 2b or 3, we restricted the group of favorable outcomes to those with an mTICI score of 3. This approach, at the same time, avoids the problem of group 2b (antegrade partial perfusion of ≥50% of the downstream ischemic territory) being quite heterogeneous with respect to functional outcome (depending on the location of the remaining thrombus material).
Clinical outcome, as opposed to angiographic outcome, was not significantly associated with occlusion length or any of the other imaging predictors. The only outcome parameter that was available for all patients in this study was mRS on discharge. The more meaningful outcome, mRS at 90 days, was only available in about 60% of the patients. However, these results should be taken seriously because the actual goal of all reperfusion therapies remains a better clinical outcome for the patients. Nevertheless, as it has already been shown that vascular recanalization is a major prerequisite for a favorable clinical outcome and reduced mortality in acute ischemic stroke,18 it will remain the first interim goal of endovascular therapy.
There are limitations to our study, which need to be taken into account when interpreting the data. First, the clinical outcome after 90 days was not available in all patients. Second, this is a single-center study with a limited number of patients. In view of the small sample size, some of the nonsignificant findings in this study such as the presence of carotid T occlusion might actually be important predictors of recanalization outcome. Therefore, further studies with larger patient cohorts will need to be conducted, which would also enable a finer graduation of recanalization outcome.
Conclusions
A shorter occlusion length as determined by dynamic CT angiography is an independent predictor of a favorable outcome of stent retriever thrombectomy in patients with acute ischemic stroke. Given that future studies show a correlation with clinical outcomes as well, dynamic CT angiography may be considered for patient selection.
ABBREVIATIONS:
- IVT
intravenous thrombolysis
- mTICI
modified Thrombolysis in Cerebral Infarction scale
- spCTA
single-phase CT angiography
- WB-CTP
whole-brain CT perfusion
Footnotes
Disclosures: Felix G. Meinel—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): b.e.imaging (speaker's honoraria). Maximilian F. Reiser—UNRELATED: German Research Society (Deutsche Forschungsgemeinschaft) (November 2006–2016, Principal Investigator, Munich Center of Advanced Photonics, Ludwig-Maximilians-University of Munich-Technical University of Munich, Germany) “Cluster of Excellence”*; Euro-BioImaging (December 2010–2016, Principal Site Investigator for Euro-BioImaging: European Commission, SP4 Capacities, FP7-Infrastructures-2010–1 [GA 262023], Ludwig-Maximilians-University of Munich, Germany)*; German National Cohort (2011–2016 Co-Principal Investigator, German National Cohort, Imaging Working Group, Whole-Body MRI Phenotyping, BMBF/Helmholtz Society, Germany)*; Munich Cluster of Excellence, M4 Imaging (2011–2016 Co-Principal Investigator: Imaging, Personalized Medicine, a new dimension of drug development)*; BMBF German Center for Lung Diseases (2010–2015 Principal Investigator)*; BMBF German Center for Cardiovascular Diseases (2010–2015 Principal Investigator),* Birgit Ertl-Wagner—UNRELATED: Board Membership: Philips Healthcare, Bracco, Springer Medical Publishing; Consultancy: Munich Medical International, Philips Healthcare; Grants/Grants Pending: Eli Lilly,* Genentech,* Guerbet,* Merck Serono,* Novartis*; Payment for Lectures (including service on Speakers Bureaus): Siemens, Bayer Schering; Payment for Manuscript Preparation: Siemens, Springer Medical Publishing, Thieme Medical Publishers, Bracco; Royalties: Springer Medical Publishing, Thieme Medical Publishers; Payment for Development of Educational Presentations: Siemens, Bracco, Springer, Thieme; Stock/Stock Options: Siemens (stock owned by spouse); Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Siemens. *Money paid to the institution.
REFERENCES
- 1. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2. Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 3. Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 4. Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 5. Liebeskind DS, Tomsick TA, Foster LD, et al. ; IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014;45:759–64 10.1161/STROKEAHA.113.004072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legrand L, Naggara O, Turc G, et al. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke 2013;44:1878–84 10.1161/STROKEAHA.113.001026 [DOI] [PubMed] [Google Scholar]
- 7. Sillanpaa N, Saarinen JT, Rusanen H, et al. The clot burden score, the Boston Acute Stroke Imaging Scale, the cerebral blood volume ASPECTS, and two novel imaging parameters in the prediction of clinical outcome of ischemic stroke patients receiving intravenous thrombolytic therapy. Neuroradiology 2012;54:663–72 10.1007/s00234-011-0954-z [DOI] [PubMed] [Google Scholar]
- 8. Gralla J, Burkhardt M, Schroth G, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol 2008;29:247–52 10.3174/ajnr.A0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Froehler MT, Tateshima S, Duckwiler G, et al. ; UCLA Stroke Investigators. The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointerv Surg 2013;5:289–93 10.1136/neurintsurg-2012-010313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mokin M, Morr S, Natarajan SK, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg 2015;7:104–07 10.1136/neurintsurg-2013-011017 [DOI] [PubMed] [Google Scholar]
- 11. Spiotta AM, Vargas J, Hawk H, et al. Hounsfield unit value and clot length in the acutely occluded vessel and time required to achieve thrombectomy, complications and outcome. J Neurointerv Surg 2014;6:423–27 10.1136/neurintsurg-2013-010765 [DOI] [PubMed] [Google Scholar]
- 12. Menon BK, O'Brien B, Bivard A, et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2013;33:365–71 10.1038/jcbfm.2012.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calleja AI, Cortijo E, Garcia-Bermejo P, et al. Collateral circulation on perfusion-computed tomography-source images predicts the response to stroke intravenous thrombolysis. Eur J Neurol 2013;20:795–802 10.1111/ene.12063 [DOI] [PubMed] [Google Scholar]
- 14. Frölich AM, Wolff SL, Psychogios MN, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol 2014;24:390–96 10.1007/s00330-013-3024-6 [DOI] [PubMed] [Google Scholar]
- 15. Beyer SE, Thierfelder KM, von Baumgarten L, et al. Strategies of collateral blood flow assessment in ischemic stroke: prediction of the follow-up infarct volume in conventional and dynamic CTA. AJNR Am J Neuroradiol 2015;36:488–94 10.3174/ajnr.A4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frölich AM, Schrader D, Klotz E, et al. 4D CT angiography more closely defines intracranial thrombus burden than single-phase CT angiography. AJNR Am J Neuroradiol 2013;34:1908–13 10.3174/ajnr.A3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyer SE, von Baumgarten L, Thierfelder KM, et al. Predictive value of the velocity of collateral filling in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2015;35:206–12 10.1038/jcbfm.2014.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 10.1161/01.STR.0000258112.14918.24 [DOI] [PubMed] [Google Scholar]
- 19. Smit EJ, Vonken EJ, van der Schaaf IC, et al. Timing-invariant reconstruction for deriving high-quality CT angiographic data from cerebral CT perfusion data. Radiology 2012;263:216–25 10.1148/radiol.11111068 [DOI] [PubMed] [Google Scholar]
- 20. Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–42 [PMC free article] [PubMed] [Google Scholar]
- 21. Royalty K, Manhart M, Pulfer K, et al. C-arm CT measurement of cerebral blood volume and cerebral blood flow using a novel high-speed acquisition and a single intravenous contrast injection. AJNR Am J Neuroradiol 2013;34:2131–38 10.3174/ajnr.A3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo AJ, Simonsen CZ, Prabhakaran S, et al. ; Cerebral Angiographic Revascularization Grading Collaborators. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke 2013;44:2509–12 10.1161/STROKEAHA.113.001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011;42:1775–77 10.1161/STROKEAHA.110.609693 [DOI] [PubMed] [Google Scholar]
- 24. Mokin M, Morr S, Natarajan SK, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg 2015;7:104–07 10.1136/neurintsurg-2013-011017 [DOI] [PubMed] [Google Scholar]
- 25. Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237–43 10.1161/STROKEAHA.110.605576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim EY, Heo JH, Lee SK, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology 2006;67:1846–48 10.1212/01.wnl.0000244492.99737.a8 [DOI] [PubMed] [Google Scholar]
- 27. Pulli B, Schaefer PW, Hakimelahi R, et al. Acute ischemic stroke: infarct core estimation on CT angiography source images depends on CT angiography protocol. Radiology 2012;262:593–604 10.1148/radiol.11110896 [DOI] [PMC free article] [PubMed] [Google Scholar]