Abstract
Alterations in signalling due to bidirectional transactivation of G protein-coupled receptor (GPCRs) and receptor tyrosine kinases (RTKs) are well established. Transactivation significantly diversifies signalling networks within a cell and has been implicated in promoting both advantageous and disadvantageous physiological and pathophysiological outcomes, making the GPCR/RTK interactions attractive new targets for drug discovery programmes. Transactivation has been observed for a plethora of receptor pairings in multiple cell types; however, the precise molecular mechanisms and signalling effectors involved can vary with receptor pairings and cell type. This short review will discuss the recent applications of proximity-based assays, such as resonance energy transfer and fluorescence-based imaging in investigating the dynamics of GPCR/RTK complex formation, subsequent effector protein recruitment and the cellular locations of complexes in living cells.
Keywords: G protein-coupled receptor, Receptor tyrosine kinase, Transactivation, Resonance energy transfer, Endocytosis, Oligomeric complexes
Abbreviations: 5-hydroxytryptamine receptor 1A, (5-HT1A); adrenoceptors, (AR); bioluminescence resonance energy transfer, (BRET); cannabinoid receptor 2, (CB2R); disintegrin and metalloproteinases, (ADAMs); epidermal growth factor, (EGF); epidermal growth factor receptor, (EGFR); fibroblast growth factor receptor, (FGFR); fluorescence correlation spectroscopy, (FCS); formyl peptide receptor, (FPR); Förster Resonance Energy Transfer, (FRET); free fatty acid, (FFA); G protein-coupled receptors, (GPCRs); GPCR kinases, (GRKs); heparin binding EGF, (Hb-EGF); hepatocyte growth factor, (HGF); human umbilical vein endothelial cells, (HUVECs); insulin growth factor receptor-1, (IGFR-1); insulin receptor, (IR); lysophosphatidic acid receptor 1, (LPA); matrix metalloproteinases, (MMPs); platelet-derived growth factor receptor, (PDGFR); proximity ligation assay, (PLA); reactive oxygen species, (ROS); receptor tyrosine kinases, (RTKs); sphingosine-1-phosphate receptor, (S1PR); tetrahydrocannabinol, (THC); total internal reflection fluorescence microscopy, (TIRF-M); vascular endothelial growth factor, (VEGF); vascular endothelial growth factor receptor 2, (VEGFR2); vasopressin 2 receptor, (V2R)
Transactivation: a mechanism to increase the signalling diversity of activated G protein-coupled receptors and receptor tyrosine kinases
G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) are major classes of cell surface receptors extensively targeted in drug discovery programmes due to their critical roles in health and disease. GPCRs are seven transmembrane spanning receptors that bind a structurally diverse range of ligands [1]. Activation stabilises GPCR conformations favouring downstream signalling via heterotrimeric G proteins (Gα and Gβγ subunits). Four main classes of G proteins exist: Gs, Gi/o, Gq/11, G12/13 that direct signalling via distinct effector proteins such as adenylyl cyclase, phospholipase C and Rho GTPases. GPCR activation also promotes the recruitment of GPCR kinases (GRKs) that phosphorylate the GPCR C terminus. This in turn enhances recruitment of β-arrestin which uncouples GPCR/G protein complexes, promoting GPCR desensitisation and endocytosis in addition to G protein-independent signalling pathways; however, the functional significance of this in physiology has been debated [2]. RTKs typically consist of a large extracellular ligand binding domain, a transmembrane domain and an intracellular catalytic kinase domain. RTKs notably bind growth factors such as epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF). Ligand binding typically induces dimerisation of receptor monomers triggering trans-autophosphorylation of C terminal tyrosine residues that act as recruitment sites for intracellular adaptor proteins, such as Src, phosphoinositide 3-kinases (PI3K) and phospholipase C (PLC). These adaptors can themselves be phosphorylated due to the intrinsic kinase activity of RTKs, increasing and diversifying the network of signalling pathways available from activation of a single receptor. RTK-mediated signalling is typically responsible for driving cell proliferation, migration and survival via extracellular signal-regulated kinases 1/2 (ERK1/2), focal adhesion kinase (FAK) and protein kinase A/Akt mediators [3].
GPCRs and RTKs were believed to act as independent signalling entities, until seminal work by Ullrich and colleagues [4] revealed rapid tyrosine phosphorylation of the epidermal growth factor receptor (EGFR) in response to known GPCR agonists. This phenomenon, termed transactivation, is characterized by altered RTK activation and downstream signalling directly attributable to GPCR/RTK interactions. Transactivation offers a mechanism to increase the number and breadth of signalling networks available within each cell, by integrating the diversity of GPCRs and GPCR ligands with the vast signalling networks mediated by activated RTKs [5].
Transactivation of the EGFR has been observed with Class A and Class B GPCR partners including but not limited to the β1, β2 and α1-adrenoceptors (AR), adenosine A1 and A3 receptors, μ opioid receptor, muscarinic M1 and the AT1R angiotensin receptor [reviewed in 6]. Evidence of transactivation has been observed in a range of cell types for other RTK family members, such as the vascular endothelial growth factor receptors (VEGFRs), fibroblast growth factor receptors (FGFRs), platelet-derived growth factor receptor (PDGFR), insulin-like growth factor receptor-1 (IGFR-1) and the insulin receptor (IR) [7]. Recent reviews have extensively covered the beneficial roles of transactivation in regulating cardioprotection [6] and vital central nervous system functions [3]. However, disadvantageous signalling as a consequence of transactivation has been identified such as progression from acute to chronic pain (μ opioid/EGFR in opioid-induced hyperalgesia [8]), proliferation of human hyperplastic prostatic cells (α1-AR/EGFR [9]), gastric cancer cell migration (CXCR4/EGFR [10]), poor patient prognosis and increased lymphatic spread in HER2+ breast cancer patients (cannabinoid receptor 2 (CB2R/HER2 [11]) and underlying tumour re-occurrence following anti-VEGF/VEGFR2 therapeutics (sphingosine 1-phosphate receptor/VEGFR2 [12]). In this short review, we focus on recent examples revealing new insights into the molecular mechanisms involved, and highlight some of the new technologies, beyond traditional biochemical techniques, used to investigate transactivation.
Ligand-dependent and independent mechanisms of transactivation
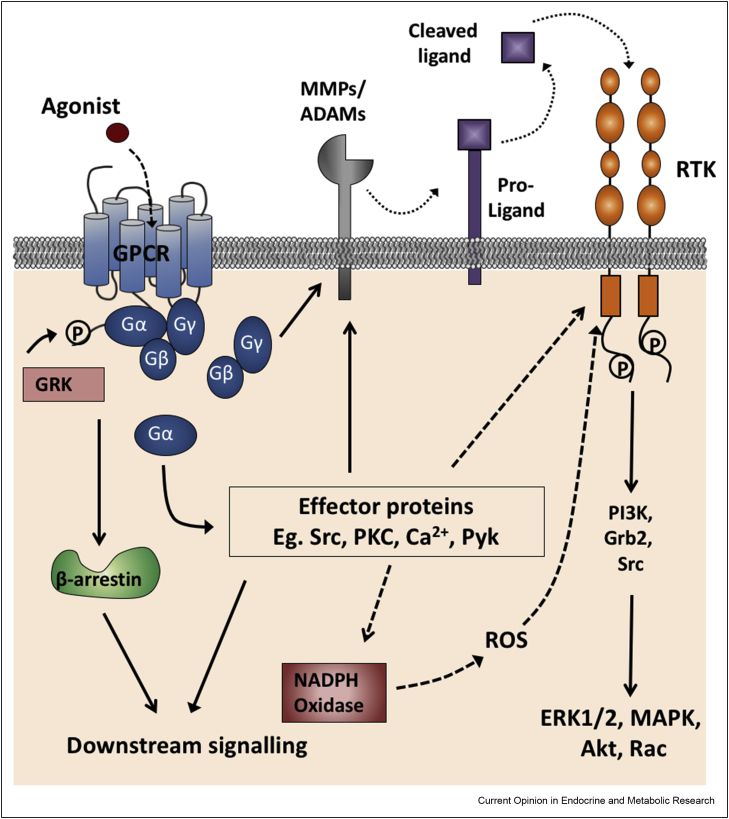
RTKs can be activated by GPCRs in a ligand-dependent or independent manner (Figure 1). Ligand-dependent transactivation occurs via matrix metalloproteinases (MMPs) or a disintegrin and metalloproteinases (ADAMs) and has been extensively characterised for the EGFR [5]. MMPs or ADAMs cleave RTK pro-ligands bound to extracellular matrix components such as heparin binding EGF (Hb-EGF). These cleaved ligands then bind to cell surface RTKs triggering downstream signalling. Activation of MMPs or ADAMs occurs as a consequence of GPCR activation; however, the exact mechanisms are not fully known but are proposed to involve Gβγ subunits [13] or Src [14, 15, 16].
Figure 1.
Ligand-dependent and independent transactivation mechanisms. GPCRs are seven transmembrane spanning receptors that are activated by agonist binding. This stabilises GPCR conformations favouring the activation and subsequent dissociation of heterotrimeric G proteins (Gα and Gβγ subunits). Gα can mediate signalling via effector proteins such as Src and PKC. In ligand-dependent transactivation, effector proteins activated by GPCR signalling such as Src can themselves induce the activation of matrix metalloproteinases (MMPs) or a disintegrin and metalloproteinases (ADAMs). Evidence also exists suggesting Gβγ subunits may activate MMPs. MMPs and ADAMs cleave pro-forms of RTK ligands that are bound to components of the extracellular matrix (dotted lines). These pro-ligands are then free to diffuse to bind to their cognate RTK. A stylised version of a RTK structure is shown here. RTK ligand binding activates the receptor, triggering monomer dimerisation, auto-transphosphorylation and subsequent downstream signalling pathways. Transactivation can also occur through ligand-independent mechanisms (dashed arrows). Effector proteins activated following GPCR activation, such as Src, PKC and Pyk can directly activate RTKs via phosphorylation of tyrosine residues in the C terminus. Additionally secondary messenger molecules generated via effector protein mediated signalling (e.g. reactive oxygen species (ROS) produced by NADPH oxidase) can also mediate direct activation of RTKs.
As GPCRs lack intrinsic tyrosine kinase activity, ligand-bound GPCRs indirectly activate RTKs via intracellular protein kinases such as Src, PI3K and Pyk [3]. These effector proteins directly induce RTK activation via phosphorylation of tyrosine or serine/threonine residues. For example, Src-mediated phosphorylation of EGFR has been observed following activation of the corticotropin releasing factor receptor 1 (CRF1R) with this transactivation critical for CRF stimulated ERK1/2 signalling [17]. Mediators can also play simultaneous roles in direct RTK activation and transactivation (e.g. Src at CXCR4 and EGFR [15]).
Another mechanism of transactivation is via production of second messengers, notably reactive oxygen species (ROS). NADPH Oxidase produced ROS has been shown to mediate transactivation between formyl peptide receptor 1 (FPR1) with VEGFR2 [18], EGFR [19] and TrkA [20] as well as the formyl peptide receptor 2 (FPR2) with HGF [21]. The multiple RTKs activated by ROS have led to speculation that ROS may be a mechanism for global transactivation [5] supported by recent evidence of ROS-mediated dual transactivation of EGFR and HER2 by neurotensin 1 receptors [22].
Though currently less extensively observed, transactivation can be bidirectional. For example, the lysophosphatidic acid receptor 1 (LPA) and EGFR can reciprocally transactivate and induce proliferation of prostate cancer cells [23]. However, this same positive crosstalk can be suppressed by activation of another GPCR, the free fatty acid receptor (FFA4 [23]). Formation of RTK/GPCR complexes can also alter effector protein coupling to the GPCR partner as seen for the CB2R. In response to tetrahydrocannabinol (THC), the CB2R typically couples to Gq/11; however, when complexed with HER2, CB2R coupling switches to Gi or Gz subtypes [11]. This suggests HER2/CB2R is a unique pharmacological entity compared to CB2R that promotes pro-tumoural signalling. RTK-mediated GPCR transactivation is typically more complex than GPCR/RTK and can include tyrosine phosphorylation of GPCRs and GRKs by RTKs or modulation of GPCR serine/threonine phosphorylation by protein kinases (reviewed in Ref. [24]). A recent example of this is internalised EGFR (induced by EGF), which can indirectly mediate inhibition of dopamine D3 receptor signalling by promoting tyrosine phosphorylation of GRK2, subsequently inhibiting D3 signalling, endocytosis and degradation [25]. To add further complexity, different RTKs can induce phosphorylation of the same GPCR but at differing residues (in this instance tyrosines), as observed at the β2-AR following ligand activation of the IR [26] or IGF-1R [27].
Challenges remain in unravelling signalling directly attributable to transactivation alone, complicated by RTKs also utilising ‘classical’ GPCR signalling mediators such as G proteins, β-arrestins and GRKs (reviewed in Ref. [7]). For example, Gαi is critical to VEGFR2 clathrin-mediated endocytosis, with knockout of Gαi retaining VEGFR2 at the cell surface and decreasing downstream VEGFR2 driven signalling [28]. Gβγ, in conjunction with Src, is implicated in regulating EGFR endocytosis, and mediating interaction of internalised EGFR/Src/GRK complexes [25]. GRKs can also directly regulate RTK-driven signalling (seen for the IGF-1R), with different GRK subtypes exhibiting opposing effects at the same receptor [29], potentially by modulating changes in the lifespan of β-arrestin association. Interestingly, following transactivation (Src and MMP dependent) of the IGF-1R by the vasopressin 2 receptor (V2R), it is the engagement of β-arrestin with IGF-1R and not V2R that is critical for vasopressin stimulated ERK1/2 signalling [16], with suggestions that RTK/β-arrestin interactions may be applicable to other GPCRs.
Many signalling effectors, such as Src, PI3K, ERK1/2 and MAPK can act as convergence points for multiple signalling pathways, including those that are GPCR or RTK mediated, making it more difficult to tease out signalling events directly attributable to transactivation. RTK inhibitors, such as AG1478 (EGFR), have been useful in ‘silencing’ the RTK component of transactivation; however, they often lack selectivity. Transactivation has largely been confirmed using indirect biochemical measures of signalling pathways (e.g. phosphorylation ERK1/2) at endogenous unmodified receptors. Although, they often lack dynamic, temporal or spatial resolution, these readouts can still reveal static spatial detail such as differential subcellular ERK1/2 and Akt activation in fractionated mice hearts and cardiomyocytes as a result of isoprenaline-induced βAR-mediated EGFR transactivation [30].
The use of resonance energy transfer techniques to measure the real-time recruitment of adaptor proteins in transactivation
There remains a need to quantify the real-time location and dynamics of transactivation specific signalling. Proximity-based techniques such as bioluminescence resonance energy transfer (BRET) or Förster Resonance Energy Transfer (FRET) offer exquisite spatial and temporal sensitivity for investigating protein–protein interactions in live cells due to the need for close proximity of donor/acceptor pairings (within 10 nm of each other) [31]. The use of a FRET-based biosensor illustrated the importance of phosphorylation by PI3K in regulating Src activity during transactivation of β2-AR/EGFR [32]. Furthermore, a BRET-based assay has highlighted the complexity of AT1R transactivation of insulin receptors as the protein kinase (ERK1/2 vs. PKC) mediator was found to differ between insulin receptor substrates [33]. BRET has also investigated the real-time kinetics of fluorescently tagged β-arrestin2 recruitment to β2-AR/IR complexes in response to isoprenaline [34] and at NanoLuc tagged β2-AR in the presence of VEGFR2 (Figure 2A [35]). The profile of β-arrestin2 recruitment to β2-AR was altered following agonist co-stimulation when compared to β2-AR agonist alone and required the presence of activated VEGFR2 (Figure 3A). In both cases β-arrestin was only seen with GPCR stimulation [34,35]. However Grb2 recruitment to AT1R/EGFR complexes measured using BRET revealed different extents depending on which receptor partner was activated, with rapid recruitment seen with EGF and only partial recruitment with the AT1R agonist angiotensin II [36]. Grb2 recruitment to AT1R/EGFR complexes in these cells (HEK293T) was shown to be independent of Gq/11 or β-arrestin, whereas previous observations in COS-7 cells or ventricular cardiomyocytes showed dependence on Gq/11 activation for AT1R/EGFR mediated hypertrophy [37]. These discrepancies may reflect differences in proximal (direct effector protein recruitment) versus indirect (e.g. downstream pathway activation) measures of transactivation [36].
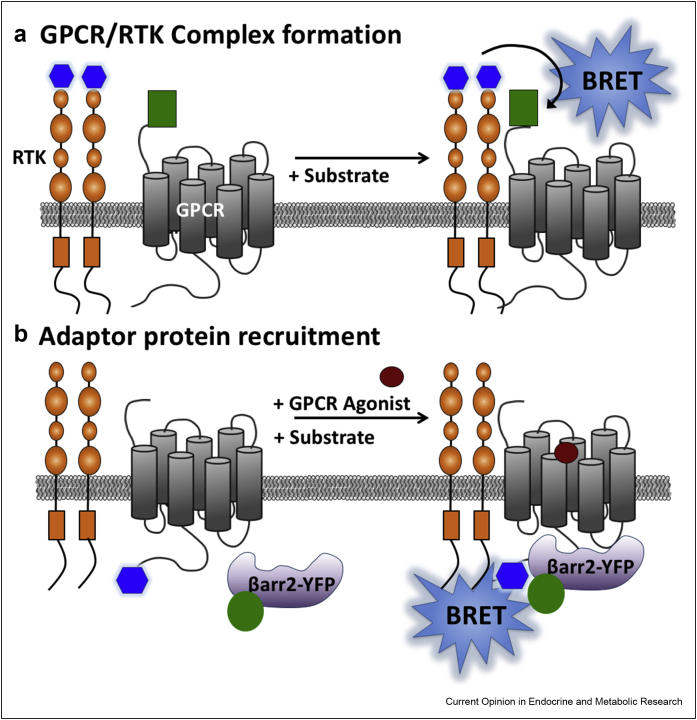
Figure 2.
Using bioluminescence resonance energy transfer to investigate GPCR/RTK complex formation and adaptor protein recruitment. RTKs can be tagged at their N terminus with a luminescent protein (e.g. NanoLuc; termed the ‘donor’; blue hexagon) at both monomers, whereas a fluorescent tag (e.g. ‘SnapTag’ termed the ‘acceptor’; green rectangle) can be attached to the N terminus of a GPCR (a). The substrate for the luminescent protein is then oxidised, producing energy in the form of photons. If donor and acceptor tagged receptors are in sufficiently close proximity (<10 nm), non-radiative transfer of this energy occurs to excite the acceptor fluorophore. The ratio of fluorescence and luminescence emissions allows a BRET ratio to be determined. BRET can also be used to investigate adaptor protein recruitment to a GPCR/RTK complex (b), for example, using a GPCR tagged at its C terminus with a luminescence protein (blue hexagon) and a fluorescently tagged adaptor protein (in this case β-arrestin2-YFP; green circle).
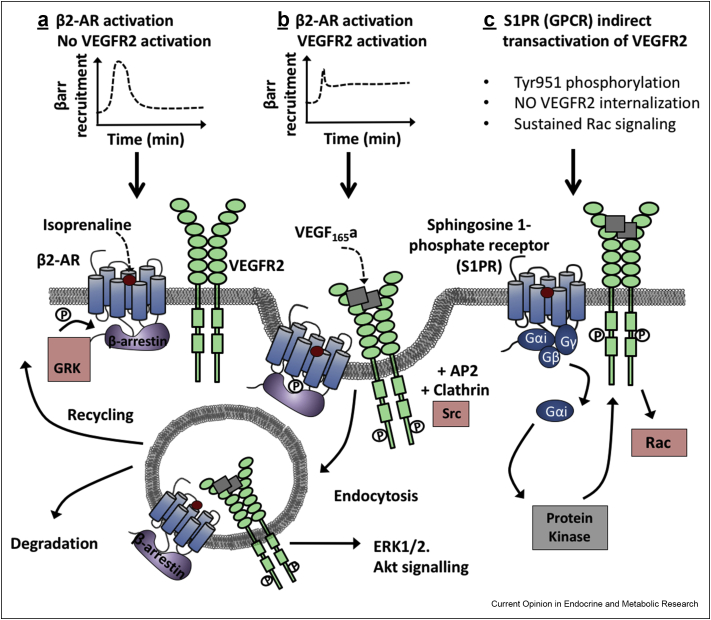
Figure 3.
Potential effects of transactivation of GPCR and vascular endothelial growth factor receptor 2 (VEGFR2) on effector protein recruitment or endocytosis. Schematics (a) and (b) are based on findings detailed in Ref. [35] using BRET to measure β-arrestin2 recruitment to β2-adrenoceptor when co-expressed with the vascular endothelial growth factor receptor 2 (VEGFR2). β2-Adrenoceptors were stimulated with the agonist isoprenaline, resulting in GRK phosphorylation of the β2-adrenoceptor C terminus and subsequent rapid recruitment of β-arrestin2, which then subsided within minutes (a). However co-stimulated with isoprenaline and the VEGFR2 prototypical agonist VEGF165a altered the profile of β-arrestin2 recruitment (b). Although peak responses were truncated and then partially dropped, BRET signals did not return to baseline. This suggested that the presence of ligand-activated VEGFR2 lead to sustained β-arrestin2 coupling to β2-adrenoceptor. These data also reconciled with observations also seen in Ref. [35] that β2-adrenoceptors and VEGFR2 co-internalise into the same Rab5+ endosomal compartments following stimulation with either receptor agonist. This has the potential for modulation of signalling, in respect to pathway activation, kinetics of signalling or intracellular fate of receptors (recycling or degradation) when compared to endocytosis of either receptor alone. GPCRs have also been shown to indirectly modulate cell surface expression and endocytosis of RTKs resulting in altered signalling outcomes. The example depicted here is derived from data in Ref. [12] (c). Ligand-induced activation of a GPCR leads to Gαi-mediated activation of intracellular protein kinases, which can then directly phosphorylate specific tyrosine residues on the C terminus of the RTK. The example depicted here is in respect to sphingosine 1 phosphate receptor (S1P1 R)-mediated regulation of VEGFR2 endocytosis and involves the intracellular protein kinase c-Abl, phosphorylating VEGFR2 at tyrosine residue 951 (as opposed to the prototypical activation residue of Tyr1175) ultimately inhibiting VEGFR2 endocytosis. This leads to sustained VEGFR2-mediated Rac signalling that drives endothelial cell proliferation and enhances tumour growth.
However, it is also becoming increasingly clear that transactivation mechanisms may differ between cell types due to changes in the expression levels or repertoire of signalling components present. Functional genomic approaches (at AT1R/EGFR complexes in HMEC-LST cells [38]) and DNA microarray gene expression studies (α2B-AR in vascular smooth muscle cells [39]) have begun to provide new unbiased methods for identifying mediators involved.
Physical complex formation between GPCRs and RTKs
The formation of oligomeric complexes between GPCRs and RTKs is now accepted as a regulator of transactivation [2]. These complexes may represent a mechanism to localise signalling components together to increase the efficiency of transactivation and resultant downstream signalling. Discrete complexes also raise the potential for cooperativity across putative GPCR/RTK interfaces; however, evidence for this is still largely speculative. Observations of complex formation have largely been derived from co-immunoprecipitation assays, which cannot definitively confirm physical complexes, their cellular location or lifespan. In contrast, the exquisite spatial sensitivity and dynamism of BRET and FRET techniques have recently been used to investigate GPCR or RTK oligomerisation in real time (reviewed in Ref. [31], summarised in Table 1).
Table 1.
Summary of GPCR/RTK complexes detected using fluorescence- or luminescence-based techniques.
| GPCR | RTK | Cell type | Technique used | Reference |
|---|---|---|---|---|
| 5-HT1A | FGFR1 | HEK293 cells (FRET, PLA), rat dorsal and median raphe nuclei (PLA) | FRET, PLA | [40] |
| 5-HT1A | FGFR1 | HEK293T cells, rat hippocampal cultures | PLA | [41] |
| 5-HT1A | FGFR1 | Rat brain dorsal hippocampus (astrocytes) | PLA | [45] |
| M1 | FGFR1 | Rat hippocampus and cerebral cortex | PLA | [46] |
| TSHR | IGF-1R | Graves orbital fibroblasts | PLA | [47] |
| β2-AR | IR | HEK293T cells | BRET | [42] |
| β2-AR | EGFR | HEK293T cells | FRET | [32] |
| AT1R | EGFR | HEK293T cells, CHO K1 cells, NIH-3T3, primary vascular smooth muscle cells | BRET | [36] |
| β2-AR | VEGFR2 | HEK293 cells, HUVECs | BRET | [35] |
| Adenosine A2A | FGFR1 | HEK293T cells | BRET | [43] |
| CB2R | HER2 | HEK293T cells (BRET, bimolecular fluorescence complementation), Her2+ breast cancer patient biopsies (PLA) |
BRET, PLA, bimolecular fluorescence complementation | [11] |
BRET = bioluminescence resonance energy transfer.
FRET = fluorescence resonance energy transfer.
PLA = proximity ligation assay.
FRET has confirmed the formation of 5-hydroxytryptamine receptor 1 A (5-HT1A; GPCR) complexes with FGFR1 [40] supporting physiological evidence for these complexes and their role in neuronal plasticity [41]. BRET studies have also revealed the formation of heteromeric complexes between the β2-AR and IR that could underlie the counter-regulatory effects of insulin and catecholamines in glucose metabolism [42]. FRET has also shown isoprenaline-induced dissociation of β2-AR/EGFR complexes which internalise to distinct endocytic compartments [32]. Constitutive and dynamic agonist-induced complexes of AT1R/EGFR [36] and β2-AR/VEGFR2 [35] have also been revealed using BRET. β2-AR/VEGFR2 complexes, as measured by BRET, were also observed with endogenously expressed β2-AR (using CRISPR/Cas9 gene edited HEK293T cells) and in human umbilical vein endothelial cells (HUVECs [35]). Interestingly significantly increased BRET was observed between adenosine A2A and FGF1 following concomitant agonist stimulation [43], consistent with previous biochemical observations in PC12 adrenal medulla cells where synergistic ERK1/2 phosphorylation was only observed with dual activation of A2A and FGFR-1 [44]. Dissociation of complexes upon GPCR stimulation has also been observed with BRET studies of CB2R/HER2 in response to THC [11].
A disadvantage of RET-based studies is they cannot necessarily show the cellular location of GPCR/RTK complexes. Fluorescence imaging of co-localised GPCRs with RTKs has been limited by the paucity of selective antibodies for GPCR subtypes. The use of proximity ligation assays (PLA) has circumvented this in some ways with notable recent observations of endogenous heterocomplexes of 5-HT1A/FGFR-1 in rat hippocampal pyramidal neurons [40] and rat hippocampal astrocytes [45], muscarinic acetylcholine receptor 1 (M1)/FGFR1 complexes in hippocampal neurons [46], constitutive thyroid stimulating hormone receptor (TSHR) and IGF-1R in Graves orbital fibroblasts [47] and CB2R/HER2 complexes in HER2+ breast cancer patient biopsies [11]. Although PLA can provide improved spatial resolution, it is limited to use with fixed permeabilized cells and cannot reveal real-time changes. The use of genetically encoded fluorescent protein tags (e.g. GFP), or exogenously labelled tags (e.g. SnapTag or HaloTag) has allowed cellular co-localisation of GPCR/RTKs to be visualised both in absence or presence of ligands [35]. Questions remain as to whether changes in endocytosis of one partner may modulate transactivation. Stimulation with insulin can induce insulin receptor mediated internalisation of the β2-AR [42]. Insulin, acting via the IR, has been shown to stimulate internalisation of the β2-AR via IR-mediated phosphorylation of specific tyrosine residues in the β2-AR C terminus enhancing association with endocytosis components such as Grb2 [48]. Similarly, the β1 agonist dobutamine has been shown to induce partial internalisation of EGFR (β1-AR/EGFR [14]). The S1PR is able to promote VEGFR2 angiogenic signalling by regulating selective tyrosine phosphorylation of VEGFR2 via Gαi activation of the protein kinase c-Abl. Interestingly this retains VEGFR2 at the cell surface altering the kinetics of VEGFR2 driven Rac signalling from a transient to sustained profile. This results in increased migration of tumour-associated endothelial cells and ultimately tumour angiogenesis [12; Figure 3C]. Dual labelling of β2-AR/VEGFR2 revealed constitutive cell surface co-localisation [35]. Stimulation with receptor selective ligands resulted in co-endocytosis into early endosomal compartments which co-localised with immunolabelled Rab5 endosomes and reconciled with BRET data showing altered and sustained β-arrestin2 recruitment at these complexes (Figure 3B). This is interesting in light of the increasing appreciation of the importance of endosomal signalling in the spatiotemporal control of signalling for GPCRs [49] and RTKs [50].
RET and imaging studies have mostly used model cell systems due to the need to modify receptors with luminescent or fluorescent labels, which risk artefacts of receptor overexpression, although future use with CRISPR/Cas9 may mitigate this. In endogenous systems the extent of transactivation may be dependent on expression levels of each partner; however, interestingly BRET studies of angiotensin II-induced transactivation of EGFR by AT1R in HEK293T cells using overexpressed receptors revealed transactivation only represented a subset of the total signalling capacity of EGFR (estimated at ∼20% [36]). GPCR/RTK complex formation may therefore represent a subset of RTKs arranged in membrane microdomains or within intracellular compartments that facilitate close proximity and the formation of discrete complexes with their partner GPCR. Advanced imaging techniques with single cell/receptor sensitivity such as fluorescence correlation spectroscopy (FCS) and total internal reflection fluorescence microscopy (TIRF-M) have illustrated that receptors are not homogenously expressed on the surface of cells, but are within discrete membrane regions [51]. This localises components of signalling within microdomains, bringing different signalling mediators into close proximity, facilitating greater efficiency of receptor/effector coupling. These specialised microdomains termed ‘lipid rafts’ are linked to the actin cytoskeleton [52]. Signalling as a consequence of transactivation of CBR1/FGFR1 complexes has been shown to emanate from lipid rafts in embryonic cortical neurons [53]. As many GPCRs and RTKs are known to localise to caveolin containing lipid rafts [54], it is likely that other GPCR/RTK complexes may also exist here.
Unravelling GPCR/RTK complex formation is further complicated by RTK heterodimerisation. Recent FRET studies have indicated that RTK homo and heterodimers have similar strength of interactions, highlighting the potential influence that RTK heterodimer may have upon transactivation [55]. The increasing acceptance of GPCR homo and heteromerisation (albeit likely to be relatively transient) may also further complicate understanding of transactivation signalling networks [56]. GPCR/RTK complexes may also be components of larger macromolecular complexes containing other membrane bound proteins such as integrins, extracellular matrix glycoproteins and co-receptors (e.g. Neuropilin-1 for VEGFR2 [57]). Investigation of the influence of these proteins on GPCR/RTK complex formation, organisation, lifetime and signalling is still in its infancy. The altered signalling seen with GPCR/RTK transactivation suggests that co-targeting of GPCR/RTK macromolecular complexes may represent new therapeutic avenues; wholesale inhibition of RTK signalling can often result in considerable off-target effects due to the integral role RTKs play in physiological processes. The use of lower concentrations of RTK inhibitors in conjunction with ‘trans-inhibition’ of GPCR partners may provide a mechanism to modulate RTK-driven signalling to overcome some of these off-target issues.
Conclusion
GPCRs have been shown to exploit the intrinsic kinase activity and vast signalling networks available to RTKs, whereas proteins previously defined as ‘GPCR signalling mediators’ are now known to also be integral signalling partners for RTKs (e.g. G proteins, β-arrestins). This bidirectional transactivation between GPCRs and RTKs allows integration of signalling inputs to increase the number and diversity of signalling outcomes available. The advancement of fluorescence- and luminescence-based techniques has allowed the identification of GPCR/RTK complexes whose dynamics, localisation and distinct pharmacological profiles can be quantified in real time. Studies of cooperativity across GPCR and RTK interfaces are still relatively understudied; however, advancements in techniques that offer increased real-time spatial and temporal resolution will allow this phenomenon to be teased apart from signalling crosstalk and may open up new opportunities to co-target GPCR/RTK complexes in drug discovery.
Conflict of interest statement
Nothing declared.
Acknowledgements
This project was funded by the Medical Research Council [grant number MR/N020081/1] and the Centre of Membrane Proteins and Receptors (COMPARE). LEK holds an Anne McClaren Fellowship from the University of Nottingham.
This review comes from a themed issue on G protein-coupled receptors (GPCRs)
Edited by Aylin Hanyaloglu and Eric Reiter
Contributor Information
Laura E. Kilpatrick, Email: laura.kilpatrick@nottingham.ac.uk.
Stephen J. Hill, Email: steve.hill@nottingham.ac.uk.
References
- 1.Hilger D., Masureel M., Kobilka B.K. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol. 2018;25:4–12. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurevich V.V., Gurevich E.V. Arrestin mediated signaling: is there a controversy? World J Biol Chem. 2018;9:25–35. doi: 10.4331/wjbc.v9.i3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Liberto V., Mudò G., Belluardo N. Crosstalk between receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCR) in the brain: focus on heteroreceptor complexes and related functional neurotrophic effects. Neuropharmacology. 2019;152:67–77. doi: 10.1016/j.neuropharm.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Daub H., Weiss F.U., Wallasch C., Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo F., Guerra G., Parisi M. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci. 2014;15:19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grisanti L.A., Guo S., Tilley D.G. Cardiac GPCR-mediated EGFR transactivation: impact and therapeutic implications. J Cardiovasc Pharmacol. 2017;70:3–9. doi: 10.1097/FJC.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crudden C., Shibano T., Song D., Suleymanova N., Girnita A., Girnita L. Blurring boundaries: receptor tyrosine kinases as functional G protein-coupled receptors. Int Rev Cell Mol Biol. 2018;339:1–40. doi: 10.1016/bs.ircmb.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Araldi D., Ferrari L.F., Levine J.D. Role of GPCR (mu-opioid)-receptor tyrosine kinase (epidermal growth factor) crosstalk in opioid-induced hyperalgesic priming (type II) Pain. 2018;159:864–875. doi: 10.1097/j.pain.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nascimento-Viana J.B., Alcántara-Hernández R., Oliveira-Barros E. The α1-adrenoceptor-mediated human hyperplastic prostate cells proliferation is impaired by EGF receptor inhibition. Life Sci. 2019;239:117048. doi: 10.1016/j.lfs.2019.117048. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y., Qu J., Che X. CXCL12/SDF-1α induces migration via SRC-mediated CXCR4-EGFR cross-talk in gastric cancer cells. Oncol Lett. 2017;14:2103–2110. doi: 10.3892/ol.2017.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasco-Benito S., Moreno E., Seijo-Vila M. Therapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancer [published correction appears in Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6505] Proc Natl Acad Sci U S A. 2019;116:3863–3872. doi: 10.1073/pnas.1815034116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper used PLA to show endogenous HER2/CB2R complexes in HER2+ breast cancer patient biopsies that correlate with poor patient prognoses. The authors elegantly used BRET to investigate the molecular mechanisms regulating these complexes and how they underlie pro-tumoral signalling outcomes. These data showed that in vitro and in vivo, HER2/CB2R complexes decreased following incubation with tetrahydrocannabinol. This was due to phosphorylation of HER2 at a specific tyrosine residue (Tyr1248) that disrupted HER2 homodimerisation, complex formation with CB2R and promoted HER2 degradation. This paper also showed how GPCR effector protein coupling can alter with RTK complex formation; CB2R G protein coupling in response to THC switched from Gq/11 coupling to Gi and Gz in the presence of HER2. This suggested HER2/CB2R were unique signalling entities that promote signalling pathways beneficial to tumors whereas CB2R directed signalling which favours anti tumoral pathways.
- 12.Balaji Ragunathrao V.A., Anwar M., Akhter M.Z. Sphingosine-1-Phosphate receptor 1 activity promotes tumor growth by amplifying VEGF-VEGFR2 angiogenic signaling. Cell Rep. 2019;29:3472–3487. doi: 10.1016/j.celrep.2019.11.036. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication is a great illustration of how the cellular location of RTKs can influence their signalling and that RTK localisation can be indirectly modulated by GPCRs. Activation of Gαi following ligand binding to a GPCR (the sphingosine 1 phosphate) activates the intracellular protein kinase c-Abl. Differential phosphorylation of VEGFR2 by c-Abl at Tyr951 (as opposed to at the prototypical activation residue of Tyr1175), prevents VEGFR2 endocytosis. Stimulation of cell surface pTyr951 VEGFR2 with VEGF results in altered signalling to that observed for internalized VEGFR2 most notably a switch in the kinetics of Rac signalling from a transient profile to sustained signalling for pTyr951 VEGFR2. A schematic summarising these results is seen in Figure 3C.
- 13.Overland A.C., Insel P.A. Heterotrimeric G proteins directly regulate MMP14/membrane type-1 matrix metalloprotease: a novel mechanism for GPCR-EGFR transactivation. J Biol Chem. 2015;290:9941–9947. doi: 10.1074/jbc.C115.647073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noma T., Lemaire A., Naga Prasad S.V. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasina S., Scherle P.A., Hall C.L., Macoska J.A. ADAM-mediated amphiregulin shedding and EGFR transactivation. Cell Prolif. 2009;42:799–812. doi: 10.1111/j.1365-2184.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oligny-Longpré G., Corbani M., Zhou J., Hogue M., Guillon G., Bouvier M. Engagement of β-arrestin by transactivated insulin-like growth factor receptor is needed for V2 vasopressin receptor-stimulated ERK1/2 activation. Proc Natl Acad Sci Unit States Am. 2012;109:6374–6375. doi: 10.1073/pnas.1112422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parra-Mercado G.K., Fuentes-Gonzalez A.M., Hernandez-Aranda J., Diaz-Coranguez M., Dautzenberg F.M., Catt K.J., Hauger R.L., Olivares-Reyes J.A. CRF1 receptor signaling via the ERK1/2-MAP and Akt kinase cascades: roles of Src, EGF receptor, and PI3-kinase mechanisms. Front Endocrinol. 2019;10:869. doi: 10.3389/fendo.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattaneo F., Castaldo M., Parisi M., Faraonio R., Esposito G., Ammendola R. Formyl peptide receptor 1 modulates endothelial cell functions by NADPH oxidase-dependent VEGFR2 transactivation. Oxid Med Cell Longev. 2018;2018:2609847. doi: 10.1155/2018/2609847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaneo F., Iaccio A., Guerra G., Montagnani S., Ammendola R. NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic Biol Med. 2011;51:1126–1136. doi: 10.1016/j.freeradbiomed.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Castaldo M., Zollo C., Esposito G., Ammendola R., Cattaneo F. NOX2-Dependent reactive oxygen species regulate formyl-peptide receptor 1-mediated TrkA transactivation in SH-SY5Y cells. Oxid Med Cell Longev. 2019;2019:2051235. doi: 10.1155/2019/2051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattaneo F., Parisi M., Ammendola R. WKYMVm-induced cross-talk between FPR2 and HGF receptor in human prostate epithelial cell line PNT1A. FEBS Lett. 2013;587:1536–1542. doi: 10.1016/j.febslet.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Moody T.W., Lee L., Ramos-Alvarez I., Jensen R.T. Neurotensin receptors regulate transactivation of the EGFR and HER2 in a reactive oxygen species-dependent manner. Eur J Pharmacol. 2019;865:172735. doi: 10.1016/j.ejphar.2019.172735. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper highlights how the activation of one GPCR (neurotensin receptor 1) can effectively lead to the activation of multiple RTKs (in this case in non small cell lung carcinoma cells). The authors therefore suggest that GPCRs antagonists used in conjunction with RT inhibitors may have improved therapeutic potential in the treatment of cancer. These data also support the idea of secondary messengers like ROS, being global mediators of transactivation and may have considerable influence on signalling in pathological conditions characterised by elevated ROS levels such as cancer.
- 23.Hopkins M.M., Liu Z., Meier K.E. Positive and negative cross-talk between lysophosphatidic acid receptor 1, free fatty acid receptor 4, and epidermal growth factor receptor in human prostate cancer cells. J Pharmacol Exp Therapeut. 2016;359:124–133. doi: 10.1124/jpet.116.233379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Sáinz J.A., Romero-Ávila M.T., Medina Ldel C. Dissecting how receptor tyrosine kinases modulate G protein-coupled receptor function. Eur J Pharmacol. 2010;648:1–5. doi: 10.1016/j.ejphar.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 25.Sun N., Zhang X., Guo S., Le H.T., Zhang X., Kim K.M. Molecular mechanisms involved in epidermal growth factor receptor-mediated inhibition of dopamine D3 receptor signaling. Biochim Biophys Acta Mol Cell Res. 2018;1865:1187–1200. doi: 10.1016/j.bbamcr.2018.06.001. [DOI] [PubMed] [Google Scholar]; This paper is a great illustration of how a RTK (the EGFR) can negatively modulate a GPCR (Dopamine D3 receptor) via a protein typically associated with GPCRs – the Gβγ subunit. In order to mediate its inhibition of the D3 receptor, the EGFR (activated by EGF) must first internalise which requires Src recruitment to EGFR that is mediated by Gβγ. Complexes of internalised EGFR and Src (facilitated by Gβγ) phosphorylate GRK2 at tyrosine residues. pTyr-GRK2 then binds to the dopamine D3 at a specific threonine residue (Thr142) in the second intracellular loop, which uncouples D3R from G proteins and promotes receptor endocytosis and lysosomal degradation.
- 26.Valiquette M., Parent S., Loisel T.P., Bouvier M. Mutation of tyrosine-141 inhibits insulin-promoted tyrosine phosphorylation and increased responsiveness of the human beta 2-adrenergic receptor. EMBO J. 1995;14:5542–5549. doi: 10.1002/j.1460-2075.1995.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karoor V., Malbon C. Insulin-like growth factor receptor-1 stimulates phosphorylation of the β2-adrenergic receptor in vivo on sites distinct from those phosphorylated in response to insulin. JBC. 1996;271:29347–29352. doi: 10.1074/jbc.271.46.29347. [DOI] [PubMed] [Google Scholar]
- 28.Sun J., Huang W., Yang S.F. Gαi1 and Gαi3mediate VEGF-induced VEGFR2 endocytosis, signaling and angiogenesis. Theranostics. 2018;8:4695–4709. doi: 10.7150/thno.26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H., Worrall C., Shen H. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci U S A. 2012;109:7055–7060. doi: 10.1073/pnas.1118359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisanti L.A., Talarico J.A., Carter R.L. β-Adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of ERK1/2 and Akt. J Mol Cell Cardiol. 2014;72:39–51. doi: 10.1016/j.yjmcc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoddart L.A., Kilpatrick L.E., Hill S.J. NanoBRET approaches to study ligand binding to GPCRs and RTKs. Trends Pharmacol Sci. 2018;39:136–147. doi: 10.1016/j.tips.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Watson L.J., Alexander K.M., Mohan M.L. Phosphorylation of Src by phosphoinositide 3-kinase regulates beta-adrenergic receptor-mediated EGFR transactivation. Cell Signal. 2016;28:1580–1592. doi: 10.1016/j.cellsig.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanni Samra Joke, Kulahin Nikolaj, Jorgensen Rasmus, Lyngsø Christina, Gammeltoft Steen, Hansen Jakob Lerche. A bioluminescence resonance energy transfer 2 (BRET2) assay for monitoring seven transmembrane receptor and insulin receptor crosstalk. Journal of Receptors and Signal Transduction. 2017;37:590–599. doi: 10.1080/10799893.2017.1369123. [DOI] [PubMed] [Google Scholar]
- 34.Susec M., Sencanski M., Glisic S. Functional characterization of β2-adrenergic and insulin receptor heteromers. Neuropharmacology. 2019;152:78–89. doi: 10.1016/j.neuropharm.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick L.E., Alcobia D.C., White C.W. complex formation between VEGFR2 and the β2-adrenoceptor. Cell Chem Biol. 2019;26:830–841. doi: 10.1016/j.chembiol.2019.02.014. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien S.L., Johnstone E.K.M., Devost D. BRET-based assay to monitor EGFR transactivation by the AT1R reveals Gq/11protein-independent activation and AT1R-EGFR complexes. Biochem Pharmacol. 2018;158:232–242. doi: 10.1016/j.bcp.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication is a great example of the sensitivity that BRET can provide in investiging the real time molecular mechanisms governing transactivation in terms of effector protein recruitment and GPCR/RTK complex formation in response to agonist stimulation. The authors show using BRET that stimulation with the AT1R agonist angiotensin II (AngII) can lead to rapid recruitment of Grb2 to the EGFR. Interestingly this recruitment is ∼20% that seen with EGF stimulation suggesting only a proportion of total EGFR may be regulated by transactivation. Constitutive and AngII induced complexes of AT1R and EGFR were also observed that did not require EGFR kinase activity of Gq/11. Conversely EGF mediated AT1R/EGFR complexes were sensitive to inhibition of EGFR kinase activity suggesting ligand dependent mechanisms may modulate transactivation. This paper also elegantly highlights how the molecular mechanisms governing transactivation may differ with the experimental readout used. For example using western blotting, the authors observed attenuation of AngII induced ERK1/2 phosphorylation using an inhibitor of EGFR kinase activity or Gq/11. However when measuring AngII induced Grb2 recruitment to EGFR as a readout of transactivation using BRET, the same Gq/11 inhibitor showed no effect. These BRET experiments of transactivation were supported by clever use of mutagenesis and CRISPR/Cas9 cell lines with Gq/11 or β-arrestin knockouts suggesting neither protein is required. The authors state that these discrepancies may reflect proximal (ie. measurements of direct effector protein recruitment such as Grb2) or indirect (ie. downstream pathway activation such as ERK1/2 phosphorylation) measurements of transactivation.
- 37.Smith N.J., Chan H.W., Qian H. Determination of the exact molecular requirements for type 1 angiotensin receptor epidermal growth factor receptor transactivation and cardiomyocyte hypertrophy. Hypertension. 2011;57:973–980. doi: 10.1161/HYPERTENSIONAHA.110.166710. [DOI] [PubMed] [Google Scholar]
- 38.George A.J., Purdue B.W., Gould C.M. A functional siRNA screen identifies genes modulating angiotensin II-mediated EGFR transactivation. J Cell Sci. 2013;126:5377–5390. doi: 10.1242/jcs.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huhtinen A., Hongisto V., Laiho A., Löyttyniemi E., Pijnenburg D., Scheinin M. Gene expression profiles and signaling mechanisms in α2B-adrenoceptor-evoked proliferation of vascular smooth muscle cells. BMC Syst Biol. 2017;11:65. doi: 10.1186/s12918-017-0439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borroto-Escuela D.O., Narvaez M., Pérez-Alea M. Evidence for the existence of FGFR1-5-HT1A heteroreceptor complexes in the midbrain raphe 5-HT system. Biochem Biophys Res Commun. 2015;456:489–493. doi: 10.1016/j.bbrc.2014.11.112. [DOI] [PubMed] [Google Scholar]
- 41.Borroto-Escuela D.O., Romero-Fernandez W., Mudó G. Fibroblast growth factor receptor 1- 5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity 2012 Jul 15;72(2):164] Biol Psychiatr. 2012;71:84–91. doi: 10.1016/j.biopsych.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Mandić M., Drinovec L., Glisic S., Veljkovic N., Nøhr J., Vrecl M. Demonstration of a direct interaction between β2-adrenergic receptor and insulin receptor by BRET and bioinformatics. PloS One. 2014;9 doi: 10.1371/journal.pone.0112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borroto-Escuela D.O., Flajolet M., Agnati L.F., Greengard P., Fuxe K. Bioluminescence resonance energy transfer methods to study G protein-coupled receptor-receptor tyrosine kinase heteroreceptor complexes. Methods Cell Biol. 2013;117:141–164. doi: 10.1016/B978-0-12-408143-7.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flajolet M., Wang Z., Futter M., Shen W., Nuangchamnong N., Bendor J., Wllach I., Nairn A., Surmeier D.J., Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narváez M., Andrade-Talavera Y., Valladolid-Acebes I. Existence of FGFR1-5-HT1AR heteroreceptor complexes in hippocampal astrocytes. Putative link to 5-HT and FGF2 modulation of hippocampal gamma oscillations. Neuropharmacology. 2020;170:108070. doi: 10.1016/j.neuropharm.2020.108070. [DOI] [PubMed] [Google Scholar]
- 46.Di Liberto V., Borroto-Escuela D.O., Frinchi M., Verdi V., Fuxe K., Belluardo N., Mudò G. Existence of muscarinic acetylcholine receptor (mAChR) and fibroblast growth factor receptor (FGFR) heteroreceptor complexes and their enhancement of neurite outgrowth in neural hippocampal cultures. Biochim Biophys Acta Gen Subj. 2017;1861:235–245. doi: 10.1016/j.bbagen.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Krieger C.C., Boutin A., Jang D. Arrestin-β-1 physically scaffolds TSH and IGF1 receptors to enable crosstalk. Endocrinology. 2019;160:1468–1479. doi: 10.1210/en.2019-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karoor V., Wang L., Wang H.Y., Malbon C.C. Insulin stimulates sequestration of beta-adrenergic receptors and enhanced association of beta-adrenergic receptors with Grb2 via tyrosine 350. J Biol Chem. 1998;273:33035–33041. doi: 10.1074/jbc.273.49.33035. [DOI] [PubMed] [Google Scholar]
- 49.Thomsen A.R.B., Jensen D.D., Hicks G.A., Bunnett N.W. Therapeutic targeting of endosomal G-protein-coupled receptors. Trends Pharmacol Sci. 2018;39:879–891. doi: 10.1016/j.tips.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weddell J.C., Imoukhuede P.I. Integrative meta-modeling identifies endocytic vesicles, late endosome and the nucleus as the cellular compartments primarily directing RTK signaling. Integr Biol (Camb). 2017;9:464–484. doi: 10.1039/c7ib00011a. DOI: 10.1016/j.tips.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Briddon S.J., Kilpatrick L.E., Hill S.J. Studying GPCR pharmacology in membrane microdomains: fluorescence correlation spectroscopy comes of age. Trends Pharmacol Sci. 2018;39:158–174. doi: 10.1016/j.tips.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Head B.P., Patel H.H., Insel P.A. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asimaki O., Leondaritis G., Lois G., Sakellaridis N., Mangoura D. Cannabinoid 1 receptor-dependent transactivation of fibroblast growth factor receptor 1 emanates from lipid rafts and amplifies extracellular signal-regulated kinase 1/2 activation in embryonic cortical neurons. J Neurochem. 2011;116:866–873. doi: 10.1111/j.1471-4159.2010.07030.x. [DOI] [PubMed] [Google Scholar]
- 54.Forrester S.J., Kawai T., O'Brien S., Thomas W., Harris R.C., Eguchi S. Epidermal growth factor receptor transactivation: mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu Rev Pharmacol Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul M.D., Grubb H.N., Hristova K. Quantifying the strength of heterointeractions among receptor tyrosine kinases from different subfamilies: implications for cell signaling [published online ahead of print, 2020 May 27] J Biol Chem. 2020 doi: 10.1074/jbc.RA120.013639. jbc.RA120.013639. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use FRET to show that RTK heterodimers have comparable strengths of interaction to that of homodimers. This suggests heterodimers are likely to be found physiologically and the authors speculate that pathological conditions associated with high RTK expression but decreased ligand availability (eg. cancer) may promote increased heterodimerisation versus homodimerisation. Although this publication is focused on RTK interactions, the interaction of GPCR with RTK heterodimers has largely been overlooked is likely to add further complexity to transactivation mediated signalling particularly in pathology.
- 56.Ferré S., Casadó V., Devi L.A. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niland S., Eble J.A. Neuropilins in the context of tumor vasculature. Int J Mol Sci. 2019;20:639. doi: 10.3390/ijms20030639. [DOI] [PMC free article] [PubMed] [Google Scholar]