Supplemental Digital Content is available in the text
Keywords: COVID-19, inflammation, lactic dehydrogenase-lymphocyte ratio, prognosis
Abstract
To develop a useful score for predicting the prognosis of severe corona virus disease 2019 (COVID-19) patients.
We retrospectively analyzed patients with severe COVID-19 who were admitted from February 10, 2020 to April 5, 2020. First, all patients were randomly assigned to a training cohort or a validation cohort. By univariate analysis of the training cohort, we developed combination scores and screened the superior score for predicting the prognosis. Subsequently, we identified the independent factors influencing prognosis. Finally, we demonstrated the predictive efficiency of the score in validation cohort.
A total of 145 patients were enrolled. In the training cohort, nonsurvivors had higher levels of lactic dehydrogenase than survivors. Among the 7 combination scores that were developed, lactic dehydrogenase-lymphocyte ratio (LLR) had the highest area under the curve (AUC) value for predicting prognosis, and it was associated with the incidence of liver injury, renal injury, and higher disseminated intravascular coagulation (DIC) score on admission. Univariate logistic regression analysis revealed that C-reactive protein, DIC score ≥2 and LLR >345 were the factors associated with prognosis. Multivariate analysis showed that only LLR >345 was an independent risk factor for prognosis (odds ratio [OR] = 9.176, 95% confidence interval [CI]: 2.674–31.487, P < .001). Lastly, we confirmed that LLR was also an independent risk factor for prognosis in severe COVID-19 patients in the validation cohort where the AUC was 0.857 (95% CI: 0.718–0.997).
LLR is an accurate predictive score for poor prognosis of severe COVID-19 patients.
1. Introduction
The coronavirus disease 2019 (COVID-19) has become an urgent public health problem due to the increasing number of infections worldwide. Since the Chinese government took appropriate and timely measures, the pandemic is currently under control in China. However, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread rapidly abroad and remains a serious issue globally, posing numerous challenges.
The majority of COVID-19 patients have mild to moderate symptoms including fever, dry cough, and fatigue, but a sub-cohort will develop severe disease, which often presents with acute respiratory distress syndrome (ARDS), coagulation dysfunction, septic shock, and multiple organ failure.[1] The overall mortality of COVID-19 patients is 2.5% in China,[2] but the mortality of severe and critically severe patients is as high as 16.6%.[3] Elevation of C-reactive protein (CRP) and lactic dehydrogenase (LDH), lymphopenia, and leukocytopenia are common laboratory changes seen in COVID-19.[4] It has been reported that age was an important factor influencing the outcome of COVID-19,[1] for instance, the time from the occurrence of the first symptom to death in patients who were older than 70 years was significantly shorter than in those who were younger than 70 years.[5] Moreover, older age, comorbid conditions, lymphopenia, higher levels of LDH, and D-dimer have been correlated with a greater risk of intensive care unit admission and death.[1,6,7] Comprehensive therapy and intensive supportive care of patients at high risk of death may prevent disease progression and distribute the limited health care resources more appropriately. Unfortunately, there is lack of a useful tool that independently predicts the progression of this disease. Therefore, it is essential to find an accurate and practical biomarker or score that can help clinicians to identify patients at high risk of death, especially among patients with severe COVID-19.
Systemic inflammation and host immune response play an important role in COVID-19.[8] The decrease in the lymphocyte count observed in COVID-19 suggests that the immune system comes under attack. Subsequently, excessive inflammation and uncontrolled immune activation result in organ or tissue injury.[8] Thus, inflammatory parameters such as lymphocyte count, neutrophils, and CRP, cellular enzymes such as LDH, creatine kinase (CK), and the biomarker combination scores such as neutrophil-lymphocyte ratio (NLR), CRP-lymphocyte ratio (CLR) could be prognostic biomarkers for predicting the prognosis of severe COVID-19. However, there are limited studies about clinical biomarkers or scores for predicting the prognosis of COVID-19. Therefore, this study aimed to analyze the clinical characteristics of severe COVID-19 patients in order to develop useful predictive scores associated with in-hospital deaths in these COVID-19 patients.
2. Methods
2.1. Patients and data collection
We carried out a retrospective analysis of COVID-19 patients who were admitted in Huoshenshan hospital and Taikang Tongji hospital from February 10, 2020, to April 5, 2020. Besides clinical symptoms, the diagnosis of COVID-19 was confirmed by positive results of SARS-CoV-2 nucleic acid detection tests from nasal and/or pharyngeal swab specimens of the patients. Additionally, all the patients were severely ill, which was defined to include any of the following conditions: respiratory rate ≥30 per/minute, peripheral oxygen saturation ≤93%, and arterial blood oxygen partial pressure/fraction of inspiration oxygen ≤300 mm Hg. Patients without complete clinical data were excluded from this study. This study was approved by the ethics committee of Wuhan Huoshenshan hospital (epicenter Wuhan, China). As this was a retrospective study and all subjects were anonymized, the requirement for informed consent was waived.
Clinical data including sex, age, comorbidities, outcome, and laboratory parameters of each patient on admission were recorded. Liver injury was defined when alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutamyl transpeptidase, or total bilirubin levels increased by at least twice the upper limits of the normal range. Renal injury was defined as blood creatinine >133 μmol/L. Coagulation was assessed by disseminated intravascular coagulation (DIC) score (Supplementary Table 1, http://links.lww.com/MD/F585).[9]
The entire cohort was randomly assigned to a training cohort (2/3) and a validation cohort (1/3) through computer generated random number. First, based on univariate analysis of the training cohort, we identified key factors which were up-regulated or down-regulated, and then developed the biomarker combination scores. By comparing the area under the curve (AUC) of these combination scores, we got the valuable ratio which had the best predictive efficiency for prognosis of severe COVID-19 patients. Second, we analyzed the correlation between this score and prognosis of severe COVID-19 patients and identified the independent factors for prognosis. Third, we demonstrated the predictive efficiency of the score in the validation cohort.
2.2. Patient and public involvement
There was no patient and public involvement in this study.
2.3. Statistics
Descriptive data were presented as mean ± standard deviation for normal distribution variables or median and interquartile ranges for skewed distribution variables. Categorical data were presented as proportions. The t test, nonparametric Mann–Whitney test, and Pearson Chi-Squared test were used to compare the normal distribution variables, skewed distribution variables, and categorical variables, respectively. Logistic regression analysis was utilized to identify the factors influencing prognosis. The factors with P < .10 in univariate logistic regression analysis were entered into the multivariate logistic regression analysis. The optimal cut-off value was evaluated by the receiver operator characteristic (ROC) curve. Odds ratios (OR) and 95% confident intervals (95% CI) were calculated by log-rank tests. Statistical analyses were performed using SPSS 23.0 software (IBM, Armonk, NY). P < .05 was considered to be statistically significant.
3. Results
3.1. Patients
A total of 159 patients were diagnosed with severe COVID-19. Fourteen patients were excluded because of incomplete data. Subsequently, 145 patients consisting of 70 men and 75 women were enrolled in this study. All the patients were randomly assigned to the training cohort (97 patients) or validation cohort (48 patients) (Supplementary Fig. 1, http://links.lww.com/MD/F584). None of the clinical characteristics were significantly different between the training cohort and the validation cohort (Table 1). The median age of these patients was 69 years (interquartile ranges: 63.00, 78.00). One hundred four (71.72%) patients had comorbidities including hypertension (46.21%), diabetes (18.62%), coronary heart disease (13.10%), chronic obstructive pulmonary disease (8.97%), and cerebrovascular disease (8.28%). Hypertension was the most common comorbidity. In the entire cohort, 57 (39.31%) patients died, and the median levels of several laboratory parameters including LDH, CRP, and D-dimer were higher than normal range in these patients. However, the median levels of other parameters including white blood cell count, neutrophil count, platelet count, liver function parameters, renal function parameters, etc. were in the normal range (Table 1).
Table 1.
Baseline characteristics of patients with COVID-19.
| Variables | Entire cohort (n = 145) | Training cohort (n = 97) | Validation cohort (n = 48) | P value |
| Sex (male, n%) | 70 (48.28%) | 49 (50.52%) | 21 (43.75%) | .44∗ |
| Age (y) | 69.00 (63.00, 78.00) | 69.00 (63.50, 77.50) | 69.50 (62.00, 78.75) | .80‡ |
| Comorbidities | 104 (71.72%) | 72 (74.23%) | 32 (66.67%) | .34∗ |
| Hypertension | 67 (46.21%) | 43 (44.33%) | 24 (50.00%) | .52∗ |
| Diabetes | 27 (18.62%) | 22 (22.68%) | 5 (10.42%) | .07∗ |
| Coronary heart disease | 19 (13.10%) | 14 (14.43%) | 5 (10.42%) | .50∗ |
| Chronic obstructive pulmonary disease | 13 (8.97%) | 10 (10.31%) | 3 (6.15%) | .42∗ |
| Cerebrovascular disease | 12 (8.28%) | 7 (7.22%) | 5 (10.42%) | .51∗ |
| White blood cell count (×109/L) | 7.20 (5.45, 10.50) | 6.73 (5.38, 9.75) | 7.68 (5.80, 12.60) | .09‡ |
| Neutrophil count (×109/L) | 5.46 (3.42, 8.72) | 5.27 (3.30, 8.31) | 5.94 (4.03, 10.33) | .15‡ |
| Lymphocyte count (×109/L) | 0.94 (0.56, 1.36) | 0.89 (0.56, 1.32) | 1.06 (0.54, 1.39) | .29‡ |
| Haemoglobin (g/L) | 115.60 ± 19.96 | 115.04 ± 21.02 | 116.73 ± 17.77 | .63† |
| Platelet count (×109/L) | 205.05 ± 98.65 | 199.43 ± 93.94 | 216.40 ± 107.68 | .33† |
| Albumin (g/L) | 32.52 ± 4.56 | 32.45 ± 4.24 | 32.66 ± 5.19 | .81† |
| Alanine aminotransferase (U/L) | 23.60 (15.21, 45.90) | 21.10 (14.75, 46.75) | 25.95 (17.39, 45.30) | .42‡ |
| Aspartate aminotransferase (U/L) | 24.31 (16.95, 39.02) | 23.80 (16.85, 39.80) | 26.05 (16.87, 39.03) | .71‡ |
| Alkaline phosphatase (U/L) | 76.60 (62.70, 98.50) | 76.20 (64.66, 98.50) | 77.21 (56.65, 99.74) | .59‡ |
| Glutamyl transpeptidase (U/L) | 34.70 (21.20, 58.20) | 34.00 (20.45, 56.70) | 36.25 (25.12, 74.93) | .50‡ |
| Total bilirubin (μmol/L) | 11.20 (8.40, 17.30) | 11.80 (8.55, 18.00) | 10.64 (8.03, 14.43) | .14‡ |
| Urea nitrogen (mmol/L) | 5.24 (3.82, 8.05) | 5.15 (3.86, 7.44) | 6.21 (3.80, 8.92) | .35‡ |
| Creatinine (μmol/L) | 67.60 (54.35, 86.40) | 68.20 (54.87, 87.67) | 64.83 (53.20, 84.68) | .49‡ |
| Lactate dehydrogenase (U/L) | 278.70 (198.95, 414.75) | 293.00 (207.25, 442.94) | 261.50 (170.95, 380.30) | .13‡ |
| Creatine kinase (U/L) | 54.00 (31.20, 89.60) | 51.90 (30.90, 89.60) | 57.45 (31.95, 89.60) | .96‡ |
| C-reactive protein (mg/L) | 29.31 (3.23, 84.15) | 37.43 (3.48, 89.36) | 27.19 (2.58, 78.79) | .76‡ |
| Prothrombin time (s) | 13.70 (12.60, 15.47) | 13.87 (12.60, 15.68) | 13.44 (12.50, 15.45) | .43‡ |
| Activated partial thromboplastin time (s) | 28.29 (26.05, 30.74) | 28.46 (26.22, 30.74) | 28.26 (25.82, 30.93) | .99‡ |
| D-dimer (mg/L) | 1.54 (0.62, 3.36) | 1.54 (0.68, 3.35) | 1.54 (0.56, 3.39) | .64‡ |
| Death (n%) | 57 (39.31%) | 39 (40.21%) | 18 (37.50%) | .75∗ |
COVID-19, corona virus disease 2019. P value was the result of comparison between training set and validation set.
Pearson Chi-Squared test.
t test.
Mann–Whitney test.
3.2. Univariate analysis of survivors and nonsurvivors in the training cohort
In the training cohort, 39 (40.21%) patients died. The nonsurvivors were older, and had higher levels of neutrophil count, LDH, CK, CRP, prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer, and lower levels of lymphocyte count, platelet count, and serum albumin than survivors. The incidence of renal injury in nonsurvivors was higher than in survivors (17.95% vs 5.17%, P = .04), and the median of the DIC scores in nonsurvivors was also higher. The incidence rates of comorbidities were not significantly different between survivors and nonsurvivors (Table 2).
Table 2.
Univariate analysis of the training cohort.
| Variables | Survivor (n = 58) | Nonsurvivor (n = 39) | P value |
| Sex (male, n%) | 27 (46.55%) | 22 (56.41%) | .34∗ |
| Age (y) | 68.00 (61.00, 73.25) | 71.00 (67.00, 84.00) | .01‡ |
| Comorbidities | 40 (68.97%) | 32 (82.05%) | .15∗ |
| Hypertension | 23 (39.66%) | 20 (51.28%) | .26∗ |
| Diabetes | 13 (22.41%) | 9 (23.08%) | .94∗ |
| Coronary heart disease | 6 (10.34%) | 8 (20.51%) | .16∗ |
| Chronic obstructive pulmonary disease | 4 (6.90%) | 6 (15.38%) | .18∗ |
| Cerebrovascular disease | 3 (5.17%) | 4 (10.26%) | .34∗ |
| White blood cell count (×109/L) | 6.40 (5.30, 9.18) | 8.20 (6.00, 12.00) | .08‡ |
| Neutrophil count (×109/L) | 4.43 (3.20, 7.05) | 6.75 (4.89, 10.54) | .01‡ |
| Lymphocyte count (×109/L) | 1.10 (0.71, 1.58) | 0.56 (0.35, 0.81) | <.001‡ |
| Haemoglobin (g/L) | 117.97 ± 18.29 | 110.69 ± 24.12 | .09† |
| Platelet count (×109/L) | 223.83 ± 80.81 | 163.15 ± 101.20 | <.01† |
| Albumin (g/L) | 33.42 ± 3.77 | 31.01 ± 4.53 | .01† |
| Lactate dehydrogenase (U/L) | 242.63 (180.30, 329.13) | 410.40 (294.96, 616.00) | <.001‡ |
| Creatine kinase (U/L) | 43.15 (27.38, 69.19) | 76.40 (44.50, 157.00) | <.001‡ |
| C-reactive protein (mg/L) | 11.55 (2.34, 62.15) | 66.85 (11.81, 134.76) | <.01‡ |
| Prothrombin time (s) | 13.27 (12.38, 14.35) | 15.40 (13.26, 16.46) | <.001‡ |
| Activated partial thromboplastin time (s) | 27.31 (25.49, 29.33) | 30.41 (26.61, 33.70) | <.01‡ |
| D-dimer (mg/L) | 1.04 (0.51, 1.96) | 2.98 (1.37, 6.57) | <.001‡ |
| Liver injury | 12 (20.69%) | 10 (25.64%) | .57∗ |
| Renal injury | 3 (5.17%) | 7 (17.95%) | .04∗ |
| DIC score | 0 (0, 0) | 1 (0, 3) | <.001‡ |
DIC, disseminated intravascular coagulation.
Pearson Chi-Squared test.
t test.
Mann–Whitney test.
3.3. Predicted efficiency of the combination scores
Since ten key parameters (up-regulated in nonsurvivors: neutrophil count, LDH, CK, CRP, PT, APTT, D-dimer; down-regulated in nonsurvivors: lymphocyte count, platelet count, albumin) were identified, we developed new scoring systems which were combinations of the inflammation-related parameters. These scoring systems were calculated by the up-regulated parameters divided by the most common down-regulated parameter, which was the lymphocyte count.
Almost all the combined scores could predict in-hospital death in COVID-19 with a relatively high accuracy, but the LDH-lymphocyte ratio (LLR) had the highest accuracy compared with other scores including NLR, CLR, CK-lymphocyte ratio, PT-lymphocyte ratio, APTT-lymphocyte ratio, and D-dimer-lymphocyte ratio [AUC of LLR = 0.866 (95% CI: 0.795–0.938)] (Fig. 1). Thereafter, we got the optimal cut-off value of LLR, which was 345.
Figure 1.
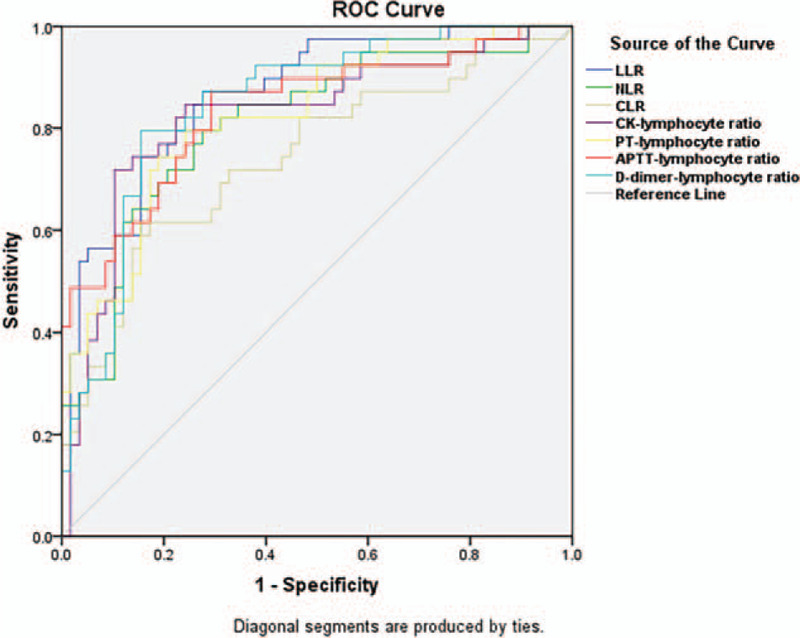
Receiver operating characteristic (ROC) curves of different predicted scores in severe COVID-19 patients from the training cohort. The area under the curve (AUC) values of lactic dehydrogenase-lymphocyte ratio (LLR), neutrophil-lymphocyte ratio (NLR), C-reactive protein-lymphocyte ratio (CLR), creatine kinase (CK)-lymphocyte ratio, prothrombin time (PT)-lymphocyte ratio, activated partial thromboplastin time (APTT)-lymphocyte ratio, and D-dimer-lymphocyte ratio were 0.866 [95% confidential interval (95% CI): 0.795–0.938], 0.808 (95% CI: 0.717–0.898), 0.742 (95% CI: 0.639–0.846), 0.823 (95% CI: 0.732–0.913), 0.823 (95% CI: 0.739–0.907), 0.836 (95% CI: 0.751–0.921), and 0.847 (95% CI: 0.769–0.925), respectively.
3.4. Elevated LLR correlated significantly with the prognosis of severe COVID-19
Next, we assessed the correlation between LLR and the prognosis of severe COVID-19 patients in the training cohort. We discovered that higher levels of LLR were significantly associated with the incidence of common complications on admission including liver injury, renal injury, and higher DIC scores (P < .05). Moreover, the nonsurvivors had a higher level of LLR than survivors [885.85 (446.91, 1242.44) vs 178.04 (128.05, 400.70), P < .001]. However, higher levels of LLR were not associated with sex and age more than 70 years (P > .05) (Table 3).
Table 3.
Lactate dehydrogenase-lymphocyte ratio and organ injury on admission in training cohort.
| Variables | n | LLR | P value |
| Sex | |||
| Male | 49 | 371.02 (156.06, 921.68) | .55‡ |
| Female | 48 | 321.52 (170.39, 699.92) | |
| Age (y) | |||
| <70 | 50 | 287.88 (139.90, 740.69) | .14‡ |
| ≥70 | 47 | 379.33 (175.50, 917.24) | |
| Liver injury | |||
| No | 75 | 299.68 (149.55, 720.00) | <.01‡ |
| Yes | 22 | 697.52 (302.22, 1372.58) | |
| Renal injury | |||
| No | 87 | 333.20 (162.57, 735.86) | .03‡ |
| Yes | 10 | 1219.97 (212.11, 1710.88) | |
| DIC score | |||
| < 2 | 73 | 240.80 (147.60, 595.58) | <.001‡ |
| ≥ 2 | 24 | 873.92 (451.60, 1808.54) | |
| Prognosis | |||
| Survivor | 58 | 178.04 (128.05, 400.70) | <.001‡ |
| Nonsurvivor | 39 | 885.85 (446.91, 1242.44) | |
DIC, disseminated intravascular coagulation; LLR, lactate dehydrogenase-lymphocyte ratio.
Mann–Whitney test.
3.5. LLR was an independent prognostic factor for severe COVID-19 in both training and validation cohorts
Furthermore, univariate logistic regression analysis in the training cohort revealed that DIC score ≥2, CRP, and LLR >345 were the factors associated with prognosis of severe COVID-19 patients. Sex, age, liver injury, and renal injury were not associated with the prognosis of severe COVID-19. However, multivariate logistic regression analysis showed that only LLR was an independent risk factor for prognosis of severe COVID-19 [OR = 9.176, 95% CI: 2.674–31.487, P < .001], and DIC score ≥2 and CRP were not independent factors (P > .05) (Table 4).
Table 4.
Univariate and multivariate logistic regression analyses to determine the prognostic factors of severe COVID-19 in the training cohort.
| Univariate | Multivariate | |||||
| Variables | OR | 95% CI | P value | OR | 95% CI | P value |
| Sex (male) | 1.486 | 0.657–3.362 | .34 | |||
| Age (≥ 70) | 2.036 | 0.893–4.646 | .09 | 2.598 | 0.860–7.845 | .09 |
| Liver injury (present) | 1.322 | 0.506–3.450 | .57 | |||
| Renal injury (present) | 4.010 | 0.968–16.608 | .06 | 4.425 | 0.705–27.769 | .11 |
| DIC score (≥ 2) | 7.429 | 2.590–21.309 | <.001 | 3.548 | 0.996–12.638 | .05 |
| CRP | 1.013 | 1.005–1.021 | <.01 | 1.008 | 0.998–1.017 | .13 |
| LLR (> 345) | 7.850 | 15.934–53.693 | <.001 | 9.176 | 2.674–31.487 | <.001 |
COVID-19 = corona virus disease 2019, CRP = C-reactive protein, DIC = disseminated intravascular coagulation, LLR = lactate dehydrogenase-lymphocyte ratio, OR = odds ratio.
Finally, to determine the predictive potential of LLR in severe COVID-19, we performed logistic regression analysis in the validation cohort using the same cut-off value of LLR as in the training cohort. The results of multivariate logistic regression analysis revealed that LLR >345 was still an independent risk factor for prognosis (OR = 17.453, 95% CI: 1.568–194.306, P = .02, supplementary Table 2, http://links.lww.com/MD/F586). In addition, the AUC of LLR in the validation cohort was 0.857 (95% CI: 0.718–0.997) (Fig. 2).
Figure 2.
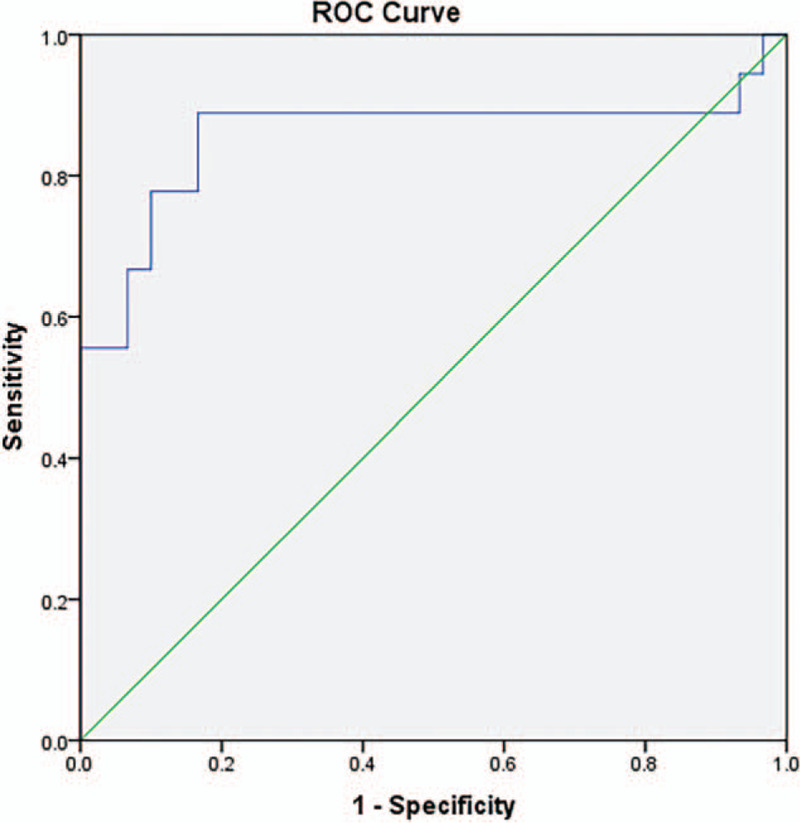
Receiver operating characteristic curve (ROC) of lactic dehydrogenase-lymphocyte ratio (LLR) in severe COVID-19 patients from the validation cohort. The area under the curve (AUC) value of LLR was 0.857 [95% confidential interval (95% CI): 0.718–0.997].
4. Discussion
COVID-19 is an infectious viral disease, usually accompanied by local or systemic inflammation which may even lead to multiple organ injury. Despite antiviral therapy and supportive care, the mortality of COVID-19 remains high, being lower than SARS, but higher than seasonal flu.[3,10] However, an accurate predictive model or biomarker for prognosis of COVID-19 is not available yet. Therefore, we performed this study to develop a new score and validate its predictive efficiency.
In accordance with previous reports,[1,11] the median age of nonsurvivors was greater than that of survivors, but advanced age (≥70 years) was not an independent risk factor for death (Table 4). Although male sex and comorbidities, particularly hypertension, have been considered to be risk factors for death in COVID-19,[11,12] we did not find any significant difference between survivors and nonsurvivors in severe COVID-19. Reduced lymphocyte count is a key laboratory parameter for the diagnosis of COVID-19, and 83.2% of the patients presented with a decreasing lymphocyte count.[13] Furthermore, the lymphocyte count of COVID-19 patients who were transferred to the intensive care unit was significantly lower than those who were not transferred.[14] In our study, nonsurvivors had a markedly reduced lymphocyte count compared to survivors. Recent studies have demonstrated that lymphopenia can become significant after the occurrence of a cytokine storm,[13] and cellular immune damage could be the pathophysiological mechanism of COVID-19 since the SARS-CoV-2 impaired the functions of CD4+ T-cells and exhausted the CD8+ T-cells.[8,13] Consequently, we developed a new predictive marker based on the lymphocyte count.
From the results of the analysis in the training cohort, we found that the inflammatory and coagulation parameters of nonsurvivors with severe COVID-19 were elevated more significantly than survivors. Moreover, the patients with immune system dysregulation were prone to bacterial infections. Therefore, inflammatory parameters such as neutrophil count and serum CRP were elevated more prominently in nonsurvivors. Notably, the severe patients had a more marked increase in CRP than the nonsevere ones.[13] However, CRP was not an independent risk factor for prognosis (Table 4), and the lack of specificity of inflammatory parameters including CRP and neutrophil count has been regarded as a disadvantage. Inflammation has been linked to coagulation abnormalities, and this was a common pathological manifestation in severe COVID-19 patients,[13,15] because hypoxia induced by the viral infection leads to endothelial cell damage and activates the blood coagulation reaction. D-dimer originates from fibrin cleavage and reflects the activation of coagulation and fibrinolysis. Therefore, elevated D-dimer levels indicate a hypercoagulable state. Since the in-hospital mortality was significantly higher in patients with D-dimer ≥2.0 mg/L than those with D-dimer <2.0 mg/L on admission,[16] some researchers have suggested that D-dimer could be an early and helpful predictive marker for prognosis of COVID-19. Remarkably elevated D-dimer levels were observed in COVID-19 nonsurvivors.[15] Similarly, our study results showed that nonsurvivors had higher D-dimer levels, and longer PT and APTT. Moreover, DIC score ≥2 was a risk factor for in-hospital death in univariate logistic regression analysis (OR = 7.429, 95% CI: 2.590–21.309, P < .001, Table 4). Compared to D-dimer, DIC scores could reflect the coagulable state more comprehensively and thus identify DIC early. Unfortunately, it was not found to be an independent risk factor in multivariate logistic regression analysis. Therefore, we believe that coagulation biomarkers may reflect the severity of COVID-19, but might not predict in-hospital death independently.
Patients with severe COVID-19 can develop complications such as organ failure. This is consistent with the extensive distribution of the angiotensin-converting enzyme 2 (ACE2), which is the SARS-CoV-2 receptors, and is widely expressed in various organs including the lungs, kidneys, cardiovascular system, and gastrointestinal tract.[17] Among the various cellular enzymes, only the median LDH was elevated beyond the normal range in COVID-19 patients in our study (Table 1). LDH is an enzyme that is present in almost all major organs and tissues, especially the myocardium, skeletal muscles, and erythrocytes, and it serves as an indicator of disturbances in the cellular integrity and viability due to inflammation or other pathological conditions.[18] For instance, the elevation of serum LDH level has been associated with pulmonary disorders such as interstitial lung disease, chronic obstructive pulmonary disease, pulmonary embolism, and microbial pulmonary disease, and pleural fluid LDH has been used to determine pulmonary cell injury and inflammation.[18] Serum LDH level was found to be associated with 28-day mortality in patients with sepsis because glucose metabolic reprogramming in immune cells plays an important role in the aggravation of sepsis and LDH catalyzes the last step of glycolysis.[19] Published studies have reported that 40% of COVID-19 patients present with elevated LDH, which has been associated with a higher risk of ARDS and death.[13] As well as respiratory system, the cardiovascular system is often involved early in COVID-19. COVID-19 patients can develop cardiovascular complications such as cardiomyopathy, heart failure, and cardiac arrhythmias.[20] Elevation of LDH also indicates cardiac injury. In a prospective study which enrolled 416 consecutive patients, 8% to 28% of COVID-19 patients developed cardiac injury with elevated troponin, and the patients with cardiac injury had a lower lymphocyte count than those without cardiac injury.[21]
Although abnormalities in the liver function parameters were frequently observed in COVID-19 patients, they were not a prominent feature of the disease because majority of the patients presented with mild abnormalities and acute liver failure seldom occurred.[22] Previous studies have found that the levels of serum aminotransferases demonstrated a statistically significant elevation in severe COVID-19 patients compared with mild patients.[22] Our study showed that the incidence of liver injury was not different between survivors and nonsurvivors (P = .57, Table 2). This may be because ACE2 is weakly expressed in hepatocytes.[17] Kidney injury may be the result of intrarenal inflammation, renal medullary hypoxia, volume insufficiency and cardiomyopathy.[23] The prevalence of acute renal injury in COVID-19 patients was 0.5% to 7%.[23] In the training cohort, the incidence of renal injury was higher in nonsurvivors than in survivors (17.95% vs 5.17%, P = .04, Table 2). Although it has been reported that kidney disease on admission, especially acute kidney injury stage 3 was associated with in-hospital death,[24] our study results revealed that renal injury was not an independent risk factor for in-hospital death (Table 4).
The results of our study indicated that LDH was a sensitive parameter for evaluating organ injury in COVID-19. Therefore, we proposed that LLR could be a prognostic factor, since it could reflect organ injury and immune status of host simultaneously. Additionally, our results showed that an elevation of LLR was associated with liver injury, renal injury, DIC score ≥2, and poor outcome (Table 3). However, liver injury, renal injury, or DIC score ≥2 were not independent risk factors for prognosis, only LLR was an independent risk factor (Table 4). Subsequently, by comparing the predictive efficiency of LLR, NLR, and other combination scores in the training cohort, we demonstrated that LLR had a better predictive efficiency (Fig. 1). NLR is a useful diagnostic marker of infectious diseases and has been widely applied to the severity assessment of bacterial infection,[25] but its AUC value was lower than that of LLR. Furthermore, we verified that LLR still had a high AUC value in the validation cohort (Figure 2). Therefore, we found that LLR was accurate for predicting the prognosis of severe COVID-19 patients. Importantly, it was convenient because LDH and lymphocyte counts are easily available in clinical practice. We believe that LLR has substantial potential for prediction of severe COVID-19.
In conclusion, our study demonstrated that LLR may be a valuable predictive score for poor prognosis of severe COVID-19 patients. Utilization of LLR may help clinicians to optimally allocate medical resources. Therefore, patients with high LLR levels could be provided additional medical resources. Further studies are required to assess the clinical applications of LLR in predicting prognosis of severe COVID-19 patients.
Acknowledgments
We would like to thank Professor Qing Chen from the department of institute of toxicology, college of preventive medicine in Army Medical University for his selfless dedication to the study.
Author contributions
Guojun Li designed the study, performed statistical analysis, and wrote the manuscript. Fumin Xu collected the data. Xinru Yin participated in data collection and interpretation. Na Wu guided the statistical analysis. Yuanjie Li and Tinghong Zhang contributed to the discussion. Dongfeng Chen participated in data interpretation and revised the discussion. Kaijun Liu provided the data and revised the manuscript. Qiu Qiu participated in designing the study and revised the manuscript. All authors have read and approved the final manuscript.
Conceptualization: Guojun Li, Qiu Qiu.
Data curation: Fumin Xu, Xinru Yin, Kaijun Liu.
Formal analysis: Na Wu.
Investigation: Yuanjie Li, Tinghong Zhang.
Methodology: Guojun Li, Na Wu.
Project administration: Yuanjie Li, Tinghong Zhang.
Software: Na Wu.
Supervision: Dongfeng Chen, Qiu Qiu.
Writing – original draft: Guojun Li.
Writing – review & editing: Kaijun Liu, Qiu Qiu.
Glossary
Abbreviations: ACE2 = angiotensin-converting enzyme 2, APTT = activated partial thromboplastin time, ARDS = acute respiratory distress syndrome, AUC = area under the curve, CI = confidence interval, CK = creatine kinase, CLR = CRP-lymphocyte ratio, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, DIC = disseminated intravascular coagulation, LDH = lactic dehydrogenase, LLR = lactic dehydrogenase-lymphocyte ratio, NLR = neutrophil-lymphocyte ratio, OR = odds ratio, PT = prothrombin time, ROC = receiver operator characteristic, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2.
References
- [1].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 2020;80:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol 2020;92:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. [DOI] [PubMed] [Google Scholar]
- [8].Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol 2020;72:1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Geriatrics CSo. Diagnosis and treatment of infection-induced multiple organ dysfunction syndrome in the elderly in China, 2019. Chin J Mult Organ Dis Elderly 2019;18:801–38. [Google Scholar]
- [10].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [11].Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020;95:834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275:33238–43. [DOI] [PubMed] [Google Scholar]
- [18].Drent M, Cobben NA, Henderson RF, et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 1996;9:1736–42. [DOI] [PubMed] [Google Scholar]
- [19].Lu J, Wei Z, Jiang H, et al. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: a retrospective observational study. J Surg Res 2018;228:314–21. [DOI] [PubMed] [Google Scholar]
- [20].Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;142:68–78. [DOI] [PubMed] [Google Scholar]
- [21].Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int 2020;40:2095–103. [DOI] [PubMed] [Google Scholar]
- [23].Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol 2020;16:308–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Russell CD, Parajuli A, Gale HJ, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect 2019;78:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


