Abstract
BACKGROUND AND PURPOSE:
Gadolinium-enhanced MR imaging is currently the reference standard for detecting active inflammatory lesions in patients with multiple sclerosis. The sensitivity of MR imaging for this purpose may vary according to the physicochemical characteristics of the contrast agent used and the acquisition strategy. The purpose of this study was to compare detection of gadolinium-enhancing lesions or active disease following a single or cumulative dose of a macrocyclic gadolinium-based contrast agent with different image acquisition delays in patients with clinically isolated syndrome or relapsing multiple sclerosis.
MATERIALS AND METHODS:
All patients received a first dose (0.1 mmol/kg) of gadobutrol and, 20 minutes later, a second dose (0.1 mmol/kg), with a cumulative dose of 0.2 mmol/kg. Two contrast-enhanced T1-weighted sequences were performed at 5 and 15 minutes after the first contrast administration, and 2 additional T1-weighted sequences at 5 and 15 minutes after the second contrast administration with a 3T magnet.
RESULTS:
One hundred fifteen patients were considered evaluable. A significantly larger number of lesions were detected in scans obtained at 5 and 15 minutes after the second contrast injection compared with scans obtained at 5 and 15 minutes after the first injection (P < .001). The number of patients with active lesions on MR imaging was significantly higher after the second dose administration (52.0%, first dose versus 59.2%, second dose; P < .001).
CONCLUSIONS:
Cumulative dosing of a macrocyclic gadolinium-based contrast agent increases detection of enhancing lesions and patients with active lesions. These data could be considered in the design of MR imaging protocols aimed at detecting active multiple sclerosis lesions.
Gadolinium-enhanced MR imaging is currently the reference standard for detecting inflammatory demyelinating lesions associated with increased permeability of the blood-brain barrier in patients with multiple sclerosis, and is commonly used as a marker of acute focal inflammatory activity.1,2 The sensitivity of the technique for this purpose may vary according to the physicochemical characteristics of the contrast agent used and the acquisition strategy (eg, delay between injection and image acquisition, contrast dose, field strength, and parameters of the postinjection T1-weighted sequence).3–12 A large body of evidence has indicated that various approaches can increase the visibility of contrast-enhancing lesions and lead to a notable improvement in sensitivity.3,4,8,9,12–15 One potential strategy that has not yet been explored is the combination of an increased contrast dose and a longer delay time at 3T MR imaging with a 2D gradient recalled-echo (GRE) T1-weighted sequence. To examine this option, we designed the present open-label, prospective study to assess the advantages of combining a high-field-strength MR imaging magnet (3T) and a cumulative gadolinium dose (0.1 mmol/kg + 0.1 mmol/kg) at different delay times compared with a single dose (0.1 mmol/kg) to detect active lesions in patients with clinically isolated syndrome (CIS) or relapsing MS. The hypothesis was that the combined advantages of a cumulative gadolinium dose and a longer delay time would significantly increase the detection rate of active lesions and the percentage of patients showing disease activity, measures that have a strong impact for the diagnosis of the disease, therapy optimization, and predicting disease course and treatment response.1,2,16
Materials and Methods
Patients
From January 2010 to December 2011, a prospective, single-center, open-label experimental phase IV study was performed at Vall d'Hebron University Hospital. The study compared a single dose and cumulative double dose of a macrocyclic gadolinium-based contrast agent (GBCA) (gadobutrol, Gadovist; Bayer Schering Pharma, Berlin, Germany) administration with different delay times at 3T to detect enhancing lesions in patients with CIS and relapsing MS using blinded, centralized MR imaging assessment.
Patients 18–50 years of age with CIS suggestive of central nervous system demyelination not attributable to other diseases and those with a diagnosis of relapsing MS based on the McDonald 2010 criteria17 showing at least 2 brain T2 lesions of the type seen in MS were eligible for inclusion in the study.
The exclusion criteria were as follows: pregnant or nursing women, patients having a pacemaker or any other factor that would preclude proximity to a strong magnetic field, those with severe claustrophobia or a known allergy to GBCAs, previous participants in a clinical trial of an investigational drug within 30 days before MR imaging, those with any medical condition that might decrease the chance of obtaining reliable data, and patients with known impaired renal function (glomerular filtration rate, <60 mL/min/1.73 m2).
The study protocol and consent documents were approved by the Clinical Research Ethics Committee of Hospital Vall d'Hebron, and the procedures were in accordance with the ethical standards of the Declaration of Helsinki, as revised in 2000. The blinded read was conducted in compliance with the International Conference on Harmonization of Good Clinical Practice guidelines and all applicable regulatory requirements. Before participation, eligible patients were informed of all aspects of the study and provided written informed consent.
Study Design and MR Imaging Protocol
Two visits took place before MR imaging acquisition. During the initial visit, consecutive patients with CIS (demonstrating on a previous MR imaging at least 2 focal brain lesions suggestive of MS) or relapsing MS were identified. All these patients were referred to the MR imaging unit as part of the diagnostic work-up in patients with CIS and of the monitoring process in patients with relapsing MS with suspected disease activity or progression.
A total of 122 patients were initially identified, though 7 (6%) were excluded because 5 did not have either CIS or relapsing MS and 2 had exclusion criteria. Ultimately, 115 patients were included in the study and analyzed; approximately one-quarter had CIS and three-quarters had relapsing MS.
During the second visit (visit 0), a pregnancy test was performed within 24 hours before MR imaging acquisition in women with childbearing potential, and demographic and clinical data were recorded. These included the patient's medical history, CIS or relapsing MS diagnosis, current medication, and neurologic examination results, including the Expanded Disability Status Scale score.
During visit 1, the MR imaging acquisition visit, all patients were examined on a 3T MR imaging scanner equipped with a 12-channel phased array head coil. The imaging protocol included the following sequences: 1) transverse proton density and T2-weighted fast spin-echo (TR, 2500 ms; TE, 16–91 ms; NEX, 1); and 2) transverse fast T2-FLAIR (TR, 9000 ms; TE, 100 ms; TI, 2500 ms; NEX, 2; flip angle, 120°). Both sequences were acquired with an FOV of 250 mm, and contiguous, 3-mm sections covering the whole brain (voxel size, 0.78 × 0.78 × 3.0 mm).
In patients in whom the previous sequences confirmed the presence of at least 2 T2 lesions, an unenhanced 2D GRE T1-weighted sequence was performed before the first GBCA dose, which was manually administered as an intravenous bolus at a rate of 1 mL/s. This first 0.1-mmol/kg dose (equivalent to 0.1 mL/kg) was followed by a 20-mL saline flush, and a first contrast-enhanced 2D GRE T1-weighted sequence was performed 5 minutes after contrast administration (scan A), and a second contrast-enhanced 2D GRE T1-weighted sequence was performed 10 minutes after scan A (scan B). Twenty minutes after the start of the first contrast administration, a second dose of GBCA (0.1 mmol/kg) was administered, followed by another saline flush, resulting in a cumulative dose of 0.2 mmol/kg (0.2 mL/kg). A third contrast-enhanced 2D GRE T1-weighted sequence was performed 5 minutes later (scan C), and a fourth contrast-enhanced 2D GRE T1-weighted sequence, 10 minutes after scan C (scan D).
The following parameters were used in all the 2D GRE T1-weighted sequences: TR, 297 ms; TE, 2.46 ms; NEX, 3; flip angle, 70°; FOV, 250 mm, acquiring contiguous 3-mm sections covering the whole brain (voxel size, 0.78 × 0.78 × 3.0 mm3).
Image Analysis
To determine the number of enhancing lesions, we independently evaluated each of the 4 sets of contrast-enhanced 2D GRE T1-weighted images in a random fashion. Three neuroradiologists with >10 years of experience (not affiliated with the center where the scans were obtained) read the images, blinded to the time point at which the enhanced T1-weighted sequences had been obtained and using proton density/T2, T2-FLAIR, and unenhanced T1-weighted sequences as references.
The 3 readers independently recorded the number and topography of enhancing lesions in each contrast-enhanced T1-weighted scan. The number of lesions per patient was restricted to a maximum of 20 lesions for each scan. Patients with MR imaging–active lesions were defined as those showing at least 1 enhancing lesion in any of the contrast-enhanced T1-weighted sequences. In addition, the readers graded the quality of the images in 3 groups, good, average, or poor, and expressed their level of confidence in the detection of each enhancing lesion in each scan as very confident, confident, or not confident.
Efficacy Analysis: End Points
The end points of the study were the number of enhancing lesions and the number of patients with MR imaging–active lesions detected with a cumulative dose of a macrocyclic GBCA versus a single dose obtained at different time delays.
Adverse Events
Adverse events were recorded and classified into mild, moderate, or severe. The observation period for recording adverse events ran from the start of administration of the first gadolinium dose up to 24 hours after administration of the second dose.
Sample Size Calculation
To assess differences in active lesion detection in the 2 types of patients following administration of a single or cumulative contrast dose, we would have to include a minimum of 115 patients in the study. The sample size was determined on the basis of an expected mean of 2.1 enhancing lesions detected with a single dose of gadolinium (1.5 lesions in CIS and 2.7 in relapsing MS)18,19 and a 15% minimum increase in the number of lesions detected after the second dose,12,20 considering that 15% of patients initially included in the study would be excluded from the final analysis.
Statistical Analysis
Descriptive analyses (mean, median, SD, minimum, and maximum) were performed for the quantitative variables, and frequency counts by category were calculated for the qualitative variables. Confidence intervals, given when appropriate, were 2-sided in each case and provided 95% confidence.
The total number of enhancing lesions per patient between each time point was compared with Poisson regression based on generalized estimations. The number of patients with active lesions at each time point was compared using a χ2 test. Statistical significance was set at a P value of <.05. κ coefficients were calculated to assess the agreement among the readers' assessments in pair-wise comparisons. Statistical analyses were performed with SAS, Version 9.3 (SAS Institute, Cary, North Carolina).
Results
Baseline demographic and clinical characteristics were homogeneous between the groups, with more women than men in both the CIS and relapsing MS groups (Table 1). Six (5.2%) patients had ≤8 T2 lesions, whereas 109 patients (94.8%) had >8 T2 lesions. Three (11.5%) patients with CIS and 47 (52.8%) with relapsing MS were under a disease-modifying treatment at the time of the study. There was 1 mild adverse event (nausea after the first contrast injection), which did not require any treatment other than observation.
Table 1:
Baseline characteristics
| Patients with CIS (n = 26) | Patients with MS (n = 89) | Total (n = 115) | |
|---|---|---|---|
| Sex (No.) (%) | |||
| Female | 17 (65.4) | 66 (74.2) | 83 (72.2) |
| Male | 9 (34.6) | 23 (25.8) | 32 (27.8) |
| Age (yr) | |||
| Mean (SD) | 32.9 (7.1) | 36.0 (6.5) | 35.3 (6.7) |
| Median | 31.0 | 36.0 | 35.0 |
| Min, max | (25.0, 47.0) | (23.0, 50.0) | (23.0, 50.0) |
| EDSS score | |||
| Mean (SD) | 1.6 (1.1) | 3.0 (1.7) | 2.7 (1.7) |
| Median | 1.5 | 3 | 2.5 |
| Min, max | (0.0, 4.0) | (0.0, 7.5) | (0.0, 7.5) |
Note:—EDSS indicates Expanded Disability Status Scale; Min, minimum; max, maximum.
Contrast-Enhancing Lesions
All 115 evaluable patients received the first and second gadolinium injections. The mean time interval between the first and second injections was 20.3 ± 1.7 minutes (19.8 ± 1.0 in CIS and 20.4 ± 1.8 in relapsing MS). After the first gadolinium dose, the 2D GRE T1-weighted scans were obtained at a mean postinjection time of 5.0 ± 0.2 minutes (scan A) and 15.1 ± 0.7 minutes (scan B). After the second dose, the scans were obtained at a mean postinjection time of 5.0 ± 0.0 minutes (scan C) and 15.1 ± 0.8 minutes (scan D).
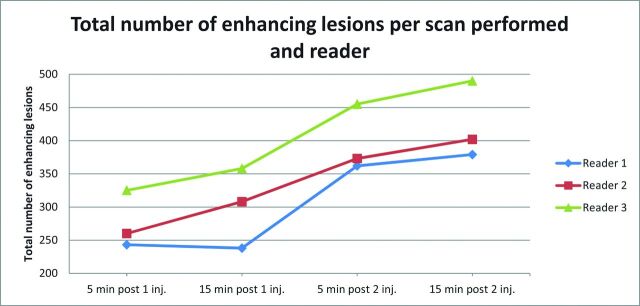
The number of enhancing lesions detected on each MR imaging scan did not significantly differ among the 3 readers (Table 2). The total number of enhancing lesions was similar in images obtained at 5 (scan A) and 15 minutes (scan B) after the first contrast injection (276 versus 301) and at 5 (scan C) and 15 (scan D) minutes after the second dose (397 versus 424) (Fig 1). However, a significantly larger number of lesions were detected in scans obtained at 5 (mean, 5.8) and 15 minutes (mean, 6.2) after the second contrast injection than at 5 (mean, 4.5) and 15 minutes after the first injection (mean, 4.9) (P < .001). The increase in total lesion count between the single-dose scan obtained at 5 minutes and the cumulative scan at 15 minutes was 58% (Table 3). This increase was only 8.7% between the 5- and 15-minute single-dose scans, and 6.6% between the 5- and 15-minute cumulative-dose scans, but an increase of 27.3% was seen between the 15-minute single-dose scan and the 5-minute cumulative-dose scan. Examples of lesion detection on images obtained at different time points are shown in Figs 2–4.
Table 2:
Enhancing lesion count per scan and readera
| Scanb | Lesion No. |
|---|---|
| Reader 1 | |
| A | 243 |
| B | 238 |
| C | 362 |
| D | 379 |
| Reader 2 | |
| A | 260 |
| B | 308 |
| C | 373 |
| D | 402 |
| Reader 3 | |
| A | 325 |
| B | 358 |
| C | 455 |
| D | 490 |
| Total lesion No. (mean) | |
| A | 828 (2.4) |
| B | 904 (2.6) |
| C | 1190 (3.5) |
| D | 1271 (3.7) |
The number of enhancing lesions detected in each MRI scan did not significantly differ among the 3 readers (P = .4280).
A, early single dose; B, delayed single dose; C, early cumulative dose; D, delayed cumulative dose.
Fig 1.
Total number of enhancing lesions per scan obtained and reader.
Table 3:
Number of enhancing lesions in patients with active lesions by scan and readera
| Reader, Scanb | No.c | Total | Median (mean) | Minimum | Maximumd |
|---|---|---|---|---|---|
| 1 | |||||
| A | 56 | 243 | 2.5 (4.3) | 1.000 | 20.000 |
| B | 54 | 238 | 2.0 (4.4) | 1.000 | 20.000 |
| C | 61 | 362 | 3.0 (5.9) | 1.000 | 20.000 |
| D | 63 | 379 | 3.0 (6.0) | 1.000 | 20.000 |
| 2 | |||||
| A | 58 | 260 | 2.0 (4.5) | 1.000 | 20.000 |
| B | 61 | 308 | 3.0 (5.0) | 1.000 | 20.000 |
| C | 68 | 373 | 3.0 (5.5) | 1.000 | 20.000 |
| D | 64 | 402 | 3.0 (6.3) | 1.000 | 20.000 |
| 3 | |||||
| A | 69 | 325 | 2.0 (4.7) | 1.000 | 20.000 |
| B | 67 | 358 | 3.0 (5.3) | 1.000 | 20.000 |
| C | 76 | 455 | 3.0 (6.0) | 1.000 | 20.000 |
| D | 79 | 490 | 3.0 (6.2) | 1.000 | 20.000 |
A higher number of enhancing lesions was detected for cumulative-versus-single dose scans (P < .001).
A, early single dose; B, delayed single dose; C, early cumulative dose; D, delayed cumulative dose.
No., number of patients with active lesions.
The number of lesions was restricted to a maximum of 20 per patient.
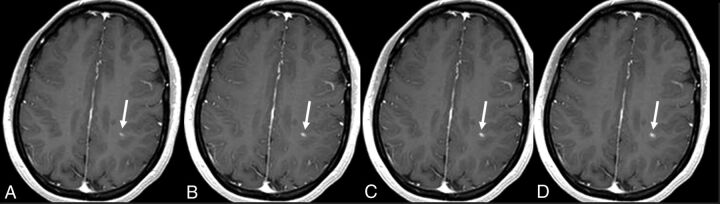
Fig 2.
A 26-year-old woman with active relapsing-remitting multiple sclerosis. A small juxtacortical peri-Rolandic lesion located in the left brain hemisphere is identified on the 4 contrast-enhanced T1-weighted scans (arrows). Observe the increase in lesion size in the delayed single-dose scan (B) and cumulative-dose scans (C and D) compared with the early single-dose scan (A). Nonetheless, lesion detection is comparable in all 4 scan conditions.
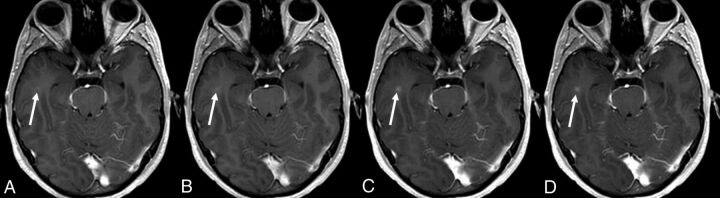
Fig 3.
A 26-year-old man presenting with clinically isolated syndrome. A small enhancing lesion located in the right temporal subcortical white matter is seen on the 2 cumulative-dose contrast-enhanced T1-weighted scans (arrows in C and D) but is initially missed on the 2 single-dose scans. Mild enhancement is seen in the early and delayed single-dose scans (arrows in A and B) only in retrospect.
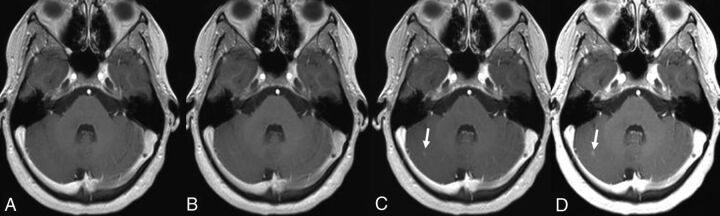
Fig 4.
A 25-year-old woman presenting with clinically isolated syndrome. A nodular enhancing lesion located in the right cerebellar hemisphere is seen on the 2 cumulative-dose contrast-enhanced T1-weighted scans (arrows in C and D) but was not identified on the early and delayed single-dose scans (A and B). The intensity of lesion enhancement is higher on the delayed cumulative-dose scan (D).
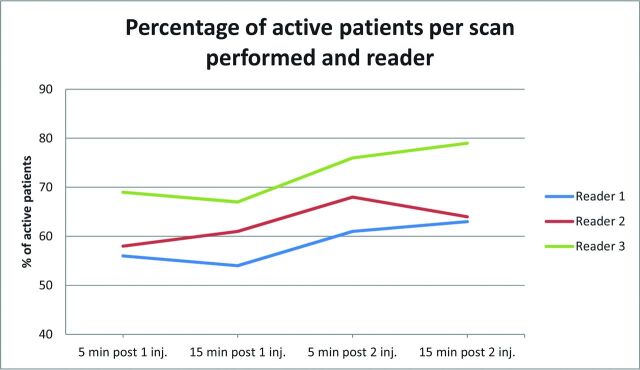
The number of patients showing at least 1 gadolinium-enhancing lesion (MR imaging–active) increased from 52% in the single-dose scans to 58.9%–59.2% in the 2 cumulative-dose scans (P < .001) (Fig 5).
Fig 5.
Percentage of patients with active lesions per scan performed and reader.
As to reproducibility in the assessment of patients with active lesions, agreement between readers 1 and 2 was highest for scan D (Table 4). Agreement between reader 3 and the other 2 readers did not show a high level of concordance for either the cumulative or delay scans.
Table 4:
κ coefficients of the interreader agreement for the number of patients with active lesions
| Postgadolinium T1-Weighted Scana | κ (95% CI) |
||
|---|---|---|---|
| Reader 1 vs Reader 2 | Reader 1 vs Reader 3 | Reader 2 vs Reader 3 | |
| A | 0.79 (0.68–0.90) | 0.71 (0.58–0.83) | 0.67 (0.54–0.80) |
| B | 0.85 (0.75–0.94) | 0.71 (0.59–0.83) | 0.83 (0.72–0.93) |
| C | 0.81 (0.70–0.92) | 0.70 (0.58–0.83) | 0.78 (0.67–0.90) |
| D | 0.95 (0.89–1.00) | 0.68 (0.55–0.81) | 0.70 (0.57–0.82) |
A, early single dose; B, delayed single dose; C, early cumulative dose; D, delayed cumulative dose.
Postcontrast Image Quality
Image quality was rated as good for most images in all 4 scans obtained: reader 1, 93.97%; reader 2, 84.95%; and reader 3, 97.20% of the total. The readers showed no differences in their rating of image quality among the 4 scans. No scan was rated as poor; therefore, all images obtained were included in the analysis. Readers expressed a higher percentage of very confident assessments for scans performed after the second injection and 15 minutes' delay (reader 1, P = .022; reader 2, P = .004; and reader 3, P = .005) (Table 3). Also across readers, significant differences were found between the image sets and the readers' assessment (P < .001), but not for the interaction between image set and reader.
The mean percentages of enhancing lesions in the 4 sets of images for which the observers rated identification as very confident were 41%, 45%, 54%, and 54% (A, B, C, and D, respectively), with higher values in the sets with longer delay times and higher doses (P < .001).
Discussion
This study shows that a cumulative dose (0.2 mmol/kg) of a macrocyclic GBCA resulted in a significant increase in the sensitivity of contrast-enhanced 3T MR imaging for detecting active lesions in patients with CIS or relapsing MS, compared with a single contrast dose. Furthermore, this approach led to a higher percentage of patients with active lesions—that is, showing at least 1 gadolinium-enhancing lesion, which is an essential feature in the initial evaluation of patients suspected of having MS and a highly relevant prognostic marker, particularly in patients with MS under disease-modifying treatments.21,22 The readers found no differences in the quality of image sets obtained under different conditions of contrast dose and delay, whereas they conveyed higher confidence for detecting contrast-enhancing lesions with the cumulative-dose delayed scan. Moreover, a higher concordance in identifying patients with active lesions was observed between the first and second readers with the cumulative-dose delayed scan, but not between the third reader and the other 2, with a low level of concordance observed in all 4 scans. These discrepant results are because reader 3 detected a larger number of active lesions in each scan compared with readers 1 and 2, though this difference did not reach significance.
The results of our study are in line with those in previous studies, in which greater lesion detection was found with the use of higher gadolinium doses or time delays on 2D spin-echo sequences at 1.5T.3,6,7,12,14,20 However, this study is the first to demonstrate this effect with 2D GRE sequences at 3T, a common scanning strategy used in clinical practice. In the present study, we selected a 2D GRE sequence because it reduces the flow artifacts seen with 2D spin-echo sequences, which are more problematic at 3T,23 leading to a decrease in background noise and better lesion detection.
Gadolinium-enhanced brain MR imaging is the most sensitive tool for detecting focal inflammatory activity in MS and is essential for establishing an early MS diagnosis and for predicting the disease course.17 Furthermore, the existence and degree of inflammatory activity are often used as an outcome measure in clinical trials, to select patients for initiation of disease-modifying treatment, to predict the risk of disability progression,24 and to monitor and predict treatment effect.25–28 In fact, several studies have attempted to define criteria and models for the early identification of incomplete response in individual patients via a combination of clinical and MR imaging measures during the first 6–12 months after treatment initiation, which are partially or completely based on the detection of disease activity.28–33 All these data support the pivotal role of the presence and number of gadolinium-enhancing lesions to assess radiologic disease activity, which is used, in turn, for diagnosing and guiding therapeutic strategies and as a surrogate marker to evaluate treatment efficacy in clinical trials. Thus, reliable detection of enhancing lesions is an important clinical and research goal in patients with CIS and MS, and considerable effort is required to optimize the parameters used in standard techniques to maximize the sensitivity of MR imaging for this purpose.
One strategy to increase the sensitivity of enhanced MR imaging for detecting active brain lesions in patients with MS is the use of high doses of contrast agent.3,6,8 Our findings indicate that this factor is the main driver of the improved sensitivity found. In our patients with CIS and MS, the sensitivity increase was mainly due to cumulative contrast dosing, whereas the influence of time was much weaker: Significant differences were detected between scans performed with single and cumulative doses, but not between the different delay times (5 and 15 minutes) used in each of the dosing regimens.
In a previous study, use of 1.0 mol/L (0.2 mmol/kg) of GBCA allowed detection of a larger number of enhancing lesions and patients with active disease than the use of 0.5 mol/L at the same dose, but the number of enhancing lesions did not differ significantly between images obtained 5 and 10 minutes after the injection.8 In contrast to that study, in which a single dose was followed by a double dose administered 24–48 hours later, we used 2 consecutive doses of GBCA, resulting in a cumulative dose of 0.2 mmol/kg. Our data support the results of other authors reporting that the combination of high contrast doses with other strategies, such as delayed acquisition, can increase the sensitivity of lesion detection compared with standard approaches.3,8 In addition, the contrast administration schedule we used was not associated with severe adverse effects in the population studied.
The design used in this study would be very difficult to implement in clinical practice (2 consecutive injections of gadolinium to achieve the cumulative double dose). However, on the basis of the results of the present study that show a significant increase in the detection of active lesions and scans showing active lesions after the second injection of contrast, we may suggest the use of a single injection of a double dose of gadolinium and a minimum delay of 5 minutes before the acquisition of the T1-weighted sequence. This “dead” time can be used to perform the T2-FLAIR sequences, so that the total acquisition time is not lengthened.21 This suggestion can facilitate the use of this strategy in clinical practice.
We also have to consider recent literature showing that with repeat contrast administration, gadolinium accumulates in the brain despite an intact blood-brain barrier and normal renal function, producing an increase in signal intensity in certain areas, such as the dentate nucleus and globus pallidus.34 This effect, which has not been associated with histopathologic findings,35,36 has mainly been detected with linear GBCAs.37–39 Only 1 study has shown signal changes in the dentate nucleus and globus pallidus after several administrations of a macrocyclic GBCA.40 However, in this study, signal changes could not be detected visually (only quantitatively), and the design of the study could not control for or exclude the use of other GBCAs, including linear agents. Moreover, the results of this study contradict other clinical studies that could not find signal-intensity changes in patients receiving several doses of the same macrocyclic GBCA used in our study.41–46 All these data have been recently analyzed in a systematic review article based on the analysis of 25 original publications,39 showing that the signal changes in certain central nervous system structures identified with MR imaging correlated positively with the exposure to linear agents but negatively with the stability of contrast agents, which is higher with macrocyclic agents. Nonclinical studies have indicated that all types of gadolinium can be deposited in different tissues, though the detected residual gadolinium concentration in the brain is approximately 1- to 15-fold higher for linear than for macrocyclic GBCAs.36,47
Although no symptoms or diseases linked to brain gadolinium deposition have been reported,35 data on long-term effects are still limited.
This study has limitations. First, the design did not enable us to establish the relative influences of cumulative dose and delayed scanning on the increase in sensitivity, though the results obtained indicate that the differences were mainly driven by the cumulative gadolinium dose. Second, the high variability in the detection of active lesions by one of the readers limits the interpretation of the higher concordance observed in cumulative and delayed scans compared with single-dose early scans between the 2 other readers.
Conclusions
The results of this study indicate that a cumulative dose (0.2 mmol/kg) of macrocyclic GBCA administered as an intravenous bolus in 2 injections of 0.1 + 0.1 mmol/kg is a safe procedure that significantly increases the detection rate of gadolinium-enhancing lesions in patients with CIS or relapsing MS. Furthermore, this approach enables identification of a larger number of patients with active lesions and provides higher confidence in lesion detection. On the basis of these results, the use of a single injection of a double dose of macrocyclic gadolinium-based contrast and a delay time of 5 minutes could be considered in brain MR imaging studies aimed at detecting active MS lesions whenever this purpose could have relevant therapeutic implications. However, on the basis of the limited data on the long-term effects of gadolinium deposition in the brain, which could also occur with macrocyclic GBCAs, the use of this strategy in all routine MR imaging examinations of patients with MS is not recommended.
ABBREVIATIONS:
- CIS
clinically isolated syndrome
- GBCA
gadolinium-based contrast agent
- GRE
gradient recalled-echo
Footnotes
Disclosures: Alex Rovira—RELATED: Grant: Bayer HealthCare, Comments: This research was funded by Bayer HealthCare Pharmaceuticals*; UNRELATED: Board Membership: Biogen Idec, Novartis, Sanofi Genzyme, Olea Medical; Consultancy: Biogen Idec, Novartis, Sanofi Genzyme, Olea Medical; Payment for Lectures Including Service on Speakers Bureaus: Bayer HealthCare Pharmaceuticals, Sanofi Genzyme, Bracco, Merck Serono, Teva Pharmaceutical Industries Ltd, Novartis, Biogen Idec; Payment for Development of Educational Presentations: Bayer HealthCare Pharmaceuticals, Bracco, Teva Pharmaceutical Industries Ltd, Novartis, Biogen Idec. Cristina Auger—RELATED: Grant: Bayer HealthCare Pharmaceuticals*; UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Novartis, Biogen Idec, Stendhal America SA. Elena Huerga—RELATED: Grant: Bayer HealthCare Pharmaceuticals.* Juan Francisco Corral—RELATED: Grant: Bayer HealthCare Pharmaceuticals.* Raquel Mitjana—RELATED: Grant: Bayer HealthCare Pharmaceuticals.* Jaume Sastre-Garriga—RELATED: Grant: Bayer HealthCare Pharmaceuticals*; Consultancy: Novartis, Biogen Idec, Celgene; Grants/Grants Pending: Sanofi Genzyme*; Payment for Lectures Including Service on Speakers Bureaus: Teva Pharmaceutical Industries Ltd, Almirall, Novartis, Biogen Idec, Merck Serono, Sanofi Genzyme; Payment for Development of Educational Presentations: Novartis, Biogen Idec; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Novartis, Merck Serono.* Mar Tintoré—UNRELATED: Board Membership: Biogen Idec, Sanofi Genzyme, Hoffmann-La Roche; Grants/Grants Pending: Biogen Idec, Novartis, Sanofi Genzyme, Merck Serono*; Payment for Lectures Including Service on Speakers Bureaus: Amirall, Bayer HealthCare Pharmaceuticals, Biogen Idec, Sanofi Genzyme, Merck Serono, Novartis, Sanofi Aventis, Hoffmann-La Roche, Teva Pharmaceutical Industries Ltd; Payment for Development of Educational Presentations: Biogen Idec. Xavier Montalban—RELATED: Grant: Bayer HealthCare Pharmaceuticals*; UNRELATED: Consultancy: Actelion, Bayer HealthCare Pharmaceuticals, Biogen Idec, Celgene, Sanofi Genzyme, Merck Serono, Novartis, Oryzon Genomics, Hoffmann-La Roche, Sanofi Genzyme, Teva Pharmaceutical Industries Ltd. *Money paid to the institution.
This work was funded by Bayer HealthCare Pharmaceuticals.
REFERENCES
- 1. Filippi M, Rocca MA. MR imaging of multiple sclerosis. Radiology 2011;259:659–81 10.1148/radiol.11101362 [DOI] [PubMed] [Google Scholar]
- 2. Zivadinov R, Stosic M, Cox JL, et al. The place of conventional MRI and newly emerging MRI techniques in monitoring different aspects of treatment outcome. J Neurol 2008;255(suppl 1):61–74 10.1007/s00415-008-1009-1 [DOI] [PubMed] [Google Scholar]
- 3. Wolansky LJ, Finden SG, Chang R, et al. Gadoteridol in multiple sclerosis patients: a comparison of single and triple dose with immediate vs. delayed imaging. Clin Imaging 1998;22:385–92 10.1016/S0899-7071(98)00072-2 [DOI] [PubMed] [Google Scholar]
- 4. Filippi M, Rovaris M, Capra R, et al. A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis: implications for phase II clinical trials. Brain 1998;121:2011–20 10.1093/brain/121.10.2011 [DOI] [PubMed] [Google Scholar]
- 5. Rovaris M, Codella M, Moiola L, et al. Effect of glatiramer acetate on MS lesions enhancing at different gadolinium doses. Neurology 2002;59:1429–32 10.1212/01.WNL.0000033800.93899.E1 [DOI] [PubMed] [Google Scholar]
- 6. Sardanelli F, Iozzelli A, Losacco C, et al. Three subsequent single doses of gadolinium chelate for brain MR imaging in multiple sclerosis. AJNR Am J Neuroradiol 2003;24:658–62 [PMC free article] [PubMed] [Google Scholar]
- 7. Stecco A, Migazzo E, Saponaro A, et al. Gadolinium dose optimisation in patients with multiple sclerosis: intra- and inter-individual comparisons. Eur J Radiol 2006;57:37–42 10.1016/j.ejrad.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 8. Uysal E, Erturk SM, Yildirim H, et al. Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 2007;188:697–702 10.2214/AJR.05.2212 [DOI] [PubMed] [Google Scholar]
- 9. Sicotte NL, Voskuhl RR, Bouvier S, et al. Comparison of multiple sclerosis lesions at 1.5 and 3.0 Tesla. Invest Radiol 2003;38:423–27 [DOI] [PubMed] [Google Scholar]
- 10. Hodel J, Outteryck O, Ryo E, et al. Accuracy of postcontrast 3D turbo spin-echo MR sequence for the detection of enhanced inflammatory lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol 2014;35:519–23 10.3174/ajnr.A3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crombé A, Saranathan M, Ruet A, et al. MS lesions are better detected with 3D T1 gradient-echo than with 2D T1 spin-echo gadolinium-enhanced imaging at 3T. AJNR Am J Neuroradiol 2015;36:501–07 10.3174/ajnr.A4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silver NC, Good CD, Barker GJ, et al. Sensitivity of contrast enhanced MRI in multiple sclerosis: effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 1997;120:1149–61 10.1093/brain/120.7.1149 [DOI] [PubMed] [Google Scholar]
- 13. Silver NC, Good CD, Sormani MP, et al. A modified protocol to improve the detection of enhancing brain and spinal cord lesions in multiple sclerosis. J Neurol 2001;248:215–24 10.1007/s004150170229 [DOI] [PubMed] [Google Scholar]
- 14. Filippi M, Capra R, Campi A, et al. Triple dose of gadolinium-DTPA and delayed MRI in patients with benign multiple sclerosis. J Neurol Neurosurg Psychiatry 1996;60:526–30 10.1136/jnnp.60.5.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Waesberghe JH, Castelijns JA, Roser W, et al. Single-dose gadolinium with magnetization transfer versus triple-dose gadolinium in the MR detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 1997;18:1279–85 [PMC free article] [PubMed] [Google Scholar]
- 16. Tourdias T, Dousset V. Neuroinflammatory imaging biomarkers: relevance to multiple sclerosis and its therapy. Neurotherapeutics 2013;10:111–23 10.1007/s13311-012-0155-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comi G, Martinelli V, Rodegher M, et al. ; PreCISe study group. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009;374:1503–11 10.1016/S0140-6736(09)61259-9 [DOI] [PubMed] [Google Scholar]
- 19. Fox RJ, Miller DH, Phillips JT, et al. ; CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–97 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 20. Filippi M, Yousry T, Rocca MA, et al. Sensitivity of delayed gadolinium-enhanced MRI in multiple sclerosis. Acta Neurol Scand 1997;95:331–34 10.1111/j.1600-0404.1997.tb00220.x [DOI] [PubMed] [Google Scholar]
- 21. Rovira À, Wattjes MP, Tintoré M, et al. ; MAGNIMS study group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol 2015;11:471–82 10.1038/nrneurol.2015.106 [DOI] [PubMed] [Google Scholar]
- 22. Wattjes MP, Rovira À, Miller D, et al. ; MAGNIMS study group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis–establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015;11:597–606 10.1038/nrneurol.2015.157 [DOI] [PubMed] [Google Scholar]
- 23. Drangova M, Pelc NJ. Artifacts and signal loss due to flow in the presence of B(o) inhomogeneity. Magn Reson Med 1996;35:126–30 10.1002/mrm.1910350116 [DOI] [PubMed] [Google Scholar]
- 24. Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann Neurol 2013;73:95–103 10.1002/ana.23758 [DOI] [PubMed] [Google Scholar]
- 25. Kappos L, Moeri D, Radue EW, et al. ; Gadolinium MRI Meta-Analysis Group. Predictive value of gadolinium-enhanced magnetic resonance imaging for relapse rate and changes in disability or impairment in multiple sclerosis: a meta-analysis. Lancet 1999;353:964–69 10.1016/S0140-6736(98)03053-0 [DOI] [PubMed] [Google Scholar]
- 26. Polman C, Kappos L, Freedman MS, et al. ; BENEFIT investigators. Subgroups of the BENEFIT study: risk of developing MS and treatment effect of interferon beta-1b. J Neurol 2008;255:480–87 10.1007/s00415-007-0733-2 [DOI] [PubMed] [Google Scholar]
- 27. Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 2013;12:669–76 10.1016/S1474-4422(13)70103-0 [DOI] [PubMed] [Google Scholar]
- 28. Río J, Castilló J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009;15:848–53 10.1177/1352458509104591 [DOI] [PubMed] [Google Scholar]
- 29. Sormani MP, Rio J, Tintorè M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 2013;19:605–12 10.1177/1352458512460605 [DOI] [PubMed] [Google Scholar]
- 30. Prosperini L, Mancinelli CR, De Giglio L, et al. Interferon beta failure predicted by EMA criteria or isolated MRI activity in multiple sclerosis. Mult Scler 2014;20:566–76 10.1177/1352458513502399 [DOI] [PubMed] [Google Scholar]
- 31. Freedman MS, Selchen D, Arnold DL, et al. ; Canadian Multiple Sclerosis Working Group. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci 2013;40:307–23 10.1017/S0317167100014244 [DOI] [PubMed] [Google Scholar]
- 32. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of ‘no evidence of disease activity' in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord 2015;8:3–13 10.1177/1756285614560733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Healy BC, Glanz BI, Stankiewicz J, et al. A method for evaluating treatment switching criteria in multiple sclerosis. Mult Scler 2010;16:1483–89 10.1177/1352458510379245 [DOI] [PubMed] [Google Scholar]
- 34. Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 35. McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–82 10.1148/radiol.15150025 [DOI] [PubMed] [Google Scholar]
- 36. Lohrke J, Frisk AL, Frenzel T, et al. Histology and gadolinium distribution in the rodent brain after the administration of cumulative high doses of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 2017;52:324–33 10.1097/RLI.0000000000000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramalho J, Semelka RC, Ramalho M, et al. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 2016;37:1192–98 10.3174/ajnr.A4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol 2016;51:273–79 [DOI] [PubMed] [Google Scholar]
- 39. Olchowy C, Cebulski K, Lasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLoS One 2017;12:e0171704 10.1371/journal.pone.0171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stojanov DA, Aracki-Trenkic A, Vojinovic S, et al. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 2016;26:807–15 10.1007/s00330-015-3879-9 [DOI] [PubMed] [Google Scholar]
- 41. Cao Y, Huang DQ, Shih G, et al. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 2016;206:414–19 10.2214/AJR.15.15327 [DOI] [PubMed] [Google Scholar]
- 42. Radbruch A, Haase R, Kickingereder P, et al. Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology 2017;283:828–36 10.1148/radiol.2017162980 [DOI] [PubMed] [Google Scholar]
- 43. Radbruch A, Haase R, Kieslich PJ, et al. No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology 2017;282:699–707 10.1148/radiol.2016162241 [DOI] [PubMed] [Google Scholar]
- 44. Radbruch A, Weberling LD, Kieslich PJ, et al. Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 2016;51:683–90 10.1097/RLI.0000000000000308 [DOI] [PubMed] [Google Scholar]
- 45. Schlemm L, Chien C, Bellmann-Strobl J, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler 2017;23:963–72 10.1177/1352458516670738 [DOI] [PubMed] [Google Scholar]
- 46. Langner S, Kromrey ML, Kuehn JP. Repeated intravenous administration of gadobutrol does not lead to increased signal intensity on unenhanced T1-weighted images-a voxel-based whole brain analysis. Eur Radiol 2017. March 13. [Epub ahead of print] 10.1007/s00330-017-4777-0 [DOI] [PubMed] [Google Scholar]
- 47. Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 2015;50:473–80 10.1097/RLI.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]