Abstract
Background:
Postoperative atrial fibrillation (POAF) is the most common complication following cardiac surgery, and is associated with increased morbidity and mortality. Inflammation has been implicated as an etiology of POAF. Mitochondrial DNA (mtDNA) has been shown to initiate inflammation. This study analyzed inflammatory mechanisms of POAF by evaluating mtDNA, neutrophils, and cytokines/chemokines in the pericardial fluid (PCF) and blood following cardiac surgery.
Methods:
Blood and PCF from patients who underwent coronary bypass and/or heart valve surgery were collected intraoperatively and at 4, 12, 24, and 48 hours postoperatively. Real-Time PCR was used to quantify mtDNA in the PCF and blood. A luminex assay was used to study cytokine and chemokine levels. Flow cytometry was employed to analyze neutrophil infiltration and activation in the PCF.
Results:
Samples from 100 patients were available for analysis. Postoperatively, mtDNA and multiple cytokine levels were higher in the PCF versus blood. Patients who developed POAF had significantly higher levels of mtDNA in the PCF compared to those who did not (p<0.0001, AUC =0.74). There was no difference in the mtDNA concentration in the blood between the POAF and non-POAF groups (p=0.897). Neutrophil concentration increased in the PCF over time from a baseline of 0.8% to 56% at 48 hours (p<0.01).
Conclusions:
The pericardial space has a high concentration of inflammatory mediators postoperatively. mtDNA in the PCF was strongly associated with the development of POAF. This finding provides insight into a possible mechanism of inflammation that may contribute to POAF, and may offer novel therapeutic targets.
Postoperative atrial fibrillation (POAF) is one of the most common complications following cardiac surgery (1). POAF can lead to a number of complications including congestive heart failure, stroke, and increased length of hospital stay and cost (1). Inflammation likely contributes to the onset of POAF following cardiac surgery. For example, Ishii et al. showed that the degree of inflammation of the atrium was associated with a proportional increase in the inhomogeneity of atrial conduction and atrial fibrillation duration (2). Additionally, the pericardial fluid (PCF) after cardiac surgery has been shown to be highly inflammatory and enriched with neutrophils, macrophages, and cytokines (3).
Mitochondria evolved from saprophytic bacteria and their genome contains CpG (cytosine-phospdiester-guanine) DNA repeats that code for formylated peptides (5). Mitochondrial DNA (mtDNA) and formylated peptides act as inflammatory mediators known as damage associated molecular patterns (DAMPs) (5). In lung transplant patients, mitochondrial DNA and formylated peptides are released following damage to mitochondria, and initiate inflammation via the pattern recognition receptors TLR9 and FPR1, respectively (6). Scozzi et al. showed that release of DAMPs from mitochondria results in primary graft dysfunction in lung transplant patients, and, ultimately, that neutrophil trafficking and effector response to damaged mitochondria result in this dysfunction (6). Furthermore, studies have demonstrated that endogenous mtDNA in the extracellular space is a danger signal that has detrimental effects on cardiomyocytes and that elevated serum levels are highly associated with the development of POAF following cardiac surgery (7–9).
Here we sought to determine if inflammatory mediators contributed to POAF following cardiac surgery. Specifically, we assessed whether or not mtDNA, neutrophils, and cytokines released into the pericardial fluid and blood were associated with the development of POAF.
Material and Methods
Patients and samples
This study was approved by the Washington University in St. Louis Institutional Review Board prior to initiation, and patients gave informed consent prior to enrollment. We designed a retrospective study using prospectively collected blood and PCF from 100 patients without history of atrial arrhythmia undergoing elective coronary artery bypass grafting and/or valve replacement or repair. Specimens were collected intraoperatively and at 4, 12, 24, and 48 hours postoperatively. Intraoperatively, pericardial fluid was collected immediately upon opening the pericardium. Postoperatively, PCF was obtained from the mediastinal chest tube. Blood was collected from existing peripheral intravenous catheters. The specimens were immediately centrifuged at 1350 RPMs following collection. The supernatant was transferred to conical tubes and stored in a −80° C freezer until PCR and Luminex analysis. Cell pellets were cryopreserved with 10% DMSO, 20% fetal bovine serum, and 70% RPMI, gradually cooled to −80°C, and then stored in liquid nitrogen for flow cytometry analysis. The primary outcome measure was development of postoperative atrial fibrillation. Postoperative atrial fibrillation was defined as atrial fibrillation sustained for 30 seconds or more. Electrocardiograms and telemetry were analyzed for each patient postoperatively up to 30 days to determine their rhythm status. Exclusion criteria were redo operation, preoperative arrhythmia, history of inflammatory condition (e.g. rheumatoid arthritis), chronic anti-inflammatory treatment, and preoperative steroid use.
Quantitative PCR
Real time polymerase chain reaction (PCR) was used to quantify mtDNA in PCF and blood. This was performed in a BioRad CFX-Connect machine using a reaction mixture containing 0.3 μL of sample, 36 μL iTAQ supermix, 1.8 μL of forward and reverse primers, and 32 μL sterile H2O. Assays were performed in triplicate under the following conditions: 1 cycle at 95°C for 3 minutes, 40 cycles at 95°C for 10 seconds, 55°C for 30 seconds, and then a melt curve from 65°C to 95°C. Primers for human cytochrome B and cytochrome C oxidase subunit III were utilized. The primer sequences cytochrome B forward, 5’-ATGACCCCAATACGCAAAAT-3’, cytochrome B reverse, 5’-CGAAGTTTCATCATGCGGAG-3’, cytochrome C forward, 5’-ATGACCCACCAATCACATGC-3’, and cytochrome C reverse, 5’-ATCACATGGCTAGGCCGGAG-3’ were used to amplify the corresponding DNA segment. Standard curves were developed to determine concentrations of mtDNA.
Flow cytometry
Flow cytometry was used to characterize cell populations in the pericardial fluid. Cell pellets were thawed and re-suspended in phosphate buffered saline. Red blood cell lysis was then performed with ACK lysing buffer. Single cell suspensions were obtained by passing through a 40μM wire mesh, and cells were incubated with 10μg/mL of Fc Block. Samples were then stained with fluorochrome-labeled anti-CD45, anti-CD11b, anti-CD66b, and anti-CD16 using methods previously described (10).
Cytokine assay
Luminex assays were used to analyze cytokines and chemokines. Human serum and pericardial fluid samples were thawed on ice, centrifuged at 10,000 G for 5 minutes at 4 °C, then serially diluted with assay buffer according to the manufacturer (MilliporeSigma, St. Louis, MO) protocol. Twenty-five microliters of diluted sample or prepared standard and controls were added to the appropriate wells in duplicate with 25μL of assay buffer and 25μL of premixed beads. The plate was sealed and placed on a rotary shaker overnight at 4 °C. The following morning the assay was completed according to the manufacturer protocol and using a hand-held magnetic plate washer. A FLEXMAP3D Luminex analyzer was used to determine Mean Fluorescence Intensity (MFI) for 50 beads per analyte. The MFIs were compared to the standard curve that generated pg/ml values for each analyte using a 5-parameter logistic regression analysis program (Milliplex Analyst 5.1.0, Vigene Tech Inc.). Thirty-six cytokines and/or chemokines were analyzed which included: tumor necrosis factor-α, tumor necrosis factor-β, interleukin-1 receptor antagonist, myoglobin, growth differentiation factor-15, myeloperoxidase, fractalkine, adamts13, brain natriuretic peptide, creatinine kinase-MB, c-reactive protein, C-X-C Motif chemokine ligand 6, d-dimer, fibrinogen, granulocyte-colony stimulator factor, granulocyte-macrophage colony-stimulating factor, haptoglobin, interferon-α, interferon-β, interferon-γ, interleukin-1α, interleukin-1β, interleukin-3, interleukin-6, interleukin-8, interleukin-10, lipocalin, monocyte chemoattractant protein 1, oncostatin M, platelet factor 4, placental growth factor, serum amyloid A, saponin-1, soluble intercellular adhesion molecule-1, sp-selectin, serum soluble adhesion molecule-1, vascular endothelial growth factor, macrophage inflammatory protein, and von Willebrand factor.
Statistics
Based on a preliminary study of 30 patients looking at 52 different cytokines and chemokines, a 20% standard deviation could be expected in these measurements with a POAF rate of about 30%. Given a power of 80% and an alpha of 0.05, we calculated that ~50/group patients would be needed to detect a 20% difference. (G*Power Ver 3.1.9.2) Continuous data were reported as mean± SD, or median [interquartile range] as appropriate, and were compared between groups using the Students t-test and Mann-Whitney U test, respectively. Categorical variables were compared using chi-squared analysis. The expected maximization method was used to estimate missing data. Repeated measures analysis was used to determine if there was an association between mtDNA, cytokines/chemokines, and POAF over time. Multivariate stepwise logistic regression was used to determine preoperative and intraoperative risk factors that were predictors of POAF development. Variables entered into the multivariable analysis were pericardial mtDNA at 24 hours, age, hypertension, mitral valve surgery, aortic valve surgery, intraaortic balloon pump, and history of congestive heart failure (11). Statistics were done with STATA Version 15.0 (STATA Corp, College Station, Texas). A P-value of ≤0.05 was considered statistically significant.
Results
Eight-hundred eighty eight (464 PCF and 424 blood) samples were available for analysis. Specifically, samples from the pericardial fluid in the POAF group were Intra-op-39, 4 hours-39, 12 hours-40, 24 hours-36, and 48 hours-28. For the Non-POAF group, there were 54, 54, 57, 57, and 45 samples for 0, 4, 12, 24, and 48 hours, respectively. Forty of 100 patients developed POAF with a median onset time of 1.8 days postoperatively. There were no significant differences in any baseline or intraoperative variables between patients who developed POAF and those who did not (Table 1). Patients who developed POAF had significantly higher levels of mtDNA in the PCF compared to those who did not develop POAF at every time point after surgery (Figure 1A). There was no difference in mtDNA concentration in the blood between the POAF and non-POAF groups (Figure 1B). Analysis of the receiver operating characteristic curve demonstrated the predictive value of mtDNA concentration for POAF was 0.74.
Table 1:
Baseline and intraoperative variables for POAF and non POAF groups. Values represented as number (%) or mean±SD. BMI-Body mass index. NYHA-New York Heart Association. CPB-Cardiopulmonary bypass. CABG-Coronary artery bypass grafting. AVR- Aortic valve replacement
| Baseline characteristic | POAF (n=40) | No POAF (n=60) | P-Value |
|---|---|---|---|
| Age | 65 ±9 | 61 (±10) | 0.06 |
| Gender (male) | 24 (60) | 33 (55) | 0.68 |
| Race (white) | 37 (92) | 51 (85) | 0.25 |
| BMI (kg/m2) | 32 ±9 | 29 (±8) | 0.12 |
| Recent MI | 4 (10) | 6 (10) | 0.92 |
| Smoking History | 24 (60) | 42 (70) | 0.28 |
| Beta blockers | 23 (56) | 37 (61) | 0.66 |
| Statin | 20 (50) | 38 (63) | 0.31 |
| Left ventricular function | 62±10 | 61±11 | 0.88 |
| Left atrial diameter (cm) | 3.9±0.65 | 4.1±0.66 | 0.51 |
| Pulmonary artery mean pressure | 34±12 | 27±12 | 0.09 |
| NYHA III or IV | 12 (30) | 16 (27) | 0.82 |
| Hypertension | 33 (83) | 46 (77) | 0.62 |
| Dyslipidemia | 19 (48) | 41 (68) | 0.06 |
| Diabetes | 15 (38) | 20 (33) | 0.68 |
| Chronic lung disease | 4 (10) | 5 (8) | 1.00 |
| Renal Failure | 1 (3) | 0 (0) | 0.40 |
| Peripheral vascular disease | 1 (3) | 1 (2) | 1.00 |
| Myocardial infarction | 4 (10) | 6 (10) | 0.64 |
| Intraoperative Characteristic | |||
| CPB time (min) | 106 (±32) | 103 (±28) | 0.65 |
| Cross clamp time (min) | 74 (±25) | 70 (±24) | 0.52 |
| CABG | 12 (30) | 28 (47) | 0.14 |
| AVR | 18 (45) | 21 (35) | 0.40 |
| Mitral valve replacement | 5 (13) | 3 (5) | 0.62 |
| Mitral valve repair | 10 (25) | 11 (18) | 0.46 |
| Tricuspid valve repair | 0 (0) | 2 (3) | 0.52 |
Figure 1:
Mitochondrial DNA levels are higher in pericardial fluid than blood of patients who develop POAF after cardiac surgery. A) mtDNA levels in the pericardial fluid of patients who developed POAF and those who did not. B) mtDNA levels in the blood of patients who developed POAF and those who did not. POAF-Postoperative atrial fibrillation. mtDNA-Mitochondrial DNA
There was a significant increase in neutrophil influx into the pericardial fluid over time at all postoperative time points compared to baseline (Figure 2, 3, 4). A representative example of flow cytometry at 24 hours post-surgery showed that 25% of cells were cd11b+ high, and 92% of this population was cd16+cd66b+ (Figure 2). Intraoperatively, baseline neutrophils comprised 0.7%± 0.6% of inflammatory cells. At 4, 24 and 48 hours postoperatively, neutrophils comprised 39±21%, 53±22%, and 56±18 of inflammatory cells, respectively (Figure 3 and 4). Absolute numbers of neutrophils at 0, 4, 24, and 48 hours were 87±59 cells/mL, 353±232 cells/mL, 904±390 cells/mL, and 1004±516 cells/mL, respectively (n=6). In the eleven patients who had flow cytometry to analyze the number and type of inflammatory cells in the pericardial fluid, there was a trend toward higher percentage of neutrophils in the pericardial fluid at 24 hours for those who developed POAF (63±12% vs 43±22%, p=0.06, Figure 4). Serum levels of WBC were not different between the two groups.
Figure 2:
Neutrophil influx into pericardial fluid after cardiac surgery. Representative plots depicting the gating strategy for determining neutrophil content in pericardial fluid. After live/dead and single/doublet exclusion, samples were gated on (A) CD11b(hi) cells, followed by (B) CD16+CD66b+ cells. Numbers represent the percentage of cells within a given gate.
Figure 3:
Percentage of total cells that are neutrophils within the pericardial fluid at the time of measurement intraoperatively and at 4, 24 and 48 hours post-operatively. Error bars represent the standard deviation of the mean. The upper and lower borders of the box represent upper and lower quartiles, respectively. The middle horizontal line represents the median. Each circle represents one patient. ****p-value<0.001.
Figure 4:
Percentage of total cells that are neutrophils in the pericardial fluid. Each line represents a single patient. Red represents patients who developed POAF and blue represents those who did not develop POAF.
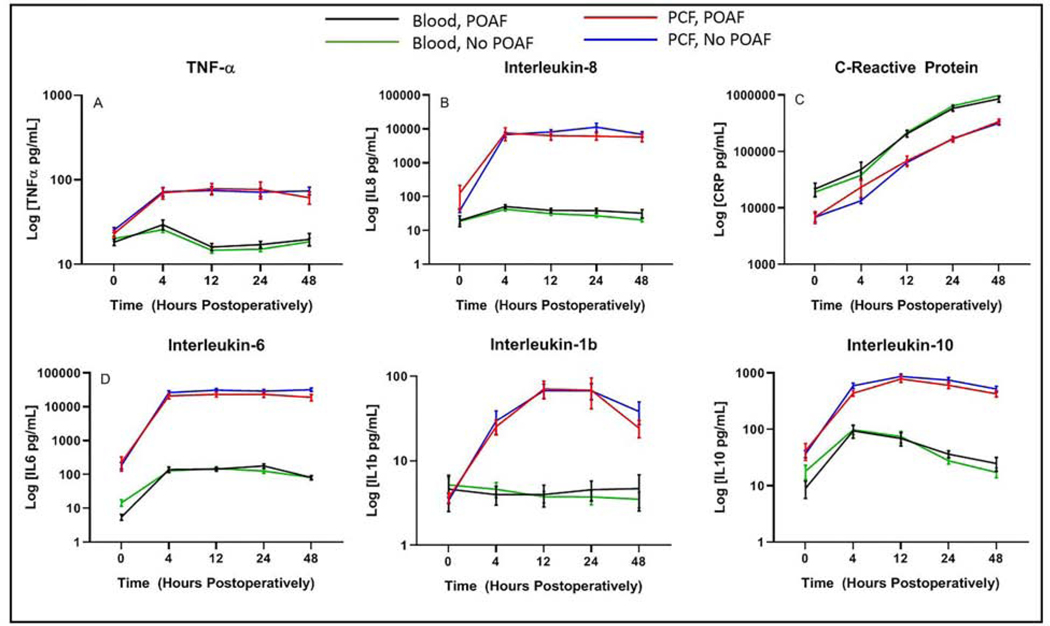
In the analysis of 36 cytokines and chemokines, we found that the levels of most cytokines (tumor necrosis factor-α, interleukin 1 receptor antagonist, myoglobin, myeloperoxidase, growth differentiation factor-15, D-Dimer, adamts13, soluble intercellular adhesion molecule-1, Sp-selectin, lipocalin, vascular endothelial growth factor, monocyte chemoattractant protein 1, interleukin-1α, interleukin-6, interleukin-8, interleukin-10, interleukin-1β, macrophage inflammatory protein, granulocyte-colony stimulating factor, creatinine kinase-MB, C-X-C Motif chemokine ligand 6, placental growth factor, oncostatin M) were much higher in the pericardial fluid than in the blood (data not shown). A few had higher levels in blood (serum soluble adhesion molecule-1, serum amyloid A, fibrinogen, haptoglobin, c-reactive protein, saponin-1, interferon-γ, interferon-α, brain natriuretic peptide), and some were no different or too low to detect (von Willebrand factor, interleukin 3, granulocyte-macrophage colony stimulating factor, tumor necrosis factor-β, Interferon-β). There was no association with any cytokine and the development of POAF. Six representative cytokines/chemokines (interleukin-6, interleukin-8, interleukin-1b, interleukin-10, TNF-α, and C-reactive protein) can be found in Figure 5. Interleukin-6, interleukin-8, interleukin-10, interleukin-1b and TNF-α levels were significantly more elevated in the PCF than in the blood at all time points, and there was a significant increase in concentration of interleukin-6, interleukin-8, interleukin-1b, interleukin-10 and TNFα over time in the PCF (p<0.0001, Figure 5). On the contrary, C-reactive protein was significantly more elevated in the blood than the PCF (p<0.0001, Figure 5).
Figure 5:
Concentrations of representative inflammatory mediators (TNF-α, Interleukin-8, C-Reactive Protein, Interleukin-6, Interleukin-1b, Interleukin-10) in the pericardial fluid and blood at time of measurement intraoperatively and at 0, 4, 12, 24, and 48 hours postoperatively. X-axis represents time in hours and y axis represents the log of the concentration in pg/mL.
Multivariable analysis (AUC=0.708, McFadden Rho-squared=0.105) showed that only age (OR: 1.04 [1.00–1.09], P=0.02) and mtDNA levels at 24 hours (OR: 1.07 [1.019–1.127], p=0.007) were significant predictors of development of POAF (Table 2). The thirty-day mortality for the POAF and non-POAF groups was 3/40 (8%) and 0/60 (0%), respectively. The average concentration of mtDNA at 24 hours for the POAF group was 0.00694 and 0.00411 pg/uL for the non-POAF group.
Table 2:
Univariable and multivariable analysis of factors that have previously been shown to be predictors of postoperative atrial fibrillation in addition to mtDNA levels at all time points. mtDNA-Mitochondrial DNA
| Univariable Analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P value | Odds Ratio ((95% CI) | P value |
| Age | 1.041 (0.997,1.081) | 0.037 | 1.044 (1.00–1.09) | 0.049 |
| Race | 4.26 (0.493, 36.966) | 0.180 | ||
| Hypertension | 0.697 (0.253, 1.916) | 0.484 | ||
| Mitral valve surgery | 0.932 (0.411, 2.115) | 0.867 | ||
| Aortic valve surgery | 0.611 (0.269, 1.391) | 0.240 | ||
| Congestive heart failure | 0.867 (0.354, 2.125) | 0.754 | ||
| [mtDNA] 0 hours | 1.126 (0.907,1.399) | 0.282 | ||
| [mtDNA] 4 hours | 1.050 (1.009,1.093) | 0.017 | ||
| [mtDNA] 12 hours | 1.035 (1.005, 1.066) | 0.022 | ||
| [mtDNA] 24 hours | 1.066 (1.016, 1.117) | 0.008 | 1.071 (1.019,1.127) | 0.007 |
| [mtDNA] 48 hours | 1.056 (1.011, 1.104) | 0.014 | ||
Comment
Elevated levels of mtDNA in the pericardial fluid, but not peripheral blood, were highly associated with POAF following cardiac surgery. There was a significant increase of neutrophils and cytokines/chemokines into the pericardial fluid postoperatively. These data suggest there was a local release of mtDNA (and not the cytokines studied) into the pericardial space that may have contributed to POAF following cardiac surgery. This release of mtDNA could have been from the surgical wound, neutrophils, or directly from cardiomyocytes.
Inflammation has been shown to contribute to the onset of POAF following cardiac surgery. Ishii et al. divided canines into 4 groups consisting of anesthesia alone, pericardiotomy group, lateral right atriotomy group, and lateral right atriotomy with anti-inflammatory therapy. They examined right atrial activation after surgery using inhomogeneity of conduction. They initiated atrial fibrillation through burst pacing, and then measured myeloperoxidase activity and neutrophil cell infiltration in the atrial myocardium to quantify the degree of inflammation (2). They ultimately found that the inhomogeneity of atrial conduction of the atriotomy and pericardiotomy groups was higher than control. When they administered anti-inflammatory medications, the inhomogeneity of atrial conduction after atriotomy was decreased (2). They also found that there were higher amounts of myeloperoxidase and neutrophils in the atrial tissue of the atriotomy and pericardiotomy groups (2). This data suggests that inflammation plays a large role in the onset of atrial fibrillation following surgery.
Mitochondria are evolutionary endosymbionts that express molecular patterns that are reminiscent of their bacterial ancestry (12). An example of a molecular pattern expressed by mitochondria is a hypomethylated CpG motif, which is recognized by several pattern recognition receptors that trigger potent inflammatory responses implicated in tissue damage (13). In response to trauma or ischemia, mitochondria can become damaged and release mitochondrial DNA into the extracellular environment (13). Due to their dependence on oxidative phosphorylation, cardiomyocytes are highly enriched with mitochondria. Notably, recent observations have shown that cardiac cells following hemodynamic stress release mtDNA (14). The release of mtDNA is therefore likely different from other inflammatory markers in that it is released locally instead of systemically like the majority of cytokines and chemokines. Furthermore, Zhang et al. conducted a prospective study in which they collected blood preoperatively from patients who were undergoing off-pump coronary artery bypass grafting. They measured mtDNA levels to determine if there was an association with the development of POAF. Ultimately, they found a strong positive correlation between mtDNA copy number and POAF following cardiac surgery (15). Our data is similar to Zhang et al. in that there was a higher level of preoperative peripheral blood mtDNA level in POAF vs non POAF patients, but it did not reach statistical significance. This was likely a result of a smaller sample size and more heterogenous patient population.
Recent work by our group has shown that the local cardiac environment of post-surgical pericardial fluid is a rich source of myeloid cells and acute phase cytokines suggesting its analysis may yield useful insights into the underlying inflammatory mechanisms of POAF (3). Here we asked if mtDNA release into the pericardial fluid and blood was associated with POAF development following cardiac surgery. Patients who developed POAF had significantly higher levels of mtDNA in the pericardial fluid compared to those did not develop POAF at all time points after surgery (Figure 1A). This was not the case with mtDNA levels in the blood as there was no significant association with the development of POAF (Figure 1B). This suggests there was likely a local release of mtDNA into the pericardial space that may have contributed to the onset of atrial fibrillation. A potential mechanism for mtDNA’s contribution to atrial fibrillation is that mtDNA is released into the pericardial space following trauma, reperfusion injury and neutrophil death during surgery, and then binds to TLR9 which is a receptor found on immune cells, neurons and cardiomyocytes. When mtDNA binds to TLR9 on immune cells, it elicits an inflammatory response causing release of cytokines, chemokines, and neutrophils (17). This is important because targeted therapy could be administered into the pericardial space to block the mechanism by which mtDNA causes inflammation. This could be accomplished by using known agents such as oligodeoxynucleotides or cyclosporine H (5,6). Specifically, cyclosporine H has been demonstrated to block formylated peptide receptor 1 (FPR1). In the body, FPR1 detects products of mitochondrial damage (N-formylated peptides) and promotes neutrophil migration thus perpetuating the inflammatory response (6).
Neutrophil infiltration into the pericardial fluid was also examined. Neutrophils have been shown to be pro-arrhythmic. Hoffman et al. found that when neutrophils bound to cardiac myocytes they caused prompt and consistent changes in myocyte electrical activity (4). We found that there was a significant increase in neutrophils into the pericardial fluid following cardiac surgery (Figure 3). CpG motifs on mitochondrial DNA are recognized by toll like receptor 9 (14). Mitochondrial DNA activates neutrophils through these CpG/toll like receptor 9 interactions (5). Specifically, mtDNA promotes neutrophil calcium flux and phosphorylation of mitogen-activated protein kinases which leads to neutrophil migration and degranulation (5).
There are limitations of this study. The sample size was small; however, it was sufficient to detect differences in mtDNA concentrations. Furthermore, the population was heterogenous and included CABG and/or valve surgery performed by multiple surgeons. Additionally, the study was observational in nature and may have been subject to residual confounding by unmeasured factors. There was also an above average rate of postoperative atrial fibrillation; however, this may have been the result of the heterogeneity of case types, or higher detection rates given patients were enrolled in a study.
In conclusion, we have demonstrated that elevated levels of mtDNA in the pericardial fluid, but not peripheral blood, were associated with POAF after cardiac surgery. This suggests that there was a local release of mtDNA into the pericardial space that was associated with the development of POAF. We also observed that pericardial fluid mtDNA levels in POAF patients were sharply elevated at 24 hours postoperatively. Because POAF occurs most frequently between 24–48 hours after cardiac surgery, quantitating mtDNA concentration after surgery may be a useful approach to assess POAF risk. Postoperative atrial fibrillation is the most common complication and arrhythmia seen after cardiac surgery, yet the etiology remains poorly understood. These findings suggest that the inflammatory mechanism might be associated with the release of mtDNA, which could yield targets for prevention and/or therapy. For example, agents that target the mtDNA in the inflammatory cascade could be a potential treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. [DOI] [PubMed] [Google Scholar]
- 2.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005; 111: 2881–2888 [DOI] [PubMed] [Google Scholar]
- 3.Kramer PA, Chacko BK, Ravi S, et al. Hemoglobin-associated oxidative stress in the pericardial compartment of postoperative cardiac surgery patients. Laboratory investigation. 2015; 95: 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman BF, Feinmark SJ, Guo SD. Electrophysiologic effects of interactions between activated canine neutrophils and cardiac myocytes. J Cardiovasc Electrophysiol; 8: 679–687 [DOI] [PubMed] [Google Scholar]
- 5.Zang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010; 464: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scozzi D, Ibrahim M, Liao, et al. Mitochondrial damage-associated molecular patterns released by lung transplants are associated with primary graft dysfunction. Am J Transplant. 2019; 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliksoen M, Mariero LH, Torp MK, et al. Extracellular mtDNA activates NF-kB via toll-like receptor 9 and induces cell death in cardiomyoctes. Basic Res Cardiol.2016; 111: 42. [DOI] [PubMed] [Google Scholar]
- 8.Sandler N, Kaczmarek E, Itagaki K, et al. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart, lung and circulation. 2017. Article in press [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Xu S, Xu Y, et al. Relation of mitochondrial DNA copy number in peripheral blood to postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting. Am J Cardiol. 2017; 119:473–477 [DOI] [PubMed] [Google Scholar]
- 10.Gelman AE, Okazaki M, Lai J, et al. CD4+ T lymphocytes are not necessary for the acuterejection of vascularized mouse lung transplants. The Journal of Immunology. 2008;180(7):4754–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen J, Lall S, Zheng V, et al. The persistent problem of new-onset postoperative atrial fibrillation: A single-institution experience over two decades. J Thorac Cardiovasc Surg 2011; 141:559–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray MW, Burger G, Lang BF. Mitochondrial Evolution. Science. 1999; 283: 1476–1481 [DOI] [PubMed] [Google Scholar]
- 13.Scozzi D, Ibrahim M, Liao, et al. Mitochondrial damage-associated molecular patterns released by lung transplants are associated with primary graft dysfunction. Am J Transplant. 2019; 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka T, Hikoso S, Yamaguchi, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012; 485: 251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Xu S, Xu Y, et al. Relation of mitochondrial DNA copy number in peripheral blood to postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting. Am J Cardiol 2017; 119:473–477 [DOI] [PubMed] [Google Scholar]
- 16.Butts B, et al. Increased Inflammation in Pericardial Fluid Persists 48 Hours After Cardiac Surgery. Circulation 2017; 136:2284–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliksoen M. Extracellular mtDNA activates NF-kB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic Res Cardiol 2016; 111:42. [DOI] [PubMed] [Google Scholar]