SUMMARY:
Minimally invasive percutaneous imaging-guided techniques have been shown to be safe and effective for the treatment of benign tumors of the spine. Techniques available include a variety of tumor ablation technologies, including radiofrequency ablation, cryoablation, microwave ablation, alcohol ablation, and laser photocoagulation. Vertebral augmentation may be performed after ablation as part of the same procedure for fracture stabilization or prevention. Typically, the treatment goal in benign spine lesions is definitive cure. Painful benign spine lesions commonly encountered in daily practice include osteoid osteoma, osteoblastoma, vertebral hemangioma, aneurysmal bone cyst, Paget disease, and subacute/chronic Schmorl node. This review discusses the most recent advancement and use of minimally invasive percutaneous therapeutic options for the management of benign spine lesions.
The role of minimally invasive percutaneous imaging-guided techniques for the treatment of benign tumors of the spine has increased during the past decade. Although previously limited by the complex anatomy of the vertebral column and the proximity of neural elements, new technologies, including radiofrequency ablation (RFA), cryoablation, microwave ablation, alcohol ablation, and laser photocoagulation, now provide an attractive alternative or adjunct therapeutic options for the treatment of benign spinal tumors beyond medical pain management, surgery, radiation therapy, and standard vertebral augmentation.
Benign tumors compose only 4%–13% of spinal lesions1 and are treated with curative intent. Most commonly, the pain secondary to benign spine lesions is managed with nonsteroidal anti-inflammatory drugs and opioids titrated to achieve pain relief while attempting to minimize side effects.2 In the absence of neurologic deficits and spinal instability, radiation therapy may be the standard of care when medical management is insufficient.3–6 Although described in malignant spine lesions, pain relief following radiation therapy may be delayed and transient.7 Surgical interventions are invasive and result in increased morbidity, which, in the absence of pending neurologic compromise and instability, is undesirable for these patients.
Minimally invasive, percutaneous, image-guided interventions for the management of benign spine lesions are indicated when pharmacologic therapy (analgesics and nonsteroidal anti-inflammatory drugs) is inadequate or contraindicated (such as side effects and pregnancy), radiation therapy is contraindicated or not desired by the patient, and surgical intervention is not recommended (absence of spinal instability and neurologic compromise). The contraindications include spinal instability with or without concomitant pathologic fracture, focal neurologic deficit, advanced coagulopathy, and systemic infection. Although no published literature exists directly comparing an operation with percutaneous ablation, these minimally invasive percutaneous procedures may be more cost-effective compared with traditional surgical interventions. This review details the armamentarium available and the most recent advances in minimally invasive, image-guided percutaneous techniques for the treatment of benign spinal lesions.
General Considerations, Procedural Setup, and Patient Care
Preprocedural consultation with the patient is mandatory and should include an explanation of the benefits, risks, potential complications, and alternative treatment options. In addition, the patient's clinical status and procedural indications should be discussed with the referring clinicians. Initial preprocedural work-up includes a history and physical examination to confirm focal pain/tenderness at the lesion site and neurologic examination to assess potential focal neurologic deficits. Laboratory tests are used to evaluate the coagulation status (platelet count of >50,000 per microliter and international normalized ratio of <1.5 are generally acceptable) and, at times, the possibility of systemic infection (complete blood count, erythrocyte sedimentation rate, C-reactive protein level, and cultures, if clinically indicated). Thermal ablation of bone and soft-tissue tumors is painful and requires moderate sedation or general anesthesia, in particular with technically challenging spinal ablations, to minimize the risk of voluntary patient movement and potential subsequent complications.
Preprocedural imaging includes dedicated weight-bearing spine radiographs, CT, and/or MR imaging. Radiographs are used to determine the presence and extent of vertebral compression fractures, kyphosis, scoliosis, the integrity of the osseous central canal, and the suitability of fluoroscopy for imaging guidance. CT is used to determine the bone lesion density (osteolytic, osteoblastic, or mixed), which has implications for the choice of thermal ablation technique, and to identify cortical discontinuities due to tumor erosion and pathologic fracture clefts, which are sources of pain and sites where cement is most likely to leak during postablation cementoplasty. MR imaging is important for delineating the extent of the tumor, including the presence of marrow replacement and extraosseous tumor extension that may be occult on CT. Pretreatment imaging also provides information regarding procedural planning in terms of the choice of thermal ablation technology, approach, probe placement, and the need for thermal protection and cementation. Vertebral augmentation is often performed under fluoroscopic guidance, which is readily available and allows near-real-time monitoring of cement distribution. Thermal ablation of benign lesions of the spine is most commonly performed under CT guidance. Postprocedural care should include a follow-up physical examination and questionnaire to determine the efficacy of treatment and identify potentially recurrent symptoms. For benign spine lesions, contrast-enhanced MR imaging is the technique of choice for posttreatment imaging and is performed at the discretion of the referring physician to evaluate treatment adequacy or when symptoms recur. Postprocedural imaging should be performed 6–8 weeks after treatment to allow ablation-related inflammation to subside.
Minimally Invasive Percutaneous Techniques
Vertebral Augmentation.
Percutaneous vertebral augmentation (PVA) encompasses several techniques aimed at internal vertebral body stabilization with bone cement. Traditional vertebroplasty involves instillation of methyl methacrylate cement directly into the vertebral body, while kyphoplasty involves first creating a cavity or several cavities with inflatable balloons, bone tamps, or osteotomes to attempt more controlled cement delivery and improved cement interdigitation.8–11 Although both vertebroplasty and kyphoplasty offer durable pain relief, improved quality of life, and improved spine alignment, no randomized clinical trials exist for direct comparison of these techniques.12 Indirect comparison of these techniques based on systematic reviews shows no substantial difference in patient outcome.12 However, the rate of asymptomatic cement leakage is higher in vertebroplasty.12 Bone cement is delivered under real-time conventional fluoroscopy or CT-fluoroscopic guidance to minimize cement leakage by using a transpedicular approach. Several randomized controlled trials have demonstrated the safety, efficacy, and durability of PVA for the management of benign and malignant spine compression fractures.8–11 It is the position of multiple radiologic and neurosurgical societies that PVA remains an appropriate therapy for the treatment of painful vertebral compression fractures refractory to nonoperative medical therapy and for vertebrae weakened by neoplasia when performed for the medical indications outlined in the published standards.9
Recently, radiofrequency (RF)-induced high-viscosity cement has been used for PVA to potentially reduce intervertebral disc and venous cement leakage.13,14 Anselmetti et al13 performed PVA in 60 patients (190 vertebrae) and reported asymptomatic venous leak in 8.2% versus 41.3% of patients and intervertebral disc cement leakage in 6.1% versus 13% of patients (high-viscosity cement versus standard viscosity cement). Several mechanisms have been postulated to contribute to pain relief following PVA, including stabilization of vertebral body macro- and microfractures, exothermic reaction associated with the setting of cement to thermally ablate nerve endings, and a possible embolization effect with reduction of vascular congestion due to cement filling of enlarged bone marrow spaces.
Radiofrequency Ablation.
In RFA, a RF generator is used to deliver high-frequency, alternating current (375–600 kHz) to the patient through an RF probe. The current passes through the exposed active tip of the probe and results in oscillation of charged tissue molecules (ions) within the ablation zone, producing frictional heat. The thermal effect depends on the electrical conducting properties of the treated tissue and the characteristics of the RF probe.15 When local tissue temperature between 60°C and 100°C is reached, there is protein denaturation and immediate coagulative necrosis.15 Several RFA probe-design technologies exist, attempting to achieve an improved ablation zone.15 Unipolar systems use dispersing grounding pads placed on patient skin near the ablation site to serve as the receiving limb of the electrical circuit to prevent potential skin burns while bipolar systems use built-in transmitting and receiving electrical elements within the probe; this feature eliminates the need for grounding pads and the possibility of skin burns.15 Internally cooled probes decrease tissue char (carbonization) by maintaining a lower temperature surrounding the active probe tip.15
More recently navigational bipolar RFA probes with built-in thermocouples have become available, which allow real-time monitoring of the ablation zone size by measuring the temperatures along the periphery of the ablation zone during the procedure.16 The navigating tip of the probe can be articulated in different orientations through the same entry site; this procedure is beneficial for accessing lesions in challenging locations and achieving larger ablation zones.16 The choice of the RF probe depends in large part on the volume of tissue to be ablated and the proximity to vital structures.15,16 RFA is typically used for the treatment of vertebral lesions with no or small extraosseous components. The main advantage of RFA is precise determination of the geometry of the ablation zone beyond which tissues are safe from thermal injury.15,16 Intact cortical bone also serves as a boundary for undesired RF energy propagation.15,16 RFA is primarily used for the treatment of lesions that are mainly osteolytic because the higher intrinsic impedance of sclerotic bone lesions prevents the radiofrequency circuit from generating sufficiently high temperatures to ensure cell death and renders RFA ineffective.17 Limitations of RFA include nonvisualization of the ablation margin with CT, pain associated with the procedure, and, frequently, increased pain during the immediate posttreatment period.
Cryoablation.
In closed cryoablation systems used in percutaneous tumor ablation, a liquid gas, commonly argon, is used to rapidly cool the tip of the cryoprobe (taking advantage of the Joule-Thomson effect—that is, pressurized gas, when allowed to expand, results in a drop in temperature), forming an enlarging ice ball with time followed by a “thawing” phase, commonly achieved with helium gas, resulting in an osmotic gradient.15,18 Formation of extracellular ice results in a relative imbalance of solutes between the intra- and extracellular environment, subsequent intracellular water extraction by osmosis, and cellular dehydration. The increase in intracellular concentration of solutes results in damage to both the enzymatic machinery of the cell and the cell membrane.15,18 The formation of intracellular ice crystals damages the cellular organelles. Thawing results in an osmotic gradient and consequent cell membrane injury.15,18 A temperature of −40°C or lower is necessary to ensure complete cell death.15 At present, the cryoprobes of 2 major manufacturers are used in spines with diameters of 1.2 mm (17-ga) and 1.7 mm (13-ga), generating predictable ablation zones using at least 1 freeze/active thaw/freeze cycle (typically 1/5/10 minutes).19
Cryoablation is typically used for benign lesions with large soft-tissue components or large lesions involving the posterior vertebral elements and is preferred to RFA for the management of osteoblastic lesions. Advantages of cryoablation include formation of a hypoattenuating ice ball, which is readily identified by CT,20 beyond which tissues are safe from thermal injury; decreased intraprocedural and postprocedural pain compared with RFA or microwave ablation; and the ability to use multiple probes in various orientations to achieve additive overlapping ablation zones. An interprobe distance of 1.5–2 cm is typically recommended with probe tips at bone-tumor or tumor–soft-tissue interfaces.19 In addition, the outer margin of the ice ball corresponds to 0°C, which is usually not sufficient for permanent tumor destruction. Reliable cell death is achieved at 3 mm from the outer edge; therefore, the ice ball should extend beyond the tumor margins.18 MR imaging–compatible cryoprobes offer an alternative imaging technique, which may permit safe ablation of spinal lesions, given the proximity of the neural elements.21
Laser Ablation.
Laser ablation uses optical fibers to transmit infrared light energy into a tumor to produce rapid temperature elevation, protein denaturation, and coagulative necrosis. A continuous wave semiconductor diode laser with an 805-nm wavelength delivers energy to the tumor by using a flexible single-use bare-tipped 400-μm optical fiber with polymer cladding placed coaxially.22–25 The amount of energy to be delivered is calculated according to the formula [Nidus Size (mm) × 100 Joules + 200 Joules], and the duration of the ablation is typically 200–600 seconds, depending on the nidus size.22–25 The size of the induced ablation zone depends on the laser wavelength, thermal and optical properties of the tissue, total duration of energy delivery, laser power, diameter of the laser fiber, and the number of fibers used.26 The maximum coagulation effect is reached at 1000–1200 J, and more energy at the same location does not increase the volume of coagulation.26 Spine laser ablation has been mainly used in the treatment of osteoid osteomas and osteoblastomas,22–25 and an energy amount of 1200 J is usually adequate. However, for larger lesions, a greater amount of energy may be necessary.22 The advantages of laser ablation include a predictable size of the ablation in proportion to the energy delivered, lack of the need to use a neutral electrode, lack of interaction with stimulators and pacemakers, and the relatively low cost of disposable laser fiber.22–25 A disadvantage is the lack of visualization of the ablation zone with CT.
Microwave Ablation.
Microwave ablation uses antennae to deliver electromagnetic microwaves (approximately 900 MHz) to target tissue, which result in agitation of ionic molecules and frictional heat and, subsequently, tissue coagulative necrosis. It is theorized that microwave ablation is less influenced by variable tissue impedance and perfusion-mediated tissue cooling; this feature potentially results in higher intratumoral temperatures and creates a larger, more uniform ablation zone and faster ablation times by using a single probe.27 Additional advantages of microwave ablation include efficacy in the management of osteoblastic lesions due to less susceptibility to increased impedance of attenuated bone, diminished heat sink phenomena, and lack of the need for grounding pads and thus diminished risk of skin burns.27–29 The antenna shaft cooling system implemented in the latest generations of microwave ablation equipment eliminates the risk of back-heating phenomena.28,29 The ability to rapidly deliver high amounts of power (up to 100 Watts) to large ablation zones might be a disadvantage when applied to spinal lesions because surrounding overheating could potentially lead to injuries to adjacent neural elements.28,29 Although hypoattenuating ablated tissue is often identified on CT, the margins of the ablation zone are not well-defined; this feature is considered a disadvantage of spinal microwave ablation. The literature regarding the efficacy and safety of spine microwave ablation is sparse, and this technology should be used with caution for spinal applications. While 2 retrospective studies have shown its safety and efficacy for the management of spine metastases,28,29 this technology has not been used for the management of benign spine lesions.
Alcohol Ablation.
Alcohol ablation is a relatively inexpensive percutaneous tumor ablation technique that causes tumor necrosis directly through cellular dehydration and indirectly through vascular thrombosis and tissue ischemia.30 Instillation of iodinated contrast or venography, before alcohol ablation, to delineate the extent of ethanol diffusion has been described as a strategy for reducing the risk of the vascular intravasation and potential injury to adjacent structures.30–32 In alcohol ablation, volumes of 3–30 mL are directly injected into the target tissue.30–32 The most important limitation of alcohol ablation is the poorly predictable size and configuration of the ablation zone due to poorly reproducible diffusion of alcohol through tumoral (particularly cortical bone) and peritumoral tissues.30
Benign Spinal Lesions
Osteoid Osteoma.
Osteoid osteoma is a benign painful bone-forming lesion that typically occurs in patients younger than 30 years of age with a male predilection (2–4:1). In the spine, these lesions classically involve the posterior elements and in 60% of cases are located in the lumbar spine. A central nidus with or without central mineralization (typically <15 mm) and surrounding osseous sclerosis are the typical imaging manifestations. Osteoid osteomas have been traditionally treated with surgical excision, which has substantial morbidity, including spinal instability, nerve injury, infection, and blood loss as well as cost burden.33 Spinal osteoid osteomas account for 10%–20% of cases, and although spontaneous regression of these tumors has been reported, 70% of untreated patients develop painful scoliosis.22,34 The entire nidus of osteoid osteoma must be ablated to ensure complete treatment. Due to the proximity of neural elements, percutaneous ablation of spinal osteoid osteoma may require logistic considerations. However, thermal protective effects of intact cortical bone, flow of CSF, and small vessels in the epidural space have been hypothesized.35,36
Given the small size of spinal osteoid osteomas (typically <15 mm), RFA and laser ablation are recommended as treatment options with no need for cementation. Both RFA and laser ablation have similar efficacy and safety profiles,26 and the choice is at the discretion of the operator. Imaging guidance is typically achieved with CT with thin sections (1–2 mm) and 3D reconstructions to allow precise positioning of the probe at the center of the nidus. Since the initial reports of RFA for the treatment of osteoid osteoma,33 this technique has almost completely supplanted surgical resection. If RFA is to be used, monopolar noncooled straight electrodes are recommended with application of RF energy to achieve temperature of 90°C for 5–6 minutes (Fig 1). Recently, navigational bipolar RFA probes have been successfully used to treat spinal osteoid osteomas (Fig 2).16 These probes allow real-time monitoring of the ablation zone size by using built-in thermocouples and are beneficial for larger lesions and challenging locations due to the articulating tip, which may be placed in various orientations by using a single skin-entry site, obviating grounding pads. Adequate ablation is achieved in 2–3 minutes.16 Investigators have successfully used RFA for management of spinal osteoid osteomas by using thermoprotective techniques for lesions as close as 1 mm to neural elements without complications (Figs 1 and 2).15,16,22,35–40 During RFA, patients under general anesthesia have a fairly predictable response with elevated heart (average increase, 40%) and respiratory rates (average increase, 50%) during both the biopsy and ablation portions of the treatment.41
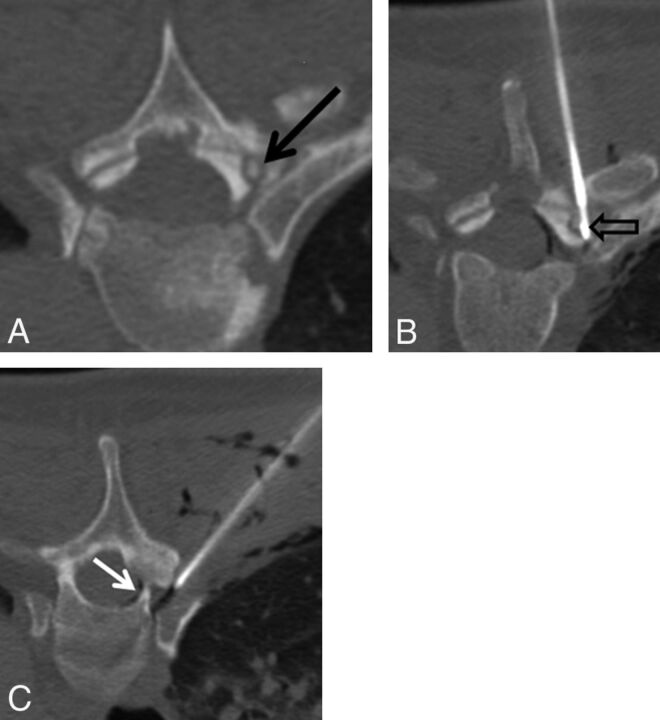
Fig 1.
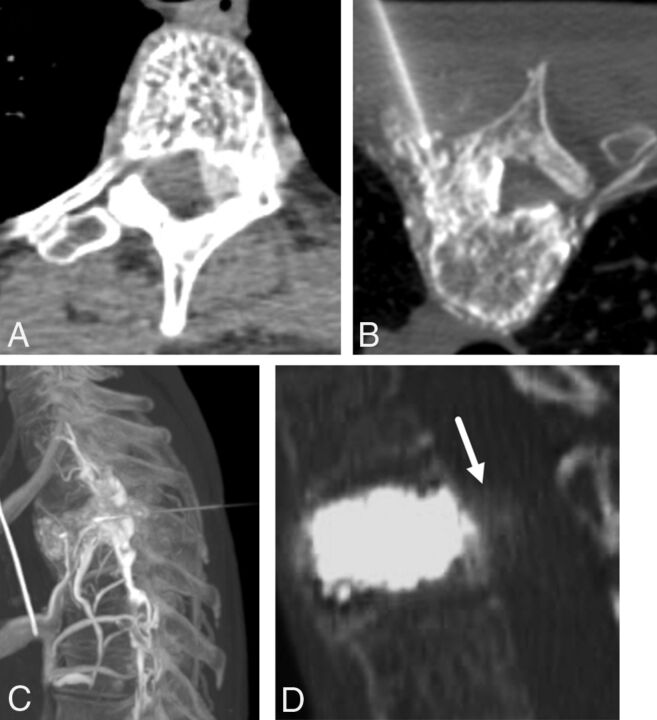
A 45-year-old man with several months of night-time predominant, right-sided midthoracic pain relieved by ibuprofen. A, Prone axial noncontrast CT image shows a small osteolytic lesion with a central mineralization in the right T5 superior articular facet (black arrow). B, Prone axial noncontrast CT image shows the radiofrequency ablation probe in the nidus of the osteoid osteoma (black open arrow). C, Prone axial noncontrast CT image shows an 18-ga spinal needle placed in the right T4–T5 neural foramen for temperature monitoring, carbon dioxide injection, and cooled dextrose 5% in water infusion. Note the gas tracking into the soft tissue and within the epidural space (white arrow).
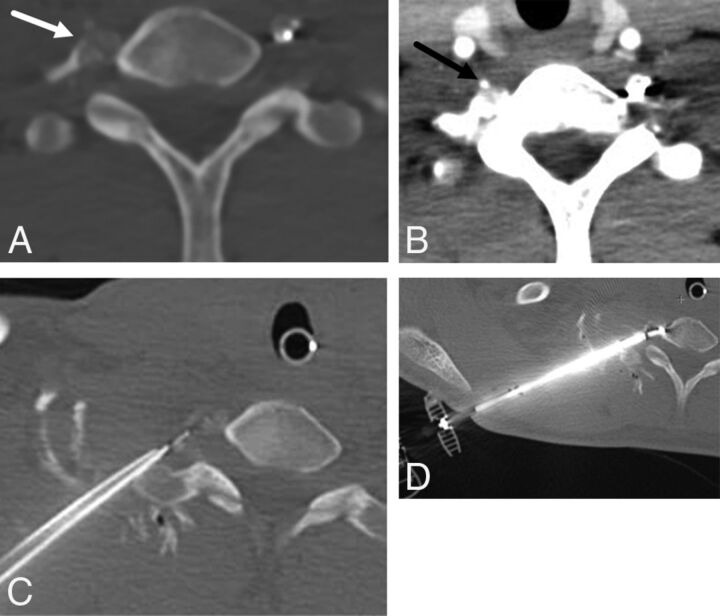
Fig 2.
A 14-year-old boy with painful right transverse process C7 osteoid osteoma (A, arrow). Preprocedural neck CT angiography demonstrates the course and location of the right vertebral artery (B, arrow). C, Thermal monitoring and protection are achieved by placement of a thermocouple and spinal needle in the right C7–T1 neuroforamen. D, RF ablation is performed by using a bipolar navigational probe with slight posterior articulation of the probe tip for optimal positioning.
Morassi et al40 treated 13 patients with spinal osteoid osteomas (11 in the posterior elements and 2 in the vertebral bodies) by using a non-cool-tip unipolar system with a 5-mm active tip (90°C for 6 minutes) using thermoprotection, achieving pain relief in 11 patients with no complications. In case of laser ablation, typically a single flexible bare-tip laser fiber is adequate; it is placed in the center of the nidus coaxially through an 18-ga spinal needle, with a delivered energy of approximately 1200 J for 200–600 seconds.23 However, for larger lesions, 2 fibers and larger energy amounts may be necessary to ensure the adequacy of the ablation. Tsoumakidou et al23 reported successful laser ablation of spine osteoid osteomas in 57 patients (vertebral body, n = 18, and posterior elements/facet joints, n = 36) by using thermoprotection with most lesions located closer than 5 mm to the neural structures. Sixty-one ablations (mean delivered energy, 1271 J) were performed with a technical success rate of 100% and a primary clinical success rate of 98.2% (at 1 month) with no major complications.23 Cryoablation has been successfully used for management of a spine osteoid osteoma.42
Osteoblastoma.
Osteoblastomas are rare benign tumors with striking histologic similarity to osteoid osteomas. There is a male predilection (2.5–1). They are typically larger (>2 cm) and expansile with less sclerotic components compared with osteoid osteomas with thin peripheral sclerosis and may be associated with aneurysmal bone cyst.43,44 On MR imaging, they demonstrate avid osseous and extraosseous enhancement. Spinal osteoblastomas compose approximately 40% of cases and often involve the posterior elements and are located in the cervical spine in 10%–40% of cases. Most untreated lesions lead to painful scoliosis, and surgical excision has traditionally been the treatment of choice for spinal osteoblastomas, which is associated with morbidity, especially given the size of the osseous defect.43,44 The entire osteolytic component and the soft-tissue component (if present) must be ablated for definitive cure. The imaging guidance (CT) and thermoprotective measures are similar to those in osteoid osteoma ablation. Typically, there is no need for postablation vertebral augmentation. Given the larger size of osteoblastomas and particularly with involvement of posterior elements and potential soft-tissue components, cryoablation could be safely and efficiently performed with simultaneous placement of multiple probes with visualization of the hypoattenuating ice ball.26
Both RFA and laser ablation are also recommended for management of these lesions.15,26,43,44 In case of RFA, considering lesion size, ≥2 ablations with straight unipolar probes should be performed to cover the entire lesion (90°C for 6 minutes each). Alternatively, a navigational bipolar RFA probe could be used to articulate the probe tip in different orientations through a single entry site, with precise determination of the size of the ablation zone by using built-in thermocouples. Although the RF ablation zone could not be visualized on CT, the intact surrounding cortical bone serves as a barrier for undesired RF energy propagation.26 Similarly, with laser ablation, at least 2 probes and more energy deposition are required to achieve a cure (Fig 3). A substantial inflammatory reaction may occur following thermal ablation of osteoblastomas, and nonsteroidal anti-inflammatory drugs may be administered for management. Weber et al44 successfully managed 2 spinal osteoblastomas with a unipolar cool-tip RFA system (90°C for approximately 400 seconds) by using >1 ablation to cover the entire nidus with no complications.
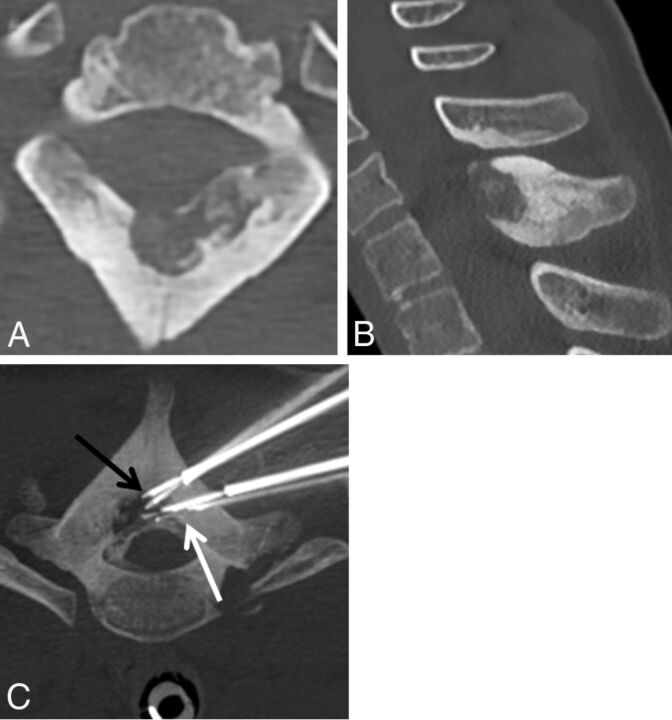
Fig 3.
A 13-year-old girl who had cervicothoracic junction pain due to a T1 osteoblastoma. Axial (A) and sagittal (B) CT demonstrates an osteolytic lesion within the anterior aspect of the T1 spinous process with cortical thickening and sclerosis surrounding the lesion. C, Prone axial maximum-intensity-projection image of a T1 osteoblastoma during laser ablation demonstrates 2 posterior laser photoelectrodes (black arrow) within the lesion and 2 anterior spinal needles (white arrow) placed for temperature monitoring.
Aneurysmal Bone Cyst.
Aneurysmal bone cyst is a benign expansile lesion of uncertain etiology comprising numerous blood-filled channels. It occurs in patients younger than 20 years of age in 80% of cases. Aneurysmal bone cyst involves the vertebral column in 20%–30% of cases (typically the posterior elements), of which about 40% involve the vertebral body. Aneurysmal bone cysts are expansile osteolytic lesions with fluid-fluid levels on MR imaging and thin peripheral/septal enhancement. These lesions may result in pain and neurologic compromise, prompting treatment. Standard surgical management depends on the lesion size and includes curettage, bone grafting, and internal fixation as well as en bloc or wide excision.45,46 Although no large studies exist to evaluate the efficacy of thermal ablation for the treatment of spinal aneurysmal bone cysts, given their larger size, typical involvement of posterior elements, and possible soft-tissue components, cryoablation is suggested as the thermal ablation technique of choice, and thermoprotection is recommended in all cases (Fig 4).26,45,46 Preablation embolization is also suggested to reduce the risk of hemorrhage and heat sink effect in large lesions.46 In case of pathologic fracture or extensive involvement of the vertebral body or pedicles, postablation cementation is recommended for stabilization.26,45 Griauzde et al46 reported the case of a spinal aneurysmal bone cyst involving the posterior elements of C6–T1 and the right C8 nerve root managed by cryoablation 4 days following embolization with N-butyl cyanoacrylate and lipiodol. The patient developed sensory neuropathy, which improved at 16-month follow-up, and imaging demonstrated no evidence of recurrence.46
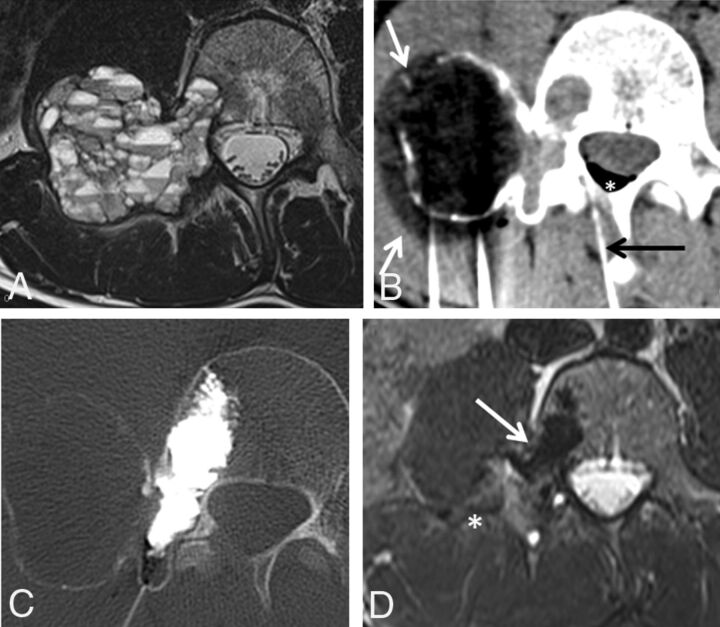
Fig 4.
A 17-year-old boy with low back pack due to a right L4 aneurysmal bone cyst. A, Axial T2-weighted image demonstrates an expansile lesion in the right L4 transverse process, pedicle, and posterior vertebral body with multiple fluid-fluid levels, most compatible with aneurysmal bone cyst. B, Axial CT image during cryoablation of the transverse process portion of the lesion. Note the hypoattenuating ice ball (white arrows) extending beyond portions of the lesion into the soft tissues. A spinal needle (black arrow) was placed into the epidural space for temperature monitoring and carbon dioxide injection (white asterisk) into the epidural space. C, Axial CT image during cementoplasty of the right L4 pedicle and posterior vertebral body components of the lesion. D, Axial T2-weighted image 3 months posttreatment demonstrates near-complete resolution of the expansile component of the tumor (white asterisk) and hypointense cement (white arrow) in the right pedicle and posterior vertebral body.
Hemangioma.
Vertebral hemangiomas are the most common benign vertebral neoplasms, with a slight female predilection, with most located in the thoracic spine. Most of these lesions are asymptomatic and incidentally identified on imaging. Classic imaging features include a polka-dotted appearance on CT and T1 hyperintensity and enhancement on MR imaging. Painful vertebral hemangiomas are typically due to impingement of the central canal or neuroforamina or concomitant pathologic fractures. Surgical intervention for vertebral hemangiomas is reserved for patients with neurologic compromise and spinal instability, and radiation therapy is used when immobilization fails to resolve neurologic symptoms or in cases of lesion progression following immobilization.47,48 PVA has been successfully used for minimally invasive management of symptomatic vertebral hemangiomas in the absence of neurologic compromise and spinal instability.47,48 PVA is associated with substantially less morbidity compared with an operation and radiation therapy and is more cost-effective. Liu et al,48 treated 33 symptomatic vertebral hemangiomas in 31 patients with PVA (mean follow-up, 15.8 months) and evaluated the clinical effects by using the Visual Analog Scale and Roland-Morris Disability Questionnaire at 1 week,1 month, and final follow-up. The authors reported substantial improvement in the Visual Analog Scale and Roland-Morris Disability Questionnaire scores and no major complications. Aggressive spinal hemangiomas, which are associated with extraosseous epidural and paravertebral components, compose approximately 1% of spinal hemangiomas and can be successfully treated with PVA.49,50 In a case series of 16 patients, Cloran et al49 treated 4 patients with symptomatic aggressive vertebral hemangiomas without neurologic compromise by using PVA, achieving complete pain resolution in all patients with no major complications.
Alcohol ablation followed by PVA has also been successfully used for management of symptomatic vertebral hemangiomas, including aggressive hemangiomas (Fig 5).30–32 As indicated previously, preablation venography with iodinated contrast should be performed by placement of a needle within the vertebral body to delineate the extent of ethanol diffusion to reduce the risk of the vascular intravasation and potential injury to adjacent structures. Doppman et al32 treated 11 patients with vertebral hemangiomas and neurologic symptoms using alcohol ablation with no immediate complications. Radiculopathy improved in 4 of 5 patients. Two patients who received the largest volumes of ethanol, 42 and 50 mL, developed pathologic compression fractures 4 and 16 weeks following treatment, respectively.32
Fig 5.
A 46-year-old man with upper back pain due to an aggressive T3 hemangioma. A, Axial contrast-enhanced CT scan demonstrates an aggressive T3 hemangioma with intraosseous and extraosseous components. B, Axial prone intraprocedural CT scan during a venogram performed via an 18-gauge needle in the left pedicle/vertebral body. Note extensive vascularity in the vertebral body and in the soft tissues surrounding the vertebral body, including the epidural space. C, Sagittal maximum-intensity-projection CT of a T3 venogram before alcohol ablation demonstrates a vascular lesion with epidural flow. Ethanol ablation was followed by vertebral augmentation for stabilization of the vertebral body. D, Post-alcohol ablation and vertebral augmentation CT demonstrates cement filling the vertebral body and the hyperattenuating ablated extraosseous component posterior to the vertebral body (white arrow).
Paget Disease.
Approximately 35%–50% of patients with Paget disease have spinal involvement, half of whom experience back pain.51 It can be seen in up to 10% of patients older than 80 years of age. The classic features of vertebral Paget disease include a “picture frame” appearance and squaring of vertebral bodies. Findings on MR imaging can be variable on the basis of the disease phase. Conservative management of painful vertebral Paget disease includes a combination of bisphosphonate therapy and analgesics.51–54 Patients with spinal instability and concomitant neurologic compromise are managed by surgical intervention. Patients refractory to conservative management, in the absence of spinal instability and neurologic deficit, have been successfully managed by PVA with no major complications (Fig 6).51–54 Pedicelli et al51 treated a patient with painful L4 Paget disease refractory to opioid analgesics and bisphosphonates with PVA and achieved substantially improved Visual Analog Scale pain and Roland-Morris Disability Questionnaire scores at day 1 and 6-month follow-up, with no complications.
Fig 6.
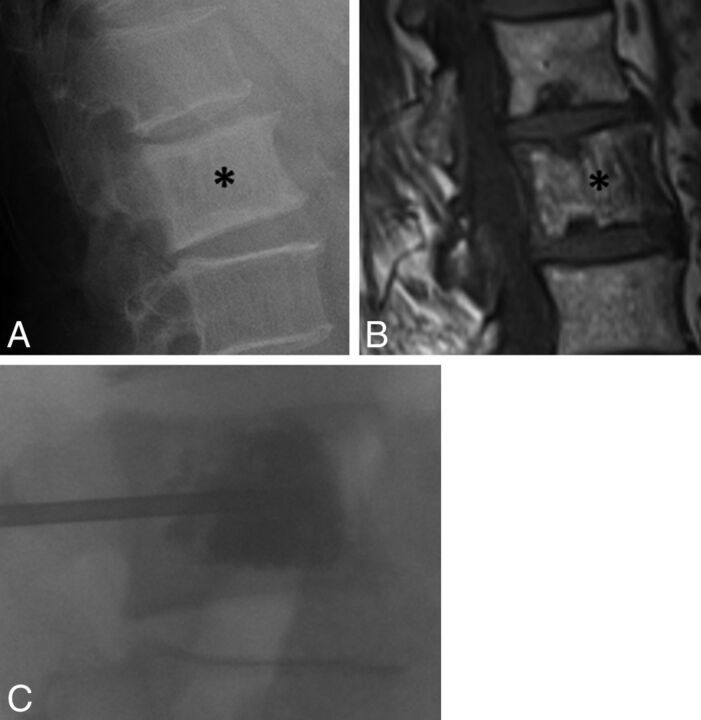
A 71-year-old man with chronic low back pain. A, Lateral radiograph of the lumbar spine shows an enlarged L2 vertebral body with cortical and trabecular thickening (asterisk), with a presumed diagnosis of Paget disease. B, Sagittal T1-weighted MR image of the lumbar spine shows vertically oriented trabecular thickening and enlargement of the L2 vertebral body (asterisk). C, Fluoroscopic image during vertebral augmentation shows cement filling the L2 vertebral body. At the conclusion of the procedure, the patient reported complete resolution of back pain.
Schmorl Nodes.
Schmorl nodes are common in all ages and may be associated with trauma. Symptomatic Schmorl nodes reflect acute or subacute vertebral endplate fracture, typically along the posterior margin, allowing vertical disc herniation and nuclear migration.55 Schmorl nodes as pain generators and their management remain a controversial topic. In cases of symptomatic Schmorl nodes, fluid-sensitive MR imaging sequences typically demonstrate hyperintensity along the node and vertebral endplate, which has been hypothesized as the source of pain.55 PVA may be an alternative option for the management of painful Schmorl nodes and degenerative Modic changes of the spine refractory to analgesics and conservative management (Fig 7).55,56 Masala et al55 managed 23 patients with painful Schmorl nodes refractory to medial and conservative management by using PVA. Pain improvement was achieved in 18 patients as evaluated 4 hours after the procedure (mean Visual Analog Scale score, 8.4 versus 2.3). The authors reported no cement leakage into the Schmorl nodes or intervertebral disc space (Fig 8).55 In addition, a Rami communicans nerve block has been successfully used for the management of symptomatic Schmorl nodes.57
Fig 7.
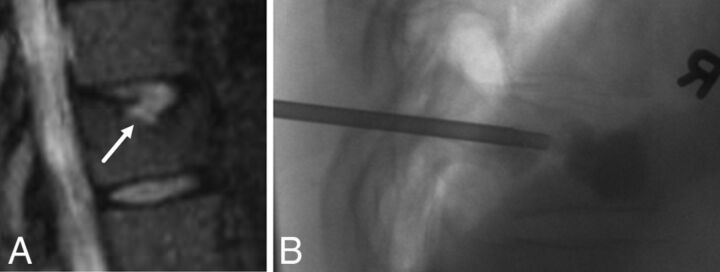
A 49-year-old woman with chronic midback pain. A, Sagittal STIR MR image of the thoracic spine demonstrates a large Schmorl node (arrow) along the superior endplate of the T10 vertebral body with associated hyperintensity. B, Lateral fluoroscopic image of the lower thoracic spine during kyphoplasty demonstrates filling of the anterior half of T10 vertebral body extending to surround the Schmorl node. Before the procedure, the patient had 6/10 pain. After the procedure, there was near-complete resolution of pain.
Fig 8.
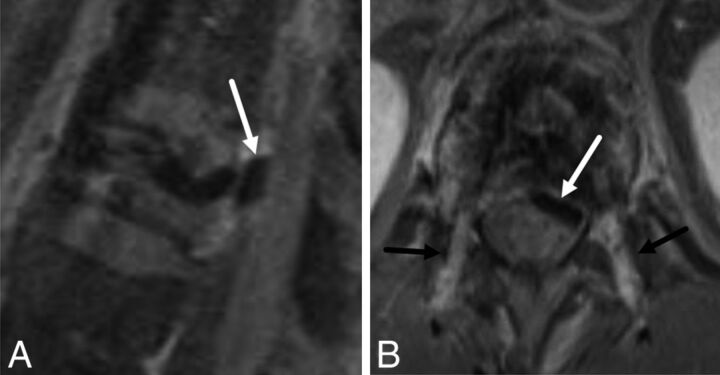
A 55-year-old man with cement leakage following radiofrequency ablation and vertebral augmentation. Sagittal (A) and axial (B) fat-suppressed T1-weighted contrast-enhanced MR images show hypointense cement within the epidural space, compatible with a leak (A and B, white arrow). Note enhancing granulation tissue along the transpedicular needle tracts (B, black arrows).
Other Benign Spine Lesions.
Several other benign spinal lesions have been successfully treated by using minimally invasive percutaneous thermal ablation and PVA with no major complications (Fig 9).58–62 However, the available literature is scant and limited to case reports, including PVA for the management of eosinophilic granuloma,58,59 RFA for the management of intraosseous glomus tumor and hibernoma,60,61 and cryoablation for the management of epithelioid hemangioendothelioma.62
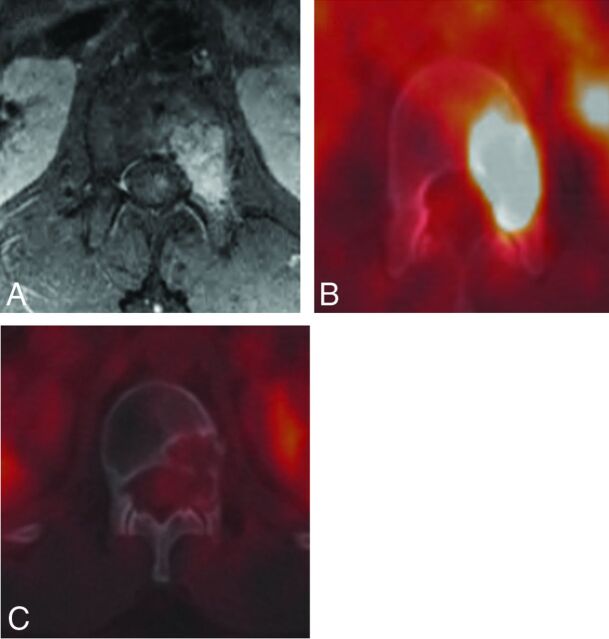
Fig 9.
A 21-year-old man with L1 pseudomyogenic hemangioendothelioma. A and B, MR imaging and PET-CT demonstrate an enhancing hypermetabolic bone marrow–replacing lesion within the L1 vertebra involving the left pedicle and posterior vertebral body. C, PET-CT performed 1 year following RFA demonstrates no evidence of residual or recurrent tumor.
Complications
The most important potential complication of percutaneous thermal ablation of the spine is injury to the spinal cord and nerve roots due to the proximity of the ablation zone to neural elements. In addition, there remains a risk of thermal skin injury. Furthermore, in ablation of vertebral body or pedicle lesions or large tumors, there is a potential risk of ablation-related fracture, which may be minimized with cementation.
Posttreatment Imaging
As stated earlier, contrast-enhanced MR imaging is the technique of choice for posttreatment imaging of benign spine lesions, which is performed at the discretion of the referring physician, if symptoms recur. The necrotic ablated volume shows no enhancement on postcontrast imaging, with variable signal intensity on T1- and T2-weighted sequences, depending on the relative amounts of sclerotic bone, residual vascular and yellow marrow, and hemorrhagic products.63 The ablated volume is surrounded by a T1-hypointense, T2-hyperintense rim that enhances after contrast administration and corresponds histologically to granulation tissue or vascular fibrosis.63 Cement appears as a signal void in the ablation cavity on all pulse sequences. Residual or recurrent tumor typically appears as T2-hyperintense, enhancing tissue at the margin of the ablation cavity; however, granulation tissue and vascular fibrosis can have identical MR imaging signal and enhancement characteristics,63 and the decision to proceed with further treatment should be based on clinical evaluation.
Thermoprotection and Thermal Monitoring
Percutaneous thermal tumor ablation in the spine poses an inherent risk of injury to the spinal cord and nerve roots due to the proximity of the ablation zone to the susceptible neural elements, which is the most important potential complication of these procedures. Numerous parameters affect the extent and severity of neurologic thermal injury, including absolute temperature, duration of thermal effect, distance from margins of ablation zone, presence or absence of intact osseous cortex, and type of nerve fiber.26 A slight motor function loss becomes evident at 10°C, and mild sensory loss, at 7°C, while both functions disappear between 5°C and 0°C.26
Current practice to prevent thermal injury during thermal spine ablation procedures involves the use of thermal insulation, and temperature and neurophysiologic monitoring.22,26,64,65 Thermal insulation can be achieved by hydrodissection or instillation of warm or cool liquid, which actively modifies the temperature surrounding the structure at risk. Hydrodissection in RFA procedures should be performed by using nonionic solutions such as dextrose 5% in water. Saline solutions should be avoided with RFA because the electrical conductivity may result in expansion of the ablation zone and creation of a plasma field.22,26 Carbon dioxide insufflation of the epidural space or neuroforamina can also be used to dissect and actively insulate the neural structures.22,26,64,65 In addition to insulation, continuous real-time and precise temperature monitoring may be undertaken during spine ablations by placing thermocouples close to the threatened structures, typically within the neuroforamina.22,26,64,65 In clinical practice, active thermoprotection (Figs 1–4) is initiated once the temperature reaches 45°C (heat) and 10°C (cold).22,26,64,65
Investigators have implemented neurophysiologic monitoring and nerve electrostimulation during spine thermal ablation procedures by using estimations of motor- and somatosensory-evoked potential amplitudes.65,66 Substantial reduction in the amplitude of evoked potential amplitudes affords early detection of impending neurologic injury, which should prompt active thermoprotection or modification in the ablation procedure. Skin injury is another potential complication of percutaneous thermal ablation. Careful assessment of the boundaries of the ablation zone minimizes the risk of skin injury. Active skin thermoprotection such as surface application of warm saline during cryoablation should be implemented to minimize skin injury. In the case of RFA, using a bipolar system inherently obviates the risk of skin burn, and use of grounding pads with unipolar systems decreases the risk of skin injury.
Summary
Continuously evolving image-guided percutaneous vertebral augmentation and spine thermal ablation procedures have been proved safe and effective tools in minimally invasive management of selected patients with benign spine lesions (Table). With the progressively increasing role of these procedures in clinical practice, radiologists should be familiar with the indications, techniques, potential complications, and most recent advances of these procedures for optimal patient care. Special attention to the details of procedural techniques, including the choice of treatment technology; thermoprotection; and the adequacy of treatment will translate into improved patient outcomes.
Summary of benign spine lesions and preferred treatment modalities
| Benign Spine Lesion | Recommended Treatment Modality |
|---|---|
| Osteoid osteoma | Radiofrequency ablation, laser ablation |
| Osteoblastoma | Cryoablation (if large or coexisting soft-tissue component) Radiofrequency ablation, laser ablation |
| Aneurysmal bone cyst | Cryoablation (due to involvement of posterior elements, soft-tissue component, and large size) |
| Cementation if extensive vertebral body involvement or pathologic fracture | |
| Preablation embolization suggested | |
| Hemangioma | Vertebral augmentation, alcohol ablation |
| Paget disease | Vertebral augmentation |
| Schmorl node | Vertebral augmentation |
ABBREVIATIONS:
- PVA
percutaneous vertebral augmentation
- RF
radiofrequency
- RFA
radiofrequency ablation
Footnotes
Disclosures: Adam N. Wallace—UNRELATED: Support for Travel to Meetings for the Study or other Purposes: DFINE,* Comments: DFINE paid my travel to a meeting to discuss a prospective clinical trial funded by the company; Other: DFINE,* Galil Medical,* Medtronic,* Comments: These companies donated ablation probes for an animal research study I am conducting. Jack W. Jennings—UNRELATED: Grant: DFINE, Comments: Principal Investigator for the STARRT trial*; Consulting Fee or Honorarium: Medtronic, Galil Medical, Comments: I have done consulting work with Galil Medical for almost a year. *Money paid to the institution.
REFERENCES
- 1. Kelley SP, Ashford RU, Rao AS, et al. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J 2007;16:405–09 10.1007/s00586-006-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hara S. Opioids for metastatic bone pain. Oncology 2008;74(suppl 1):52–54 [DOI] [PubMed] [Google Scholar]
- 3. Jiang L, Liu XG, Yuan HS, et al. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. Spine J 2014;14:944–54 10.1016/j.spinee.2013.07.450 [DOI] [PubMed] [Google Scholar]
- 4. Boyaci B, Hornicek FJ, Nielson GP, et al. Epithelioid hemangioma of the spine: a case series of six patients and review of the literature. Spine J 2013;13:e7–13 10.1016/j.spinee.2013.06.070 [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Liu X, Jiang L, et al. Treatments for primary aneurysmal bone cysts of the cervical spine: experience of 14 cases. Chin Med J (Engl) 2014;127:4082–86 [PubMed] [Google Scholar]
- 6. Pennekamp W, Peters S, Schinkel C, et al. Aneurysmal bone cyst of the cervical spine (2008:7b). Eur Radiol 2008;18:2356–60 10.1007/s00330-008-0944-7 [DOI] [PubMed] [Google Scholar]
- 7. Lutz S, Berk L, Chang E, et al. ; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965–76 10.1016/j.ijrobp.2010.11.026 [DOI] [PubMed] [Google Scholar]
- 8. McConnell CT Jr, Wippold FJ 2nd, Ray CE Jr, et al. ACR appropriateness criteria management of vertebral compression fractures. J Am Coll Radiol 2014;11:757–63 10.1016/j.jacr.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 9. Barr JD, Jensen ME, Hirsch JA, et al. ; Society of Interventional Radiology, American Association of Neurological Surgeons, Congress of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, American Society of Spine Radiology, Canadian Interventional Radiology Association, Society of Neurointerventional Surgery. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J Vasc Interv Radiol 2014;25:171–81 10.1016/j.jvir.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 10. Schupfner R, Stoevelaar HJ, Blattert T, et al. Treatment of osteoporotic vertebral compression fractures: applicability of appropriateness criteria in clinical practice. Pain Physician 2016;19:E113–20 [PubMed] [Google Scholar]
- 11. Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373:1016–24 10.1016/S0140-6736(09)60010-6 [DOI] [PubMed] [Google Scholar]
- 12. Yimin Y, Zhiwei R, Wei M, et al. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty: a review. Med Sci Monit 2013;19:826–36 10.12659/MSM.889479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anselmetti GC, Zoarski G, Manca A, et al. Percutaneous vertebroplasty and bone cement leakage: clinical experience with a new high-viscosity bone cement and delivery system for vertebral augmentation in benign and malignant compression fractures. Cardiovasc Intervent Radiol 2008;31:937–47 10.1007/s00270-008-9324-6 [DOI] [PubMed] [Google Scholar]
- 14. Trumm CG, Jakobs TF, Stahl R, et al. CT fluoroscopy-guided vertebral augmentation with a radiofrequency-induced, high-viscosity bone cement (StabiliT®): technical results and polymethylmethacrylate leakage in 25 patients. Skeletal Radiol 2013;42:113–20 10.1007/s00256-012-1386-5 [DOI] [PubMed] [Google Scholar]
- 15. Rybak L. Fire and ice: thermal ablation of musculoskeletal tumors. Radiol Clin North Am 2009;47:455–69 10.1016/j.rcl.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 16. Wallace AN, Tomasian A, Chang RO, et al. Treatment of osteoid osteomas using a navigational bipolar radiofrequency ablation system. Cardiovasc Intervent Radiol 2016;39:768–72 10.1007/s00270-015-1243-8 [DOI] [PubMed] [Google Scholar]
- 17. Singh S, Saha S. Electrical properties of bone: a review. Clin Orthop Relat Res 1984:249–71 [PubMed] [Google Scholar]
- 18. Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg 2007;205:342–56 10.1016/j.jamcollsurg.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 19. Tomasian A, Wallace A, Northrup B, et al. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol 2016;37:189–95 10.3174/ajnr.A4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saliken JC, McKinnon JG, Gray R. CT for monitoring cryotherapy. AJR Am J Roenrgenol 1996;166:853–55 10.2214/ajr.166.4.8610562 [DOI] [PubMed] [Google Scholar]
- 21. Callstrom MR, Kurup N. Percutaneous ablation for bone and soft tissue metastases: why cryoablation? Skeletal Radiol 2009;28:835–89 [DOI] [PubMed] [Google Scholar]
- 22. Rybak LD, Gangi A, Buy X, et al. Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. 2010;195:W293–98 10.2214/AJR.10.4192 [DOI] [PubMed] [Google Scholar]
- 23. Tsoumakidou G, Thénint MA, Garnon J, et al. Percutaneous image-guided laser photocoagulation of spinal osteoid osteoma: a single-institution series. Radiology 2016;278:936–43 10.1148/radiol.2015150491 [DOI] [PubMed] [Google Scholar]
- 24. Gangi A, Basile A, Buy X, et al. Radiofrequency and laser ablation of spinal lesions. Semin Ultrasound CT MR 2005;26:89–97 10.1053/j.sult.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 25. Gangi A, Alizadeh H, Wong L, et al. Osteoid osteomas: percutaneous laser ablation and follow-up in 114 patients. Radiology 2007;242:293–301 10.1148/radiol.2421041404 [DOI] [PubMed] [Google Scholar]
- 26. Tsoumakidou G, Koch G, Caudrelier J, et al. Image-guided spinal ablation: a review. Cardiovasc Intervent Radiol 2016;39:1229–38 10.1007/s00270-016-1402-6 [DOI] [PubMed] [Google Scholar]
- 27. Kurup AN, Callstrom MR. Ablation of skeletal metastases: current status. J Vasc Interv Radiol 2010;21:S242–50 10.1016/j.jvir.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 28. Kastler A, Alnassan H, Aubry S, et al. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol 2014;25:1470–75 10.1016/j.jvir.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 29. Pusceddu C, Sotgia B, Fele RM, et al. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol 2013;24:229–33 10.1016/j.jvir.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 30. Gangi A, Buy X. Percutaneous bone tumor management. Semin Intervent Radiol 2010;27:124–36 10.1055/s-0030-1253511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gabal AM. Percutaneous technique for sclerotherapy of vertebral hemangioma compressing spinal cord. Cardiovasc Intervent Radiol 2002;25:494–500 10.1007/s00270-002-1944-7 [DOI] [PubMed] [Google Scholar]
- 32. Doppman JL, Oldfield EH, Heiss JD. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology 2000;214:341–48 10.1148/radiology.214.2.r00fe46341 [DOI] [PubMed] [Google Scholar]
- 33. Rosenthal DI, Alexander A, Rosenberg AE, et al. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 1992;183:29–33 10.1148/radiology.183.1.1549690 [DOI] [PubMed] [Google Scholar]
- 34. Haibach H, Farrell C, Gaines RW. Osteoid osteoma of the spine: surgically correctable cause of painful scoliosis. CMAJ 1986;135:895–99 [PMC free article] [PubMed] [Google Scholar]
- 35. Dupuy DE, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol 2000;175:1263–66 10.2214/ajr.175.5.1751263 [DOI] [PubMed] [Google Scholar]
- 36. Cové JA, Taminiau AH, Obermann WR, et al. Osteoid osteoma of the spine treated with percutaneous computed tomography-guided thermocoagulation. Spine 2000;25:1283–86 10.1097/00007632-200005150-00014 [DOI] [PubMed] [Google Scholar]
- 37. Klass D, Marshall T, Toms A. CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol 2009;19:2238–43 10.1007/s00330-009-1404-8 [DOI] [PubMed] [Google Scholar]
- 38. Vanderschueren GM, Obermann WR, Dijkstra SP, et al. Radiofrequency ablation of spinal osteoid osteoma: clinical outcome. Spine (Phila Pa 1976) 2009;34:901–04 10.1097/BRS.0b013e3181995d39 [DOI] [PubMed] [Google Scholar]
- 39. Martel J, Bueno A, Nieto-Morales ML, et al. Osteoid osteoma of the spine: CT-guided monopolar radiofrequency ablation. Eur J Radiol 2009;71:564–69 10.1016/j.ejrad.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 40. Morassi LG, Kokkinis K, Evangelopoulos DS, et al. Percutaneous radiofrequency ablation of spinal osteoid osteoma under CT guidance. Br J Radiol 2014;87:20140003 10.1259/bjr.20140003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenthal DI, Marota JJ, Hornicek FJ. Osteoid osteoma: elevation of cardiac and respiratory rates at biopsy needle entry into tumor in 10 patients. Radiology 2003;226:125–28 10.1148/radiol.2261011993 [DOI] [PubMed] [Google Scholar]
- 42. Whitmore MJ, Hawkins CM, Prologo JD, et al. Cryoablation of osteoid osteoma in the pediatric and adolescent population. J Vasc Interv Radiol 2016;27:232–37; quiz 238 10.1016/j.jvir.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 43. Rehnitz C, Sprengel SD, Lehner B, et al. CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol 2012;81:3426–34 10.1016/j.ejrad.2012.04.037 [DOI] [PubMed] [Google Scholar]
- 44. Weber MA, Sprengel SD, Omlor GW, et al. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skeletal Radiol 2015;44:981–93 10.1007/s00256-015-2139-z [DOI] [PubMed] [Google Scholar]
- 45. Tsoumakidou G, Too CW, Garnon J, et al. Treatment of a spinal aneurysmal bone cyst using combined image-guided cryoablation and cementoplasty. Skeletal Radiol 2015;44:285–89 10.1007/s00256-014-1967-6 [DOI] [PubMed] [Google Scholar]
- 46. Griauzde J, Gemmete JJ, Farley F. Successful treatment of a Musculoskeletal Tumor Society grade 3 aneurysmal bone cyst with N-butyl cyanoacrylate embolization and percutaneous cryoablation. J Vasc Interv Radiol 2015;26:9050–59 10.1016/j.jvir.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 47. Hao J, Hu Z. Percutaneous cement vertebroplasty in the treatment of symptomatic vertebral hemangiomas. Pain Physician 2012;15:43–49 [PubMed] [Google Scholar]
- 48. Liu XW, Jin P, Wang LJ, et al. Vertebroplasty in the treatment of symptomatic vertebral haemangiomas without neurological deficit. Eur Radiol 2013;23:2575–81 10.1007/s00330-013-2843-9 [DOI] [PubMed] [Google Scholar]
- 49. Cloran FJ, Pukenas BA, Loevner LA, et al. Aggressive spinal haemangiomas: imaging correlates to clinical presentation with analysis of treatment algorithm and clinical outcomes. Br J Radiol 2015;88:20140771 10.1259/bjr.20140771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guarnieri G, Ambrosanio G, Vassallo P, et al. Vertebroplasty as treatment of aggressive and symptomatic vertebral hemangiomas: up to 4 years of follow-up. Neuroradiology 2009;51:471–76 10.1007/s00234-009-0520-0 [DOI] [PubMed] [Google Scholar]
- 51. Pedicelli A, Papacci F, Leone A, et al. Vertebroplasty for symptomatic monostotic Paget disease. J Vasc Interv Radiol 2011;22:400–03 10.1016/j.jvir.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 52. Kremer MA, Fruin A, Larson TC 3rd, et al. Vertebroplasty in focal Paget disease of the spine: case report. J Neurosurg (Spine) 2003;99:110–13 10.3171/spi.2003.99.1.0110 [DOI] [PubMed] [Google Scholar]
- 53. Tancioni F, Di Leva A, Levi D, et al. Spinal decompression and vertebroplasty in Paget's disease of the spine. Sury Neurol 2006;66:189–91 10.1016/j.surneu.2005.11.058 [DOI] [PubMed] [Google Scholar]
- 54. Wallace AN, Chang RO, Hsi AC, et al. Painful Pagetic vertebra palliated with percutaneous vertebral augmentation. Diagn Interv Imaging 2016;97:269–72 10.1016/j.diii.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 55. Masala S, Pipitone V, Tomassini M, et al. Percutaneous vertebroplasty in painful Schmorl nodes. Cardiovasc Intervent Radiol 2006;29:97–101 10.1007/s00270-005-0153-6 [DOI] [PubMed] [Google Scholar]
- 56. Masala S, Anselmetti GC, Marcia S, et al. Treatment of painful Modic type I changes by vertebral augmentation with bioactive resorbable bone cement. Neuroradiology 2014;56:637–45 10.1007/s00234-014-1372-9 [DOI] [PubMed] [Google Scholar]
- 57. Jang JS, Kwon HK, Lee JJ, et al. Rami communicans nerve block for the treatment of symptomatic Schmorl's nodes: a case report. Korean J Pain 2010;23:262–65 10.3344/kjp.2010.23.4.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan HQ, Li MH, Wu CG, et al. Percutaneous vertebroplasty for eosinophilic granuloma of the cervical spine in a child. Pediatr Radiol 2007;37:1053–57 10.1007/s00247-007-0575-1 [DOI] [PubMed] [Google Scholar]
- 59. Cardon T, Hachulla E, Flipo RM, et al. Percutaneous vertebroplasty with acrylic cement in the treatment of a Langerhans cell vertebral histiocytosis. Clin Rheumatol 1994;13:518–21 10.1007/BF02242955 [DOI] [PubMed] [Google Scholar]
- 60. Ringe KI, Imeen Ringe K, Rosenthal H, et al. Radiofrequency ablation of a rare case of an intraosseous hibernoma causing therapy-refractory pain. J Vasc Interv Radiol 2013;24:1754–56 10.1016/j.jvir.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 61. Becce F, Richarme D, Letovanec I, et al. Percutaneous radiofrequency ablation of primary intraosseous spinal glomus tumor. Skeletal Radiol 2012;41:467–72 10.1007/s00256-011-1308-y [DOI] [PubMed] [Google Scholar]
- 62. Sebastian AS, Adair MJ, Morris JM, et al. Minimally invasive treatment of a painful osteolytic lumbar lesion secondary to epithelioid hemangioendothelioma. Global Spine J 2015;5:135–39 10.1055/s-0034-1387198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wallace AN, Greenwood TJ, Jennings JW. Use of imaging in the management of metastatic spine disease with percutaneous ablation and vertebral augmentation. AJR Am J Roentgenol 2015;205:434–41 10.2214/AJR.14.14199 [DOI] [PubMed] [Google Scholar]
- 64. Buy X, Tok CH, Szwarc D, et al. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol 2009;32:529–34 10.1007/s00270-009-9524-8 [DOI] [PubMed] [Google Scholar]
- 65. Tsoumakidou G, Garnon J, Ramamurthy N, et al. Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Intervent Radiol 2013;36:1624–28 10.1007/s00270-013-0641-z [DOI] [PubMed] [Google Scholar]
- 66. Kurup AN, Morris JM, Boon AJ, et al. Motor evoked potential monitoring during cryoablation of musculoskeletal tumors. J Vasc Interv Radiol 2014;25:1657–64 10.1016/j.jvir.2014.08.006 [DOI] [PubMed] [Google Scholar]