Abstract
The staphylococcal α-hemolysin is critical for the pathogenesis of Staphylococcus aureus skin and soft tissue infection. Vaccine and infection-elicited α-hemolysin–specific antibodies protect against S. aureus–induced dermonecrosis, a key feature of skin and soft tissue infection. Many interactions between α-hemolysin and host cells have been identified that promote tissue damage and modulate immune responses, but the mechanisms by which protective adaptive responses cross talk with innate responses at the tissue level are not clear. Using an established mouse model of skin and soft tissue infection and a newly developed histopathologic scoring system, we observed pathologic correlates early after infection, predicting protection against dermonecrosis by anti–α-hemolysin antibody. Protection was characterized by robust neutrophilic inflammation and compartmentalization of bacteria into discrete abscesses, which led to the attenuation of dermonecrosis and enhancement of bacterial clearance later in the infection. The ultimate outcome of infection was driven by the recruitment of neutrophils within the first day after infection but not later. Antibody-mediated protection was dependent on toxin neutralization rather than on enhanced opsonophagocytic killing by neutrophils or protection against toxin-mediated neutrophil lysis. Together, these findings advance our understanding of the mechanisms by which the early synergism between antibody-mediated toxin neutralization and tissue-specific neutrophilic inflammation preserve tissue integrity during infection.
INTRODUCTION
Staphylococcus aureus, a bacterial pathogen that causes a variety of diseases in humans, is the most common cause of skin and soft tissue infection (SSTI) globally (Esposito et al., 2016). Several candidate vaccines have failed to prevent S. aureus infection in clinical trials (Fowler et al., 2013; Rupp et al., 2007), prompting the need to better understand the mechanisms of protective immunity.
One vaccine candidate for preventing S. aureus infection is α-hemolysin (Hla). This pore-forming toxin activates or lyses various cell types that express the Hla receptor, ADAM10 (e.g., erythrocytes, platelets, leukocytes, keratinocytes, and endothelial cells) (Seilie and Bubeck Wardenburg, 2017). Active or passive immunization with an Hla mutant (HlaH35L) protected against S. aureus SSTI in mice (Kennedy et al., 2010). Protection against recurrent S. aureus SSTI in mice was also at least partly dependent on Hla-specific antibody responses (Montgomery et al., 2014). Antibody-mediated protection specific for Hla may also be clinically relevant because anti-Hla IgG levels correlated with a lower risk of recurrent S. aureus SSTI in children (Fritz et al., 2013).
Innate immunity is the first line of defense against S. aureus SSTI, with the characteristic neutrophil-dominated abscess compartmentalizing bacteria to allow for effective clearance (Kobayashi et al., 2015). However, the mechanisms by which protective antibodies interact with innate immune responses to promote protection against S. aureus infection are not well-understood. Whereas Hla-specific antibodies appear to promote increased neutrophil recruitment and improve outcomes in experimental S. aureus skin infection (Abtin et al., 2014), the role of neutrophils appears to be dynamic depending on the phase of the infection (Kim et al., 2011). The objective of this study was to determine the mechanisms by which protective anti-Hla antibody interacts with tissue-specific innate immune cells to prevent tissue damage and promote the resolution of infection. Our results demonstrate that protection against tissue necrosis was mediated through early robust neutrophilic inflammation in conjunction with antibody-mediated toxin neutralization rather than enhanced opsonophagocytic killing or neutrophil survival.
RESULTS
Antibody-mediated protection augmented inflammatory response and attenuated tissue necrosis
To investigate the tissue-protective effects of anti-Hla antibody, we used a passive transfer model in which naive mice received serum from mice vaccinated with HlaH35L 1 day before infection with S. aureus. The anti-Hla antibody level was confirmed in the skin lysates and blood from the infected mice receiving the protective antibody (Figure 1a). Recipients of naive serum developed small areas of dermonecrosis at 6 hours after infection (hai), which peaked in size on 1 day after infection (dai), and gradually decreased in size with crust formation by 7 dpi. As previously reported (Kennedy et al., 2010), the mice receiving protective antibody developed small abscesses with minimal dermonecrosis after infection (Figure 1b and c). Although there were no significant differences in the number of bacteria recovered from the lesions early after infection (1 dai), there were fewer bacteria on 7 dai in mice receiving protective antibody (Figure 1d). In contrast, the level of myeloperoxidase, a marker for neutrophil activity, demonstrated a higher trend in the presence of the protective antibody early after infection but not later (Figure 1e).
Figure 1. Antibody-mediated protection against Staphylococcus aureus dermonecrosis augmented inflammation.
Naive or Hla-specific sera were passively transferred before SSTI (n = 5 mice per group). (a) Anti-Hla IgG levels in the peripheral blood and skin 2 d after serum transfer and 1 d after infection. (b) Lesion photographs: cutaneous edema (red arrows), dermonecrosis (black arrows), and abscess (arrowhead). Bar = 0.5 cm. (c) Lesion size. (d) CFU per ml. (e) MPO levels. (f) Histopathologic scores. (g) Photomicrographs on 1 d and 7 d after infection: bacteria (arrows), peripheral abscesses (arrowheads), fibrinoid necrosis with thrombus (asterisks), and intracellular or extracellular bacteria (insets). H&E and Gram stain. Bar = 500 μm for the first and fourth rows, 100 μm for the second row, and 20 μm for the third row. Data were compared using (c) two-way ANOVA with repeated measures and Tukey’s post-hoc test or (a, d–f) Mann–Whitney U test. Data are presented as (a, c–e) mean ± SEM or (f) boxplots with median (horizontal line), interquartile range (box), and maximum and/or minimum (whiskers). *P < 0.05, **P < 0.01, ****P < 0.0001. CFU, colony-forming unit; d, day; h, hour; Hla, α-hemolysin; MPO, myeloperoxidase; NS, not significant; OD, optical density; SSTI, skin and soft tissue infection.
To objectively quantify tissue damage and inflammation, a histopathologic scoring system was developed with four elements: inflammation, necrosis, vascular damage, and bacterial burden (Supplementary Table S1 and Supplementary Figures S1 and S2). Mice receiving naive serum had more severe necrosis, vascular damage, and bacterial burden on 1 and 7 dai, as reflected by higher histopathologic scores, than mice receiving protective antibody (Figure 1f). In contrast, mice receiving protective antibody had more severe inflammation early after infection, but these differences resolved later during infection. Microscopically, in the absence of the protective antibody on 1 day after infection, mice developed characteristic widespread epidermal coagulative necrosis with minimal inflammation and a band of extracellular bacteria at the panniculus carnosus, surrounded by small peripheral abscesses (Figures 1g). Most of the intralesional vessels exhibited circumferential fibrinoid necrosis with occasional thrombi, but the vessels at the periphery of the lesions were viable. In contrast, protective antibody resulted in the development of large, well-demarcated, subcutaneous abscesses with inflammatory cells infiltrating the viable overlying epidermis and dermis and intracellular bacteria within the abscess detected through Gram stain. The intralesional vessels were mostly viable with rare fibrinoid necrosis.
On 7 dai, without protective antibody, the mice developed severe full-thickness cutaneous coagulative to liquefactive necrosis and soft tissue fat and muscular necrosis with multifocal infiltration of mixed inflammatory cell admixed with clusters of cocci. In contrast, the mice receiving protective antibody had abscesses partially replaced by fibrin and granulation tissue, with dermal infiltration of mixed inflammatory cells and hyperplastic epidermis. Intracellular bacteria were rarely identified. These results support the hypothesis that protective antibody induces an early, organized inflammatory response to attenuate tissue necrosis and to compartmentalize bacteria, which eventually promotes bacterial clearance and resolution of infection.
Early neutrophilic infiltration dominates in antibody-mediated protection
We next quantified the recruited immune cells in the lesions by immunohistochemical staining and flow cytometry. On 1 dai, regardless of whether mice received protective antibody, there was an infiltration of Ly-6C+/Ly-6G+ cells with segmented nuclei (neutrophils) predominantly within and adjacent to the abscess (Figure 2a). Consistent with the inflammation severity scores, there were more neutrophils in the lesions of mice receiving protective antibody on 1 dai but not in those on 7 dai through flow cytometry (Figure 2b and c). Mice receiving protective antibody also had more F4/80+ cells throughout the skin on 1 and 7 dai (Figure 2a) as well as increased newly recruited monocytes and dermal macrophages, albeit in much lower numbers than neutrophils. The numbers of γδT cells were higher in the mice receiving protective antibody on 1 ddai, supported by the presence of more intact epidermal CD3+ cells observed through immunohistochemistry. These findings demonstrate that neutrophils are the predominant inflammatory cells associated with antibody-mediated protection, suggesting an important role in protection against necrosis.
Figure 2. Early neutrophilic infiltration dominated in antibody-mediated protection.
Immune cells were quantified after SSTI in mice receiving naive or Hla-specific sera. (a) Immunohistochemical staining of lesions 1 d and 7 d after infection at the abscess periphery (upper panels) and the overlying skin (lower panels). Insets demonstrated higher magnification of the highlighted areas. Epidermal F4/80+ cells (Langerhans cells and/or macrophages) and CD3+ cells (dendritic epidermal T cells or γδT cells) were intact (protective insets) or had lost distinct cell borders and staining (naive insets). Bar = 100 μm. (b) Inflammatory cells in the lesions were quantified by flow cytometry (n = 5 mice per group). (c) Representative dot plots. Data were compared using Mann–Whitney U test and presented as mean ± SEM. *P < 0.05, **P < 0.01. Hash (#) indicates the number of cells per lesion. d, day; Hla, α-hemolysin; MHC, major histocompatibility complex; NS, not significant; SSTI, skin and soft tissue infection.
Transcriptomic profiles of inflammation are increased in antibody-mediated protection
To determine whether the increased inflammation early after the infection was associated with a commensurate increase in transcription of proinflammatory genes, we quantified the early kinetics of gene expression in the skin lesions using quantitative RT-PCR analysis. Gene expression of proinflammatory cytokines (Tnf and Infg), neutrophil trafficking chemokines (Cxcl2), and cytokines and chemokines related to T helper type 17 (Il-17a, Il-17f, Il-22, Il-23a, and Ccl20), T helper type 1 (Il-12, Cxcl9, and Cxcl11), and T helper type 2 (Il-4 and Ccl22) type responses in the mice receiving protective antibody were higher on 1 dai than those in the controls, normalized to uninfected mice (Figure 3 and Supplementary Figure S3). Therefore, the neutrophilic inflammation we observed by histopathology was associated with increases in inflammatory gene transcription.
Figure 3. Neutrophil-recruiting cytokines and chemokines were increased in antibody-mediated protection.
RNA was extracted from infected and healthy skin for RT-PCR (n = 4 mice per group). (a) Heatmap of mRNA expression of cytokines and chemokines in Staphylococcus aureus SSTI at 3 h, 6 h, and 24 h after infection in mice receiving naive (−) or protective (+) sera. Gene expression was quantified as fold changes relative to healthy uninfected skin. (b) Representative cytokines and chemokines. The Benjamini–Hochberg procedure was performed for multiple comparisons using the false discovery rate correction with a q-value < 0.05. Data are presented as mean ± SEM. *P < 0.05. h, hour; NS, not significant; SSTI, skin and soft tissue infection.
Early neutrophil recruitment is essential for antibody-mediated protection
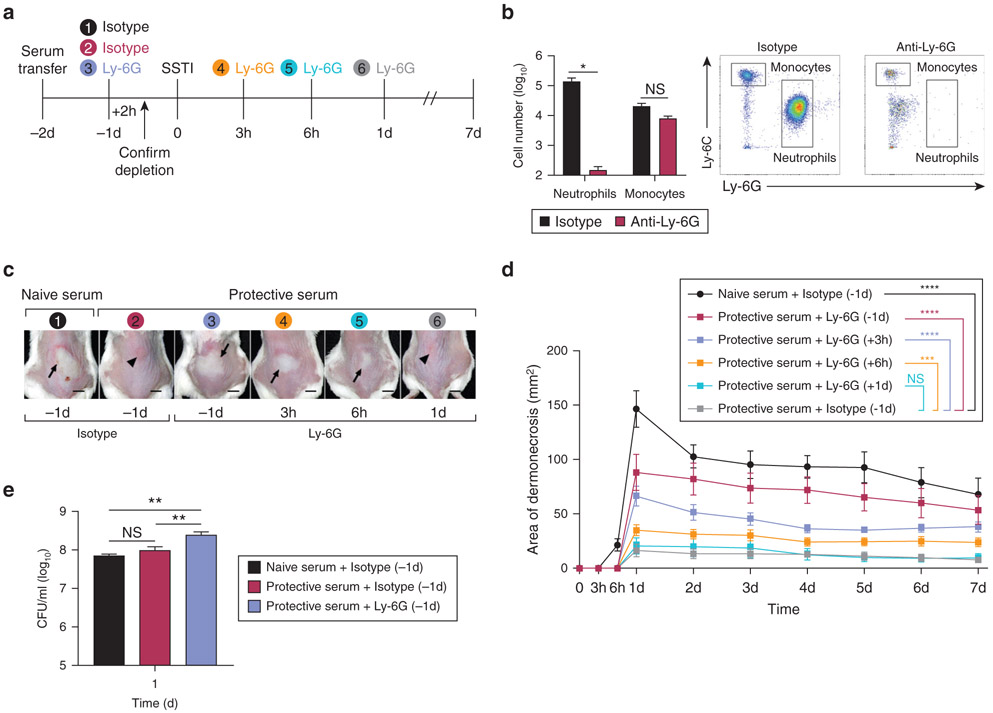
These findings suggested that early neutrophil recruitment within the first 24 hai was critical for protection against tissue necrosis. To test whether neutrophils had a dynamic role in the protection, the mice received a passive transfer of serum followed by neutrophil depletion using anti–Ly-6G antibody at different times before or after infection (Figure 4a). Even in the presence of protective antibody, lesion size correlated with how early neutrophils were depleted, with the largest lesions observed when neutrophils were depleted at 24 hours before infection, which was associated with an increased bacterial burden (Figure 4b-e). The lesions were also larger when neutrophils were depleted at 3 and 6 hai, compared with those that received isotype control, but smaller than those depleted before infection. Importantly, there were no significant differences in lesion size between the mice that had neutrophils depleted at 1 dai and the control mice. Because macrophages are important for neutrophil recruitment in S. aureus SSTI (Abtin et al., 2014) and we observed increased numbers of newly recruited monocytes and macrophages in the mice that received protective antibody, we tested their importance in our model. Depletion of both neutrophils and recruited monocytes using anti–Ly-6C/Ly-6G antibody (clone 1A8 or RB6-8C5, BioXCell, West Lebanon, NH) resulted in a similar kinetic profile as with anti–Ly-6G alone (Supplementary Figure S4a and b). Consistent with a dominant role for neutrophils, depletion of recruited monocytes to the skin using clodronate liposomes had no impact on antibody-mediated protection (Supplementary Figure S4c and d). Taken together, these findings demonstrate that unlike recruited monocytes to the tissue, recruitment of neutrophils within the first 24 hai, but not thereafter, determines the fate of infection in antibody-mediated protection against SSTI.
Figure 4. Antibody-mediated protection was dependent on neutrophil recruitment within 24 h.
Mice received a passive transfer of serum followed by neutrophil depletion using anti–Ly-6G antibody and SSTI (n = 5 mice per group). (a) Experimental timeline. Treatments of different groups were shown in different colors. (b) Depletion efficacy was 99.9% in the blood. Total cell number and representative flow cytometry dot plots. (c) Lesion photographs at 1 d after infection: dermonecrosis (arrows) and abscess (arrowheads). Bars = 0.5 cm. (d) Lesion size. (e) CFU per ml on 1 d after infection. Data were compared using (b) Mann–Whitney U test, (d) two-way ANOVA with repeated measures with Tukey’s post-hoc test, and (e) Kruskal–Wallis test with Dunn’s post-test. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with isotype control receiving protective serum. CFU, colony-forming unit; d, day; h, hour; NS, not significant; SSTI, skin and soft tissue infection.
Depletion of neutrophils abolishes antibody-mediated protection against cutaneous necrosis
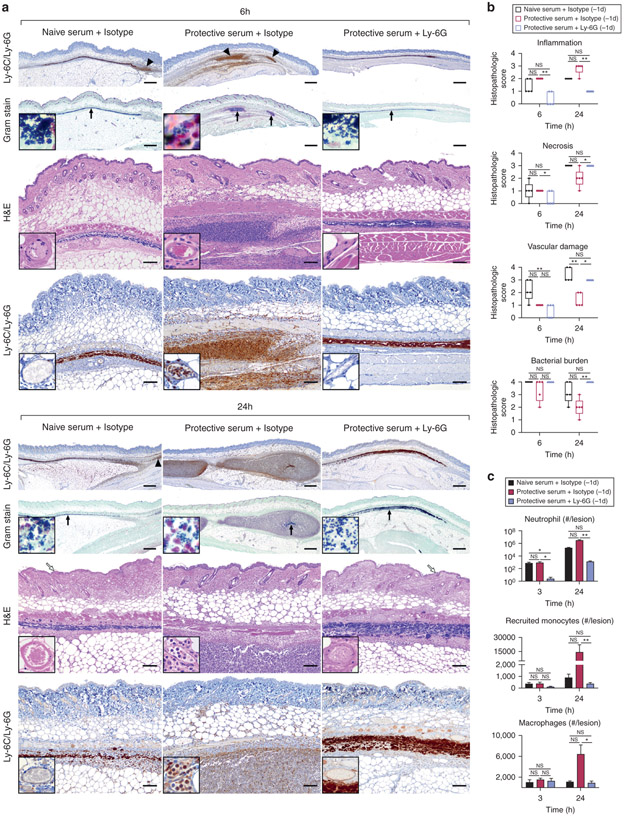
To understand the early cellular events promoting antibody-mediated protection against necrosis, neutrophils were depleted 1 day before infection, followed by quantification of histopathologic severity and immune cells within the first 24 hai. The depletion of neutrophils abolished antibody-mediated protection against tissue necrosis; the protective histopathologic phenotype reverted to the nonprotective phenotype by 1 dai (Figure 5a and b). At 6 hai, in the absence of protective antibody, there was necrosis of intralesional vessels with minimal infiltration of inflammatory cells and abundant extracellular bacteria in a band-like pattern, but the epidermis was viable. In contrast, there was leukocyte extravasation and early formation of poorly demarcated abscesses containing bacteria in the presence of the protective antibody. Despite neutrophil depletion, there was minimal vascular damage and epidermal necrosis at 6 hai in the presence of the protective antibody. At 24 hai, consistent with our previous results, extensive epidermal and vascular necrosis developed in the absence of the protective antibody. Even in the presence of the protective antibody, depletion of neutrophils resulted in the nonprotective histopathologic phenotype, with widespread epidermal and vascular necrosis, minimal inflammation, and abundant extracellular bacteria. The higher numbers of neutrophils in mice that received the protective antibody at 24 hai were not apparent at 3 hai, suggesting early inhibition of neutrophil recruitment (Figure 5c and Supplementary Figure S5). These findings underscore the importance of understanding how early histopathologic changes predict the ultimate fate of infection.
Figure 5. Depletion of neutrophils abolished antibody-mediated protection against cutaneous necrosis.
Mice received a passive transfer of serum followed by neutrophil depletion using anti–Ly-6G antibody and SSTI (n = 5 mice per group). (a) Photomicrographs at 6 h and 24 h after infection. Ly-6C/Ly-6G: Abscess (arrowheads) and intralesional vessels (insets). Gram stain: Bacteria (arrows), high magnification of extracellular or intracellular bacteria (insets). H&E: Intralesional vessels (insets) and epidermal necrosis (open arrows). Bar = 500 μm (low magnification) and 100 μm (high magnification). (b) Histopathologic scores. (c) Inflammatory cells quantified by flow cytometry. Data were compared using (b, c) Kruskal–Wallis test with Dunn’s post-hoc test. Data are presented as (b) boxplots with median (horizontal line), interquartile range (box), and maximum and/or minimum (whiskers) or (c) mean ± SEM. *P < 0.05, **P < 0.01. Hash (#) indicates the number of cells per lesion. d, day; h, hour; NS, not significant; SSTI, skin and soft tissue infection.
Antibody protects at the tissue level by a toxin neutralization
We next investigated the mechanisms by which the Hla-specific antibody-mediated protection. Because we observed enhanced bacterial clearance late after infection (7 dai) but not early (1 dai), we hypothesized that the rapid protection by the antibody is independent of enhanced opsonophagocytosis. To test this, we opsonized bacteria with serum, incubated with neutrophils to allow bacterial killing, and quantified bacterial survival. ΔSpa deletion mutant of strain 923 was used to avoid interference of protein A with opsonophagocytosis. Opsonization of bacteria with antiserum against an S. aureus cell wall protein, IsaA, was included as a control, which demonstrated increased bacterial killing compared with the no serum control group. However, there were no significant differences in the ability of mouse neutrophils to kill the bacteria opsonized with naive or protective sera (Figure 6a). Similarly, there were no significant differences in the ability of human neutrophils to kill S. aureus, regardless of opsonization with naive or anti-Hla sera.
Figure 6. Antibody-mediated toxin neutralization protected against dermonecrosis.
(a) Bacteria were opsonized by mice serum and incubated with mouse or human neutrophils for bacterial killing, and bacterial survival was quantified. (b) Mice serum was incubated with Hla before incubation with mouse or human neutrophils. Neutrophil viability was quantified. (c) Mice serum was incubated with 1 μM Hla before incubation with mouse or human monocytes from the peripheral blood. (d) Mice received a passive transfer of naive serum, IgG, or Fabs before SSTI. (n = 4–5). Data were compared using (a, c) one-way ANOVA with Tukey’s test, (b) Kruskal–Wallis test with Dunn’s post-hoc test, or (d) two-way ANOVA with repeated measurements with Tukey’s test. Results in a–c were a compilation of 2–5 independent experiments using 5–15 mice in each experiment and blood from a total of six different healthy human donors. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***p < 0.001, ****P < 0.0001. Hash (#) indicates the number of cells per lesion. Fab, antigen-binding fragment; Hla, α-hemolysin; NS, not significant; SSTI, skin and soft tissue infection.
One possible explanation for antibody-mediated protection against the cell-poor necrotic tissue phenotype is that protective antibody inhibits direct Hla-mediated cytotoxicity against neutrophils. Therefore, neutrophils were incubated with serum and Hla, followed by quantification of neutrophil viability. However, we observed very little killing of either mouse or human neutrophils by Hla, consistent with low ADAM10 expression on these cells (Abtin et al., 2014). Moreover, there were no significant differences in neutrophil viability regardless of treatment with serum (Figure 6b). In contrast, antibody inhibited Hla-induced cytotoxicity of mouse and human monocytes (Figure 6c), consistent with the higher ADAM10 expression in these cells (Abtin et al., 2014). Because antibody neither promoted bacterial killing by neutrophils nor protected neutrophils from Hla-induced cytotoxicity, we hypothesized that antibody protected against necrosis by neutralizing toxin at the tissue level. To test this, IgG was purified from protective serum followed by papain cleavage to generate antigen-binding fragments (Fabs), which are sufficient for toxin neutralization but not for opsonophagocytosis. Fabs were passively transferred to mice before SSTI. Consistent with our hypothesis, passive transfer of Hla-specific Fabs resulted in protection that was comparable with that of purified IgG (Figure 6d). Therefore, Hla-specific antibody protects against tissue necrosis by direct toxin neutralization.
DISCUSSION
In this study, we found that antibody-mediated protection against S. aureus dermonecrosis is characterized by early inflammation and enhanced bacterial clearance later in the infection. The fate of infection is determined early after infection and depends on both toxin neutralization and effective neutrophil recruitment to compartmentalize bacteria into discrete abscesses and prevent dermonecrosis. Protection is independent of enhanced opsonophagocytosis or neutrophil survival. Therefore, antibody-mediated protection at the tissue level represents a synergy between innate and adaptive immunity.
Neutrophils were the predominant inflammatory cells associated with protection against dermonecrosis, and their recruitment within the first 24 hours was critical, which was consistent with a time-dependent role of neutrophils in a mouse model with S. aureus wound superinfection (Kim et al., 2011). Importantly, the histopathologic changes in this study were observed as early as 6 hai, which predicted the outcome of infection. In antibody-mediated protection, the early abscess formation and requisite confinement of bacteria resulted subsequently in the attenuation of dermonecrosis. In contrast, without a protective antibody, there was a striking central cell-poor zone of tissue necrosis above the necrotic vessels with abundant uncontained bacteria. However, the cutaneous tissue at the lesion periphery was viable, reminiscent of a segmental ischemic injury. These differences in early histopathologic findings suggest that infection could inhibit protective inflammation by multiple mechanisms, including direct Hla-induced cytotoxicity, inhibition of leukocyte recruitment, and/or a bystander effect wherein regional tissue ischemia and/or necrosis results in resident cell death.
One possibility that could reconcile these observations with the early vascular necrosis and delayed infiltration of neutrophils in the absence of a protective antibody is Hla-mediated endothelial injury. In this study, vascular necrosis was observed early at 6 hai, whereas extensive epidermal necrosis was not observed until 1 dai. In support of this, the injection of purified Hla into the mice induced endothelial damage in as few as 3 hours (Powers et al., 2012). However, another group reported that Hla-induced endothelial damage was not present at 6 hai, and they concluded that the Hla-induced lysis of perivascular macrophages resulted in the inhibition of neutrophil recruitment (Abtin et al., 2014). We similarly observed lysis of F4/80+ cutaneous macrophages but could not differentiate whether this was due to direct Hla-mediated toxicity or as a consequence of necrosis of the blood vessels with which these cells are closely associated. In addition, we cannot completely exclude the role of macrophages in our model because the mouse dermal macrophages are a heterogeneous population (Tamoutounour et al., 2013), resulting in incomplete depletion by clodronate liposomes. Another possible explanation for the inhibition of neutrophil recruitment could be Hla-induced lysis of dendritic epidermal T cells (Sampedro, 2018). We also found lysis of dendritic epidermal T cells scattered within the necrotic epidermis, but we could not determine whether the cell lysis was due to direct Hla toxicity or secondary to epidermal necrosis. Given the methodological differences among the studies, it seems likely that Hla-induced toxicity to vascular endothelial cells, perivascular macrophages, and dendritic epidermal T cells collectively contribute to the inhibition of neutrophil recruitment. Further study is necessary to define the kinetics of the host–pathogen interaction that result in cell lysis, abscess formation, and bacterial clearance.
We found that antibody-mediated protection was independent of the enhancement of opsonophagocytosis. However, phagocytosis and compartmentalization of bacteria within abscesses were still critical to prevent tissue necrosis in the context of antibody-mediated protection. This was demonstrated by the strikingly different bacterial distribution histologically early after infection between the mice receiving naive and those receiving protective sera despite similar lesional bacterial burden. This was further supported by the observation of epidermal necrosis in neutrophil-depleted mice despite the presence of a protective antibody that should prevent toxin-mediated epidermal necrosis. Our findings support a model wherein protective antibody acts early by neutralizing the toxic effects of Hla on multiple cell types in the skin and vasculature, enabling leukocyte recruitment and abscess formation, which together contribute to the attenuation of cutaneous necrosis. However, given the important differences in the pathogenesis of and immune response to S. aureus infections in mice and humans, the translatability of these findings must be demonstrated. This limitation notwithstanding, these findings suggest that immunotherapeutic strategies to prevent toxin-mediated S. aureus infections such as skin infection and pneumonia should prioritize targeting toxin neutralization as a means to protect against tissue damage.
In conclusion, these findings advance our understanding of the early events that shape the tissue microenvironment to protect against S. aureus–induced dermonecrosis and demonstrate that optimal protection results from the synergy of antibody-mediated toxin neutralization and an early robust neutrophilic inflammation.
MATERIALS AND METHODS
Vaccination and serum transfer
All animal experiments were conducted according to the regulations of the Institutional Animal Care and Use Committee at the Abigail Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH. The plasmid for HlaH35L purification was a gift from Dr Juliane Bubeck Wardenburg (Washington University, St. Louis, MO). IsaA was cloned into pET28a plasmid and purified using previously described methods (Si et al., 2020). Female BALB/c mice (6–8 weeks old; Jackson Laboratories, Bar Harbor, ME) were vaccinated with HlaH35L three times at 2-week intervals (Bubeck Wardenburg and Schneewind, 2008) or with IsaA once and euthanized 2 weeks later by carbon dioxide inhalation. Blood was collected through cardiac puncture. IgG and Fabs were prepared using protein A/G columns and Fab Preparation Kit (Thermo Fisher, Waltham, MA), respectively. Serum, purified IgG, or Fabs were passively transferred to mice through retro-orbital injection 1–2 days before infection. Anti-Hla antibody levels in the blood and skin were quantified using ELISA (Hycult Biotech, Wayne, PA). Then, 96-well plates were coated with HlaH35L (5 μg/ml), and mouse serum or supernatant from homogenized skin lesion were diluted and added to the wells. Detection was performed using alkaline phosphatase–conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO), and absorbance values at 405 nm were measured.
Bacterial preparation
USA300 S. aureus strain 923 (Montgomery et al., 2008) and ΔSpa deletion mutant of strain 923 (Zhao et al., 2016) were grown overnight at 37 °C on a tryptic soy agar and tryptic soy agar containing 5 μg/ml tetracycline, respectively. A colony was selected and inoculated into tryptic soy broth and grown overnight in a shaking incubator (250 r.p.m.) at 37 °C. On the day of the infection, the bacterial culture was diluted to 1:100 and grown at 37 °C for 3 hours. The bacteria were pelleted, washed, and adjusted to the desired concentration. The inoculum was confirmed by plating on tryptic soy agar.
Mouse model of S. aureus SSTI
As previously described (Montgomery et al., 2014), mice were inoculated subcutaneously in the dorsal lumbosacral area with 1.5 × 107 colony-forming units of S. aureus per 50 μl. Lesion size was measured daily through digital photography. Lesions were homogenized and plated on mannitol salt agar for colony-forming unit enumeration. Myeloperoxidases in the supernatant from homogenized lesion were quantified using ELISA. Mice were treated intraperitoneally with anti–Ly-6G (100 μg) or Ly-6C/Ly-6G antibody (50 μg) 1 day before infection or 3, 6, or 24 hai to deplete neutrophils. Depletion efficacy was confirmed in the blood at 2 hours after depletion (Figure 4b and Supplementary Figure S4a). Control mice received a corresponding isotype antibody (clone 2A3 or LTF-2, BioXCell). For macrophage depletion, mice were treated intraperitoneally with 50 μl of clodronate liposomes or control liposomes (Encapsula NanoSciences, Brentwood, TN) 2 days before infection. The skin was digested 2 days after depletion to confirm depletion efficacy (Supplementary Figure S4c).
Histopathologic scoring and immunohistochemistry
Skin lesions were excised, fixed in 10% neutral-buffered formalin, processed, embedded in paraffin, sectioned, and stained with H&E and Hucker-Twort Gram stain. Skin lesions were scored blindly and independently by a veterinary anatomic pathology resident (CY) and a board-certified veterinary anatomic pathologist (RNJ) using the scoring system described in this study (Supplementary Table S1 and Supplementary Figures S1 and S2). After independent review and scoring, a meeting was held to resolve differences in the histopathologic score while blinded to the group identity, and a consensus score was reached. The Ohio State University Comparative Pathology and Mouse Phenotyping Shared Resource performed the immunohistochemical staining (Supplementary Table S2).
Quantification of leukocytes
Skin lesions were dissected and digested with 100 μg/ml Liberase TM (Roche, Mannheim, Germany) and 0.1 mg/ml DNase I in RPMI medium at 37 °C for 1.5 hours. The digested tissue was filtered, resuspended in 40% buffered Percoll (Sigma-Aldrich), and layered on 70% buffered Percoll, followed by centrifugation at 930g for 20 minutes at room temperature. The cells at the interface were collected, stained with fluorescently conjugated antibodies (Supplementary Table S2), and quantified using counting beads (Thermo Fisher). Blood leukocytes were isolated using PolymorphPrep (Progen, Heidelberg, Germany). The cells were acquired on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA) and analyzed using Flow Jo software (gating strategies are in Supplementary Figure S6).
RNA isolation and qPCR
RNA was extracted from healthy and infected skin stabilized in RNAlater (Thermo Fisher) using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA quantity and quality were assessed using a 2100 Bioanalyzer (Agilent, Santa Clara, CA). RNA (1 μg) was reverse transcribed to cDNA using a high-capacity cDNA archive kit (Thermo Fisher). qPCR of gene expression was performed using a customized plate (SABioscience, Frederick, MD) with an ABI Prism 7500 Series Real-time PCR Thermocycler (Thermo Fisher). Relative gene expression in the skin lesion was normalized against the internal control Ldha and then compared with that of the healthy skin using the ΔΔCt method (Montgomery and Daum, 2009).
Bacterial opsonophagocytic killing and toxin neutralization assays
Mice were given 1 ml 4% thioglycollate intraperitoneally, and the peritoneum was flushed at 16 hours later to collect neutrophils. Informed consent was obtained from six healthy human donors before blood collection. Mouse peritoneal and human blood neutrophils were positively selected using anti–Ly-6G magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) and Human Neutrophil Isolation Kits (STEMCELL Technology, Vancouver, Canada), respectively, and confirmed through flow cytometry. Heat-inactivated diluted serum (1:5) was incubated with ΔSpa for 30 minutes, and neutrophils with RPMI plus 2% fetal bovine serum were added with a multiplicity of infection of 10 for human neutrophils and one for mouse neutrophils. Antibody against a cell wall protein of S. aureus, IsaA, was used as a control for opsonophagocytic killing. After incubating for 45 minutes at 37 °C to allow bacterial uptake, gentamicin (0.2 mg/ml) was added for 30 minutes to kill the extracellular bacteria. Neutrophils were washed, resuspended, and lysed using 0.1% Triton. The released intracellular bacteria were plated on mannitol salt agar for colony-forming units (T0). The remaining wells were incubated for another 1 hour to allow bacterial killing, and neutrophils were lysed followed by plating for colony-forming units (T1). The killing percentage was calculated by [1–(T1/T0)] × 100. For the toxin neutralization assay, different concentrations of Hla (Sigma-Aldrich) were incubated with diluted serum (1:5) for 30 minutes and then for another 1 hour at 37 °C after adding purified neutrophils. Mouse and human monocytes from the blood were isolated using PolymorphPrep and used as controls for the toxin neutralization assay. Neutrophils and monocytes were stained with Sytox for cell viability, and analysis by flow cytometry using the gating strategy in Supplementary Figure S6 was performed.
Statistical analysis
Data were analyzed using GraphPad Prism software. Data were tested for normal distribution with the D’Agostino–Pearson normality test. Normally distributed data of multiple groups were compared using one-way ANOVA with Tukey’s post-hoc test. Data that were not normally distributed were analyzed using Mann–Whitney U test or Kruskal–Wallis test with Dunn’s post-hoc test. The cutaneous lesion sizes over time were compared using two-way ANOVA with repeated measures followed by Tukey’s test. Differences were considered significant when P-values were < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Comparative Pathology and Mouse Phenotyping Shared Resource of The Ohio State University (supported in part by National Cancer Institute grant P30 CA016058 for performing the immunohistochemical staining). We thank Tim Vojt, medical illustrator for The Ohio State University College of Veterinary Medicine, for assistance with figure compilation. This work was supported by the National Institutes of Health grant R01-AI125489. JDDRR, FHRA, and SPS were supported by the Cystic Fibrosis Foundation grant PARTID18P0.
Abbreviations:
- Fab
antigen-binding fragment
- dai
day after infection
- hai
hour after infection
- Hla
α-hemolysin
- SSTI
skin and soft tissue infection
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2020.09.001.
Data availability statement
No datasets were generated or analyzed during this study.
REFERENCES
- Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 2014;15:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008;205:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis 2016;29:109–15. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013;309:1368–78. [DOI] [PubMed] [Google Scholar]
- Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013;56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010;202:1050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Granick JL, Kwok C, Walker NJ, Borjesson DL, Curry F-RE, et al. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood 2011;117:3343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 2015;185:1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia [published correction appears in J Infect Dis 2008;198:1725]. J Infect Dis 2008;198:561–70. [DOI] [PubMed] [Google Scholar]
- Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 2014;82:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery CP, Daum RS. Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: a role for panton-valentine leukocidin? Infect Immun 2009;77:2159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus α-hemolysin. J Infect Dis 2012;206:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp ME, Holley HP Jr, Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, et al. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007;51:4249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro GR. Staphylococcus aureus α-toxin damages the tissue microenvironment during skin and soft tissue infection. Dissertation. The University of Chicago; 2018. [Google Scholar]
- Seilie ES, Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol 2017;72:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Zhao F, Beesetty P, Weiskopf D, Li Z, Tian Q, et al. Inhibition of protective immunity against Staphylococcus aureus infection by MHC-restricted immunodominance is overcome by vaccination. Sci Adv 2020;6:eaaw7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoutounour S, Guilliams M, Montanana Sanchis FM, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 2013;39:925–38. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chong AS, Montgomery CP. Importance of B lymphocytes and the IgG-Binding protein Sbi in Staphylococcus aureus skin infection. Pathogens 2016;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during this study.