The authors retrospectively reviewed a consecutive series of patients with diffuse intracranial dolichoectasia and compared demographics, vascular risk factors, additional aneurysm prevalence, and clinical outcomes with a group of patients with vertebrobasilar dolichoectasia. Twenty-five patients had diffuse intracranial dolichoectasia, and 139 had vertebrobasilar dolichoectasia. Patients with diffuse intracranial dolichoectasia were older than those with vertebrobasilar dolichoectasia and had a higher prevalence of abdominal aortic aneurysms, other visceral aneurysms, and smoking history. Patients with diffuse intracranial dolichoectasia were more likely to have aneurysm growth. They conclude that the natural history of patients with diffuse intracranial dolichoectasia is significantly worse than that in those with isolated vertebrobasilar dolichoectasia.
Abstract
BACKGROUND AND PURPOSE:
Among patients with vertebrobasilar dolichoectasia is a subset of patients with disease affecting the anterior circulation as well. We hypothesized that multivessel intracranial dolichoectasia may represent a distinct phenotype from single-territory vertebrobasilar dolichoectasia. The purpose of this study was to characterize clinical characteristics and angiographic features of this proposed distinct phenotype termed “diffuse intracranial dolichoectasia” and compare them with those in patients with isolated vertebrobasilar dolichoectasia.
MATERIALS AND METHODS:
We retrospectively reviewed a consecutive series of patients with diffuse intracranial dolichoectasia and compared their demographics, vascular risk factors, additional aneurysm prevalence, and clinical outcomes with a group of patients with vertebrobasilar dolichoectasia. “Diffuse intracranial dolichoectasia” was defined as aneurysmal dilation of entire vascular segments involving ≥2 intracranial vascular beds. Categoric and continuous variables were compared by using χ2 and Student t tests, respectively.
RESULTS:
Twenty-five patients had diffuse intracranial dolichoectasia, and 139 had vertebrobasilar dolichoectasia. Patients with diffuse intracranial dolichoectasia were older than those with vertebrobasilar dolichoectasia (70.9 ± 14.2 years versus 60.4 ± 12.5 years, P = .0002) and had a higher prevalence of abdominal aortic aneurysms (62.5% versus 14.3%, P = .01), other visceral aneurysms (25.0% versus 0%, P < .0001), and smoking (68.0% versus 15.9%, P < .0001). Patients with diffuse intracranial dolichoectasia were more likely to have aneurysm growth (46.2% versus 21.5%, P = .09) and rupture (20% versus 3.5%, P = .007) at follow-up. Patients with diffuse intracranial dolichoectasia were less likely to have good neurologic function at follow-up (24.0% versus 57.6%, P = .004) and were more likely to have aneurysm-related death (24.0% versus 7.2%, P = .02).
CONCLUSIONS:
The natural history of patients with diffuse intracranial dolichoectasia is significantly worse than that in those with isolated vertebrobasilar dolichoectasia. Many patients with diffuse intracranial dolichoectasia had additional saccular and abdominal aortic aneurysms. These findings suggest that diffuse intracranial dolichoectasia may be a distinct vascular phenotype secondary to a systemic arteriopathy affecting multiple vascular beds.
Fusiform and dolichoectatic intracranial aneurysms are generally associated with a poor natural history with high rates of rupture, mass effect, and ischemic stroke.1–4 These aneurysms often form secondary to circumferential loss or weakening of the internal elastic lamina of a vessel segment and can involve an entire vessel or may be limited to a single vessel segment (eg, basilar artery, cavernous or supraclinoid ICA, proximal MCA, and so forth).5–8 Notably, most prior reports of intracranial dolichoectasia have focused on involvement of single-vessel territories, most commonly the vertebrobasilar system. These dolichoectatic aneurysms are associated with a wide range of proposed, underlying etiologies, including healed, focal arterial dissections; chronic hypertension; prior radiation therapy; connective tissue diseases; arterial tortuosity syndrome; glycogen storage diseases; infection; and myxomatous emboli.5–8
During the past several years, we have noticed in our clinical practice a subset of patients who have segmental aneurysmal dolichoectasia of multiple intracranial vascular territories in the absence of a known, underlying insult such as radiation, infection, genetic connective tissue disease, or dissection. We hypothesized that multivessel presentation of intracranial dolichoectasia may represent a process distinct from the frequently described single-territory dolichoectasia, potentially with distinct presentation, associations, and natural history. For this report, we have defined “diffuse intracranial dolichoectasia” as aneurysmal dilation of entire segments that involve ≥2 intracranial vascular beds. These criteria were chosen due to the notion that fusiform aneurysmal dilation of multiple intracranial vascular beds indicates a systemic vascular insult, while fusiform aneurysmal dilation of a single bed suggests a more localized pathologic process.
The primary purpose of this article was to fully characterize the clinical risk factors and anatomic and angiographic characteristics of this proposed distinct entity, which we term “diffuse intracranial dolichoectasia.” The secondary purpose was to compare clinical risk factors and anatomic and angiographic characteristics of patients with diffuse intracranial dolichoectasia with those in a group of patients with dolichoectasia isolated to the vertebrobasilar system.
Materials and Methods
Patient Population
Following institutional review board approval, we identified all patients with diffuse intracranial dolichoectasia evaluated at our institution from January 1, 2001, to December 31, 2014. We also identified a control group of patients with isolated vertebrobasilar dolichoectasia. To identify patients, we searched our imaging data base for reports of head MR imaging, MRA, CTA, and DSA examinations in which any one of the following terms was used in the body or conclusions of the report: “fusiform,” “aneurysmal dilation,” “dilation,” “fusiform aneurysm,” “dolichoectasia,” “dolichoectatic,” and “dolicho.” All identified imaging examinations were then reviewed by 2 radiologists in consensus. Cases with fusiform aneurysmal dilation of multiple vascular segments were then identified for additional review to determine whether they met the diagnostic criteria for diffuse intracranial dolichoectasia. Cases in which there was fusiform aneurysmal dilation with or without tortuosity isolated to the vertebrobasilar system were defined as “isolated vertebrobasilar dolichoectasia.” Of the cases that were identified for additional review as potentially representing diffuse intracranial dolichoectasia, maximum measurements were obtained of the basilar artery, supraclinoid ICAs, and bilateral M1 segments.
Diagnostic Criteria
To be included in this diffuse intracranial dolichoectasia group, patients had to meet the following diagnostic inclusion criteria: adult patients with fusiform aneurysmal dilation of entire vascular segments (ie, supraclinoid ICA, basilar artery, M1 segment of the MCA) that involved ≥2 intracranial vascular beds (ie, vertebrobasilar system, left anterior circulation, or right anterior circulation). Aneurysmal dilation of a vascular segment was defined as a diameter of at least 2 times the normal diameter of a given vascular segment as defined by an angiographic atlas.9 Isolated vertebrobasilar dolichoectasia was defined as an aneurysmal dilation 2 times the normal diameter without a definable neck involving a portion of an arterial segment (either vertebral or basilar) with any degree of tortuosity. The criteria for aneurysmal dilation of a vessel segment are summarized in Table 1. Exclusion criteria included the following: 1) pediatric patients, 2) patients with underlying connective tissue diseases (ie, Ehlers-Danlos and Marfan syndromes, Loeys-Dietz syndrome, autosomal dominant polycystic kidney disease, neurofibromatosis type 1, and so forth), 3) patients with a history of cranial radiation, 4) patients with an underlying infectious etiology (ie, varicella zoster vasculopathy, HIV, and so forth), and 5) patients with poststenotic dilations secondary to atherosclerosis or dissection. All patients in the vertebrobasilar dolichoectasia control group and 11 patients in the diffuse intracranial dolichoectasia group were also included in a prior study on the imaging natural history of patients with vertebrobasilar, nonsaccular dolichoectatic aneurysms.10
Table 1:
Definition of vascular segments and aneurysmal dilation
| Vascular Segment | Definition of Vascular Segment | Definition of Aneurysmal Dilation |
|---|---|---|
| Cavernous ICA | Entry point of ICA into the cavernous segment to the ophthalmic artery origin | ≥8.5 mm |
| Supraclinoid ICA | ICA from the ophthalmic artery origin to the ICA bifurcation | ≥8.0 mm |
| M1 segment of MCA | Origin of MCA to the MCA bifurcation | ≥5.0 mm |
| Basilar artery | Confluence of vertebral arteries to the basilar bifurcation | ≥6.0 mm |
Baseline Demographics and Clinical Risk Factors
We collected the following baseline patient demographic characteristics and comorbidities: age; sex; the presence of an ectatic abdominal aorta (maximum diameter, 2.6–2.9 cm) or an abdominal aortic aneurysm (maximum diameter, ≥3 cm); and the presence of other vascular ectasias/aneurysms, fibromuscular dysplasia, coronary artery disease, peripheral artery disease, hypertension, diabetes mellitus, hyperlipidemia, tobacco use (current, former, never), pack-years of tobacco use (if available), alcohol abuse, sympathomimetic abuse, and family history of abdominal aortic aneurysm, stroke, or aneurysm. In addition to baseline demographic and clinical risk factors, we collected data on presenting symptoms at the time of the discovery of the intracranial dolichoectasia (ie, incidental, stroke, subarachnoid hemorrhage, neuralgia, mass effect, headache, hemifacial spasm, other). Baseline demographic and clinical risk factors were compared between the diffuse intracranial dolichoectasia group and the isolated vertebrobasilar dolichoectasia group.
Imaging Characteristics
Among patients who met the diagnostic criteria for diffuse intracranial dolichoectasia and those in the isolated vertebrobasilar dolichoectasia control group, we collected the following information from their imaging examinations: the presence of a saccular aneurysm; the presence of other intracranial vascular dilations, subarachnoid hemorrhage, or intraparenchymal hemorrhage on presentation; white matter ischemic disease; and thrombus within the aneurysm. All measurements on CTA and MRA were obtained by using multiplanar reformatting software (Terarecon, San Mateo, California). The maximum cross-sectional diameter of the vessel perpendicular to the longitudinal axis of the vessel was obtained. For DSA images, the maximum diameter of the vessel perpendicular to the longitudinal axis of the vessel was obtained. Imaging characteristics were compared between the diffuse intracranial dolichoectasia group and the isolated vertebrobasilar dolichoectasia group.
Outcomes Studied
In cases in which there was serial imaging, we collected data on presence of dolichoectatic aneurysm growth or rupture and new ischemic stroke. We also collected data on the clinical status of the patient at last follow-up. Clinical status was defined as no or minimal disability (mRS ≤ 2), moderate or severe disability (mRS 3–5), and death. Among patients who died, the cause of death was noted, when available. Outcomes were compared between the diffuse intracranial dolichoectasia group and the isolated vertebrobasilar dolichoectasia group.
Statistical Analysis
All categoric variables are reported as number (percentage), while continuous variables are reported as mean ± SD. Categoric variables were compared by using χ2 tests, and continuous variables were compared by using a Student t test. To determine whether a diagnosis of diffuse intracranial dolichoectasia was independently associated with aneurysm growth, rupture, and new infarct, we performed a multivariate logistic regression analysis, adjusting for baseline factors that were different between groups (ie, age and smoking history). All analysis was performed by using the SAS-based statistical software package JMP 12.0 (SAS Institute, Cary, North Carolina).
Results
Baseline Patient Characteristics for Diffuse Intracranial Dolichoectasia
The search of radiology reports (ie, MR imaging, MRA, CTA, and DSA) yielded 890 unique patients with the terms “fusiform,” “aneurysmal dilation,” “dilation,” “fusiform aneurysm,” “dolichoectasia,” “dolichoectatic,” and “dolicho” in their reports. After further review of imaging reports and radiologic images, 25 patients met the diagnostic criteria for diffuse intracranial dolichoectasia.
The mean patient age was 70.9 ± 14.2 years. Two patients were younger than 50 years of age (8.0%), and 21 patients (84.0%) were 60 years of age or older. Eighty-four percent (21/25) of patients were men. The most common clinical presentation was acute ischemic stroke or transient ischemic attack (13/25, 52%). Of the ischemic strokes, 6 affected the posterior circulation and 7 affected the anterior circulation. Five patients (20%) had symptoms due to mass effect from the aneurysmal dilations, 3 patients (12%) had dizziness, and in the remaining 4 patients (16%), the aneurysms were discovered incidentally during evaluation for loss of consciousness, syncope, or visual blurring.
Among patients with abdominal imaging, 50% (8/16) had an abdominal aortic aneurysm and an additional 13% (2/16) had aortic ectasia. Hypertension was present in 88% (22/25) of patients, and 68% (17/25) were current or former smokers. Hyperlipidemia was present in 52% (13/25) of patients, and intracranial atherosclerosis was present in 48% (12/25). These data are summarized in Table 2.
Table 2:
Summary of patient characteristics and outcomes
| Variable | DID | VBD | P Value |
|---|---|---|---|
| No. | 25 | 139 | – |
| Mean age (SD) (yr) | 70.9 (14.2) | 60.4 (12.5) | .0002 |
| Age group (yr) | |||
| 20–29 | 1 (4.0) | 5 (3.6) | <.0001 |
| 30–39 | 0 (0.0) | 6 (4.3) | |
| 40–49 | 1 (4.0) | 6 (4.3) | |
| 50–59 | 2 (8.0) | 44 (31.6) | |
| 60–69 | 7 (28.0) | 43 (30.9) | |
| 70–79 | 6 (24.0) | 30 (21.5) | |
| ≥80 | 8 (32.0) | 5 (3.6) | |
| Sex | |||
| Male | 21 (84.0) | 105 (75.5) | .51 |
| Female | 4 (16.0) | 34 (24.5) | |
| Indication for imaging | |||
| Stroke | 13 (52.0) | 45 (32.7) | .10 |
| Mass effect | 5 (20.0) | 25 (18.0) | .81 |
| Other/incidental | 7 (28.0) | 69 (49.6) | .08 |
| Comorbidities | |||
| Abdominal aortic aneurysm or ectasia | 10 (62.5) | 12 (14.3) | .01 |
| Other visceral aneurysms | 4 (25.0) | 0 (0.0) | <.0001 |
| Fibromuscular dysplasia | 0 (0.0) | 0 (0.0) | 1.0 |
| Coronary artery disease | 10 (40.0) | 40 (29.0) | .38 |
| Peripheral artery disease | 4 (16.0) | 6 (4.3) | .07 |
| Hypertension | 22 (88.0) | 97 (70.3) | .11 |
| Diabetes mellitus | 3 (12.0) | 22 (15.9) | .85 |
| Hyperlipidemia | 13 (52.0) | 73 (52.9) | .96 |
| Smoking | 17 (68.0) | 22 (15.9) | <.0001 |
| Alcohol abuse | 1 (4.0) | 7 (5.0) | .82 |
| Sympathomimetic abuse | 0 (0.0) | 0 (0.0) | 1.0 |
| Family history | |||
| Abdominal aortic aneurysm | 3 (12.0) | 9 (6.4) | .58 |
| Ischemic stroke | 7 (28.0) | 32 (23.0) | .78 |
| Intracranial aneurysm | 1 (4.0) | 9 (6.4) | .98 |
| Other saccular aneurysms present | 7 (28.0) | 22 (15.8) | .24 |
| Growth on follow-up | 6 (46.2) | 30 (21.6) | .09 |
| SAH on follow-up | 5 (20.0) | 5 (3.5) | .007 |
| Infarct on follow-up | 7 (28.0) | 18 (13.0) | .10 |
| Clinical status at last follow-up | |||
| Good neurologic function | 6 (24.0) | 80 (57.6) | .004 |
| Poor neurologic function | 6 (24.0) | 22 (15.8) | .48 |
| Death | 13 (52.0) | 37 (26.6) | .02 |
| Aneurysm-related death | 6 (24.0) | 10 (7.2) | .02 |
Note:—VBD indicates vertebrobasilar dolichoectasia; DID, diffuse intracranial dolichoectasia.
Angiographic and Anatomic Characteristics for Diffuse Intracranial Dolichoectasia
The most common distribution of disease was dilation of the basilar artery and 1 internal carotid artery (10 patients, 40%). Five patients (20%) had diffuse dilation of the basilar artery and bilateral ICAs, and 4 patients (16%) had dilation of the bilateral ICAs without dilation of the basilar artery. Two patients (8.0%) had dilation of the basilar artery and bilateral ICAs and MCAs.
The mean maximum diameter of the dilated basilar arteries was 15.2 ± 8.3 mm. The mean maximum diameter of the dilated ICA segments was 12.7 ± 7.0 mm, and the mean maximum diameter of dilated MCA segments was 10.1 ± 4.3 mm. Seven patients (28%) had additional intracranial saccular aneurysms. These data are summarized in Table 3.
Table 3:
Angiographic characteristics and distribution of DID
| No. (%) | |
|---|---|
| Vessels involved | |
| Anterior circulation only | 4 (16.0) |
| Bilateral ICAs | 4 (16.0) |
| Anterior and posterior circulation | 21 (84.0) |
| Basilar artery + 1 ICA | 10 (40.0) |
| Basilar artery + bilateral ICAs | 5 (20.0) |
| Basilar artery + bilateral ICAs + bilateral MCAs | 2 (8.0) |
| Basilar artery + 1 ICA/ipsilateral M1 | 2 (8.0) |
| Basilar artery + 1 ICA + bilateral MCAs | 1 (4.0) |
| Basilar artery + 1 MCA | 1 (4.0) |
| Mean (SD) maximum diameter of dilated (mm) | |
| Basilar artery | 15.2 (8.3) |
| Internal carotid artery | 12.7 (7.0) |
| MCA | 10.1 (4.3) |
| Thrombus in fusiform/dolichoectatic aneurysm | 7 (28.0) |
Note:—DID indicates diffuse intracranial dolichoectasia.
Outcomes for Diffuse Intracranial Dolichoectasia
Thirteen patients had imaging follow-up (mean, 54.1 ± 78.4 months). Of these patients, 6 (46%) had growth of at least 1 aneurysmal dolichoectasia, which occurred at a mean of 56.0 ± 54.3 months. Given a total of 63.1 patient-years of imaging follow-up, the overall growth rate was 9.5%/patient-year. Seven patients (56%) had imaging evidence of a new infarct after a mean of 60.9 ± 66.5 months of follow-up, including 2 patients with brain stem perforator infarcts, 5 patients with cerebellar or thalamic infarcts, and 4 patients with anterior circulation infarcts.
Five patients (5/25, 20.0%) had imaging evidence of subarachnoid hemorrhage at a mean of 59.0 ± 73.1 months of follow-up. Of these 5 patients, 3 had rupture of 2 basilar dolichoectatic/fusiform aneurysms, 1 had rupture of a posterior inferior cerebellar artery dissecting aneurysm, and in 1 patient, the source of the subarachnoid hemorrhage was unclear (On-line Table). Including patients without imaging follow-up, the overall rate of subarachnoid hemorrhage was 5.9%/year (5 cases over 84.9 patient-years).
The mean clinical follow-up for all 25 patients was 40.8 ± 62.3 months. At last follow-up, 13 patients (52%) had died, 6 patients (24%) had poor neurologic outcome, and 6 patients (24%) had good neurologic outcome. Among the patients who died, 4 died from aneurysm rupture, 2 died from hydrocephalus secondary to mass effect from a growing basilar artery fusiform aneurysm, and 1 patient died from intraparenchymal hemorrhage, which was not aneurysm-related. The remaining deaths were noncerebrovascular in nature, including 4 patients who died from cancer and 2 patients who died from unknown causes. In 4 of the 6 patients who had poor neurologic outcome at last follow-up, the cause of the morbidity was related to diffuse intracranial dolichoectasia. Representative case examples are provided in Figs 1–4.
Fig 1.
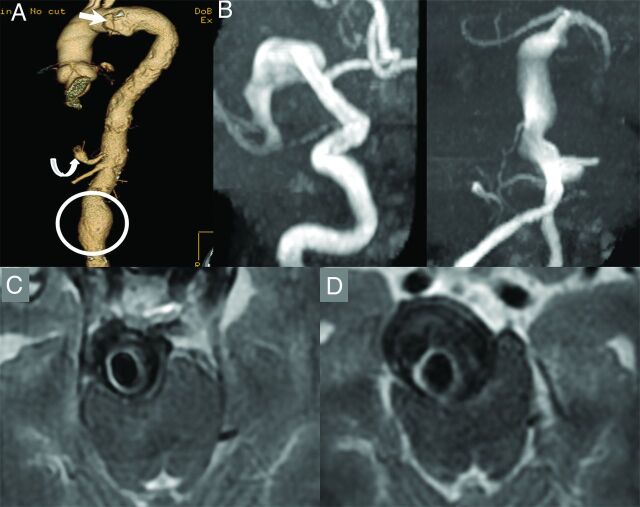
A 67-year-old man who is a former smoker with a history of a penetrating atheromatous ulcer of the aortic arch (white arrow), a 5.6-cm abdominal aortic aneurysm (circle), and a celiac artery aneurysm (curved arrow, A). The patient had an episode of dizziness and headache and underwent a noncontrast CT of the head, which demonstrated enlarged intracranial arteries. An MRA demonstrated fusiform aneurysmal dilation of the entire right M1 segment measuring 10 mm in maximum diameter and a largely thrombosed fusiform aneurysm of the basilar artery, which measured 18 mm in maximum diameter (B and C). Approximately 9 months later, the aneurysm grew to 25 mm in diameter and started causing obstructive hydrocephalus (D). The patient also had a new perforator pontine infarct at the time (not shown). A programmable ventriculoperitoneal shunt was placed; however, the patient died due to complications of hydrocephalus 3 months later.
Fig 2.
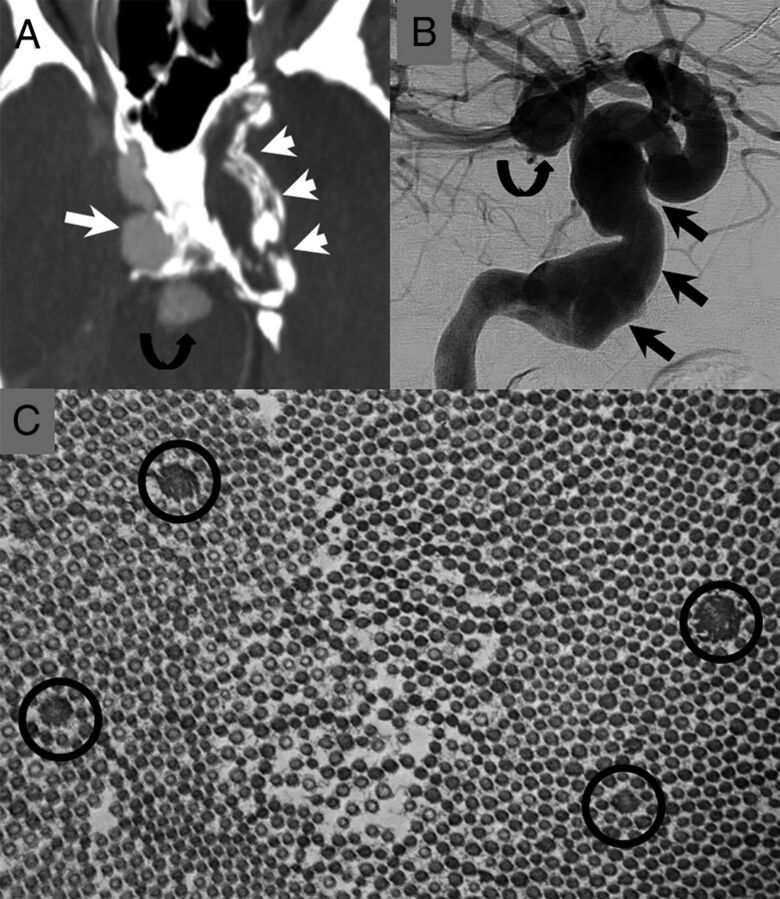
The patient is a 47-year-old man. He presented in his 20s with 2 large fusiform aneurysms of his cavernous carotid arteries. He later developed an aneurysm of his basilar artery and had a subarachnoid hemorrhage from a dissecting aneurysm of his PICA (not shown). A, CTA image demonstrates a large fusiform aneurysm of the right cavernous carotid artery (long white arrow) and a thrombosed/calcified aneurysm of the left cavernous carotid artery (short white arrows). There is also an aneurysm of the basilar tip (curved black arrow). B, Right ICA cerebral angiogram shows a large fusiform aneurysm of the right petrocavernous carotid artery (straight black arrows) and a basilar tip aneurysm (curved black arrow). C, There was suspicion for underlying connective tissue disease. The patient underwent a skin biopsy. Electron microscopy of the skin biopsy shows multiple abnormally enlarged collagen fibers (black circles) consistent with collagen flowers. These are typically seen in Ehlers-Danlos syndrome. The patient later underwent genetic testing for Loeys-Dietz, Ehlers-Danlos, and Marfan syndromes. The findings of all tests were negative.
Fig 3.
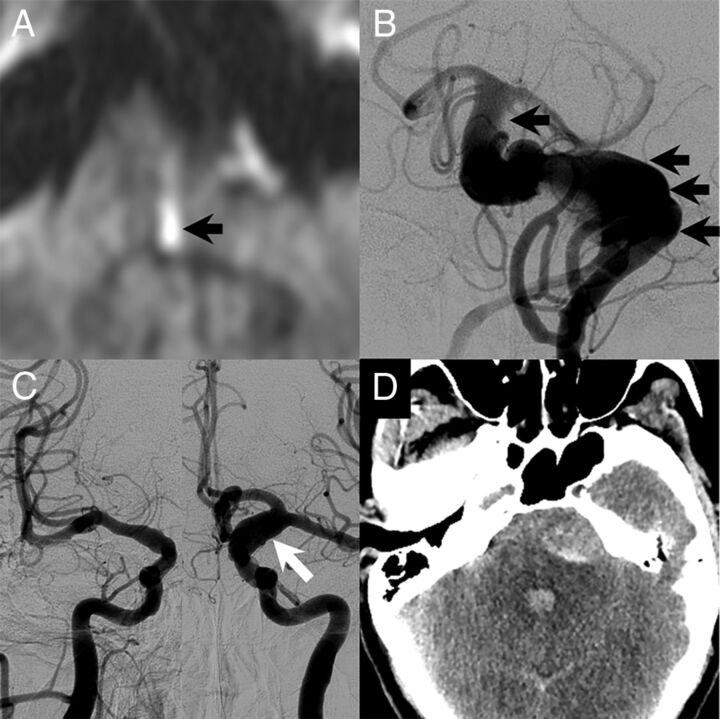
A 51-year-old man with a long history of headaches with associated nausea and vomiting. He had acute-onset left-sided weakness with a prominent left facial droop along with left face, arm, and leg numbness and slurred speech. The patient had no family history of cerebral aneurysms, though his father had an abdominal aortic aneurysm. A, MR imaging at the time of the initial evaluation showed a medial left pontine infarct (black arrow). There was evidence of a large dolichoectatic aneurysm of the basilar artery on MR imaging, and the patient underwent cerebral angiography for further evaluation. B, Cerebral angiography demonstrated a fusiform-type aneurysm of the basilar artery with a filling defect that was consistent with thrombus (black arrows). The patient also had diffuse arteriomegaly with dilation of the right supraclinoid ICA to 6 mm and dilation of the left supraclinoid ICA to 10 mm (white arrow, C). D, The day following the angiography, the patient had a 10/10 headache. Noncontrast CT at the time showed diffuse subarachnoid hemorrhage with most of the blood products surrounding the basilar artery aneurysm. He died the next day.
Fig 4.
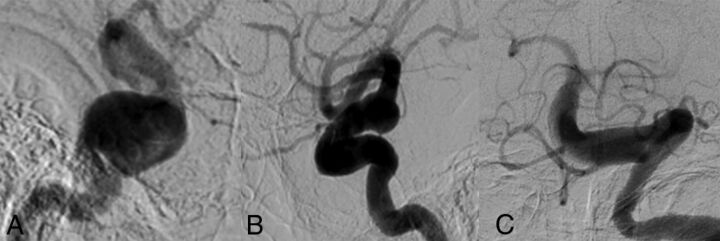
An 87-year-old man with a history of a right third-nerve palsy. A, Right ICA cerebral angiogram demonstrates a 20-mm cavernous carotid fusiform aneurysm with associated dilation of the supraclinoid ICA as well. B, Left ICA cerebral angiogram shows dilation of the left supraclinoid ICA to approximately 10 mm. C, Left vertebral artery cerebral angiogram shows diffuse dilation and tortuosity of the basilar artery measuring 9 mm in maximum diameter. The cause of the third-nerve palsy was thought to be the right cavernous aneurysm.
Comparison of Diffuse Intracranial Dolichoectasia and Isolated Vertebrobasilar Dolichoectasia Groups
A total of 139 patients had vertebrobasilar dolichoectasia with a mean follow-up of 44 ± 42.9 months. A case of vertebrobasilar dolichoectasia is demonstrated in Fig 5. None of the patients with vertebrobasilar dolichoectasia progressed to the diffuse form. Patients with diffuse intracranial dolichoectasia were older (70.9 ± 14.2 years versus 60.4 ± 12.5 years, P = .0002), and the age distribution of patients with diffuse intracranial dolichoectasia was older (P < .0001). There was a predominance of males in both patient groups (84.0% versus 75.5%, P = .51). Patients with diffuse intracranial dolichoectasia were significantly more likely to have abdominal aortic aneurysms or ectasias (62.5% versus 14.3%, P = .01) as well as visceral aneurysms (25.0% versus 0.0%, P < .0001). Patients with diffuse intracranial dolichoectasia trended toward higher rates of peripheral artery disease (16.0% versus 4.3%, P = .07) and hypertension (88.0% versus 70.3%, P = .11). Patients with diffuse intracranial dolichoectasia were significantly more likely to smoke (68.0% versus 15.9%, P < .0001).
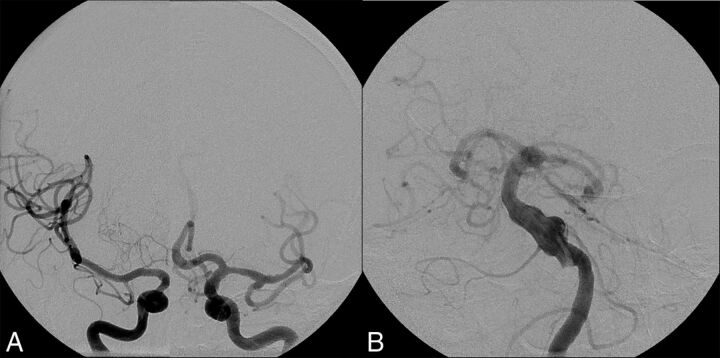
Fig 5.
Vertebrobasilar dolichoectasia in a 67-year-old man. A, Right and left ICA cerebral angiograms demonstrate normal-caliber internal carotid arteries, MCAs, and anterior cerebral arteries bilaterally. B, Left vertebral artery cerebral angiogram demonstrates an irregular dolichoectatic and fusiform aneurysm involving the entirety of the basilar artery.
Patients with diffuse intracranial dolichoectasia trended toward higher aneurysm growth rates (46.2% versus 21.6%, P = .09) and cerebral infarction rates (28.0% versus 13.0%, P = .10) and were more likely to have SAH on follow-up (20.0% versus 3.5%, P = .007). Patients with diffuse intracranial dolichoectasia were less likely to have good neurologic function at follow-up (24.0% versus 57.6%, P = .004), were more likely to die (52.0% versus 26.6%, P = .02), and were more likely to die due to aneurysm-related factors (24.0% versus 7.2%, P = .02). These data are summarized in Table 2.
On multivariate analysis, adjusting for age and smoking history, a diagnosis of diffuse intracranial dolichoectasia was independently associated with higher odds of aneurysm rupture (OR = 6.33; 95% CI, 1.23–35.31; P = .02) and higher odds of new ischemic stroke (OR = 18.62; 95% CI, 3.63–146.57; P = .0003). There was no significant difference in the odds of aneurysm growth on multivariate analysis (OR = 2.23; 95% CI, 0.57–8.68; P = .24).
Discussion
Our study of 25 patients with diffuse intracranial dolichoectasia demonstrated a number of notable findings. First, this condition is a relatively rare vascular phenotype, predominantly affecting elderly male smokers with hypertension. The natural history of this disease is dismal, with only a minority surviving with good outcome at a mean follow-up of 3.5 years. Furthermore, among patients with abdominal imaging, >60% had abdominal aortic aneurysms or ectasias and nearly 30% of patients had an additional intracranial saccular aneurysm. Compared with a control group of patients with isolated vertebrobasilar dolichoectasia, patients with diffuse intracranial dolichoectasia were more likely to have aortic and visceral aneurysms, were more likely to smoke, and were more likely to experience aneurysm growth, rupture, and morbidity and mortality on both univariate and multivariate analyses. Overall, these findings suggest that diffuse intracranial dolichoectasia may represent a distinct vascular phenotype from isolated vertebrobasilar dolichoectasia and is likely the manifestation of a diffuse, systemic arteriopathy affecting multiple vascular beds. A summary of our proposed diagnostic criteria for diffuse intracranial dolichoectasia and how they compare with current definitions of vertebrobasilar dolichoectasia is provided in Table 4.
Table 4:
Comparison of diagnostic criteria and outcomes
| Diffuse Intracranial Dolichoectasia | Vertebrobasilar Dolichoectasiaa | |
|---|---|---|
| Imaging appearance | Fusiform aneurysmal dilation of an entire vascular segment (ie, supraclinoid ICA, basilar artery, M1 segment of the MCA) | Fusiform: aneurysmal dilation without definable neck involving a portion of an arterial segment with any degree of tortuosity Dolichoectatic: uniform aneurysmal dilation of an artery involving the entire basilar or vertebral or both with any degree of tortuosity Transitional: uniform aneurysmal dilation of an artery with superimposed dilation of a portion of the involved arterial segment |
| Distribution | ≥2 Intracranial vascular beds (ie, vertebrobasilar system, left anterior circulation, or right anterior circulation) | Vertebrobasilar system only |
| Size criteria | Cavernous ICA: ≥8.5 mm Supraclinoid ICA: ≥8.0 mm MCA: ≥5.0 mm Basilar artery: ≥6.0 mm |
Basilar artery diameter of >5.0 mm |
| Growth rate | 10%/year | 7%/year2,10 |
| Ischemic stroke risk | 11%/year | 3%/year2,10 |
| Aneurysm rupture risk | 6%/year | 2%/year2,10 |
Definitions of vertebrobasilar dolichoectasia proposed by Flemming et al.2
We fully acknowledge that patients with multivessel intracranial dolichoectasia have been reported previously in the literature. However, prior reports were generally quite small and did not specifically identify multivessel involvement as a distinct entity and did not provide detailed baseline demographic, imaging, and outcome data to fully characterize our proposed subgroup. In a review of 9 studies on vertebrobasilar dolichoectasia, Gutierrez et al11 noted that approximately 45% of patients with vertebrobasilar dolichoectasia had some degree of ectasia or dilation of the anterior circulation as well. However, on closer inspection of these studies, it is apparent that the degree of dilation or tortuosity of the anterior circulation and the outcomes of patients with diffuse intracranial dolichoectasia were not reported.3,10,12–14 For example, in a case series that our group previously published, 63 of 152 patients (41.5%) with vertebrobasilar nonsaccular dolichoectatic aneurysms had concomitant dolichoectasias in the anterior circulation by visual inspection. However, only 11 of those patients (7.2%) are included in the current study because the other 52 did not meet the size criteria set forth in Table 1. In fact, even in the large surgical series reported by Anson et al15 and Drake et al16 of patients with surgically treated large fusiform aneurysms, <5 percent of cases would meet the diagnostic criteria of diffuse intracranial dolichoectasia. Other small case series and case reports have reported outcomes of patients with diffuse intracranial dolichoectasia with a well-defined underlying etiology such as prior radiation, autosomal dominant polycystic kidney disease, connective tissue disease, or infection. However, findings from these studies do not necessarily apply to the patient population included in our series due to substantial differences in risk factors and etiology.17–23
Our comparison of the baseline clinical characteristics and outcomes of patients with diffuse intracranial dolichoectasia with patients with isolated vertebrobasilar dolichoectasia suggests that at the very least, this entity is a distinctive phenotype or a more diffuse and severe form of vertebrobasilar dolichoectasia. In general, patients with diffuse intracranial dolichoectasia were older, were more likely to have hypertension and other atherosclerotic risk factors, and were more likely to smoke. Furthermore, patients in the diffuse intracranial dolichoectasia group were significantly more likely to have intracranial, abdominal aortic, and visceral arterial aneurysms than those with isolated vertebrobasilar dolichoectasia. These differences are not only isolated to patients studied at our institution. Compared with the systematic review of the literature on vertebrobasilar dolichoectasia published by Gutierrez et al,11 patients in our current series were generally older (71 versus 63 years), were more often male (84% versus 64%), and were more likely to have hypertension (88% versus 66%), smoke (68% versus 42%), and have other atherosclerotic risk factors. Furthermore, considering all patients in our series with abdominal imaging, 50% of patients had abdominal aortic aneurysms compared with an average of 11% in the vertebrobasilar dolichoectasia literature.11 The rate of concomitant saccular aneurysm formation is twice as high as that reported in the literature as well (28% versus 15%).
The natural history of patients with diffuse intracranial dolichoectasia was also substantially more aggressive than that of the isolated vertebrobasilar dolichoectasia group as well as that reported in the vertebrobasilar dolichoectasia literature. Twenty percent of patients in our series had subarachnoid hemorrhage on follow-up, for a subarachnoid hemorrhage rate of 6.9%/patient-year. This compares with rupture rates of just 1%–2% per year reported for dolichoectatic and fusiform aneurysms of both the anterior and posterior circulations.3,4,24 The growth rate for patients with diffuse intracranial dolichoectasia was also substantially higher than that reported in the literature among patients with single-vessel dolichoectatic aneurysms (9.5%/year versus 6.5%/year).10 More than 50% of the patients in the current series had ischemic stroke after a mean of approximately 5 years of follow-up. Meanwhile, the rates of ischemic stroke among previously published large series of vertebrobasilar dolichoectasia are 3%, 11%, 16%, and 38% at 1, 5, 10, and 12 years, respectively.11
Limitations
Our study has limitations. First, this is a retrospective study spanning >15 years at a single institution. Therefore, there is wide variation in the type of imaging used, clinical management of these patients, and degree of follow-up. In addition, our study is prone to substantial selection bias. Patients who presented with diffuse intracranial dolichoectasia at our tertiary referral institution were more likely to have complications related to this disease, which could explain the poor natural history seen in our study. We do not have access to pathologic specimens or gene samples from the patients included in this study. Thus, we are unable to determine whether the pathologic basis of this disease is similar to that in other fusiform aneurysms and ectasias or whether the degree of vessel wall weakening is, in fact, more severe. While none of the patients in our series had a clinical diagnosis of a genetic connective tissue disease such as Marfan syndrome, Ehlers-Danlos syndrome, or Loeys-Dietz syndrome, most of the patients included in this series did not receive any genetic testing, so we cannot rule out the presence of an undiagnosed connective tissue disease. Therefore, the total number of patients or the association to secondary diseases may not be as high as that reported in our study. In our comparison of the natural history of diffuse intracranial dolichoectasia with that of vertebrobasilar dolichoectasia, we did not account for the fact that the morphology of the vertebrobasilar dolichoectatic lesions has a significant impact on their natural history. As defined in Table 4, dolichoectatic vertebrobasilar aneurysms have a much more favorable natural history than fusiform or transitional lesions.10 Last, because this entity has not been extensively studied outside small case series and case reports, there are no well-defined diagnostic criteria. Our choice of size criteria in diagnosing diffuse intracranial dolichoectasia are somewhat arbitrary (doubling of mean values reported in the literature). Using such strict criteria could have resulted in exclusion of patients with milder or earlier forms of this disease.
Conclusions
Our study of 25 patients with diffuse intracranial dolichoectasia demonstrated that this rare disease is associated with a very poor natural history, with about 20% of patients having subarachnoid hemorrhage on follow-up, 30% of patients having ischemic stroke, and 25% of patients with aneurysm-related mass effect. Nearly two-thirds of patients with this disease have abdominal aortic aneurysms, and 25% have additional saccular intracranial aneurysms. Given the high morbidity and mortality rates associated with this disease and the high rate of concomitant intracranial saccular aneurysm and abdominal aortic aneurysms in this population, these findings suggest that diffuse intracranial dolichoectasia may represent a distinct vascular phenotype. Further research is needed to better characterize the histopathologic and genetic features of diffuse intracranial dolichoectasia and determine ideal treatment options.
Supplementary Material
Footnotes
Disclosures: Giuseppe Lanzino—UNRELATED: Consultancy: Covidien/Medtronic.* David F. Kallmes—UNRELATED: Consultancy: Minnetronix,* Comments: Data and Safety Monitoring Board; Grants/Grants Pending: Medtronic, MicroVention, Sequent Medical, Surmodics, Codman, NeuroSigma, Comments: preclinical research and clinical trials*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Medtronic, Comments: FDA panel meeting.* *Money paid to the institution.
REFERENCES
- 1. Dispensa BP, Saloner DA, Acevedo-Bolton G, et al. Estimation of fusiform intracranial aneurysm growth by serial magnetic resonance imaging. J Magn Reson Imaging 2007;26:177–83 10.1002/jmri.20944 [DOI] [PubMed] [Google Scholar]
- 2. Flemming KD, Wiebers DO, Brown RD Jr, et al. The natural history of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis 2005;20:270–79 10.1159/000087710 [DOI] [PubMed] [Google Scholar]
- 3. Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology 2008;70:66–72 10.1212/01.wnl.0000286947.89193.f3 [DOI] [PubMed] [Google Scholar]
- 4. Sacho RH, Saliou G, Kostynskyy A, et al. Natural history and outcome after treatment of unruptured intradural fusiform aneurysms. Stroke 2014;45:3251–56 10.1161/STROKEAHA.114.006292 [DOI] [PubMed] [Google Scholar]
- 5. Mizutani T. A fatal, chronically growing basilar artery: a new type of dissecting aneurysm. J Neurosurg 1996;84:962–71 10.3171/jns.1996.84.6.0962 [DOI] [PubMed] [Google Scholar]
- 6. Mizutani T, Aruga T. “Dolichoectatic” intracranial vertebrobasilar dissecting aneurysm. Neurosurgery 1992;31:765–73; discussion 773 10.1227/00006123-199210000-00024 [DOI] [PubMed] [Google Scholar]
- 7. Mizutani T, Kojima H. Clinicopathological features of non-atherosclerotic cerebral arterial trunk aneurysms. Neuropathology 2000;20:91–97 10.1046/j.1440-1789.2000.00277.x [DOI] [PubMed] [Google Scholar]
- 8. Mizutani T, Miki Y, Kojima H, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 1999;45:253–59; discussion 259–60 10.1097/00006123-199908000-00010 [DOI] [PubMed] [Google Scholar]
- 9. Mocco J, Huston J, Fargen KM, et al. An angiographic atlas of intracranial arterial diameters associated with cerebral aneurysms. J Neurointerv Surg 2014;6:533–35 10.1136/neurintsurg-2013-010838 [DOI] [PubMed] [Google Scholar]
- 10. Nasr DM, Brinjikji W, Rouchaud A, et al. Imaging characteristics of growing and ruptured vertebrobasilar non-saccular and dolichoectatic aneurysms. Stroke 2016;47:106–12 10.1161/STROKEAHA.115.011671 [DOI] [PubMed] [Google Scholar]
- 11. Gutierrez J, Sacco RL, Wright CB. Dolichoectasia-an evolving arterial disease. Nat Rev Neurol 2011;7:41–50 10.1038/nrneurol.2010.181 [DOI] [PubMed] [Google Scholar]
- 12. Passero SG, Calchetti B, Bartalini S. Intracranial bleeding in patients with vertebrobasilar dolichoectasia. Stroke 2005;36:1421–25 10.1161/01.STR.0000172311.64662.9c [DOI] [PubMed] [Google Scholar]
- 13. Yu YL, Moseley IF, Pullicino P, et al. The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry 1982;45:29–36 10.1136/jnnp.45.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakatomi H, Segawa H, Kurata A, et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: insight on the mechanism of growth. Stroke 2000;31:896–900 10.1161/01.STR.31.4.896 [DOI] [PubMed] [Google Scholar]
- 15. Anson JA, Lawton MT, Spetzler RF. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg 1996;84:185–93 10.3171/jns.1996.84.2.0185 [DOI] [PubMed] [Google Scholar]
- 16. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997;87:141–62 10.3171/jns.1997.87.2.0141 [DOI] [PubMed] [Google Scholar]
- 17. Schievink WI, Torres VE, Wiebers DO, et al. Intracranial arterial dolichoectasia in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1997;8:1298–303 [DOI] [PubMed] [Google Scholar]
- 18. Sedat J, Alvarez H, Rodesch G, et al. Multifocal cerebral fusiform aneurysms in children with immune deficiencies report of four cases. Interv Neuroradiol 1999;5:151–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naunheim MR, Walcott BP, Nahed BV, et al. Arterial tortuosity syndrome with multiple intracranial aneurysms: a case report. Arch Neurol 2011;68:369–71 10.1001/archneurol.2011.29 [DOI] [PubMed] [Google Scholar]
- 20. Azzarelli B, Moore J, Gilmor R, et al. Multiple fusiform intracranial aneurysms following curative radiation therapy for suprasellar germinoma: case report. J Neurosurg 1984;61:1141–45 10.3171/jns.1984.61.6.1141 [DOI] [PubMed] [Google Scholar]
- 21. Braunsdorf WE. Fusiform aneurysm of basilar artery and ectatic internal carotid arteries associated with glycogenosis type 2 (Pompe's disease). Neurosurgery 1987;21:748–49 10.1227/00006123-198711000-00030 [DOI] [PubMed] [Google Scholar]
- 22. Capone PM, Mechtler LL, Bates VE, et al. Multiple giant intracranial aneurysms associated with lymphomatoid granulomatosis: a magnetic resonance imaging and angiographic study. J Neuroimaging 1994;4:109–11 10.1111/jon199442109 [DOI] [PubMed] [Google Scholar]
- 23. Siqueira Neto JI, Silva GS, De Castro JD, et al. Neurofibromatosis associated with moyamoya arteriopathy and fusiform aneurysm: case report [in Portuguese]. Arq Neuropsiquiatr 1998;56:819–23 10.1590/S0004-282X1998000500019 [DOI] [PubMed] [Google Scholar]
- 24. Flemming KD, Wiebers DO, Brown RD Jr, et al. Prospective risk of hemorrhage in patients with vertebrobasilar nonsaccular intracranial aneurysm. J Neurosurg 2004;101:82–87 10.3171/jns.2004.101.1.0082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.