Abstract
Background
Livestock farmers are at risk of Q fever, a zoonotic disease transmitted to humans from animals such as cattle, sheep and goats. Australia bears substantial Q fever burden, particularly among farmers. A One Health approach engages cross-sectoral collaboration among animal, human and environmental health and is the preferred framework for Q fever prevention.
Methods
Cattle, sheep and goat farmers were invited to participate in an online survey in 2019 to gauge perceptions about Q fever and its prevention. Participants were recruited via membership newsletters and social media. Descriptive analyses and logistic regressions were performed.
Results
A total of 351 farmers completed the survey. Most respondents (80%) had been farming for ≥20 years, with sheep and beef cattle their primary stock. 71% reported knowledge of Q fever, and 85% identified transmission through contaminated dust inhalation was highly likely. The majority of respondents (97%) were aware of Q fever vaccine, and 95% agreed it was effective in preventing disease, yet 42% remained unvaccinated. Reported barriers to vaccination included poor access to a trained doctor and time and cost related to vaccination. Most farmers (≥91%) believed that subsidized vaccination and improved awareness would promote higher uptake.
Conclusion
While Q fever knowledge among respondents was good, their practices related to airborne transmission prevention were poor. Livestock farmers would benefit from adherence to dust and aerosol transmission prevention practices. One Health partnership between government and industry is needed to promote Q fever awareness and address low vaccination rates among livestock farmers by funding vaccination programs.
Keywords: Q fever, Livestock, Farmers, One Health, Vaccination, Australia
Highlights
-
•
Australian livestock farmers have a good understanding of Q fever
-
•
Knowledge of farm-level biosecurity measures is suboptimal
-
•
Livestock farmers supported a coordinated approach to vaccination
-
•
One Health with subsidized vaccination presents an optimal prevention program
1. Introduction
Coxiella burnetii causes Q fever zoonosis in humans with livestock being its principal reservoir [1,2]. Clinical manifestations span asymptomatic infections, acute disease, chronic Q fever and post Q fever fatigue syndrome [3,4]. Livestock farmers bear substantial burden of Q fever zoonosis [3,5].
Higher Q fever notifications among Australian livestock farmers in recent years [6] further support the importance of Q fever prevention for farmers. The U.S. Centers for Disease Control and Prevention (U.S. CDC) recommends non-specific Q fever prevention strategies including hand hygiene, wearing protective clothing and shoes, eye goggles and face shields, and respiratory protection using a facemask or N95 respirator when the risk of exposure is high such as handling birth fluid/placenta [4]. Vaccination as a specific disease prevention strategy significantly reduces Q fever incidence [7], and a human vaccine is registered only in Australia [8]. Despite this, high Q fever incidence among Australian livestock farmers indicates possible low uptake of vaccination [6], and/or inadequate practice of preventative measures.
Australia implemented its national Q fever immunization program during 2001–2006 among abattoir workers and farmers. An evaluation of the national program found that the vaccination was effective, but not at the desired level due to low uptake in the livestock sector [9]. Additionally, there is little evidence on whether Australian livestock farmers practice non-specific Q fever prevention measures [4]. Persistent burden of human Q fever cases in the livestock industry, following the nationally funded vaccination program in Australia highlights possible inadequacy with a single approach such as vaccination, and the need for a multifaceted disease prevention program involving key stakeholders including industry and at risk populations [2].
An integrated approach having multi-stakeholder representation is purported to provide an appropriate framework for Q fever prevention [1,10]. One Health is a framework that combines efforts from human, animal and environmental health sectors. A One Health approach has been piloted to prevent and control Q fever outbreaks internationally and in Australia [[11], [12], [13]]. Findings from these pilot studies set the ground for the large-scale application of One Health measures in the livestock industry. Piloting One Health principles in the Australian goat farm was an example where non-specific Q fever control measures were supplemented by the use of human vaccination [11]. However, countries not having an available human vaccine may benefit the most from a One Health approach utilizing non-specific measures such as environmental control and transmission prevention as shown in the U.S. goat and cattle dairy [12].
In line with the Australian goat farm example, we propose that a One Health approach, when complemented with human vaccination would provide the strongest framework for Q fever prevention. However, lack of empirical studies and the need for assessing preparedness of the Australian livestock industry for adopting a One Health approach esteemed to suggest a cross-sectional survey would be an efficient way of acquiring evidence. We aimed to assess livestock farmers' knowledge, attitudes and perceptions about Q fever and its prevention adopting a One Health approach to inform livestock industry's current Q fever prevention policies including farmers' vaccination in Australia.
2. Methods
2.1. Study design, site and population
An online cross-sectional study was targeted at all registered members of Livestock SA, a nonprofit organization that represents livestock producers in South Australia (SA). Cattle, sheep and goat farmers registered with Livestock SA in 2019 were invited to participate in the survey. Participants were recruited via the Livestock SA website, newsletters, stock journal, Facebook page and email during March 21–June 10, 2019 using SurveyMonkey platform (Supplement S4–S7). The questionnaire consisted of 23 questions (22 closed and 1 open) divided across six sections; (1) socio-demographic information, (2) knowledge and perceptions about Q fever, (3) self-reported exposure to specific animals and Q fever prevention practices, (4) vaccine awareness and recommendation for specific at-risk groups, (5) perceived challenges for vaccination, and (6) vaccination promotion strategies.
2.2. Pretesting, data collection and ethics approval
Experts external to the research group including Livestock SA representatives with knowledge of Q fever and expertise on conducting surveys pretested the legibility and coherence of the questionnaire. The questionnaire was initially made available via SurveyMonkey (www.surveymonkey.com) on Livestock SA website, Facebook page, newsletters and the stock journal (a weekly newspaper for the agricultural industry) in March-April 2019. A direct email was sent on two successive occasions two weeks apart in May 2019 to enhance recruitment. Participation in the survey was voluntary. Ethics approval was granted by the Low-Risk Human Research Ethics Review Group, The University of Adelaide (Approval No: H-2019-040).
2.3. Statistical analysis
Demographic characteristics were descriptively analyzed. The spatial distribution of livestock farmers' socio-economic positioning was examined using the Australian Bureau of Statistics (ABS) published data on Socio-Economic Indexes for Areas (SEIFA). Each SEIFA is constructed by combining specific weighted variables against postcode of usual residence ranging from one decile (lowest) to 10 deciles (highest) [14].
Livestock farmers were asked to indicate the size of their stock and categorized into small-medium and large producers: beef cattle, ≤200 animals = small-medium producer and > 200 animals = large producer; and sheep, ≤1000 animals = small-medium producer and > 1000 animals = large producer. Livestock SA confirmed the levels of classification that we used as they estimated that the average number of livestock in SA was 1600 sheep or 220 cattle per farm.
Fisher's exact tests were used where appropriate to assess whether livestock farmers' knowledge of Q fever was associated with their self-reported disease prevention practices, perceived modes of disease transmission, and suggested strategies for vaccine promotion. Although data on farmers' knowledge were collected using a four-level Likert scale i.e., a great deal, some, little and nil knowledge, responses were combined into a binary variable as “a great deal or some knowledge” and “little or nil knowledge”. Likewise, responses to exposure to specific animals were collected using a five-level Likert scale that was eventually rescaled into a four-level: high exposure = exposed always/often, moderate exposure = exposed sometimes, low exposure = exposed rarely, and nil exposure = never exposed.
Univariate logistic regression was used to calculate effect estimates for Q fever prevention practices among livestock farmers by their self-reported knowledge, stock type stratified as single vs multiple, and vaccination status. Responses to Q fever prevention practices were collected using a five-level Likert scale i.e., always, often, sometimes, rarely and never, which were subsequently collapsed and recoded as yes = practice always/often/sometimes and no = practice rarely/never. After recoding, some observations were still not sufficiently large to produce an effect estimate and hence excluded from the model.
Multivariate logistic regressions were used to estimate livestock farmers' odds of being vaccinated for selected predictors including perceptions about Q fever vaccine, barriers for vaccination, and disease impacts. A five-level Likert scale was used to collect responses to these predictors of vaccination, which was re-stratified as (1) strongly agree/agree = agree, (2) neither agree nor disagree and (3) disagree/strongly disagree = disagree. The positive level of agreement i.e., agree was considered as the reference category. All models were adjusted for age, gender, level of education and years of farming. Coefficient plots were used to display selected point estimates and their confidence intervals computed from regression models.
All statistical analyses were performed using Stata version 15 [15]. Geographic mapping of livestock farmers' postcode of residence was carried out using ESRI's ArcGIS version 10.5.1 [16].
3. Results
3.1. Study population
A total of 3513 members of Livestock SA were targeted. Members who provided their email addresses received an email: the first invite was distributed to 2586 members: 1161 (44.9%) opened the email and 294 (11.4%) clicked on the survey link. A reminder invitation was distributed to 2582 members: 1010 (39.1%) opened the email and 276 (10.7%) clicked on the link. A total of 351 livestock farmers completed the survey.
3.2. Socio-demographic characteristics
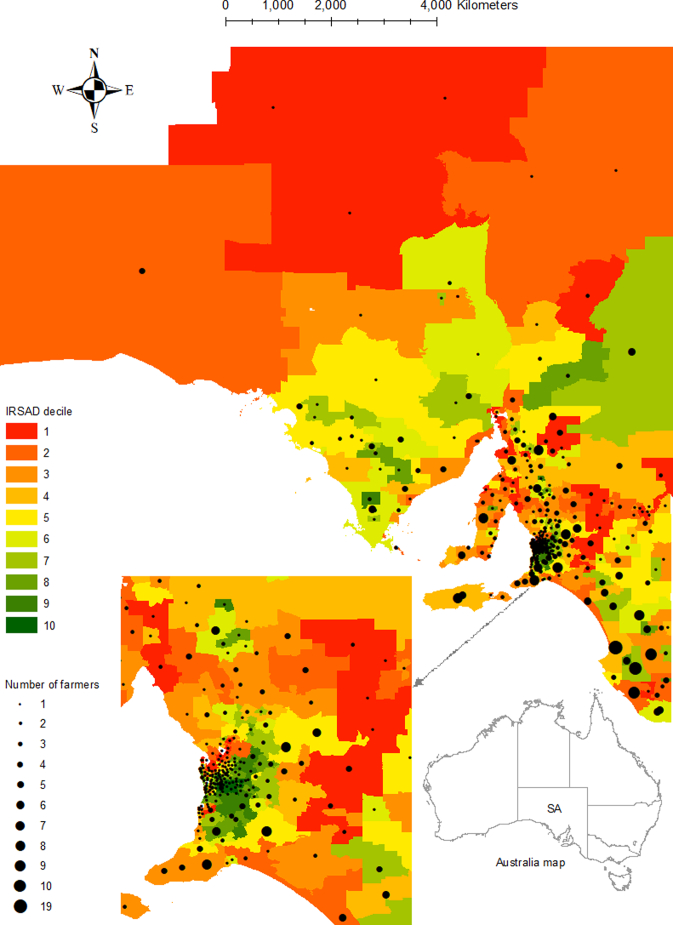
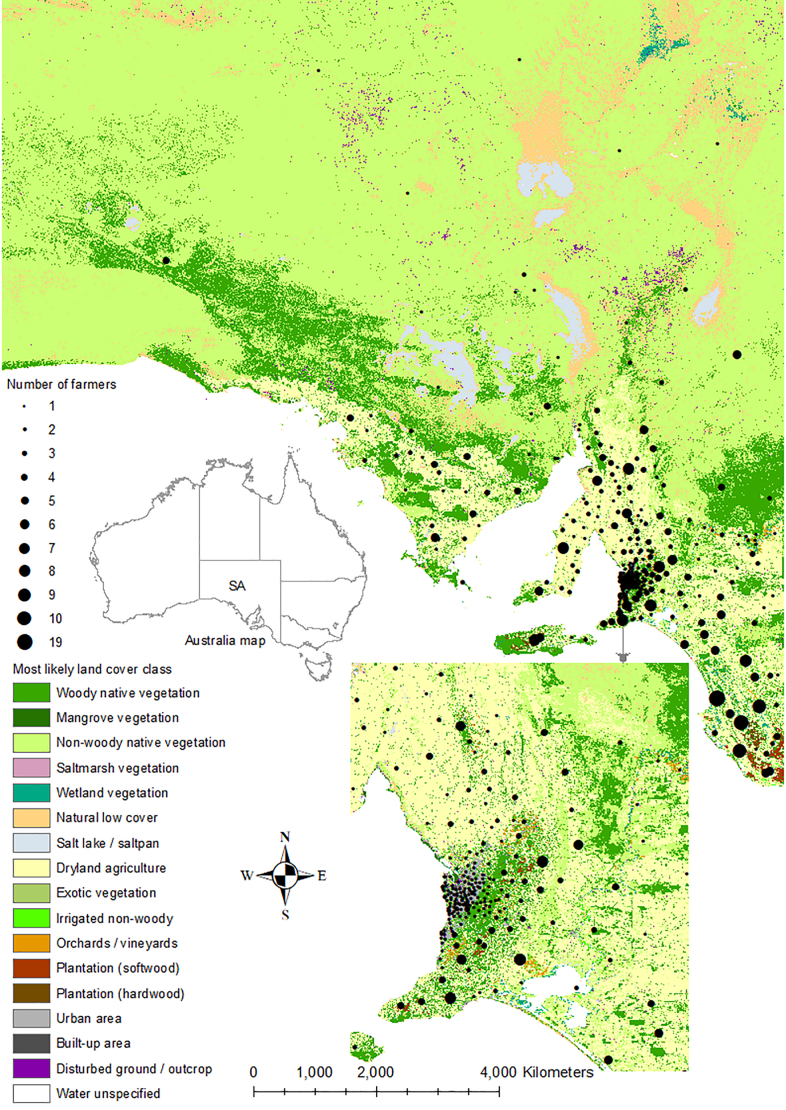
Of 351 livestock farmers, 172 (49.0%) had one type of stock and 179 (51.0%) had multiple types of stock (Table 1). Most farmers (309/350, 88.3%) were between 40 and 79 years old and the majority (227/349, 65.0%) were males. About half of the farmers (172/349, 49.3%) had a certificate/diploma/higher level of education. Most farmers (281/351, 80.1%) had been farming for >20 years. Of 200 beef cattle producers, 132 (66.0%) were small-medium producers, and of 289 sheep producers, 180 (62.3%) were large producers. The majority of farmers (238/345, 69.0%) lived in an area having the IRSAD (The Index of Relative Socio-Economic Advantage and Disadvantage) decile one to five (Table 1 and Fig. 1A). Contrastingly, farmers lived in areas of mixed vegetation and land use i.e., native (predominantly woody/non-woody native and mangrove) and non-native such as dryland agriculture, but rarely areas with other vegetation including urban or built up area (Fig. 1B).
Table 1.
Livestock farmers' characteristics, Q fever knowledge and vaccination status by stock type, 2019 (N = 351).
| Characteristics | Single stock (n = 172) | Multiple stock (n = 179) |
|---|---|---|
| Age group (%) | ||
| 20–39 years | 12 (7.0) | 25 (14.0) |
| 40–59 years | 91 (53.2) | 78 (43.6) |
| 60–79 years | 66 (38.6) | 74 (41.3) |
| ≥ 80 years | 2 (1.2) | 2 (1.1) |
| Sex (%) | ||
| Female | 55 (32.2) | 67 (37.6) |
| Male | 116 (67.8) | 111 (62.4) |
| Level of education (%) | ||
| Completed part secondary | 34 (19.8) | 29 (16.4) |
| Completed secondary | 46 (26.7) | 48 (27.1) |
| Trade / Apprenticeship | 12 (7.0) | 6 (3.4) |
| Certificate / Diploma | 43 (25.0) | 52 (29.4) |
| Bachelor degree or higher | 37 (21.5) | 42 (23.7) |
| Year of farming (%) | ||
| 1–10 | 10 (5.8) | 14 (7.8) |
| 11–20 | 25 (14.5) | 21 (11.7) |
| >20 | 137 (79.7) | 144 (80.4) |
| Beef and sheep stock status (%) | ||
| Beef only | 44 (25.6) | 11 (6.1) |
| Sheep only | 122 (70.9) | 22 (12.3) |
| Both beef and sheep | 0 | 145 (81.0) |
| Neither beef nor sheepa | 6 (3.5) | 1 (0.6) |
| Number of types of stock (%) | ||
| 1 | 172 (100.0) | 0 |
| 2 | 0 | 138 (77.1) |
| 3–5 | 0 | 41 (22.9) |
| Beef producer (%) | ||
| Small-medium producer (≤200 animals) | 32 (72.7) | 100 (64.1) |
| Large producer (>200 animals) | 12 (27.3) | 56 (35.9) |
| Sheep producer (%) | ||
| Small-medium producer (≤1000 animals) | 41 (33.6) | 68 (40.7) |
| Large producer (>1000 animals) | 81 (66.4) | 99 (59.3) |
| Socio-Economic Indexes for Areas – IRSAD (%)b | ||
| Decile 1–5 | 123 (72.3) | 115 (65.7) |
| Decile 6–10 | 47 (27.7) | 60 (34.3) |
| Q fever knowledge (%) | ||
| A great deal | 17 (10.0) | 20 (11.2) |
| Some | 98 (57.6) | 114 (63.7) |
| Little | 48 (28.2) | 41 (22.9) |
| Nil | 7 (4.1) | 4 (2.2) |
| Awareness of Q fever vaccine (%) | ||
| Aware | 150 (94.3) | 168 (98.8) |
| Not aware | 9 (5.7) | 2 (1.2) |
| Vaccination status (%) | ||
| Yes | 82 (55.0) | 102 (60.4) |
| No | 67 (45.0) | 67 (39.6) |
| Time elapsed since the vaccination (%) | ||
| 1 year | 6 (7.2) | 5 (4.9) |
| 2–5 years | 17 (20.5) | 17 (16.7) |
| > 5 years | 60 (72.3) | 77 (75.5) |
| Do not know | 0 | 3 (2.9) |
| Reason for vaccination (%) | ||
| Self-perceived risk of getting Q fever | 64 (77.1) | 72 (70.6) |
| Employer perceived risk of getting Q fever | 7 (8.4) | 15 (14.7) |
| General practitioner perceived risk of getting Q fever | 7 (8.4) | 6 (5.9) |
| Other | 5 (6.0) | 9 (8.8) |
Note: Percentages in parentheses are relative to the number of respondents for a specific characteristic, and where relevant may not add up to 100 due to rounding.
Of seven farmers who had neither beef nor sheep six had single stock (two dairy cattle; one goats; and three other stock — one pigs, horses and poultry; one layer hens; and one horses) and one multiple stocks (goats and other stock — horses), except beef cattle, dairy cattle, goats, and sheep all other livestock were classified as “other”.
IRSAD — The Index of Relative Socio-Economic Advantage and Disadvantage.
Fig. 1.
Location of livestock farmers by postcode of the usual place of residence, and corresponding Index of Relative Socio-Economic Advantage and Disadvantage (IRSAD) decile (Panel A — source: Australian Bureau of Statistics), and land cover/vegetation class (Panel B — source: Department for Environment and Water), South Australia (SA), 2019. Appearance differs between Panel A and Panel B at the northwest region, as there was no postcode information for that region in Panel A.
3.3. Knowledge and awareness of Q fever and its vaccine
Of 349 livestock farmers who reported knowledge on Q fever, 249 (71.3%) indicated a great deal or some knowledge (Table 1). Farmers' knowledge was not associated with their type of livestock (single vs multiple). Most farmers (318/329, 96.7%) were aware of a human vaccine for Q fever (Table 1). A greater proportion of farmers having had multiple stocks were aware of the vaccine compared to farmers who had single stock (Fisher's exact, p = 0.024).
3.4. Perception of Q fever transmission by the level of knowledge
Substantial variation was noted among the listed routes for Q fever transmission: from 19/265 (7.2%) farmers reporting sexual transmission between humans to 261/309 (84.5%) identifying aerosol transmission was likely (Supplementary Table S1). All listed transmission modes except transmission through culling infected animals were significantly associated with farmers' self-reported knowledge: consuming undercooked meat, consumption of unpasteurized dairy, aerosol transmission, laundering of clothes and sexual transmission.
3.5. Level of exposure to animals
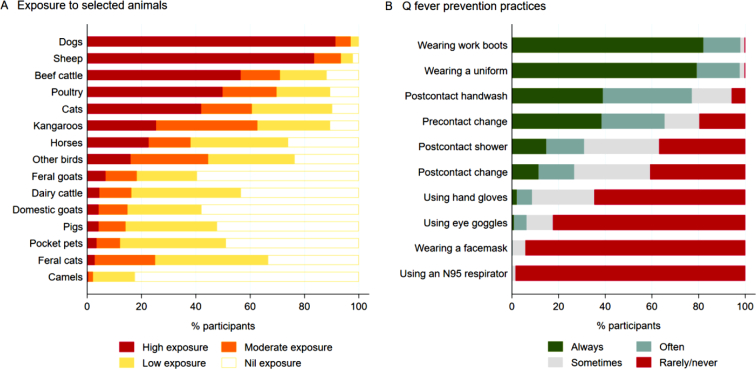
High exposure to dogs and sheep was reported by 306/335 (91.3%) and 279/334 (83.5%), and 188/310 (60.6%) – 230/324 (71.0%) of farmers had moderate-high exposure to cats, kangaroos, poultry and beef cattle, and low-nil exposure was reported by the majority for other listed animals with camels being the least reported animal (Fig. 2A).
Fig. 2.
Livestock farmers' self-reported exposure to specific animals (Panel A) and reported practices for Q fever prevention (Panel B), 2019.
3.6. Q fever prevention practices
Most livestock farmers reported frequently wearing work boots (338/345, 98.0%) and a uniform (339/347, 97.7%) when having contact with animals (Fig. 2B). The majority of farmers reported frequent handwashing after contact (267/346, 77.2%) and changing into a uniform/work boots before contact (220/336, 65.5%). Conversely, 277/336 (82.4%) – 324/329 (98.5%) reported rare or no use of eye goggles, facemasks or N95 respirators (Fig. 2B).
When livestock farmers' Q fever prevention practices were related to their self-reported knowledge, only wearing work boots and showering after contact with animals were found to be associated. Other practices such as wearing a uniform, using a facemask, handwashing after contact, changing into uniform/work boots before animal contact, changing out of uniform/work boots after animal contact, using hand gloves, using eye goggles and using an N95 respirator were not associated (Supplementary Table S2).
3.7. Prevention practices by the level of knowledge
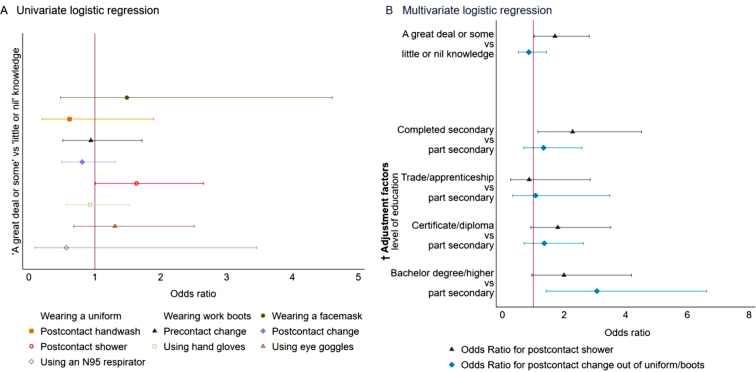
In univariate logistic regression, showering after contact with animals (OR 1.63; 95% CI, 1.01–2.65) was associated with livestock farmers' knowledge (Fig. 3A). Farmers who had a great deal or some knowledge were more likely (OR 1.7; 95% CI, 1.03–2.82) to shower after contact with animals compared with farmers who had little or nil knowledge (Fig. 3B). Likewise, farmers who completed a secondary level education were more than twice (OR 2.27; 95% CI, 1.15–4.51) as likely to shower after contact with animals compared with farmers who did not after adjusting for other covariates. However, farmers who had a bachelor or higher education were three times (OR 3.06; 95% CI, 1.42–6.62) more likely to change out of uniform/work boots after contact with animals compared with farmers with lower educational attainment (Fig. 3B).
Fig. 3.
Relationship between livestock farmers' self-reported knowledge and Q fever prevention practices, 2019.
Panel A. Unadjusted Odds Ratio for Q fever knowledge and prevention practices, wearing a uniform and wearing work boots omitted from models because of collinearity.
Panel B. Adjusted Odds Ratio for Q fever knowledge and selected prevention practices.
Ref. category – little or nil knowledge, part secondary. The vertical red line indicates Odds ratio = 1 i.e., no relationship. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.8. Prevention practices by beef and sheep producer size
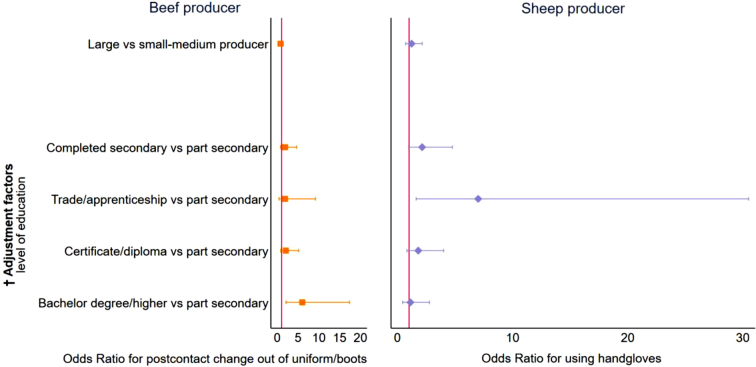
Large beef producers who had a bachelor's degree or higher education were six times (OR 5.99; 95% CI, 2.07–17.36) more likely to change out of uniform/work boots after contact with animals compared with small-medium producers (Fig. 4). In contrast, large sheep producers who had a trade or apprenticeship education were seven times (OR 7.0; 95% CI, 1.61–30.5) more likely to use hand gloves compared to small-medium producers (Fig. 4).
Fig. 4.
Relationship between livestock farmers' stock type and size, and selected Q fever prevention practices, 2019.
† Models were adjusted for age, sex, level of education and years of farming (except education other adjustment factors' confidence intervals for odds ratios included 1, hence were not plotted). Ref. category – small-medium producer (beef ≤200 animals, sheep ≤1000 animals). The vertical red line indicates Odds ratio = 1 i.e., no relationship. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.9. Perceptions of Q fever vaccine, attitudes towards vaccination and farmers' vaccination status
Livestock farmers with a great deal or some knowledge of Q fever were > 3 times (OR 3.29; 95% CI, 1.92–5.66) more likely to get vaccinated against Q fever compared to farmers with little or no knowledge after adjusting for covariates. Conversely, farmers who were neutral or disagreed with Q fever vaccine being effective were 82% (OR 0.18; 95% CI, 0.05–0.70) less likely to get vaccinated as opposed to those who agreed. Likewise, farmers who disagreed with people's belief of Q fever not being a serious illness and does not require vaccination were 53% (OR 0.47; 95% CI, 0.27–0.83) less likely to get vaccinated compared to those who agreed.
3.10. Perceived impacts of Q fever and barriers to vaccination
Most participants agreed that Q fever has health and economic impacts (≥311/322, ≥96.6%) and the human vaccine is effective (295/309, 95.5%), while 141/236 (59.8%) believing the vaccine is harmful to previously exposed individuals. More than half (167/301, 55.5%–201/301, 66.8%) of livestock farmers agreed with the listed barriers for vaccination such as people's belief of Q fever not being a serious illness, costs, time and access to an accredited vaccine provider.
3.11. Vaccination recommendations and promotion strategies
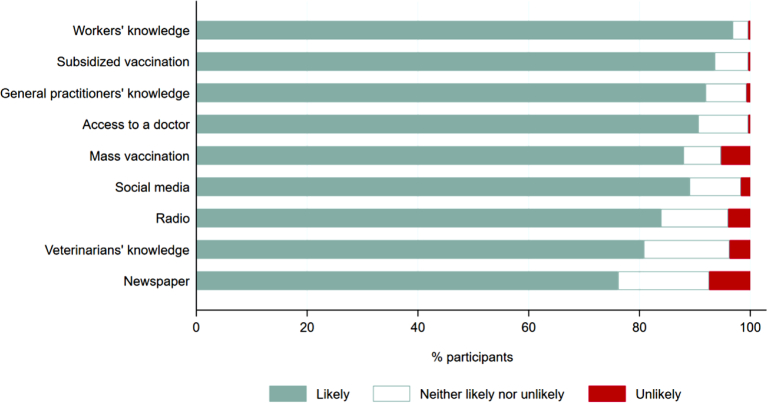
Most livestock farmers (≥274/306, ≥89.5%) recommended vaccination against Q fever for themselves, their spouses, farmhands, stockyard workers, shearers, roustabouts and veterinarians, except for others living on farms (256/304, 84.2%). Most farmers (≥278/307, ≥90.6%) suggested subsidized vaccination, improving access to a trained doctor and improving general practitioners' and workers' knowledge of Q fever are likely to help increase vaccination uptake (Fig. 5). Other strategies were also considered effective by at least 239/296 (80.7%) of farmers except for print media (226/297, 76.1%) (Fig. 5). All strategies were associated with farmers' knowledge (p ≤ 0.001) (Supplementary Table S3).
Fig. 5.
Livestock farmers' suggested Q fever vaccination promotion strategies, 2019.
4. Discussion
This is the first study involving a large sample of Australian livestock farmers representing the whole of South Australia and investigating their knowledge, attitudes and perceptions of Q fever within a One Health framework. Open rates and click rates for this study were much higher than the Food and Agriculture industry survey average in 2019 [open rate — 23.3% and click rate — 2.9%] [17].
The male preponderance in our study conforms with other Australian [18], and international studies [19]. Demographic profiles including education and usual place of residence based on the IRSAD decile, indicate Australian livestock farmers' modest overall socioeconomic status and possibly highlights the cost of vaccination as a barrier [18]. Farms with mostly dryland vegetation may mean that farmers' susceptibility to Q fever remains high because contaminated dust is a major vehicle of transmission [20]. The majority of farmers in our study had sheep stock, with sheep previously found to be associated with elevated Coxiella burnetii seroprevalence in the Netherlands [21], and increased incidence of human Q fever in Minnesota, U.S. [22]. Large stock size (defined as ≥100 cattle) was found to be associated with higher Coxiella burnetii seropositivity [19], and in our study, 64% of cattle producers had >100 animals. In addition, the majority of farmers had been farming for a prolonged period of time and had a long exposure and duration of time since vaccination, adding further complexities to their disease risks as the duration of livestock exposure was previously shown to increase susceptibility to Q fever [23].
In our study, only 3% of all farmers indicated that they did not know anything about Q fever compared to 8% of Australian goat producers [24], reflecting an overall better knowledge among sheep and cattle producers. Given a good understanding of Q fever among livestock farmers and their relatively modest socioeconomic conditions, ‘cost of vaccination’ and ‘access to accredited Q fever immunization providers’ could be a major challenge for vaccination as opposed to ‘lower than real risk perceptions’, although both were highlighted as potential barriers in Australia [25]. Our findings substantiate previously highlighted barriers, yet are novel as these were drawn from the direct perspectives of Australian livestock farmers compared to the previously reported findings from epidemiological reviews. We recommend that government and industry partners consider livestock farmers' understanding and risk perceptions about Q fever as potential enablers of vaccination, and promote subsidized vaccination through enhanced rural and remote access to accredited vaccination providers, as well as promoting good infection control practices.
In this study, we found that livestock farmers had moderately high levels of exposure to animals that are considered to be high risk for Q fever in humans through exposure to their birth products, placenta, milk, urine and faeces. These animals include sheep and cattle [26], pets such as domestic cats [27], and kangaroos [28]. Thus, Australian livestock farmers may be at increased risk of Q fever. Almost all livestock farmers reportedly practice general biosecurity measures such as wearing protective clothing and work boots during contact with animals, which are recommended to reduce indirect transmission from animals to humans [29]. Nevertheless, these biosecurity measures if not accompanied by the use of respirators, which the majority of Australian livestock farmers did not practice, are likely to be inadequate to prevent airborne transmission [4,30]. Use of respirators in a farm setting may seem less feasible and as farmers have unavoidable contact with livestock and their environment, vaccination remains the most viable option for Q fever prevention [7]. Australian livestock farmers should be vaccinated to ensure adequate protection against Q fever, in addition to practicing biosecurity measures, as the duration of immunity conferred by the vaccine is unknown.
Our study had limitations. As recruitment was only from one state, generalizability of our findings nationally and internationally is limited. It is reasonable to consider that livestock farmers' remoteness and socioeconomic background potentially could have precluded many of them from getting vaccinated against Q fever. In turn, reporting bias might have been introduced i.e., livestock farmers' willingness to pay for vaccination could have influenced the responses. Although our sample size was moderate, the breadth of our target population, the higher click rates and open rates in the Agriculture industry support the scientific validity of our findings.
Overall, livestock farmers with greater knowledge of Q fever were more likely to practice certain prevention measures than farmers with less knowledge. Some prevention practices were commoner among farmers having higher education and larger herds. Besides, farmers with higher levels of knowledge and perceptions about Q fever were more likely to be vaccinated compared with their counterparts. While vaccinating at-risk groups arguably constitutes the best case scenario as shown during the nationally subsidized vaccination campaign in Australia [9], sustaining such programs is always challenging [31]. Countries without a human vaccine may need to rely on non-specific measures such as disease surveillance, on-farm veterinary measures, environmental decontamination, and use of personal protective equipment all working in coordination [4].
5. Conclusions
Supporting all suggested Q fever prevention strategies with strong policies in a coordinated approach is more likely to be effective. We recommend an inter-sectoral approach with revision of livestock farmers' vaccination policy and enforcing strict biosecurity measures at farm levels to protect the Australian livestock sector against Q fever. A One Health partnership is required among the Government, the livestock industry, and human and animal health departments to promote Q fever awareness and address low vaccination rates among livestock workers by funded vaccination programs.
Author contributions
MRR, HM, AM and PB conceptualized the study. MRR and DC collected data. MRR analyzed data and drafted the manuscript. The manuscript was contributed to by HM, AM, DC and PB.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no competing interest.
Acknowledgements
We thank SA livestock farmers for participating in this study. We also thank Livestock SA for its help in implementing and completing the study. Special thanks to Jana Maria Bednarz from Adelaide Health Technology Assessment for her statistical inputs. M.R. Rahaman is supported by the Adelaide Scholarships International (ASI) scholarship of the University of Adelaide. H. Marshall acknowledges NHMRC support Practitioner Fellowship APP1155066.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100232.
Contributor Information
Md Rezanur Rahaman, Email: mdrezanur.rahaman@adelaide.edu.au.
Helen Marshall, Email: helen.marshall@adelaide.edu.au.
Adriana Milazzo, Email: adriana.milazzo@adelaide.edu.au.
Deane Crabb, Email: dcrabb@livestocksa.org.au.
Peng Bi, Email: peng.bi@adelaide.edu.au.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Botelho A. The Principles and Practice of Q Fever: The One Health Paradigm. 2017. Trends and challenges of Q fever control in animal and human populations; pp. 391–404. [Google Scholar]
- 2.Rahaman M.R., Milazzo A., Marshall H., Bi P. Spatial, temporal, and occupational risks of Q fever infection in South Australia, 2007-2017. J. Infect. Public Health. 2020;13:544–551. doi: 10.1016/j.jiph.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Simoes J.C.C., Anastácio S.F., da Silva G.J. 2017. The Principles and Practice of Q Fever: The One Health Paradigm; pp. 1–427. [Google Scholar]
- 4.Anderson A., Bijlmer H., Fournier P.E., Graves S., Hartzell J., Kersh G.J. CDC - National Center for Emerging and Zoonotic Infectious Diseases; Atlanta, Georgia: 2013 March 29. Diagnosis and Management of Q fever - United States, 2013: Recommendations from CDC and the Q Fever Working Group. Report No.: 3 Contract No.: 3. [PubMed] [Google Scholar]
- 5.Groten T., Kuenzer K., Moog U., Hermann B., Maier K., Boden K. Who is at risk of occupational Q fever: new insights from a multi-profession cross-sectional study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowbridge C.P., Tobin S., Seale H., Ferson M.J. Notifications of Q fever in NSW, 2001-2010. N S W Public Health Bull. 2012;23:31–35. doi: 10.1071/NB11037. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill T.J., Sargeant J.M., Poljak Z. The effectiveness of Coxiella burnetii vaccines in occupationally exposed populations: a systematic review and meta-analysis. Zoonoses Public Health. 2014;61:81–96. doi: 10.1111/zph.12054. [DOI] [PubMed] [Google Scholar]
- 8.Rahaman M.R., Milazzo A., Marshall H., Bi P. Is a one health approach utilized for Q fever control? A comprehensive literature review. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16050730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidding H.F., Wallace C., Lawrence G.L., McIntyre P.B. Australia’s national Q fever vaccination program. Vaccine. 2009;27:2037–2041. doi: 10.1016/j.vaccine.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Kahn L.H. Integrating a one health approach into epidemiology to improve public policy. Int. J. Epidemiol. 2019;0:1–3. doi: 10.1093/ije/dyz178. [DOI] [PubMed] [Google Scholar]
- 11.Bond K.A., Vincent G., Wilks C.R., Franklin L., Sutton B., Stenos J. One health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol. Infect. 2016;144:1129–1141. doi: 10.1017/S0950268815002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggs H.M., Turabelidze G., Todd S.R., Slifka K.J., Drexler N.A., Pratt D. 63rd Annual Meeting; New Orleans, LA USA: American Society of Tropical Medicine and Hygiene. 2014. Q fever outbreak on a large U.S. goat and cattle dairy: a One Health investigation; p. 199. [Google Scholar]
- 13.van der Hoek W., Morroy G., Renders N.H., Wever P.C., Hermans M.H., Leenders A.C.A.P. Epidemic Q fever in humans in the Netherlands. In: Toman R., Heinzen R.A., Samuel J.E., Mege J.-L., editors. Coxiella burnetii: Recent Advances and New Perspectives in Research of the Q Fever Bacterium. Vol. 984. Springer; Dordrecht: 2012. pp. 329–364. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 14.Australian Bureau of Statistics . Commonwealth of Australia; Canberra: 2016. Socio-Economic Indexes for Areas (SEIFA) Technical Paper. [Google Scholar]
- 15.StataCorp . StataCorp LLC; College Station, Texus: 2017. Stata Statistical Software: Release 15. [Google Scholar]
- 16.ESRI . 2017. ArcGIS Desktop: Release 10.5.1. Redlands, California. [Google Scholar]
- 17.Mailchimp . The Rocket Science Group, LLC; Atlanta, GA 30308 USA: 2019. Email Marketing Benchmarks by Industry.https://mailchimp.com/resources/email-marketing-benchmarks/ [cited 30 April 2020]. Available from. [Google Scholar]
- 18.Lower T., Corben P., Massey P., Depczynski J., Brown T., Stanley P. Farmers’ knowledge of Q fever and prevention approaches in New South Wales. Aust J Rural Health. 2017;25:306–310. doi: 10.1111/ajr.12346. [DOI] [PubMed] [Google Scholar]
- 19.Park J.H., Chu H., Yoo S.J., Hwang K.J., Lim H.S. Serologic survey and risk factors for Coxiella burnetii infection among dairy cattle farmers in Korea. J. Korean Med. Sci. 2018;33 doi: 10.3346/jkms.2018.33.e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorko E., Rimarova K., Pilipcinec E. Influence of the environment and occupational exposure on the occurrence of Q fever. Cent. Eur. J. Public Health. 2012;20:208–214. doi: 10.21101/cejph.a3754. [DOI] [PubMed] [Google Scholar]
- 21.De Lange M.M., Schimmer B., Vellema P., Hautvast J.L., Schneeberger P.M., Van Duijnhoven Y.T. Coxiella burnetii seroprevalence and risk factors in sheep farmers and farm residents in the Netherlands. Epidemiol. Infect. 2014;142:1231–1244. doi: 10.1017/S0950268813001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez J., Whitten T., Branscum A.J., Garcia-Seco T., Bender J.B., Scheftel J. Understanding Q fever risk to humans in Minnesota through the analysis of spatiotemporal trends. Vector Borne Zoonotic Dis. 2018;18:89–95. doi: 10.1089/vbz.2017.2132. [DOI] [PubMed] [Google Scholar]
- 23.Van den Brom R., Schimmer B., Schneeberger P.M., Swart W.A., van der Hoek W., Vellema P. Seroepidemiological survey for Coxiella burnetii antibodies and associated risk factors in Dutch livestock veterinarians. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunther M.J., Heller J., Hayes L., Hernandez-Jover M. Dairy goat producers’ understanding, knowledge and attitudes towards biosecurity and Q-fever in Australia. Prev Vet Med. 2019;170:104742. doi: 10.1016/j.prevetmed.2019.104742. [DOI] [PubMed] [Google Scholar]
- 25.Sloan-Gardner T.S., Massey P.D., Hutchinson P., Knope K., Fearnley E. Trends and risk factors for human Q fever in Australia, 1991-2014. Epidemiol. Infect. 2017;145:787–795. doi: 10.1017/S0950268816002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guatteo R., Seegers H., Taurel A.F., Joly A., Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: a critical review. Vet. Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro A.J., Norris J.M., Bosward K.L., Heller J. Q fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses Public Health. 2017;64:252–261. doi: 10.1111/zph.12305. [DOI] [PubMed] [Google Scholar]
- 28.Flint J., Dalton C.B., Merritt T.D., Graves S., Ferguson J.K., Osbourn M. Q fever and contact with kangaroos in New South Wales. Commun. Dis. Intell. 2016;40:E202–E203. [PubMed] [Google Scholar]
- 29.Schimmer B., Lenferink A., Schneeberger P., Aangenend H., Vellema P., Hautvast J. Seroprevalence and risk factors for Coxiella burnetii (Q fever) seropositivity in dairy goat farmers’ households in the Netherlands, 2009-2010. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenga C., Gangemi S., De Luca A., Calimeri S., Lo Giudice D., Pugliese M. Seroprevalence and occupational risk survey for Coxiella burnetii among exposed workers in Sicily, Southern Italy. Int. J. Occup. Med. Environ. Health. 2015;28:901–907. doi: 10.13075/ijomeh.1896.00448. [DOI] [PubMed] [Google Scholar]
- 31.Massey P.D., Durrheim D.N., Way A. Q-fever vaccination—unfinished business in Australia. Vaccine. 2009;27:3801. doi: 10.1016/j.vaccine.2009.04.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2