Abstract
The liver transplantation (LT) population is aging, with the need for transplant being driven by the growing prevalence of nonalcoholic steatohepatitis (NASH). Older LT recipients with NASH may be at an increased risk for adverse outcomes after LT. Our objective is to characterize outcomes in these recipients in a large multicenter cohort. All primary LT recipients ≥65 years from 2010 to 2016 at 13 centers in the Re-Evaluating Age Limits in Transplantation (REALT) consortium were included. Of 1023 LT recipients, 226 (22.1%) were over 70 years old, and 207 (20.2%) had NASH. Compared with other LT recipients, NASH recipients were older (68.0 versus 67.3 years), more likely to be female (47.3% versus 32.8%), White (78.3% versus 68.0%), Hispanic (12.1% versus 9.2%), and had higher Model for End-Stage Liver Disease–sodium (21 versus 18) at LT (P < 0.05 for all). Specific cardiac risk factors including diabetes with or without chronic complications (69.6%), hypertension (66.3%), hyperlipidemia (46.3%), coronary artery disease (36.7%), and moderate-to-severe renal disease (44.4%) were highly prevalent among NASH LT recipients. Graft survival among NASH patients was 90.3% at 1 year and 82.4% at 3 years compared with 88.9% at 1 year and 80.4% at 3 years for non-NASH patients (log-rank P = 0.58 and P = 0.59, respectively). Within 1 year after LT, the incidence of graft rejection (17.4%), biliary strictures (20.9%), and solid organ cancers (4.9%) were comparable. Rates of cardiovascular (CV) complications, renal failure, and infection were also similar in both groups. We observed similar posttransplant morbidity and mortality outcomes for NASH and non-NASH LT recipients. Certain CV risk factors were more prevalent in this population, although posttransplant outcomes within 1 year including CV events and renal failure were similar to non-NASH LT recipients.
Since the implementation of Model for End-Stage Liver Disease (MELD) scores in 2002, the proportion of older candidates awaiting and undergoing liver transplantation (LT) in the United States has increased considerably.(1) Several factors contribute to this trend including an aging population with chronic hepatitis C virus (HCV) infection and nonalcoholic steatohepatitis (NASH), as well as limited donor supply and improved pretransplant care.(2) The prevalence of NASH on the LT waiting list has doubled in the past decade and is now a leading indication for LT in the United States, among patients with and without hepatocellular carcinoma (HCC).(3,4)
Both NASH and older age are associated with increased risk of metabolic syndrome and cardiovascular (CV) disease.(5,6) These comorbid conditions may increase the risk of adverse outcomes after LT. However, data are mixed regarding mortality and morbidity outcomes in LT recipients with NASH. Several large-scale studies have demonstrated similar or even superior survival compared with other leading indications for LT, albeit with a disproportionate risk of death attributed to CV events and infection.(3,7–10) A more recent study by Nagai et al., however, reported an increased risk of death within 1 year of transplant among patients with NASH compared with HCV and alcohol-related liver disease, especially among older LT recipients.(11) The effect of increasing age on post-LT mortality was most pronounced among patients with NASH, with a hazard ratio of 2.08 (95% confidence interval [CI], 1.63–2.64) and 2.66 (95% CI, 1.98–3.57) for ages 65 to <70 and 70 years or older, respectively, compared with patients <50 years. Compared with other LT recipients, patients with NASH also experience more CV events and chronic kidney disease, particularly in the setting of post-LT metabolic syndrome and immunosuppression.(12,13) Data regarding other morbidity measures are limited.
Comprehensive granular data are needed to evaluate clinically important outcomes after LT for recipients with NASH, particularly among older adults. The objective of this study was to characterize the outcomes for older LT recipients (age 65 years or older) with NASH in a large multicenter US-based cohort.
Patients and Methods
PATIENTS AND DATA ACQUISTION
Patients aged 65 years or older who received a LT from 2010 to 2016 and were followed at 13 participating centers in the US-based Re-Evaluating Age Limits in Transplantation (REALT) consortium were included in the analysis. Recipient, donor, procurement, explant, and posttransplant outcome data were individually extracted from each center’s local electronic medical records and collected via the research electronic data capture platform. When available, transplant variables were completed via center-specific UNet (electronic platform designed, built, continuously updated and maintained by UNOS) data reports. A comprehensive list of collected variables and accompanying data definitions is provided in Supporting Table 1. The study was approved by the institutional review board at Stanford University and at each participating site.
We excluded patients who were undergoing multiple organ transplantation except for simultaneous liver-kidney transplantation (SLKT). Those with a prior history of LT were also excluded because NASH was an uncommon cause of repeat LT during this time period, and LT recipients with a prior history of LT are at differential mortality risk compared with those undergoing primary LT. All patients were followed until the date of graft failure or death, or at least until December 31, 2017.
Publicly available program-specific reports were obtained from the Scientific Registry of Transplant Recipients to ascertain the total number of patients transplanted at the 13 participating centers during the study period.(14) The aim was to better define the denominator of total transplants performed at these centers, with the caveat that aggregated data from program-specific reports include all ages and those with prior history of LT and exclude multiorgan transplants including SLKT, so they do not exactly mirror our study inclusion criteria.
DATA DEFINITIONS AND OUTCOMES
NASH was considered the primary etiology of their liver disease if the clinical suspicion for the primary cause of chronic liver disease was NASH or if there was a dual diagnosis of HCC and NASH. Patients with concurrent viral hepatitis, alpha-1-antitrypsin disease, autoimmune hepatitis, hemochromatosis, or primary biliary cholangitis were excluded. LT recipients with a primary etiology of NASH and a secondary diagnosis of alcohol-related liver disease or cryptogenic cirrhosis remained in the cohort; for these patients, the dominant etiology was clinically considered to be NASH.
Each participating transplant center followed their institutional protocols for pre-LT cardiac evaluation. These protocols varied in terms of evaluation by a cardiologist, risk stratification, and standard preoperative cardiac testing. At some centers, the presence of 2 or more cardiac risk factors, such as older age, hypertension, hyperlipidemia, diabetes, and/or tobacco use, led to left heart catheterization, whereas other centers reserved left heart catheterization for patients with stress-induced ischemia or the inability to achieve the maximum predicted heart rate on stress testing. Not all patients with known coronary artery disease (CAD) including history of coronary artery bypass graft (CABG) automatically underwent left heart catheterization; at several centers, cardiac workup in these cases was at the discretion of the cardiologist. In recent years, several centers incorporated coronary multidetector computed tomographic angiography (CTCA) to screen for CAD.
The primary outcome was time to graft failure. Secondary outcomes included the following: time to death; CV events within 1 year of LT; infection within 1 year of LT; malignancy including HCC within 1 year of LT; rejection and biliary complications; health care utilization, including intensive care unit (ICU) and length of stay; and renal failure at specified time points including 1, 3, 6, and 12 months after transplant. Estimated glomerular filtration rates (eGFRs) were calculated by the Modification of Diet in Renal Disease version 4. (3,7–10,15) Renal failure was considered a binary outcome that was true if the patient was on dialysis (any type of renal replacement therapy) or had an eGFR <30 mL/minute/1.73 m2 at the specified time points of 1, 3, 6, and 12 months. Heart failure was a clinical diagnosis that could include either systolic or diastolic heart failure. Causes of death were reported when available.
Summary scores based on existing literature were used to estimate medical comorbidity (Charlson comorbidity index); donor risk (donor risk index [DRI]); CV risk (cardiovascular risk in orthotopic liver transplantation [CAR-OLT] score); and HCC recurrence risk (Risk Estimation of Tumor Recurrence After Transplant score).(16–19)
STATISTICAL ANALYSIS
Graft survival was estimated using Kaplan-Meier methods, with patients censored at last follow-up if death had not occurred. No patients were excluded from the survival analysis. Posttransplant outcomes other than survival were reported at either the specific time points (1, 3, 6, or 12 months) or as a binary outcome. LT recipients who died during transplant or who had <1 day of follow-up were excluded from descriptive posttransplant outcomes because they were unable to contribute meaningful posttransplant follow-up time. The eGFR was plotted at 1, 3, 6, and 12 months after LT to compare renal function in NASH versus non-NASH patients. For this analysis, SLKT recipients were excluded, and patients on dialysis were defaulted to an eGFR of 10 mL/minute/1.73 m2.
Multivariate logistic regression analysis was performed to evaluate the association of NASH with select binary posttransplant outcomes, including heart failure, stroke, and renal failure at 12 months. The regression models were adjusted for age, sex, and pre-LT variables previously recognized to influence these post-LT outcomes. The outcome of heart failure was adjusted for diastolic dysfunction on echocardiogram, history of CAD with PCI or CABG, and history of atrial fibrillation; stroke was adjusted for history of any CAD, history of atrial fibrillation, pre-LT diabetes and hypertension; and renal failure was adjusted for pre-LT moderate or severe renal disease, ascites, diabetes, and hypertension. Summary scores for pretransplant comorbidity were stratified by transplant year to assess for changes during the study period.
Power calculations were performed for graft survival and occurrence of heart failure and stroke at 12 months. Considering that 20.2% of our cohort were patients with NASH (exposed cohort), that there was a median 3.44 years of follow-up time, an observed mortality rate of 11.1% in our non-NASH group at 1 year, and a censoring rate of 1.7%, our study was powered to detect statistically significant differences in 1-year graft survival with a power of 80% and a type 1 error rate of 5% with a relative hazard of 1.47. Considering the same proportion, follow-up time, a mortality rate of 19.6%, and 25.5% of patients censored for loss to follow-up over 3 years, the study was powered to detect a difference in 3-year graft survival with a relative hazard of 1.65. With a sample size of 1023 patients and an observed rate of 6.2% for heart failure and 5.0% for stroke in the non-NASH patients, our cohort was powered to detect an odds ratio of 2.19 for heart failure and 2.34 for stroke.
Sensitivity analyses were performed to analyze for the following:
An era effect related to the availability of direct- acting antiviral (DAA) therapy for HCV infection, comparing survival and post-LT outcomes before and after the approval of sofosbuvir in October 2013.
Patients with HCC.
Patients without HCC.
All statistical analyses were performed using R, version 3.6.1 (release 2019–07-05, R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a P value of <0.05 was considered significant, and all tests were 2-tailed. Variables were compared between NASH and non-NASH groups using Student t test, chi-square test, 1-way analysis of variance, and Wilcoxon rank sum test, as appropriate. Missing data were excluded from demographic tables and comparisons.
Results
During the study period, 1023 LT recipients met inclusion criteria. The median age was 67.5 years (interquartile range [IQR], 66.0–69.2 years), with 226 (22.1%) LT recipients over the age of 70 years (Table 1). On the basis of aggregated data from program-specific reports, there were 6834 transplants performed at these 13 centers during the study period, indicating that our study cohort of 1023 patients >65 years of age accounted for 15.0% of all LT recipients. By comparison, patients >65 years of age comprised 9.0% of all LTs, living or deceased donor, in the United States between 2010 and 2016.(1)
TABLE 1.
Cohort Demographics Including Selected Recipient, Donor, and Transplant Characteristics (n = 1023)
| NASH Group (n = 207) | Non-NASH Group (n = 816) | P Value | |
|---|---|---|---|
| Age at transplant, years | 68.0 (66.4–70.0) | 67.3 (66.0–69.0) | 0.04 |
| Sex, female | 98 (47.3) | 268 (32.8) | <0.001 |
| Race | <0.001 | ||
| White | 162 (78.3) | 555 (68.0) | |
| Black | 1 (0.5) | 65 (8.0) | |
| Hispanic/Latino | 25 (12.1) | 75 (9.2) | |
| Asian | 7 (3.4) | 84 (10.3) | |
| Other | 12 (5.8) | 37 (4.5) | |
| HCC | 78 (37.7) | 464 (56.9) | <0.001 |
| Ascites | 167 (80.7) | 516 (63.3) | <0.001 |
| Hepatic encephalopathy | No 132 (64.1) | 407 (49.9) | <0.001 |
| History of variceal bleed | 60 (29.0) | 162 (19.9) | 0.006 |
| TIPS | 50 (24.2) | 65 (8.0) | <0.001 |
| Spontaneous bacterial peritonitis | 36 (17.4) | 90 (11.0) | 0.02 |
| Suspected hepatorenal syndrome | 49 (23.7) | 148 (18.1) | 0.09 |
| Portal vein thrombosis | 31 (15.0) | 99 (12.1) | 0.33 |
| Allocation (UNOS) MELD-Na | 25 (19–32) | 25 (16–32) | 0.38 |
| Laboratory MELD-Na | 21 (16–29) | 18 (12–26) | <0.001 |
| Exception points | 64 (30.9) | 380 (46.6) | <0.001 |
| Medical history | |||
| MI | 11 (5.3) | 43 (5.3) | 0.99 |
| Congestive heart failure | 18 (8.7) | 47 (5.8) | 0.17 |
| Peripheral vascular disease | 8 (3.9) | 39 (4.8) | 0.71 |
| Cerebrovascular disease | 16 (7.7) | 35 (4.3) | 0.07 |
| Chronic pulmonary disease | 23 (11.1) | 93 (11.4) | 0.99 |
| Diabetes without chronic complications | 121 (58.5) | 207 (25.4) | <0.001 |
| Diabetes with chronic complications | 23 (11.1) | 43 (5.3) | 0.004 |
| Moderate-to-severe renal disease* | 92 (44.4) | 295 (36.2) | 0.04 |
| Charlson comorbidity score | 8 (6–9) | 7 (6–8) | <0.001 |
| Hypertension | 136 (66.3) | 430 (54.0) | 0.002 |
| Hyperlipidemia | 95 (46.3) | 188 (23.8) | <0.001 |
| Atrial fibrillation | 15 (7.4) | 63 (7.9) | 0.90 |
| Deep vein thrombosis | 8 (3.9) | 31 (3.9) | 0.99 |
| CABG | 21 (10.3) | 19 (2.4) | 0.03 |
| Any CAD (history of PCI or CABG, or CAD seen on CTCA/Left heart catheterization) | 76 (36.7) | 168 (20.6) | <0.001 |
| CAR-OLT score | 40 (32–49) | 34 (27–40) | <0.001 |
| Donor and transplant variables | |||
| Donor age, years | 49.0 (34.0–61.0) | 45.0 (29.0–57.3) | 0.01 |
| DCD | 19 (9.2) | 55 (6.8) | 0.30 |
| Living donor LT | 24 (11.6) | 55 (6.7) | 0.03 |
| SLKT | 16 (7.7) | 71 (8.7) | 0.76 |
| DRI | 1.79 (1.43–2.03) | 1.61 (1.30–1.95) | 0.004 |
| Center variables | |||
| Transplant center volume | 0.97 | ||
| <50/year | 5 (2.4) | 20 (2.5) | |
| 50–100/year | 85 (41.1) | 327 (40.1) | |
| >100/year | 117 (56.5) | 469 (57.5) | |
NOTE: Data are given as median (IQR) or n (%).
Moderate-to-severe renal disease is defined by clinical assessment and includes chronic kidney disease, acute kidney injury, and hepatorenal syndrome.
NASH was the primary etiology of liver disease in 207 (20.2%) patients. LT recipients without NASH included 395 (48.4%) patients with viral hepatitis, 198 (24.3%) with alcohol-related liver disease, 110 (13.5%) with autoimmune or cholestatic liver disease, and 113 (13.8%) with another or unspecified etiology. Compared with non-NASH recipients, NASH LT recipients were older (68.0 versus 67.3 years; P = 0.04), more likely female (47.3% versus 32.8%; P < 0.001), and White (78.3% versus 68.0%) or Hispanic (12.1% versus 9.2%; P < 0.001). NASH LT recipients were less likely to have HCC (37.7% versus 56.9%; P < 0.001) and had higher laboratory Model for End-Stage Liver Disease–sodium (MELD-Na) at transplant (21 versus 18; P < 0.001). Manifestations of chronic liver disease, including ascites, hepatic encephalopathy, variceal bleeding, and spontaneous bacterial peritonitis, were highly prevalent in NASH LT recipients, affecting 80.7%, 64.1%, 29.0%, and 17.4% of patients, respectively.
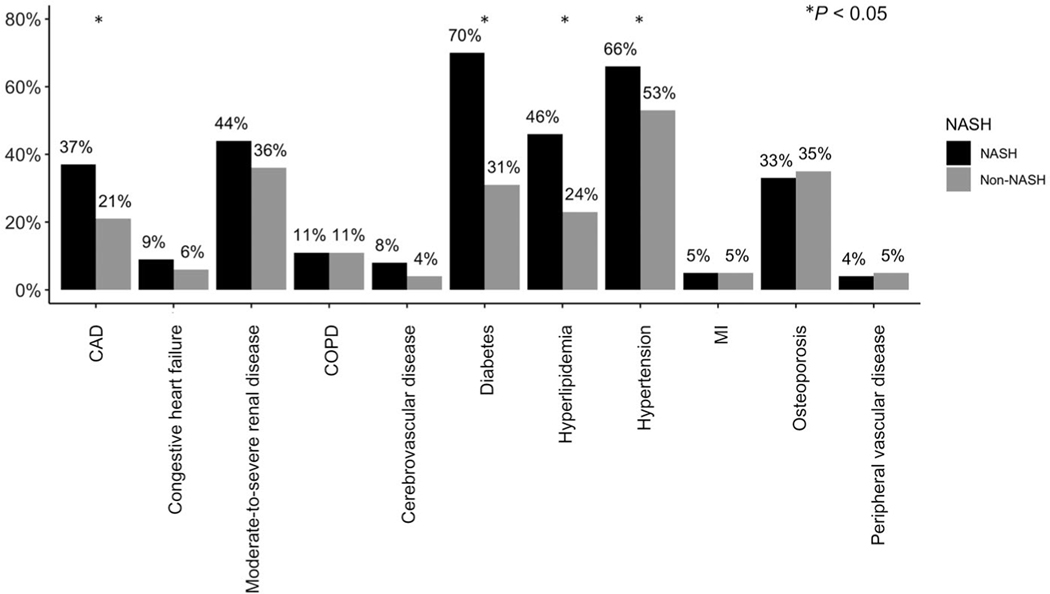
With regard to medical history, rates of cardiopulmonary disease were similar between the groups, whereas diabetes and renal disease were more prevalent among those with NASH, correlating with a marginally higher median Charlson comorbidity index (8 versus 7; P < 0.001). LT recipients with NASH were also more likely to have pre-LT hypertension, hyperlipidemia, or CAD compared with non-NASH recipients (P < 0.05; Fig. 1). Pretransplant cardiac evaluation variables were available for 96.2% of the cohort. Overall, 74.9% of patients underwent stress testing, and 37.8% underwent left heart catheterization for evaluation of CAD (Supporting Table 2). The primary type of stress testing was dobutamine stress testing (47.2%) followed by myocardial perfusion imaging (40.6%) and exercise stress testing (8.8%). Compared with non-NASH patients, those with NASH were more likely to undergo stress testing or left heart catheterization (P = 0.03 and P = 0.048, respectively).
FIG. 1.
Pretransplant comorbidities by liver disease etiology (NASH versus non-NASH; n = 1023).
Transplant characteristics were similar between the groups, with similar rates of donation after circulatory death (DCD) LT (7.2% overall) and SLKT (8.5%). NASH LT recipients received older donor organs (median donor age 49.0 versus 45.0 years; P = 0.01), translating to a higher calculated DRI compared with non-NASH recipients (1.79 versus 1.61; P = 0.004). NASH LT recipients were also more likely to receive living donor LT (11.6% versus 6.7%; P = 0.03).
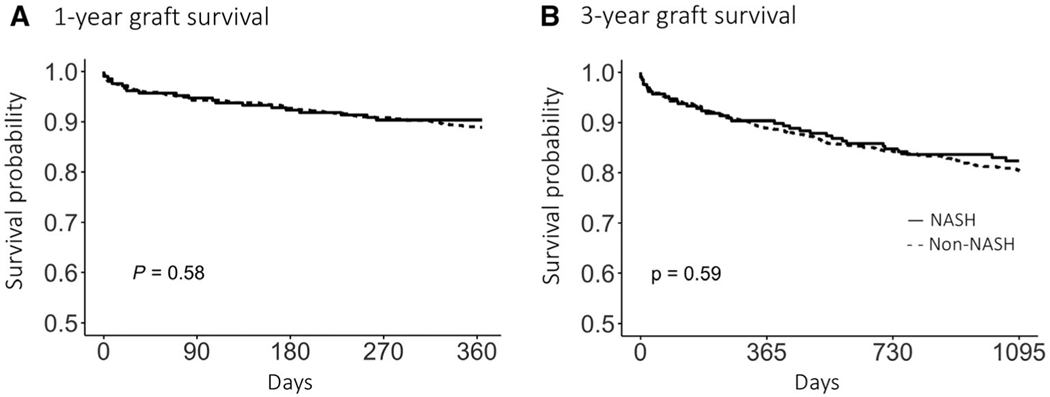
There was no difference in 1-year (90.3% versus 88.9%, log-rank P = 0.56) and 3-year (82.4% versus 80.4%, log-rank P = 0.51) graft survival between NASH and non-NASH LT etiologies, respectively (Fig. 2). Patient survival among NASH patients was 91.3% at 1 year and 83.3% at 3 years compared with 90.1% at 1 year and 81.5% at 3 years for non-NASH patients (log-rank P = 0.64 and 0.62, respectively). The cause of death was available for 163 of the 236 (69%) patients with a reported death, and the most common causes of death were infectious (24%), cardiac (16%), nonhepatic malignancy (15%), HCC or cholangiocarcinoma (12%), and graft-related complications (9%; Supporting Table 3).
FIG. 2.
Kaplan-Meier survival curves for (A) 1-year and (B) 3-year graft survival stratified by etiology of liver disease (NASH and non-NASH).
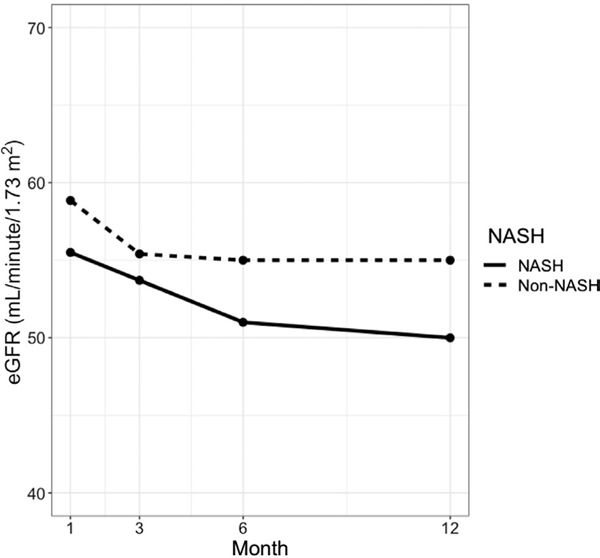
The median duration of follow-up was 1254 days (IQR, 731–1934 days). Table 2 gives the rates of selected posttransplant outcomes in NASH and non-NASH recipients. Among the 1016 (99.3%) patients who survived at least 1 day after transplant, 177 (17.4%) experienced rejection in the first year (95.5% of which were classified as acute cellular rejection); 212 (20.9%) experienced biliary stricture requiring intervention; 34 (3.4%) experienced hepatic artery thrombosis; and 28 (2.8%) experienced portal vein thrombosis. There was no significant difference in the incidence of these complications between NASH and non-NASH recipients. CV, neurological, and infectious outcomes were similar between the 2 groups, including atrial fibrillation (13.7%), myocardial infarction (MI; 2.8%), stroke (5.5%), heart failure (6.3%), delirium (12.5%), seizures (4.2%), viral infection (15.9%), bacterial infection (36.6%), and fungal infection (5.7%). Rates of malignancy within 1 year were also similar, with an incidence of 4.9% for solid organ malignancy (of which 40% was recurrent HCC), 3.8% for skin cancer, and 1.3% for posttransplant lymphoproliferative disease (PTLD). Renal outcomes were also comparable, with 4.0% of LT recipients on dialysis at 3 months and 1.5% on dialysis at 12 months. Trends in eGFR at 1, 3, 6, and 12 months after LT are shown in Fig. 3. NASH LT recipients had lower median eGFR at each recorded time point. Compared with non-NASH patients, NASH patients had higher rates of health care utilization after transplant, including post-LT intubated days (P = 0.01), days in the ICU (P = 0.05), and total length of stay (P = 0.01).
TABLE 2.
Selected Posttransplant Outcomes Excluding Those With Less Than 1 Day of Follow-up (n = 1016)
| NASH Group (n = 205) | Non-NASH Group (n = 811) | P Value | |
|---|---|---|---|
| Days of follow-up | 1285 (759–1924) | 1234 (728–1938) | 0.58 |
| Any rejection | 34 (16.7) | 143 (18.0) | 0.76 |
| Any biliary stricture | 41 (20.1) | 171 (21.4) | 0.76 |
| Hepatic artery thrombosis within 1 year | 6 (3.0) | 28 (3.5) | 0.87 |
| Portal vein thrombosis | 6 (3.0) | 22 (2.7) | 1.00 |
| Atrial fibrillation within 1 year | 28 (13.7) | 111 (13.8) | 1.00 |
| MI within 12 months | 5 (2.5) | 23 (2.9) | 0.94 |
| Stroke within 12 months | 16 (7.8) | 40 (5.0) | 0.16 |
| Heart failure within 12 months | 14 (6.9) | 50 (6.2) | 0.86 |
| Delirium with neurology consult | 29 (14.2) | 98 (12.2) | 0.52 |
| Seizures | 8 (3.9) | 35 (4.4) | 0.93 |
| Viral infection within 12 months | 32 (16.0) | 130 (16.4) | 0.99 |
| Bacterial infection within 12 months | 81 (39.9) | 291 (36.6) | 0.43 |
| Fungal infection within 12 months | 12 (6.0) | 46 (5.8) | 1.00 |
| Days from transplant to extubation | 1 (0–2) | 1 (0–1) | 0.01 |
| Days in ICU after LT | 3 (2–5) | 3 (2–5) | 0.05 |
| Length of stay after LT | 10 (7–17) | 9 (6–15) | 0.01 |
| Post-LT discharge to rehabilitation center | 49 (30.1) | 165 (24.4) | 0.16 |
| Hospital admissions within 12 months | 1 (0–3) | 1 (0–3) | 0.13 |
| Outpatient clinic visits within 12 months | 9 (6–12) | 9 (6–12) | 0.65 |
| >2 returns to the operating room within 12 months | 12 (7.1) | 38 (5.5) | 0.54 |
| Dialysis | |||
| At 1 month | 18 (9.0) | 67 (8.5) | 0.93 |
| At 3 months | 9 (4.6) | 32 (4.2) | 0.96 |
| At 6 months | 5 (2.6) | 24 (3.2) | 0.86 |
| At 12 months | 3 (1.6) | 12 (1.7) | 1.00 |
| Renal failure | |||
| At 1 month* | 35 (17.4) | 100 (12.8) | 0.11 |
| At 3 months | 31 (16.8) | 84 (11.6) | 0.08 |
| At 6 months | 31 (16.8) | 93 (13.2) | 0.25 |
| At 12 months | 31 (17.6) | 92 (13.5) | 0.20 |
| Skin cancer within 12 months | 5 (2.5) | 34 (4.3) | 0.32 |
| PTLD within 12 months | 1 (0.5) | 12 (1.5) | 0.43 |
| Solid organ cancer within 12 months | 7 (3.5) | 43 (5.5) | 0.33 |
| Recurrent HCC | 2 (1.0) | 18 (2.2) | 0.39 |
| Survival outcomes | |||
| Death | 44 (21.5) | 185 (22.8) | 0.75 |
| Retransplant | 2 (1.0) | 15 (1.8) | 0.57 |
| Alive at the end of follow-up | 161 (78.5) | 626 (77.2) | 0.75 |
NOTE: Data are given as median (IQR) or n (%).
Renal failure defined as dialysis or eGFR <30 mL/minute/1.73 m2.
FIG. 3.
Median eGFR at 1, 3, 6, and 12 months after transplant comparing NASH (solid line) to non-NASH (dashed line). SLKT recipients were excluded, and patients on dialysis were defaulted to an eGFR of 10 mL/minute/1.73 m2.
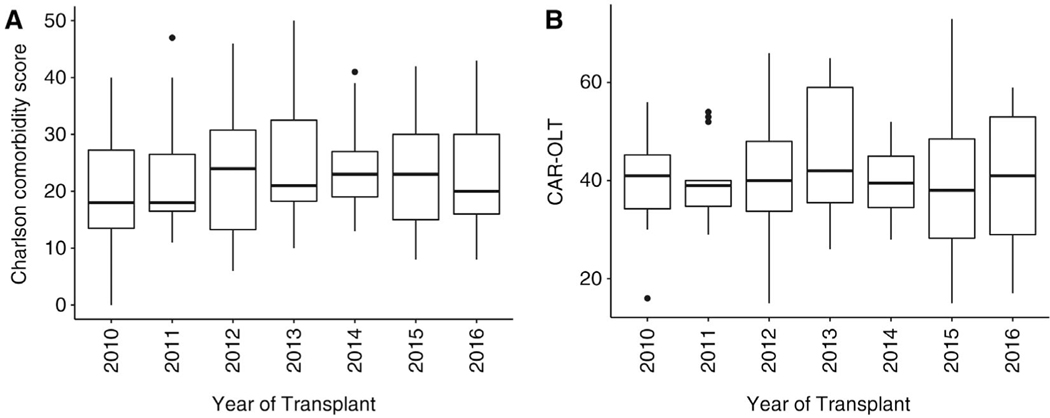
In the multivariate logistic regression, NASH was not associated with posttransplant development of heart failure, stroke, or renal failure at 12 months (Table 3). There were no significant differences in recipient characteristics or posttransplant outcomes among NASH LT recipients when stratified by age 65–69 and ≥70 years (data not shown). Figure 4 demonstrates that measures of pretransplant comorbidity, including the Charlson comorbidity index and CAR-OLT score for CV risk, were stable during the study period.
TABLE 3.
Multivariate Logistic Regression Analysis for Heart Failure Within 12 Months After LT, Stroke Within 12 Months After LT, and Renal Failure at 12 Months After LT (excluding SLKT)
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Heart failure within 12 months after LT | ||||
| Age at transplant (per year increase) | 1.06 | 0.96–1.17 | 1.06 | 0.95–1.18 |
| Sex, male (reference: female) | 0.95 | 0.56–1.59 | 0.79 | 0.45–1.38 |
| Laboratory MELD at transplant (per unit increase) | 1.02 | 1.00–1.04 | 1.03 | 1.01–1.06 |
| History of CABG | 2.95 | 1.09–7.98 | 2.35 | 0.77–7.10 |
| History of CAD with PCI | 2.74 | 1.33–5.65 | 2.59 | 1.16–5.79 |
| History of atrial fibrillation | 3.34 | 1.73–6.46 | 3.24 | 1.58–6.67 |
| Diastolic dysfunction on transthoracic echocardiogram (reference: absent) | ||||
| Present | 0.66 | 0.35–1.24 | 0.64 | 0.32–1.28 |
| Unknown or unable to measure | 0.79 | 0.43–1.47 | 0.82 | 0.43–1.56 |
| NASH | 1.19 | 0.66–2.17 | 0.92 | 0.48–1.76 |
| Stroke within 12 months after LT | ||||
| Age at transplant (per year increase) | 1.03 | 0.93–1.15 | 1.03 | 0.92–1.15 |
| Sex, male (reference: female) | 0.54 | 0.31–0.93 | 0.53 | 0.30–0.93 |
| Laboratory MELD at transplant (per unit increase) | 1.00 | 0.97–1.02 | 0.99 | 0.96–1.02 |
| History of any CAD | 1.83 | 1.04–3.22 | 1.65 | 0.90–3.03 |
| History of atrial fibrillation | 2.50 | 1.17–5.32 | 2.29 | 1.05–5.03 |
| Pre-LT diabetes | 1.31 | 0.76–2.25 | 1.08 | 0.59–2.00 |
| Pre-LT hypertension | 1.01 | 0.59–1.74 | 0.93 | 0.53–1.65 |
| NASH | 1.61 | 0.89–2.95 | 1.36 | 0.70–2.67 |
| Renal failure at 12 months after LT | ||||
| Age at transplant (per year increase) | 1.04 | 0.96–1.13 | 1.06 | 0.97–1.17 |
| Sex, male (reference: female) | 0.82 | 0.54–1.25 | 0.80 | 0.51–1.27 |
| Laboratory MELD at transplant (per unit increase) | 1.07 | 1.05–1.09 | 1.05 | 1.02–1.07 |
| Pre-LT moderate or severe renal disease | 4.37 | 2.86–6.70 | 2.82 | 1.65–4.82 |
| Pre-LT ascites | 2.70 | 1.64–4.46 | 1.18 | 0.65–2.15 |
| Pre-LT diabetes | 1.14 | 0.75–1.73 | 1.17 | 0.72–1.91 |
| Pre-LT hypertension | 0.95 | 0.63–1.44 | 0.86 | 0.54–1.37 |
| NASH | 1.13 | 0.69–1.84 | 0.83 | 0.47–1.48 |
NOTE: Bold value indicates statistical significance.
Variables were selected based on known risk factors and/or significance in the univariate analysis.
FIG. 4.
Trends in (A) Charlson comorbidity score and (B) CAR-OLT scores among NASH LT recipients during the 7-year study period. Boxplots with median are denoted by the horizontal line, IQR by the rectangle, and outliers by the whiskers (vertical lines).
In the sensitivity analysis for a DAA era effect, we observed no significant differences in posttransplant survival by era between NASH and non-NASH patients. In the pre-DAA era, 3-year graft survival was 85.5% and 79.7% for NASH and non-NASH patients (P = 0.17), respectively. In the post-DAA era, 3-year survival was 80.1% for NASH and 80.0% for non-NASH (P = 0.55; Supporting Fig.2). In a sensitivity analysis including only the 542 patients with HCC, there was no difference in 3-year graft survival between NASH and non-NASH patients (85.0% versus 81.9%; P = 0.40). Among the 481 patients without HCC, there was no difference in 3-year graft survival between NASH and non-NASH patients (80.8% versus 79.7%; P = 0.85). There were also no differences in post-LT CV or renal outcomes (Supporting Table 4).
Discussion
In our large US multicenter cohort among older LT recipients, 1- and 3-year graft survival outcomes in LT recipients with NASH were 90.3% and 82.4%, respectively, and comparable to non-NASH LT recipients. As expected, certain CV risk factors, including hypertension, diabetes, and CAD, were more prevalent in the NASH population. However, rates of CV events, graft-related complications, and infections within 1 year after transplant were similar to non-NASH LT recipients. Health care utilization measures, including intubated days, ICU stay, and length of stay after transplant, were higher among NASH patients, which is potentially related to greater severity of liver disease at the time of LT as evidenced by the higher MELD-Na scores.
Several national and international cohort-based studies have demonstrated no significant survival differences between NASH and non-NASH LT recipients.(9) However, using national data from the Organ Procurement and Transplantation Network (OPTN), Nagai et al.(11) demonstrated increased risk of 1-year mortality among NASH LT recipients compared with HCV and alcohol-related liver disease during the most recent era (January 1, 2016, to June 30, 2017). This observation was driven, however, by the fact that survival for HCV-related liver disease improved over time. In fact, 1-year survival estimates for patients with NASH in their study were relatively stable (88.1%−89.1%), corroborating the survival findings from our cohort albeit in the general population not limited to older age. In our cohort, the leading causes of death were infectious, cardiac, and malignant etiologies. A previous study using the OPTN database by VanWagner et al. showed no difference in overall all-cause mortality among 5507 patients with NASH compared with other etiologies, but the authors did find increased early (30-day) and longterm CV-specific mortality in the population in unadjusted logistic regression analysis.(20) In the multivariate analysis with adjustment for age and pretransplant cardiometabolic risk factors, including renal impairment, diabetes, and CV disease, the association between NASH and CV mortality was no longer significant. Our study focused on older adults (age ≥65 years) and did not observe an increased CV-specific mortality among those patients with NASH.
Until now, morbidity outcomes after LT have not been well described in older LT recipients with NASH. In our study, the rates of graft complications among older LT recipients with NASH including rejection (17.4%), biliary (20.9%), and vascular complications (6.2%) approximate estimates among all LT recipients from earlier Scientific Registry of Transplant Recipients–based and single-center cohort studies.(21,22) Furthermore, the frequency of specific post-LT CV complications among older LT recipients with NASH such as atrial fibrillation (13.7%), MI (2.8%), stroke (5.5%), and heart failure (6.3%) corroborate estimates from previous studies of all LT recipients.(12) It has been suggested that patients with NASH may be at increased risk of post-LT CV events, which was not observed in our cohort when patients with NASH were compared with patients with all other etiologies.(12) This may be, in part, due to the endpoints selected, as well as the older age composition of our cohort, who generally have more comorbidities and may be at high risk for CV events regardless of liver disease etiology. Pretransplant selection criteria and cardiac workup protocols at the participating centers may have also been able to mitigate the risk. Our findings regarding neurological, renal, and infectious outcomes provide additional insight into contemporary post-LT outcomes in the older LT recipient population.
In our cohort, NASH patients did have lower eGFR at 1, 3, 6, and 12 months after LT, which could be due to the greater severity of liver disease at LT, as evidenced by higher MELD-Na scores and prevalence of preexisting renal disease. In a previous study of LT recipients with NASH, renal disease (defined as an eGFR <30 mL/minute/1.73 m2 at the time of LT or dialysis within 2 weeks of LT) was associated with an increased risk of all-cause mortality but not graft loss, suggesting that the mortality was due to nonliver-related causes.(23) It is suggested that this mortality rate may have been driven by persistent kidney dysfunction after LT and increased risk of CV mortality, although details regarding cause of death, prevalence of chronic kidney disease, and immunosuppression after LT were not available for this OPTN-based study.(24) Our study also did not evaluate data on the immunosuppression regimen, which can often contribute to renal disease. Further investigation regarding post-LT chronic kidney disease will be needed to guide outcomes for research in this field going forward because this has implications for immunosuppression management and decision making regarding SLKT or kidney transplantation after LT.
Although it is well recognized that NASH and older age are associated with increased mortality and CV risk, inferior survival after LT has not been evident in our cohort or in others. This may be related to current recipient selection practices. All LT candidates typically undergo comprehensive CV evaluation prior to LT, and those with high surgical risk do not proceed to LT. In our cohort, patients with NASH were more likely to undergo stress testing and left heart catheterization prior to LT to evaluate for CAD. Thus, patients age 65 years or older with NASH ultimately undergoing LT are a selected group that may not be predisposed to inferior outcomes compared with LT recipients with other etiologies of liver disease. We demonstrate in our cohort that the Charlson comorbidity index and CAR-OLT scores were stable during the study time period, suggesting that despite increasing age and prevalence of NASH nationally, the centers in our REALT consortium did not transplant patients with additional comorbid or cardiac risks during the study period in this older LT population. In addition, longer duration of posttransplant follow-up may be needed to observe significant survival differences because CV events and mortality may take several years to manifest.
The strengths of this study include the granularity of data abstracted from the electronic health record at each participating center. Mortality measures based on large registry data are well described, yet morbidity outcomes and post-LT complications in this population are less so. These data provide contemporary large-scale estimates of common conditions not typically captured from registry databases including recipient comorbidities, graft complications, CV events, renal function, immunosuppression, infection, and malignancy. This multicenter consortium represents a spectrum of transplant centers across the United States including small, medium, and large volume with a range of listing practices.
This study does have limitations. To ensure adequate follow-up time, only LTs performed until 2016 were included. As a result, patients with HCV during this time period may be overrepresented, and outcomes for NASH compared with other etiologies may be different in an era where HCV treatment is widely available. The extensive array of collected variables may increase the risk of type 1 errors from multiple hypotheses testing, although few of the outcome comparisons were statistically significant. In addition, other factors known to contribute to post-LT outcomes, such as frailty and sarcopenia, are not captured in this study. Lastly, despite the relatively large sample size, our cohort may be underpowered to detect differences in certain outcomes.
In conclusion, our findings from the REALT consortium demonstrate acceptable graft survival and post-LT comorbid complications in older LT recipients with NASH. Nationally, NASH is increasingly prevalent and represents a growing proportion of the LT wait-list and transplant populations. The aging of the LT population is largely driven by NASH, with wider acceptance of older LT recipients and transplant centers pushing the envelope on age limits in LT. Both mortality and morbidity outcomes should continue to be closely scrutinized in this undeniably high-risk population.
Supplementary Material
Acknowledgments
Allison J. Kwong is supported by National Institute of Allergy and Infectious Diseases grant R25AI147369. Lisa VanWagner is supported by National Heart, Lung and Blood Institute grant K23 HL136891. Jennifer C. Lai is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01AG059183.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency played no role in the analysis of the data or the preparation of this manuscript.
Lisa VanWagner consults for Gilead Sciences; and is on the speakers’ bureau, has grants from, and consults for W. L. Gore & Associates. Yuval Patel consults for Intercept Pharmaceuticals. Sonali Paul has grants from Target PharmaSolutions, Intercept Pharmaceuticals, and GENFIT. Jennifer C. Lai consults for Axcella Health, Ambys Medicines, and BioMarin Pharmaceuticals.
Additional supporting information may be found in the online version of this article.
Abbreviations:
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CAR-OLT
cardiovascular risk in orthotopic liver transplantation
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CTCA
computed tomographic angiography
- CV
cardiovascular
- DAA
direct-acting antiviral
- DCD
donation after circulatory death
- DRI
donor risk index
- eGFR
estimate glomerular filtration rate
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ICU
intensive care unit
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- MI
myocardial infarction
- NASH
nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- PCI
percutaneous coronary intervention
- PTLD
posttransplant lymphoproliferative disease
- REALT
Re-Evaluating Age Limits in Transplantation
- SLK
simultaneous liver-kidney
- TIPS
transjugular intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
REFERENCES
- 1).Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant 2020;20(suppl 1):193–299. [DOI] [PubMed] [Google Scholar]
- 2).Su F, Yu L, Berry K, Liou IW, Landis CS, Rayhill SC, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology 2016;150:441–453. [DOI] [PubMed] [Google Scholar]
- 3).Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci 2017;62:2915–2922. [DOI] [PubMed] [Google Scholar]
- 4).Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188–2195. [DOI] [PubMed] [Google Scholar]
- 5).Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 6).Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 7).Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 8).Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl 2012;18:29–37. [DOI] [PubMed] [Google Scholar]
- 9).Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:394–402. [DOI] [PubMed] [Google Scholar]
- 10).Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. ; for European Liver and Intestine Transplant Association (ELITA). Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol 2019;71:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Nagai S, Collins K, Chau LC, Safwan M, Rizzari M, Yoshida A, et al. Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Clin Gastroenterol Hepatol 2019;17:2759–2768. [DOI] [PubMed] [Google Scholar]
- 12).VanWagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012;56:1741–1750. [DOI] [PubMed] [Google Scholar]
- 13).Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl 2011;17:1292–1298. [DOI] [PubMed] [Google Scholar]
- 14).Scientific Registry of Transplant Recipients. Program-specific reports. https://www.srtr.org/reports-tools/program-specific-reports/. Accessed May 9, 2020. [DOI] [PubMed]
- 15).Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 16).Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17).Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 18).VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT, et al. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: the CAR-OLT score. Hepatology 2017;66:1968–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).VanWagner LB, Lapin B, Skaro AI, Lloyd-Jones DM, Rinella ME. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int 2015;35:2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Levitsky J, Goldberg D, Smith AR, Mansfield SA, Gillespie BW, Merion RM, et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol 2017;15:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg 2009;208:896–903. [DOI] [PubMed] [Google Scholar]
- 23).Molnar MZ, Joglekar K, Jiang Y, Cholankeril G, Abdul MKM, Kedia S, et al. ; for Global NAFLD Consortium. Association of pretransplant renal function with liver graft and patient survival after liver transplantation in patients with nonalcoholic steatohepatitis. Liver Transpl 2019;25:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Piano S, Tonon M, Angeli P. Predicting outcomes of liver transplantation in patients with nonalcoholic steatohepatitis: pretransplant renal function is key. Liver Transpl 2019;25:362–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.