A cohort of 204 patients were randomly divided into 2 groups. Patients in group A (n = 102) underwent 80-kVp CTA with 30 mL of contrast agent, while patients in group B (n = 102) underwent conventional CTA (120 kVp, 60 mL of contrast agent). With DSA as a reference standard, diagnostic accuracy on a per-aneurysm basis was 89.9% for group A and 93.9% for group B. The authors conclude that in detecting intracranial aneurysms, 80-kVp/30-mL contrast CTA provides the same diagnostic accuracy as conventional CTA with substantial radiation dose and contrast agent reduction.
Abstract
BACKGROUND AND PURPOSE:
Multidetector row CTA has become the primary imaging technique for detecting intracranial aneurysms. Technical progress enables the use of cerebral CTA with lower radiation doses and contrast media. We evaluated the diagnostic accuracy of 80-kV(peak) cerebral CTA with 30 mL of contrast agent for detecting intracranial aneurysms.
MATERIALS AND METHODS:
Two hundred four patients were randomly divided into 2 groups. Patients in group A (n = 102) underwent 80-kVp CTA with 30 mL of contrast agent, while patients in group B (n = 102) underwent conventional CTA (120 kVp, 60 mL of contrast agent). All patients underwent DSA. Image quality, diagnostic accuracy, and radiation dose between the 2 groups were compared.
RESULTS:
Diagnostic image quality was obtained in 100 and 99 patients in groups A and B, respectively (P = .65). With DSA as reference standard, diagnostic accuracy on a per-aneurysm basis was 89.9% for group A and 93.9% for group B. For evaluating smaller aneurysms (<3 mm), the diagnostic accuracy of groups A and B was 86.3% and 90.8%, respectively. There was no difference in diagnostic accuracy between each CTA group and DSA (all, P > .05) or between the 2 CTA groups (all, P > .05). The effective dose in group A was reduced by 72.7% compared with group B.
CONCLUSIONS:
In detecting intracranial aneurysms with substantial radiation dose and contrast agent reduction, 80-kVp/30-mL contrast CTA provides the same diagnostic accuracy as conventional CTA.
Approximately 85% of all subarachnoid hemorrhages result from ruptured intracranial aneurysms.1 Such hemorrhages have high case fatality, particularly for relatively young patients, younger than 65 years of age.2 Clinical urgency may sometimes be difficult to assess, given that some patients only present with headache and near-normal neurologic examination findings.3 Thus early identification of underlying intracranial aneurysms seems to be especially important.
DSA is currently the criterion standard for the assessment of aneurysms but has some inherent drawbacks. This technology is invasive, time-consuming, and relatively expensive.4 Furthermore, it uses a higher radiation dose and causes permanent neurologic complications in 0.12% of patients.5 Multidetector row CT angiography has always been the primary imaging technique for the evaluation of intracranial aneurysms, especially for the critical patients presenting with subarachnoid hemorrhage, because of its wide availability, reduced imaging time, and high diagnostic accuracy.4–7 Even for the patients with headache and near-normal neurologic examination findings, CTA may be important for screening. However, radiation exposure and contrast material–induced nephropathy are inherent drawbacks of CTA. Technical progress enables performing cerebral CTA with ever lower radiation doses and contrast media volumes while maintaining image quality.8–12 However, previous studies did not fully assess the diagnostic accuracy of such gentler CTA protocols because few patients underwent DSA as a reference standard. For example, a study by Luo et al8 included 120 patients who underwent cerebral CTA with both low tube voltage and low contrast agent volume, but only 43 patients (n < 15 for the 2 low-dose groups) underwent DSA for comparison.
The purpose of our study was, therefore, to evaluate, in a large patient population, the diagnostic accuracy of 80-kV(peak) cerebral CTA with 30 mL of contrast agent for detecting intracranial aneurysms with DSA as the reference standard.
Materials and Methods
Patients
This prospective study was approved by the Jinling Hospital institutional review board. Written informed consent was provided by all patients or their legal guardians. Two hundred four patients were included in our study between September 2013 and January 2015.
Inclusion criteria for this study were the following: 1) clinically suspected intracranial aneurysm, that is, patient presentation with subarachnoid hemorrhage or a suspicion of an intracranial aneurysm after medical examinations; 2) clinical referral for both cerebral CTA and DSA when patients were able to undergo the 2 examinations; and 3) cerebral CTA performed before DSA with no more than 3 days between procedures. Patients were excluded if they were younger than 18 years of age (n = 1), had a history of prior surgical clipping or endovascular coiling (n = 2), had a history of prior reaction to iodinated contrast media, or had known renal insufficiency (creatinine level, >120 mol/L) (n = 0).
Included patients who underwent CTA with different tube voltages and different volumes of iodinated contrast agent were randomly divided into 2 groups based on a computer-generated allocation sequence. The parity of each random number determined to which group the patients examined on the same day would be assigned (ie, odd numbers for group A and even numbers for group B), to avoid the change of contrast agent before each patient's examination. Group A consisted of 102 patients, including 50 men (mean age, 49 ± 12 years) and 52 women (mean age, 53 ± 13 years); group B consisted of 102 patients, including 47 men (mean age, 52 ± 14 years) and 55 women (mean age, 58 ± 13 years). There was no significant difference in sex between the 2 groups (P > .05). The mean age of patients in group A was 51 ± 14 years (range, 21–79 years), while patients in group B were somewhat older (P = .024), with a mean age of 55 ± 14 years (range, 22–81 years). The baseline characteristics of all patients are presented in On-line Table 1.
Cerebral CT Angiography Acquisition
Cerebral CTA examinations were performed by using a dual-source CT system (Somatom Definition; Siemens, Erlangen, Germany). Routine automatic tube current modulation (CARE Dose4D; Siemens) was used at 230 mAs for each patient. The collimation was 64 × 0.6 mm, with a 0.33-second rotation time and a pitch of 1.5. Image reconstruction was performed with a 0.75-mm section thickness and 0.5-mm increment with a dedicated reconstruction algorithm (H30f).
In group A, patients received 30 mL of iodinated contrast medium (iopromide, Ultravist 300 mg I/mL; Bayer HealthCare, Berlin, Germany) and were imaged at 80 kVp (double low-dose protocol). In group B, the patients received 60 mL of the same contrast medium and were imaged at 120 kVp, which is the standard work-up CTA protocol in the clinic. The contrast agent was injected into the antecubital vein via an 18-ga catheter at the rate of 4.0 mL/s, followed by 30 mL of saline solution with the same flow rate.
Using a bolus-tracking technique, an ROI was placed in the right internal carotid artery. When the predefined threshold of 100 HU was reached, image acquisition started 2 seconds later. The acquisition lasted approximately 3–4 seconds.
DSA Acquisition and Evaluation
DSA was performed with femoral catheterization with a biplane DSA unit with rotational capabilities (Axiom Artis dTA; Siemens). A single 3D-DSA acquisition was obtained before removing the catheter only in the target vessel with confirmed or suspected aneurysms, to reduce the radiation dose. Once the procedure was completed, the angiographic datasets were transferred to an adjacent 3D workstation (Siemens) for generation of 3D reformatted images. All angiographies were performed by a group of highly experienced (>10 years of experience) interventional neuroradiologists (not authors) who also performed evaluations of the presence, location, and size of intracranial aneurysms in the DSA images. If there was a strong suspicion of an aneurysm on CTA that was not found on DSA, repeat interpretation by at least 2 interventional neuroradiologists (not authors) was performed to arbitrate this discrepancy.
Objective Image-Quality Evaluation
All CT images were transferred to a dedicated workstation (syngo MultiModality Workplace; Siemens). All CT measurements were independently performed by 2 radiologists (Q.Q.N. and S.L., with 1 and 5 years' experience in neuroradiology, respectively) twice with a 6-month interval between measurements. The CT attenuation values of vascular structures were measured by using a user-defined circular ROI with an area of 0.12–0.16 cm2 in the bilateral ICAs and 0.04–0.06 cm2 in the bilateral middle cerebral arteries on the transverse CT images. To mitigate partial volume effects and operator dependence of measurements, we prescribed 3 independent ROIs, respectively, on both sides of the cavernous segment of the ICA and the first segment of the MCA trunk. With Moyamoya disease and ICA or MCA occlusion, the CT attenuation values of vascular structures were not measured. The average attenuation values were used for statistical analysis.
Brain parenchyma was selected as the vascular background, and image noise was calculated as the SD of the attenuation values. The attenuation values of the brain parenchyma were measured by placing an ROI of 1 cm2 in the white matter above the lateral ventricles. Signal-to-noise ratio and contrast-to-noise ratio (CNR) were calculated by using the following formulas13,14:
where CTnumbera is the mean Hounsfield unit of the target artery, CTnumberb is the mean Hounsfield unit of brain parenchyma, and SD is the standard deviation of the attenuation value in the brain parenchyma.
Subjective Image-Quality Evaluation
Subjective image-quality analysis of cerebral CTA was performed by using volume-rendering, multiplanar reconstructions, and maximum intensity projections. Two experienced neuroradiologists (L.J.Z. and G.Z.C., with 16 and 4 years' experience, respectively) blinded to the acquisition technique independently scored CTA images after a dedicated formal training in 20 patients who were not included in this study. After 6 months, repeated evaluations were performed to measure intrareader agreement. In the case of disagreement, a final consensus score was determined during joint interpretation.
Overall image quality was determined on the basis of the degree of image noise and vessel sharpness on a 4-point Likert Scale15–18: 1) poor, nondiagnostic, major degree of noise, blurry vessel outlines, rendering evaluation impossible; 2) moderate, substantial noise, suboptimal vessel sharpness; 3) good, moderate image noise and good vessel sharpness; and 4) excellent, minor-to-no noise, exquisite vessel delineation. Images with overall image quality scores of ≥3 were regarded as diagnostic.
Intracranial Aneurysm Evaluation
Aneurysms were measured within the arteries of the anterior circulation (ie, anterior communicating, anterior cerebral, middle cerebral, internal carotid, and anterior choroidal arteries) and the arteries of the posterior circulation (vertebral and basilar, posterior communicating, posterior cerebral, anterior superior cerebellar, and posterior inferior cerebellar arteries).
The same 2 neuroradiologists performing the subjective image analysis independently evaluated the presence or absence of aneurysms on cerebral CTA. In case of disagreement, a third experienced neuroradiologist (C.S.Z. with 10 years' experience) arbitrated. For subarachnoid hemorrhage, other nonaneurysmal causes were also recorded.
Radiation Dose Estimation
The volume CT dose index (milligray) and dose-length product (DLP, milligray × centimeter) were recorded from the dose report. The effective dose (ED, millisieverts) was calculated by using the formula ED = DLP × κ, by using a conversion factor (κ) for head CT imaging (κ = 0.0021 mSv/mGy × cm).19
Statistical Analysis
Statistical analyses were performed by using SPSS software (Version 21; IBM, Armonk, New York). Quantitative variables were expressed as mean ± SD, and categoric data were expressed as frequencies or percentages. Quantitative variables were tested for normal distribution by using the Kolmogorov-Smirnov test. The t test was used if the quantitative variables followed normal distribution, and if the quantitative variables did not follow normal distribution, the Mann-Whitney U test was used. A χ2 or Fisher exact test was used to analyze differences in categoric data for baseline characteristics and subjective image quality between groups A and B. P < .05 indicated a statistical difference. Intraclass correlation coefficient and κ analysis were used to evaluate inter- and intrareader agreement for the measurement of subjective and objective image quality, respectively. An intraclass correlation coefficient or κ value < 0.20 indicated poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, very good agreement.15,20
In this study, 3D-DSA was regarded as the reference standard for detection of intracranial aneurysms. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated on both a per-patient and per-aneurysm basis. The confidence intervals for per-aneurysm analysis were obtained by using bootstrapping. Two-sided 95% confidence intervals based on binomial probabilities were also provided. Comparisons between the 2 CTA groups and DSA were made by using the McNemar test. The χ2 or Fisher exact test was used to compare the sensitivity, specificity, PPV, NPV, and accuracy between the 2 different CTA groups. P < .05 was a statistically significant difference.
Results
Image Quality
Mean attenuation, SNR, and CNR values for the ICA, MCA, and brain parenchyma for both groups are presented in On-line Table 2. The mean attenuation in the ICA and MCA of group A was higher than that of group B (P < .01). The mean SNRICA, CNRICA, SNRMCA, and CNRMCA of group A were lower than those of group B (P < .01). The mean image noise of group A was higher than that of group B (P < .01). Reliability analysis showed an excellent inter- and intraobserver agreement for measurements of objective image quality (all intraclass correlation coefficients, > 0.80).
Diagnostic-quality scores of ≥3 were given in 100 patients (98%) in group A and 99 patients (97%) in group B. There was no difference in diagnostic image quality in the 2 groups (χ2 = 0.21, P = .65), indicating that diagnostic image quality could be reliably obtained with either CTA protocol. The inter- and intrareader agreements for all subjective image-quality measurements were good with all κ values > 0.60.
Diagnostic Performance
Among 204 patients, 121 patients had 157 aneurysms based on 3D-DSA findings, while 83 patients had no aneurysms. Of 121 patients with aneurysms, 99 patients had a single aneurysm and 22 had multiple aneurysms. On-line Table 3 shows the aneurysm detection and other nonaneurysmal causes in each group.
Cerebral CTA correctly detected 143 aneurysms in 118 patients with 14 aneurysms missed and 6 false-positive aneurysm diagnoses against the 3D-DSA reference standard. CTA correctly demonstrated all nonaneurysmal causes in 12 patients. For group A, the sensitivity and specificity for detecting aneurysms on a per-patient basis were 96.8% and 97.5%, respectively, and 88.5% and 92.5%, respectively, on a per-aneurysm basis. For group B, sensitivity and specificity were 98.3% and 97.7% on a per-patient basis, respectively, and 94.3% and 93.3% on a per-aneurysm basis (Table 1 and Fig 1). There were no statistically significant differences in sensitivity, specificity, PPV, NPV, and diagnostic accuracy on a per-patient or per-aneurysm basis between the 2 cerebral CTA protocols and 3D-DSA (all P > .05). In addition, there was no difference in sensitivity, specificity, PPV, NPV, and diagnostic accuracy between the 2 cerebral CTA protocols (all, P > .05).
Table 1:
Aneurysm detection with cerebral CTA compared with a 3D-DSA reference standarda
| Approach | Results (No.) |
Statistical Analysis (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Per patient | |||||||||
| Group A | 60 | 39 | 1 | 2 | 96.8 (89.0–99.1) | 97.5 (87.1–99.6) | 98.4 (91.2–99.7) | 95.1 (83.9–98.7) | 97.0 (91.7–99.0) |
| Group B | 58 | 42 | 1 | 1 | 98.3 (91.0–99.7) | 97.7 (87.9–99.6) | 98.3 (91.0–99.7) | 97.7 (87.9–99.6) | 98.0 (93.1–99.5) |
| Per aneurysm | |||||||||
| Group A | 77 | 39 | 3 | 10 | 88.5 (81.6–95.4) | 92.9 (88.6–98.6) | 96.3 (91.3–100.0) | 79.6 (67.3–89.8) | 89.9 (84.5–94.6) |
| Group B | 66 | 42 | 3 | 4 | 94.3 (88.6–98.6) | 93.3 (84.4–100.0) | 95.7 (89.9–100.0) | 91.3 (82.6–97.8) | 93.9 (88.7–98.3) |
Note:—TP indicates true positive; TN, true negative; FP, false positive; FN, false negative.
The data in parentheses are 95% confidence intervals.
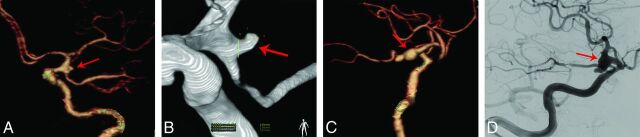
Fig 1.
Comparison of the 2 CTA protocols for detecting an aneurysm in the posterior communicating artery. A and B, An 80-kVp cerebral CTA with 30 mL of contrast agent in a 49-year-old woman. A volume-rendered digital subtraction CTA image (A) shows an aneurysm in the left posterior communicating artery (red arrow), which is confirmed by 3D-DSA (B). C and D, A 120-kVp cerebral CTA with 60 mL of contrast agent in a 66-year-old woman. C, Volume-rendered digital subtraction CTA image (C) shows an aneurysm in the right posterior communicating artery (red arrow), which is confirmed by 2D-DSA (D).
Of 157 total aneurysms, 53 were <3 mm, 84 were 3–8 mm, and 20 were >8 mm. Cerebral CTA had a sensitivity of 75.0%, 95.7%, and 100% in group A, and 81.0%, 100%, and 100% in group B for detecting aneurysms of <3 mm, 3–8 mm, and >8 mm, respectively. Diagnostic accuracy grouped by aneurysm size for group A was 86.4%, 96.5%, and 100%; for group B, it was 90.8%, 96.5%, and 100% (Table 2 and Fig 2). There was no difference in sensitivity, specificity, PPV, NPV, and diagnostic accuracy between the 2 cerebral CTA protocols for detecting aneurysms of different sizes, even for aneurysms of <3 mm (all, P > .05).
Table 2:
Aneurysm detection with cerebral CTA according to aneurysm sizea
| Aneurysm Size | Results (No.) |
Statistical Analysis (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| <3 mm | |||||||||
| Group A | 24 | 39 | 2 | 8 | 75.0 (59.4–90.6) | 95.1 (87.8–100.0) | 92.3 (80.8–100.0) | 83.0 (72.3–93.6) | 86.3 (78.1–93.2) |
| Group B | 17 | 42 | 2 | 4 | 81.0 (61.9–95.2) | 95.5 (88.6–100.0) | 89.5 (73.7–100.0) | 91.3 (82.6–97.8) | 90.8 (83.1–96.9) |
| 3–8 mm | |||||||||
| Group A | 44 | 39 | 1 | 2 | 95.7 (89.1–100.0) | 97.5 (92.5–100.0) | 97.8 (93.3–100.0) | 95.1 (87.8–100.0) | 96.5 (93.0–100.0) |
| Group B | 38 | 42 | 1 | 0 | 100 (100.0–100.0) | 97.7 (93.0–100.0) | 97.4 (92.3–100.0) | 100 (100.0–100.0) | 98.8 (96.3–100.0) |
| >8 mm | |||||||||
| Group A | 9 | 39 | 0 | 0 | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) |
| Group B | 11 | 42 | 0 | 0 | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) | 100 (100.0–100.0) |
Note:—TP indicates true positive; TN, true negative; FP, false positive; FN, false negative.
The data in parentheses are 95% confidence intervals.
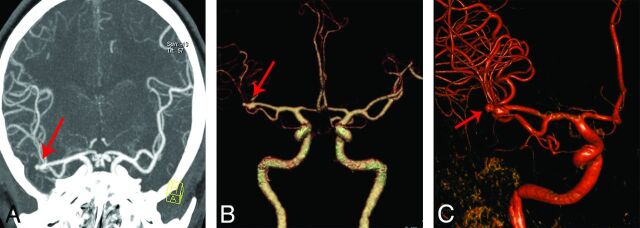
Fig 2.
An 80-kVp cerebral CTA with 30 mL of contrast agent in a 45-year-old man. Maximum-intensity-projection image (A) and a volume-rendered digital subtraction CTA image (B) show an aneurysm in the right middle cerebral artery (red arrow), which is confirmed by 3D-DSA (C).
There were 88 aneurysms in the anterior circulation arteries and 69 in the posterior circulation arteries (Table 3). The detailed distribution of intracranial aneurysms detected by DSA and CTA is presented in On-line Table 4. Cerebral CTA had a sensitivity of 86.2% and 93.1% in group A and a sensitivity of 93.3% and 95.0% in group B for determining aneurysm locations in the anterior and posterior circulation, respectively. The diagnostic accuracies for detecting aneurysms in the anterior and posterior circulation were 90.8% and 94.3% in group A and 94.6% and 96.4% in group B. There was no difference in sensitivity, specificity, PPV, NPV, and diagnostic accuracy for detecting aneurysms in different locations between the 2 cerebral CTA protocols (all, P > .05).
Table 3:
Aneurysm detection with cerebral CTA according to aneurysm locationa
| Location | Results (No.) |
Statistical Analysis (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Anterior circulation | |||||||||
| Group A | 50 | 39 | 1 | 8 | 86.2 (77.6–94.8) | 97.5 (92.5–100.0) | 98.0 (94.1–100.0) | 83.0 (70.2–93.6) | 90.8 (84.7–95.9) |
| Group B | 28 | 42 | 2 | 2 | 93.3 (83.3–100.0) | 95.5 (88.5–100.0) | 93.3 (83.3–100.0) | 95.5 (88.6–100.0) | 94.6 (89.2–98.6) |
| Posterior circulation | |||||||||
| Group A | 27 | 39 | 2 | 2 | 93.1 (82.8–100.0) | 95.1 (87.8–100.0) | 93.1 (82.8–100.0) | 95.1 (87.8–100.0) | 94.3 (88.6–98.6) |
| Group B | 38 | 42 | 1 | 2 | 95.0 (87.5–100.0) | 97.7 (93.0–100.0) | 97.4 (92.3–100.0) | 95.5 (88.6–100.0) | 96.4 (91.6–100.0) |
Note:—TP indicates true positive; TN, true negative; FP, false positive; FN, false negative.
The data in parentheses are 95% confidence intervals.
From a total of 14 false-negative aneurysms (Fig 3), 12 aneurysms were <3 mm in diameter (n = 8 in group A, n = 4 in group B). The remaining 2 aneurysms with sizes of 5 and 7 mm were missed in group A. Of the 14 false-negative aneurysms, 10 aneurysms were located in anterior circulation arteries (n = 8 in group A, n = 2 in group B) and 4 aneurysms were located in posterior cerebral arteries (n = 2 in each group).
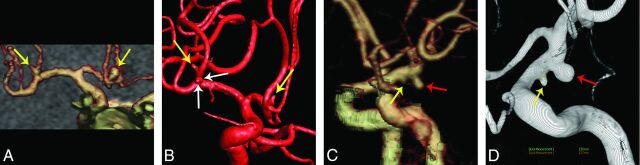
Fig 3.
False-negative aneurysms in the 2 CTA protocols. A and B, An 80-kVp cerebral CTA with 30 mL of contrast agent in a 50-year-old man. A volume-rendered digital subtraction CTA image (A) shows 2 true-positive aneurysms in the anterior communicating artery and right middle cerebral artery, respectively (yellow arrows), while another 2 small aneurysms with diameters of 1.1 and 0.6 mm were found in the right middle cerebral artery (white arrows) on 3D-DSA (B), which were not found in the CTA image. C and D, A 120-kVp cerebral CTA with 60 mL of contrast agent in a 46-year-old man. The volume-rendered digital subtraction CTA image (C) shows a true-positive aneurysm in the left anterior choroidal artery (red arrow) and a false-negative aneurysm in the left posterior communicating artery (yellow arrow). The aneurysm in the left posterior communicating artery (yellow arrow) was found at repeat interpretation. 3D-DSA shows the 2 aneurysms (D).
Of the 6 false-positive aneurysms (Fig 4), 4 aneurysms (n = 2 in each group) were <3 mm and 2 aneurysms (n = 1 for each group) were 3–8 mm. Three of the reported false-positive aneurysms (n = 1 for group A, n = 2 for group B) were located in the anterior circulation arteries, while the remaining 3 (n = 2 for group A, n = 1 for group B) were located in the posterior circulation arteries.
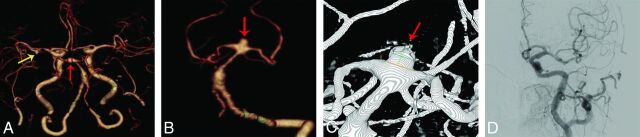
Fig 4.
A 120-kVp cerebral CTA with 60 mL of contrast agent in a 70-year-old woman. A and B, Volume-rendered digital subtraction CTA images show a true-positive aneurysm in the top of basilar artery (red arrow), which was confirmed by 3D-DSA (C) and a false-positive aneurysm in the left middle cerebral artery (yellow arrow), which was not evident in 2D-DSA.
Radiation Dose
The mean volume CT dose index, DLP, and ED of groups A and B are presented in On-line Table 5. The mean volume CT dose index, DLP, and ED of group A were 7.0 ± 0.4 mGy, 136.7 ± 8.8 mGy × cm, and 0.3 ± 0.0 mSv, respectively; for group B, these values were 25.9 ± 2.0 mGy, 507 ± 44.7 mGy × cm, and 1.1 ± 0.1 mSv, respectively. The volume CT dose index, DLP, and ED in group A were reduced by approximately 73.0%, 73.0%, and 72.7% compared with group B.
Discussion
Multidetector row CTA has been widely used to detect intracranial aneurysms due to its high diagnostic accuracy and image quality. However, radiation exposure and contrast material-induced nephropathy caused by CTA have received extensive attention. Currently, technical progress such as high-pitch technology, tube voltage, and tube current reduction enable performing cerebral CTA with ever lower radiation dose and contrast media volumes while maintaining image quality. This prospective study, conducted in a sizeable patient population with randomization, demonstrates that a low tube voltage, low contrast agent cerebral CTA protocol shows the same sensitivity and specificity as the standard protocol for detecting intracranial aneurysms compared with 3D-DSA as a reference standard. Cerebral CTA with 80 kVp/30 mL could reduce the effective radiation dose by approximately 73% and contrast agent volume by 50% without compromising diagnostic yield.
Our findings are in agreement with the results of previous investigations showing that lowering the tube voltage can reduce the effective dose without affecting diagnostic accuracy and image quality.8,11,15 In 1 study, for example, no significant difference was found in the diagnostic accuracy between low-tube-voltage (80 kVp) and conventional-tube-voltage (120 kVp) CTA protocols in 48 patients.11 In addition, low-tube voltage CTA also showed high sensitivity (75%) and specificity (100%) for detecting small aneurysms of <3 mm. In a recent study on 294 patients with spontaneous subarachnoid hemorrhage, 100-kVp cerebral CTA provided a high detection rate of 76.9% (10/13) for small aneurysms (< 3 mm), consistent with the results of our 120-kVp CTA protocol.12 However, the effective dose of 100-kVp protocol was only reduced by 35.4% compared with our 120-kVp protocol. The application of dose-reduction strategies such as lower tube voltage or current has been reported to cause a trade-off in decreased image quality.21,22 However, many technologic advances in CT techniques, such as iterative reconstruction, have been demonstrated to markedly improve the performance of low-dose CT acquisition.23,24 Additionally, lowering tube voltage enhances the contrast attenuation in target vessels, thus maintaining a diagnostic CNR. As observed in our study, the mean image noise of the 80-kVp CTA protocol was statistically higher than that of the 120-kVp protocol; however, the image quality of the 80-kVp CTA protocol did not decrease despite the increase in noise.
Unlike the above-mentioned studies, our study used a double low-dose cerebral CTA (80-kVp tube voltage and 30-mL contrast agent) protocol in a relatively large group of patients, with a 73% reduction in the effective radiation dose. We found that the diagnostic accuracy of aneurysm detection of this double low-dose CTA protocol was identical to that of the conventional CTA protocol, even for small aneurysms (<3 mm). In addition, our study showed the high negative predictive value of the double low-dose CTA protocol for intracranial aneurysm detection based on a per-patient basis, which indicated that a negative finding of this double low-dose CTA protocol can reliably rule out intracranial aneurysms in the patients with subarachnoid hemorrhage. These observations suggest that an 80-kVp/30-mL cerebral CTA protocol may be suitable for intracranial aneurysm detection in clinical practice.
The double low-dose protocol showed a reduction in sensitivity for the detection of small aneurysms (<3 mm) in comparison with the standard protocol. Of all the 14 false-negative aneurysms, 12 were <3 mm. In our study, small aneurysms were not well-identified, mostly due to smaller size or infundibular dilation. There were still 7 undetected aneurysms, despite repetitive comparison between cerebral CTA and DSA. These aneurysms were either too small or were located overlying the bone structures, causing the missed diagnoses. Moreover, threshold selection will have an effect on the visualization of small aneurysms with the use of volume-rendering to reformat images. In addition, 10 of the 14 false-negative aneurysms were in 7 patients with multiple aneurysms, in which the chances of overlooking a small dilation are significantly higher. Additionally, satisfaction of searching intracranial aneurysms in patients with multiple aneurysms is also an important source of interpretation error.
Three of the false-positive findings were diagnosed by 3D-DSA as infundibular dilations at the origin of the posterior communicating artery. This finding reflects a well-known pitfall of cerebral CTA, namely the difficulty in distinguishing an infundibular dilation from a true aneurysm at this level unless the vessel emerging at the infundibular apex can be found.25
Our study has some limitations. First, the 2 CTA protocols in our study were not compared intraindividually; this omission may have introduced some bias. In this study, no statistical difference was found in the diagnostic image quality between the double low-dose CTA group and the conventional CTA group. Second, significantly higher image noise in the double low-dose CTA group was observed. This finding is explained by the application of standard filtered back-projection as a reconstruction technique instead of the more advanced iterative reconstruction, which was not available on the scanner used for this study. Iterative reconstruction is regarded as an effective technology for improving image quality by reducing image noise,26–28 with a CNR reduction of up to 13%. Our CTA protocol also would likely benefit from the application of iterative reconstruction techniques and the predicted increase in overall image quality. Third, false-negatives with our double low-dose CTA protocol deserve special attention and careful interpretation because they are potentially lethal and subsequent DSA examination would result in additional contrast and radiation, especially for the CTA studies with false-negative findings; however, these results did not affect the diagnostic accuracy on a per-patient basis in our study. Of 10 missed aneurysms with the double low CTA protocol, 6 occurred in 3 patients with multiple (n ≥ 3) aneurysms. Conventionally, DSA would be performed once there was a positive finding on CTA in patients with suspected aneurysms. Thus, although our protocol missed some small aneurysms in patients with multiple aneurysms, the false-negative results did not ultimately affect patient management compared with the traditional CTA protocol. Finally, the diagnostic accuracy of the double low-dose protocol for detecting small aneurysms of <5 mm needs to be further confirmed in even larger studies.
Conclusions
An 80-kVp cerebral CTA with 30 mL of contrast agent has the same sensitivity and specificity for detecting intracranial aneurysms compared with a conventional cerebral CTA protocol. Furthermore, this protocol substantially reduces the radiation dose and contrast agent volume.
Supplementary Material
ABBREVIATIONS:
- CNR
contrast-to-noise ratio
- DLP
dose-length product
- ED
effective dose
- NPV
negative predictive value
- PPV
positive predictive value
Footnotes
Disclosures: U. Joseph Schoepf—UNRELATED: Consultancy: Guerbet; Grants/Grants Pending: Astellas Pharma,* Bayer Schering Pharma,* Bracco,* GE Healthcare,* Medrad,* Siemens*; Royalties: Springer, Meetings-by-Mail. *Money paid to the institution.
U.J. Shoepf is a consultant for and/or receives research support from Bayer, Bracco, GE Healthcare, Medrad, and Siemens. The other authors have no conflicts of interest to declare.
This work was supported by the Program for New Century Excellent Talents in the University (NCET-12-0260).
References
- 1. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18 10.1016/S0140-6736(07)60153-6 [DOI] [PubMed] [Google Scholar]
- 2. Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011;10:626–36 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 3. Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 2010;9:504–19 10.1016/S1474-4422(10)70087-9 [DOI] [PubMed] [Google Scholar]
- 4. Lu L, Zhang LJ, Poon CS, et al. Digital subtraction CT angiography for detection of intracranial aneurysms: comparison with three-dimensional digital subtraction angiography. Radiology 2012;262:605–12 10.1148/radiol.11110486 [DOI] [PubMed] [Google Scholar]
- 5. Moran CJ. Aneurysmal subarachnoid hemorrhage: DSA versus CT angiography—is the answer available? Radiology 2011;258:15–17 10.1148/radiol.101911 [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Xing W, Peng Y, et al. Cerebral aneurysms: accuracy of 320-detector row nonsubtracted and subtracted volumetric CT angiography for diagnosis. Radiology 2013;269:841–49 10.1148/radiol.13130191 [DOI] [PubMed] [Google Scholar]
- 7. Westerlaan HE, van Dijk J, Jansen-van der Weide MC, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis—systematic review and meta-analysis. Radiology 2011;258:134–45 10.1148/radiol.10092373 [DOI] [PubMed] [Google Scholar]
- 8. Luo S, Zhang LJ, Meinel FG, et al. Low tube voltage and low contrast material volume cerebral CT angiography. Eur Radiol 2014;24:1677–85 10.1007/s00330-014-3184-z [DOI] [PubMed] [Google Scholar]
- 9. Millon D, Derelle AL, Omoumi P, et al. Nontraumatic subarachnoid hemorrhage management: evaluation with reduced iodine volume at CT angiography. Radiology 2012;264:203–09 10.1148/radiol.12111384 [DOI] [PubMed] [Google Scholar]
- 10. Ramgren B, Björkman-Burtscher IM, Holtås S, et al. CT angiography of intracranial arterial vessels: impact of tube voltage and contrast media concentration on image quality. Acta Radiol 2012;53:929–34 10.1258/ar.2012.120218 [DOI] [PubMed] [Google Scholar]
- 11. Sun G, Ding J, Lu Y, et al. Comparison of standard-and low-tube voltage 320-detector row volume CT angiography in detection of intracranial aneurysms with digital subtraction angiography as gold standard. Acad Radiol 2012;19:281–88 10.1016/j.acra.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 12. Tang K, Li R, Lin J, et al. The value of cerebral CT angiography with low tube voltage in detection of intracranial aneurysms. Biomed Res Int 2015;2015:876796 10.1155/2015/876796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho ES, Chung TS, Oh DK, et al. Cerebral computed tomography angiography using a low tube voltage (80 kVp) and a moderate concentration of iodine contrast material: a quantitative and qualitative comparison with conventional computed tomography angiography. Invest Radiol 2012;47:142–47 10.1097/RLI.0b013e31823076a4 [DOI] [PubMed] [Google Scholar]
- 14. Furtado A, Adraktas D, Brasic N, et al. The triple rule-out for acute ischemic stroke: imaging the brain, carotid arteries, aorta, and heart. AJNR Am J Neuroradiol 2010;31:1290–96 10.3174/ajnr.A2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen GZ, Zhang LJ, Schoepf UJ, et al. Radiation dose and image quality of 70 kVp cerebral CT angiography with optimized sinogram-affirmed iterative reconstruction: comparison with 120 kVp cerebral CT angiography. Eur Radiol 2015;25:1453–63 10.1007/s00330-014-3533-y [DOI] [PubMed] [Google Scholar]
- 16. Söderman M, Holmin S, Andersson T, et al. Image noise reduction algorithm for digital subtraction angiography: clinical results. Radiology 2013;269:553–60 10.1148/radiol.13121262 [DOI] [PubMed] [Google Scholar]
- 17. Szucs-Farkas Z, Bensler S, Torrente JC, et al. Nonlinear three-dimensional noise filter with low-dose CT angiography: effect on the detection of small high-contrast objects in a phantom model. Radiology 2011;258:261–69 10.1148/radiol.10100760 [DOI] [PubMed] [Google Scholar]
- 18. Zhang WL, Li M, Zhang B, et al. CT angiography of the head-and-neck vessels acquired with low tube voltage, low iodine, and iterative image reconstruction: clinical evaluation of radiation dose and image quality. PLoS One 2013;8:e81486 10.1371/journal.pone.0081486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCollough CH, Primak AN, Braun N, et al. Strategies for reducing radiation dose in CT. Radiol Clin North Am 2009;47:27–40 10.1016/j.rcl.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Wang J, Liu X, et al. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology 2015;274:508–16 10.1148/radiol.14132687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith AB, Dillon WP, Gould R, et al. Radiation dose reduction strategies for neuroradiology CT protocols. AJNR Am J Neuroradiol 2007;28:1628–32 10.3174/ajnr.A0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graser A, Wintersperger BJ, Suess C, et al. Dose reduction and image quality in MDCT colonography using tube current modulation. AJR Am J Roentgenol 2006;187:695–701 10.2214/AJR.05.0662 [DOI] [PubMed] [Google Scholar]
- 23. Hérin E, Gardavaud F, Chiaradia M, et al. Use of Model-Based Iterative Reconstruction (MBIR) in reduced-dose CT for routine follow-up of patients with malignant lymphoma: dose savings, image quality and phantom study. Eur Radiol 2015;25:2362–70 10.1007/s00330-015-3656-9 [DOI] [PubMed] [Google Scholar]
- 24. Geyer LL, Schoepf UJ, Meinel FG, et al. State of the art: iterative CT reconstruction techniques. Radiology 2015;276:339–57 10.1148/radiol.2015132766 [DOI] [PubMed] [Google Scholar]
- 25. Pereira BJA, Holanda VM, de Holanda CVM, et al. Intracranial aneurysm arising from infundibular dilation. BMJ Case Rep 2013;2013:pii: bcr2013200115 10.1136/bcr-2013-200115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donmez H, Serifov E, Kahriman G, et al. Comparison of 16-row multislice CT angiography with conventional angiography for detection and evaluation of intracranial aneurysms. Eur J Radiol 2011;80:455–61 10.1016/j.ejrad.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 27. Korn A, Bender B, Fenchel M, et al. Sinogram affirmed iterative reconstruction in head CT: improvement of objective and subjective image quality with concomitant radiation dose reduction. Eur J Radiol 2013;82:1431–35 10.1016/j.ejrad.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 28. Moscariello A, Takx RA, Schoepf UJ, et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique—comparison with traditional filtered back projection. Eur Radiol 2011;21:2130–38 10.1007/s00330-011-2164-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.