Abstract
Although neoantigen-based cancer vaccines show great potential in cancer immunotherapy due to their ability to induce effective and long-lasting anti-tumor immunity, their development is hindered by the limitations of neoantigens identification, low immunogenicity, and weak immune response. Cyclophosphamide (CTX) not only directly kills tumors but also causes immunogenic cell death, providing a promising source of antigens for cancer vaccines. Herein, a combined immunotherapy strategy based on temperature-sensitive PLEL hydrogel is designed. First, CTX-loaded hydrogel is injected intratumorally into CT26 bearing mice to prime anti-tumor immunity, and then 3 days later, PLEL hydrogels loaded with CpG and tumor lysates are subcutaneously injected into both groins to further promote anti-tumor immune responses. The results confirm that this combined strategy reduces the toxicity of CTX, and produces the cytotoxic T lymphocyte response to effectively inhibit tumor growth, prolong survival, and significantly improve the tumor cure rate. Moreover, a long-lasting immune memory response is observed in the mice. About 90% of the cured mice survive for at least 60 days after being re-inoculated with tumors, and the distant tumor growth is also well inhibited. Hence, this PLEL-based combination therapy may provide a promising reference for the clinical promotion of chemotherapy combined with cancer vaccines.
Keywords: Immunotherapy, Thermo-responsive hydrogels, Immunogenic cell death, Cancer vaccine, Cyclophosphamide
Graphical abstract
Highlights
-
•
PLEL based-CTX hydrogel system avoided the rapid clearance of CTX and reduced systemic toxicity.
-
•
PLEL-assisted tumor lysate vaccine was cheap, safe, and contained all tumor antigens.
-
•
This strategy promoted the maturation and activation of DCs, enhanced cancer-specific CD8+ T cell responses.
-
•
PLEL-assisted combination strategy achieved a good tumor inhibition effect and generate a lasting immune memory. .
-
•
This local administration strategy could kill tumors that could not be detected or removed surgically in the clinic.
1. Introduction
Immunotherapy, which is one of the important methods for the treatment of cancer, aims to induce the body's anti-tumor immune response to eliminate tumors [1,2]. Cancer vaccines, immune checkpoint inhibitors (ICB), and adoptive T cell therapy are three key approaches to cancer immunotherapy [3]. Unlike the other two treatments, cancer vaccines have received widespread attention for their role in the early stages of the cancer-immune cycle, causing a sustained anti-tumor immune response and lasting tumor regression [[4], [5], [6]]. Although Sipuleucul-T, a therapeutic dendritic cells (DCs) cancer vaccine [7], was approved by the FDA in 2010 for the treatment of metastatic prostate cancer, it still has a low clinical response rate [8]. In most cases, vaccines against cancer antigens rely on DCs to function. DCs are the sentinels of the immune system that swallow, process, and present cancer antigens to T cells and other immune cells, triggering and guiding cancer-specific immune responses [9]. The source and type of antigen are one of the important factors affecting the effect of DCs-based cancer vaccines [10]. Neoantigens, namely tumor-specific antigens, are non-self peptides produced by tumor cell mutations in the host system [11,12]. Although neoantigens stimulate tumor-specific anti-tumor immunogenicity [13], they are limited by their expensive and complicated production process [14]. Tumor lysates (TLs) are an inexpensive and safe source of antigens that contain all of the patient's specific tumor-associated antigens (TAAs), including neoantigens, avoiding the long and expensive process of identifying and generating neoantigens [15]. A large number of tumor antigens in tumor lysates can provide multiple targets for the immune system, and these antigens can be derived from each patient to prepare a personalized cancer vaccine [16]. However, clinical trials of traditional tumor lysate vaccines have shown limited immune efficacy [17], so it is of great significance to find new strategies to improve the immune effects of tumor lysate vaccines.
Recent studies have shown that the clinical efficacy of chemotherapeutic agents is not entirely dependent on their toxicity to tumor cells [18]. The tumor cells killed by some chemotherapeutic drugs release immune-related danger signals to cause a certain degree of tumor-specific immune response, that is, immunogenic cell death (ICD) [[19], [20], [21]]. Cyclophosphamide (CTX), routinely used in cancer chemotherapy and autoimmune diseases, is also considered to be an ICD-inducing drug. For example, low-dose rhythmic cyclophosphamide treatment was proved to restore the activity of natural killer cells and T cells [22]. However, cyclophosphamide treatment alone induces only a weak anti-tumor immune response, requiring multiple or large doses [23], and is limited by systemic toxicity and drug resistance [24]. Cyclophosphamide combined with immunotherapy has been proven to be effective for cancer patients [25], but combination therapy deserves more focus on its safety and some combination programs may lead to further side effects [26,27], or the anti-tumor immune response of combination strategies is still not potent enough [28], so it is necessary to further explore safer and more effective combination therapy strategies.
Although systemic administration is commonly used in the clinic, it has obvious side effects on normal tissues [29]. In terms of immunotherapy, local administration may trigger a systemic anti-tumor immune response and may be safer than systemic administration [30,31]. The injectable intelligent hydrogel is one of the best candidates for local drug delivery [32]. They can be used not only as a chemotherapeutic sustained-release system but also as a carrier for cancer vaccines [33]. The drug-loaded hydrogel forms a local drug repository, releasing the agents continuously and slowly, which in turn activates the body's immune cells and/or protects these agents from degradation [34]. Among them, temperature-responsive hydrogels are ideal biomaterials for sustained and controlled local drug release [35]. They are sol-state at room temperature, at which time the drug could be loaded and injected into the body, and then they transform into the gel at physiological temperature to realize local sustained release of drugs [36].
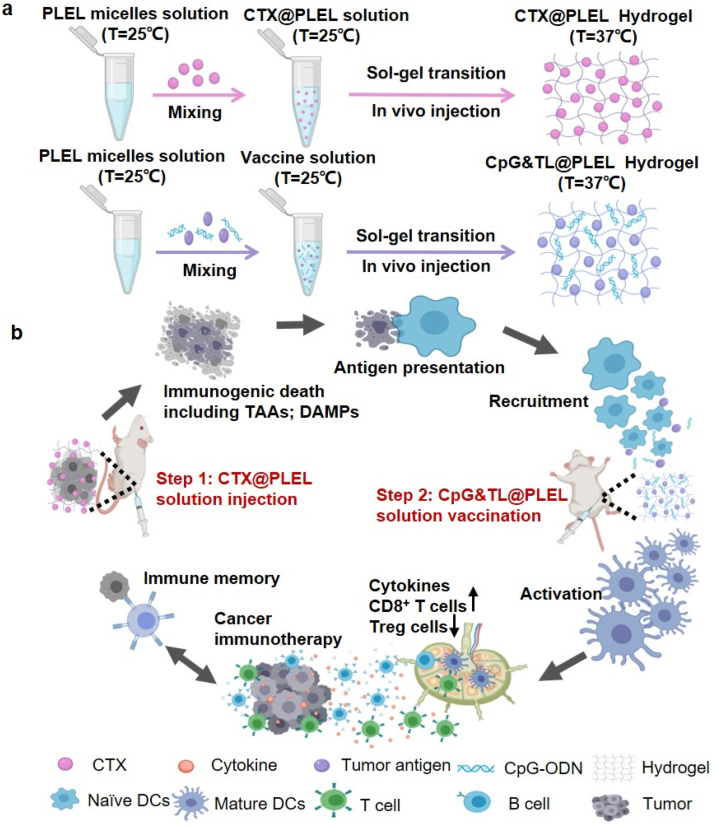
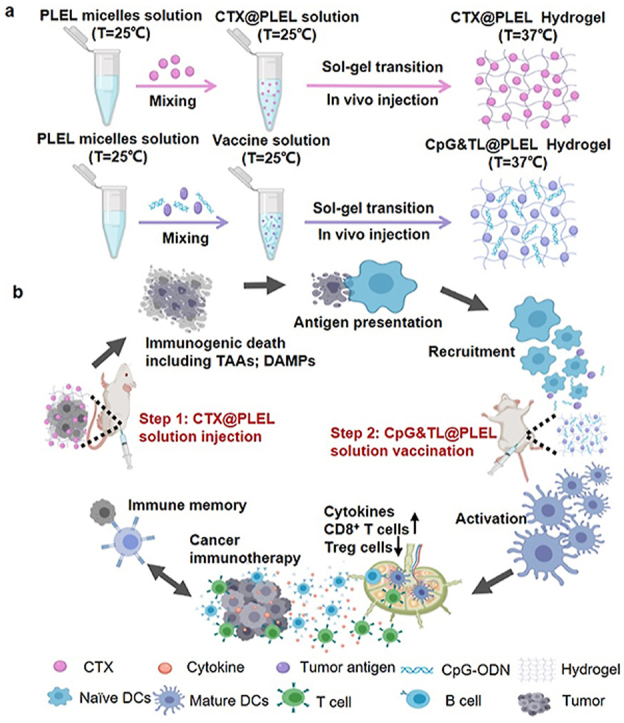
Herein, considering the advantages of the hydrogel system and the deficiencies of cancer vaccines and cyclophosphamide, we designed a prime-boost combination therapy strategy. As shown in Scheme 1, first we physically mix cyclophosphamide (CTX) and poly (D, l-lactide)-poly (ethylene glycol)-poly (D, l-lactide) (PDLLA-PEG-PDLLA, PLEL) hydrogel and then perform the intratumoral injection. Cyclophosphamide is sustained released at the tumor site to kill tumor cells, and then dead tumor cells release personalized tumor antigens (TAAs) and immune-stimulating danger signals such as damage-associated molecular patterns (DAMPs), which are taken up and recognized by dendritic cells, thereby priming anti-tumor immunity; Three days later, tumor lysates and the adjuvant cytosine-phosphate-guanine oligonucleotide (CpG-ODN) are co-loaded into the PLEL hydrogel to form a hydrogel cancer vaccine, which is injected subcutaneously into bilateral groins. After the gel is formed at body temperature, the tumor antigens and adjuvants are continuously released. This promotes the migration of dendritic cells after ingesting and recognizing tumor antigens to the hydrogel vaccine sites so that they are further activated and matured, which boosts the antitumor immunity [37]. In the previous study, we have confirmed that the PLEL cancer vaccine loaded with granulocyte-macrophage colony-stimulating factor (GM-CSF), CpG-ODN, and tumor lysate promotes effective DCs maturation and activation in lymph nodes. Orthogonal experiments were also used to screen the optimal dose of each component. However, orthogonal experiments showed that the anti-tumor effect of GM-CSF in the formula was not significant, so CpG-ODN was only used as an adjuvant in this paper [38]. As a toll-like receptor 9 agonist, CpG-ODN is more effective than other TLR ligands in improving the migration of antigen-presenting cells (APCs) [[39], [40], [41]], provoking TH1 cell-type immunity and tumor-specific CD8+ T cell immune responses [42,43]. The hydrogel acts as a barrier to prevent the degradation of CpG and antigens. The entire treatment strategy synergistically promotes tumor immunotherapy in a prime-boost manner. It is observed that compared with free cyclophosphamide combined hydrogel vaccine or soluble vaccine, PLEL-assisted combination therapy has better anti-tumor effects, better immune stimulation effects, and lower side effects. Besides, this treatment strategy promotes the maturation and activation of DCs in lymph nodes, enhances cancer-specific CD8+ T cell responses, and down-regulates the ratio of CD4+CD25+FoxP3+ Treg immunosuppressive cells in the spleen. Moreover, the PLEL-based combined treatment strategy effectively inhibits mouse CT26 colon cancer and forms immune memory to significantly reduce the tumor recurrence rate. Furthermore, this PLEL hydrogel-based strategy suppresses distant tumors through systemic anti-tumor immunity mediated by the local administration. Last but not least, our PLEL hydrogels have reversible phase transition characteristics and are easy to produce, store, and apply [44]. Since this combined treatment strategy enhances the anti-tumor effect of tumor lysates-based cancer vaccine and reduces the toxic and side effects of traditional chemotherapy, it is beneficial to cancer immunotherapy and provides a new idea for the combined use of cancer vaccine and chemotherapy in clinical practice.
Scheme 1.
Schematic illustration of the PLEL-based combination strategy to amplify cancer immunotherapy. a) the preparation process of CTX@PLEL and CpG&TL@PLEL hydrogels. b) In vivo immune mechanism of PLEL hydrogel-based combination therapy strategy (Step 1: CTX@PLEL intratumoral injection to prime antitumor immune response; step 2: CpG&TL@PLEL subcutaneous vaccination to boost tumor-specific immunity).
2. Materials and methods
Materials: Poly (ethylene glycol) (PEG, Mn = 1500, 1000, and 2000, respectively), stannous octoate (Sn (Oct)2, 95%), cyclophosphamide (CTX), and ε-caprolactone (ε-CL) were purchased from Sigma-Aldrich (USA). Penicillin–streptomycin, RMPI 1640 medium, and fetal bovine serum (FBS) were all obtained from Gibco (USA). CpG-ODN1826 (5ʹ-TCCATGACGTTCCTGACGTT-3ʹ) was obtained from Sangon Biotech (China). CD3-FITC, CD4-APC, CD8a-PE Cyanine7, CD11c-PE Cyanine7, CD44-PE, CD45-PE, CD62L-APC, CD86-APC were got from eBioscience ™ (USA). FITC anti-mouse H-2Dd Antibody and True-Nuclear™ Mouse Treg Flow™ Kit (FOXP3 Alexa Fluor®488/CD4 APC/CD25 PE) were purchased from Biolegend (USA). Mouse Inflammation CBA Kits were purchased from BD Pharmingen (USA).
Female Balb/c mice were obtained from HFK Bioscience Co., Ltd. (China) and kept under a specific-pathogen-free condition with free access to standard food and water. All animal experiments were performed following the protocols approved by the Ethics Committee of the Animal Experimental Center of West China Hospital of Sichuan University. ECAEC checking number: 20190919001 (Chengdu, P. R. China).
Preparation of CpG&TL@PLEL hydrogel vaccine and CTX@PLEL: Tumor cell lysates (TLs) were prepared following the previously reported method. In short, after 5 times repeated freeze-thaw cycles, the supernatant was collected centrifugally and the protein was quantified and then lyophilized for subsequent use. PDLLA-PEG-PDLLA (PLEL) copolymers were synthesized as described previously then dissolved in phosphate-buffered saline (PBS, pH = 8.0) [36]. CpG-ODN (50 μg) and TL (100 μg) were dissolved in PBS (pH = 7.0) and mixed with PLEL copolymer solution (10 wt%) at room temperature (T = 25 °C). Similarly, the CTX solution (100 mg kg−1) was added to the PLEL copolymer solution (5 wt%) to obtain CTX@PLEL. Dynamic rheological measurements were conducted using a HAAKE Rheostress 6000 rheometer (Thermo scientific, USA), the strain sweeps were carried out on PLEL and CTX@PLEL at 1 Hz in a strain range from 0.1% to 1000%, and the frequency sweeps were measured from 0.1 to 100 rad s−1 by using 1% strain [45].
Controlled release of CTX from PLEL hydrogel: Transwell co-culture system was used to analyze the controlled release of CTX. Briefly, CTX@PLEL was placed on the upper layer and the lower layer was PBS, which was placed in a 37 °C incubator whilst shaking. Part of the lower layer PBS was taken out at a specific time point and new PBS was added. The released CTX was quantified by HPLC.
Evaluation of anti-tumor effect in vivo: 1 × 106 CT26 tumor cells were inoculated on the lower right dorsal of female Balb/c mice, and the mice were randomized to treatment when the tumor volume reached 50–80 mm3 or progressed for 7 days. The tumor's length and width were recorded every other day or twice a week, and tumor volume was calculated as follows: 0.5 × length × (width)2. Mice were sacrificed when the length exceeded 20 mm.
To assess immune response induced by combination therapy, Balb/c mice bearing CT26 tumors were got various therapeutics, and the inguinal lymph nodes, spleens, and serum were removed for flow cytometry analysis 7 days later.
To confirm the presence of antitumor immune memory in the cured mice treated with CTX@PLEL + CpG&TL@PLEL, 1 × 106 CT26 cells were inoculated again in five normal Balb/c mice and nine cured Balb/c mice. The tumor size was measured with a vernier caliper every other day until the mice were sacrificed. The spleen cells from four mice in each group were collected for flow cytometry analysis.
To investigate the effect of the combination therapy on distant tumors, female Balb/c mice were inoculated with 1 × 106 CT26 colon cancer cells on the right lower dorsal, and the same number of CT26 cells were inoculated on the left lower dorsal 2 days later. 50 μl CTX or CTX@PLEL were injected into the tumor on the right in situ 5 days after the initial inoculation, and 100 μl CpG&TL or CpG&TL@PLEL were injected into both groins 3 days later. The tumor size was measured with a vernier caliper every other day until the mice were sacrificed.
Statistical Analysis: All data in the research were indicated as the mean ± standard error of the mean (SEM). The statistical difference analysis was evaluated by two-way ANOVA or T-test using GraphPad Prism 7 software.
3. Results
3.1. Free CTX combined with the hydrogel cancer vaccine promotes tumor inhibition but also increases systemic toxicity
According to the previous method, PLEL hydrogel was synthesized and a hydrogel cancer vaccine loaded with tumor cell lysate and adjuvant CpG was prepared [38,44]. Our previous studies had confirmed that the hydrogel vaccine loaded with GM-CSF, CpG-ODN, and tumor lysates promoted effective DCs maturation and activation in lymph nodes [38]. GM-CSF indeed activates the DCs. However, in the previous report of our group, the orthogonal experiment with three factors and three levels showed that CpG-ODN played a greater role than GM-CSF in affecting the anti-tumor effect and the production of serum anti-tumor cytokines, and GM-CSF was more expensive, so CpG-ODN was only used as an adjuvant in this work. Moreover, the characterization of the hydrogel vaccine was described in detail in our previous research [38], therefore, here we do not examine the relevant characteristics of the hydrogel vaccine. Considering that direct intratumoral injection of free cyclophosphamide is the simplest and most direct method, we first investigated the therapeutic effect of free cyclophosphamide combined with the hydrogel cancer vaccine.
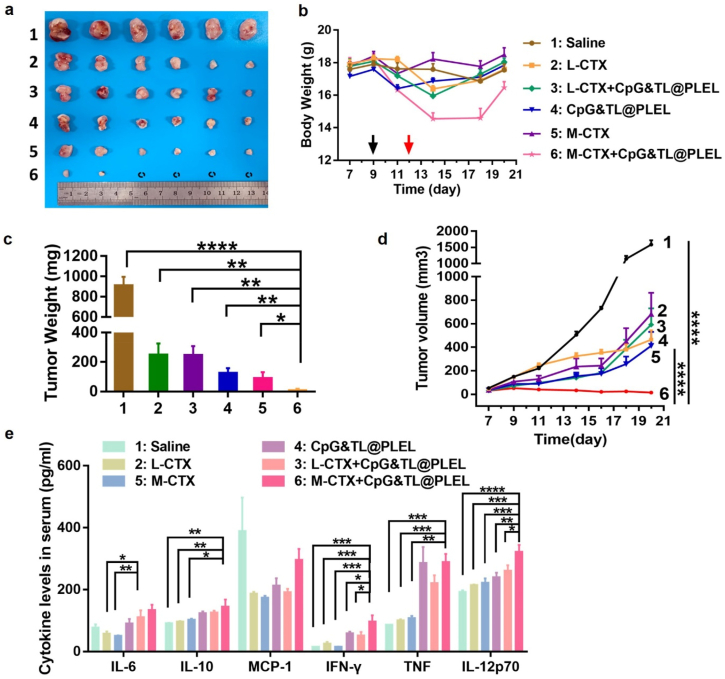
We found that the medium-dose free cyclophosphamide combined with hydrogel cancer vaccine did significantly inhibit the growth of CT26 colon cancer (Fig. 1). The tumors in the other groups (saline, low-dose free cyclophosphamide, medium-dose free cyclophosphamide, hydrogel vaccine, and low-dose free cyclophosphamide combined with hydrogel vaccine) grew progressively, while in the group of medium-dose free cyclophosphamide combined hydrogel vaccine (M-CTX + CpG&TL@PLEL), the tumors gradually decreased, and some tumors had even regressed (Fig. 1a, c, d). However, obvious weight loss was also observed in this group (Fig. 1b), suggesting a significant side effect. Besides, serum cytokine levels of the mice were detected, and the M-CTX + CpG&TL@PLEL group showed the highest levels of IFN-γ and IL-12p70 compared with other groups (Fig. 1e), suggesting its good anti-tumor effect may be related to the role of anti-tumor cytokines.
Fig. 1.
Efficacy of free cyclophosphamide combined with the hydrogel cancer vaccine. a) Photographs of tumors in different groups. b) Bodyweight curves of CT26 tumor-bearing mice over time, the black arrows represent CTX administration and the red arrows represent hydrogel vaccine administration. c) Tumor weight in all groups. d) Tumor growth curves of different treatment groups. e) Serum cytokine levels in CT26 tumor-bearing mice (n = 3–4). *p < 0.05, **p < 0.01 and ***p < 0.001.
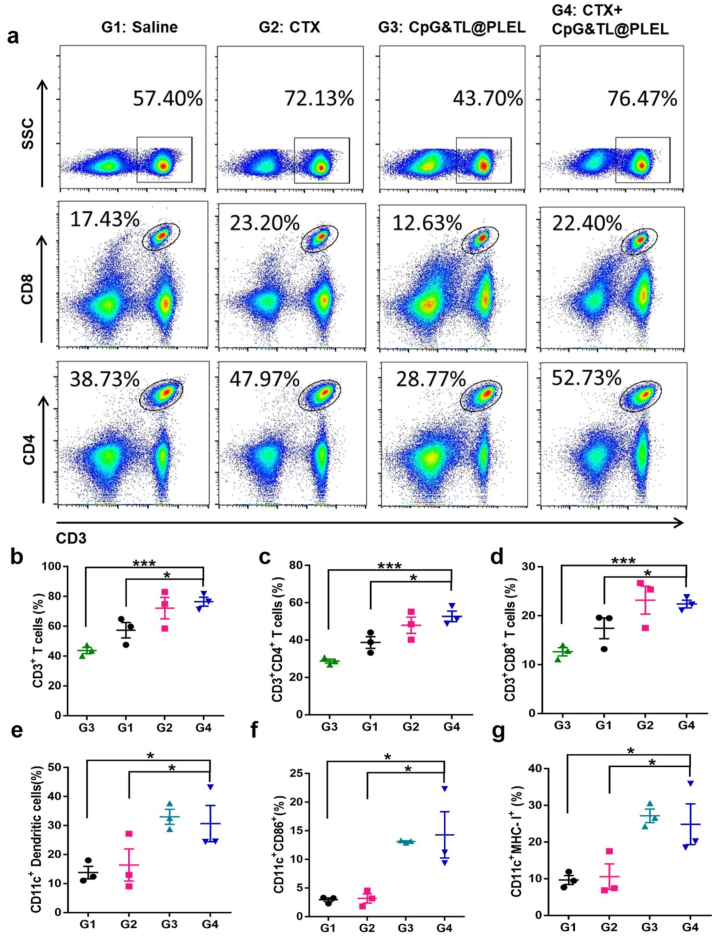
Further, we investigated the changes of DC and T cell populations in mice treated with a single medium dose of free cyclophosphamide, a single hydrogel vaccine, and a combination of the two (Fig. 2 and Fig. S2). After combined use, the proportion of CD3+, CD3+CD4+, CD3+CD8+ T lymphocytes was approximately twice that of the single hydrogel vaccine group (Fig. 2a–d); The ratio of CD11c+, CD11c + CD86+, CD11c + MHC-I + DC cells were separately twice, 4 times, and twice of that of the single free cyclophosphamide group (Fig. 2e–g), suggesting that intratumoral injection of free CTX caused immunogenic tumor cell death and promoted T lymphocyte proliferation and activation, and the hydrogel vaccine promoted the proliferation and activation of DCs. However, compared with free CTX, the combined use of hydrogel vaccine did not further synergistically promote the proliferation and activation of T lymphocytes; Compared with the single hydrogel vaccine, the combined use of free CTX did not show the effect of further promoting the proliferation and activation of DC cells. This may be due to the reduced synergies caused by the rapid degradation or removal of free CTX at the injection site [46]. In any case, the above results suggested that combination therapy does have certain advantages over monotherapy.
Fig. 2.
Immune cell activation of free cyclophosphamide combined with the hydrogel cancer vaccine. a) Representative flow cytometric analysis images. b-d) Relative quantification of CD3+, CD4+, and CD8+ T cells in inguinal lymph nodes. e-g) Relative quantification of CD11c+, CD86+, and MHC-I + DCs in inguinal lymph nodes analyzed by flow cytometry. *p < 0.05, **p < 0.01 and ***p < 0.001.
3.2. CTX@PLEL combined with the hydrogel cancer vaccine reduces the toxicity of CTX and enhances the antitumor effect
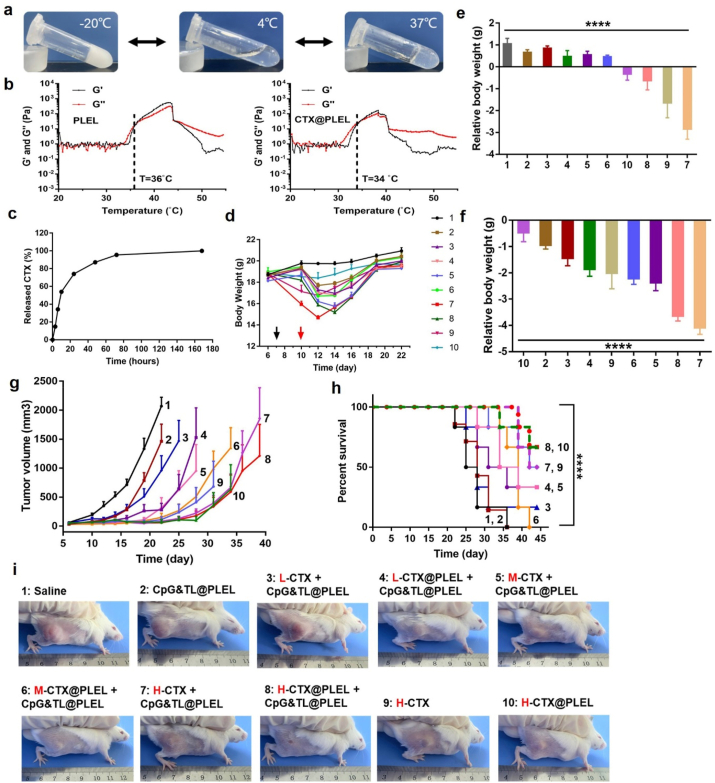
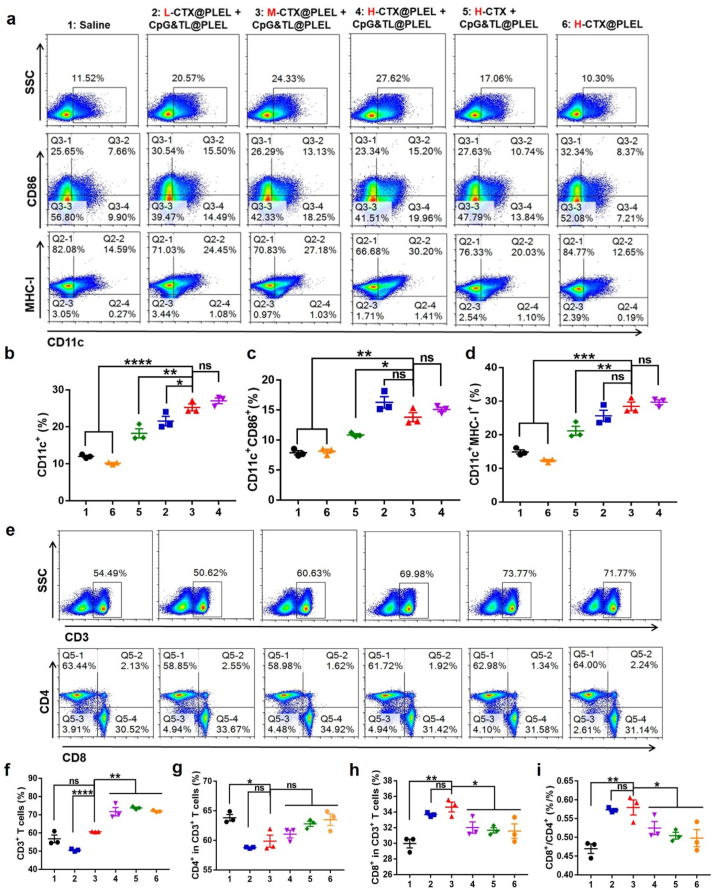
Inspired by the above experimental results, to reduce the side effects of CTX and enhance the anti-tumor efficacy, PLEL hydrogel was used to load high, medium, and low concentrations of CTX, and then combined with hydrogel vaccine to treat CT26 subcutaneous tumor (Fig. 3). In vitro experiments showed that CTX-loaded hydrogel maintained the temperature-sensitive characteristics (Fig. 3a, b, and Fig. S3). CTX@PLEL is a solid-state at −20 °C and could be easily stored, and a sol state at 25 °C, which is convenient for in vitro drug loading and in vivo injection. It is in a gel state at 37 °C, which facilitates the formation of a drug reservoir in the body to achieve slow and sustained release of drugs, thereby reducing the degradation and inactivation of drugs. As shown in Fig. S3 c, both PLEL and CTX@PLEL have linear viscoelastic regions. Under the detection conditions of oscillation frequency 1 Hz and temperature 37 °C, the storage modulus (Gʹ) of PLEL is greater than the loss modulus (Gʺ), which shows the viscoelastic solid characteristics, while Gʺ of CTX@PLEL is greater than Gʹ, which shows the viscoelastic fluid characteristics. After adding CTX, the modulus of PLEL hydrogel increased, especially the loss modulus (Gʺ). The results of frequency scanning at 1% strain and 37 °C showed that the modulus of PLEL hydrogel decreased after CTX addition in low frequency region, while the change of modulus before and after CTX addition was not obvious in high frequency region (Fig. S3 d). More than 80% of CTX was released on the third day (Fig. 3c). Under high concentration of CTX, after CTX injection, the weight loss of mice in the free CTX group was about twice that of the CTX@PLEL group (Fig. 3d and e), indicating that PLEL hydrogel could significantly reduce the toxicity of CTX. As the concentration of CTX increases, the weight of mice lost more, suggesting that the toxicity of the combination therapy increased with the concentration of CTX (Fig. 3f). But at high concentrations, the bodyweight of mice in group 8 (H-CTX@PLEL + CpG&TL@PLEL) decreased less than that in group 7 (H-CTX + CpG&TL@PLEL), indicating that the participation of PLEL can alleviate the toxicity of CTX. At the low concentration of CTX, compared with the free CTX combined with hydrogel vaccine group (L-CTX + CpG&TL@PLEL, namely group 3), on day 25 (one mouse in group 3 was sacrificed), the tumor inhibition efficacy in the combination of CTX-loaded hydrogel with hydrogel vaccine group (L-CTX@PLEL + CpG&TL@PLEL, namely group 4) is better (Fig. 3g), and the survival time of mice in group 4 is significantly prolonged (Fig. 3h); At the medium concentration of CTX, on day 28 (one mouse in group 5 (M-CTX + CpG&TL@PLEL) was sacrificed), the tumor inhibition efficacy in group 6 (M-CTX@PLEL + CpG&TL@PLEL) is better (Fig. 3g), while at the high concentration of CTX, there is no significant difference in the therapeutic effects of group 7 and 8 (Fig. 3g). These indicated that under the conditions of medium and low CTX, the auxiliary of PLEL hydrogel shows a better therapeutic effect. However, at high concentrations, CTX itself (group 9) has a very good therapeutic effect (Fig. 3g and h) and cannot show the advantages of PLEL hydrogel.
Fig. 3.
The therapeutic effect of PLEL hydrogel loaded with different concentrations of cyclophosphamide combined with hydrogel vaccine. a) Reversible sol-gel phase transition process of CTX-loaded thermosensitive PLEL hydrogel. b) Change in storage (Gʹ) and loss (Gʹʹ) modulus for blank hydrogel and CTX-loaded hydrogel with increasing temperature. c) Cumulative release of CTX from the hydrogel (n = 3). d) Bodyweight curves of CT26 tumor-bearing mice over time, the black arrows represent CTX administration and the red arrows represent hydrogel vaccine administration. e) Relative body weight after administrating CTX. f) Lowest relative body weight after administrating CTX and hydrogel vaccine. g) Tumor growth curves (when one mouse in each group was sacrificed, the tumor growth curve of that group was stopped.) and h) Survival rate of different treatment groups. i) Representative photographs of CT26 tumor-bearing mice on day 23 (The letters in red represent low, medium, and high concentrations of CTX, L: 50 mg kg−1, M: 100 mg kg−1, H: 200 mg kg−1). 1: Saline, 2: CpG&TL@PLEL, 3: L-CTX + CpG&TL@PLEL, 4: L-CTX@PLEL + CpG&TL@PLEL, 5: M-CTX + CpG&TL@PLEL, 6: M-CTX@PLEL + CpG&TL@PLEL, 7: H-CTX + CpG&TL@PLEL, 8: H-CTX@PLEL + CpG&TL@PLEL, 9: H-CTX, 10: H-CTX@PLEL. ****p < 0.0001.
3.3. Medium concentration of CTX@PLEL combined with the hydrogel cancer vaccine shows the most powerful anti-tumor immune response
Further, we used flow cytometry to analyze the lymph node immune cell typing of mice. Although the antitumor effect was similar in Fig. 3 at high concentration CTX, compared with group 5 (H-CTX + CpG&TL@PLEL) and group 6 (H-CTX@PLEL), the proportion of CD11c+, CD11c + CD86+, and CD11c + MHC-I + DCs in group 4 (H-CTX@PLEL + CpG&TL@PLEL) was significantly increased (Fig. 4a–d), which indicated that PLEL hydrogel assisted combination treatment promoted the maturation and activation of DCs. Again, this underscores the importance of hydrogels and combination therapies. Besides, compared with the low concentration (group 2), the medium and high concentration of CTX@PLEL + CpG&TL@PLEL (group 3 and 4) significantly increased the proportion of CD11c + DCs but there was no difference between medium and high concentrations (Fig. 4a and b). Although the proportion of CD3+ T cells increased with the concentration of CTX (Fig. 4e and f), compared with the high concentration, the proportion of CD8+ T cells and the ratio of CD8+ to CD4+ were remarkably increased at low and medium concentrations and there was no difference between low and medium concentrations (Fig. 4h and i). The above indicates that compared with low and high concentrations, the medium concentration of CTX@PLEL + CpG&TL@PLEL not only promoted DCs maturation but also enhanced the proliferation and activation of CD8+ T lymphocytes. Therefore, considering the toxicity and the strength of the immune response and antitumor efficacy, we chose the medium concentration of CTX for the next study.
Fig. 4.
Immune cell population in mouse lymph nodes after different treatment. a) Representative flow cytometric analysis images of DCs. b-d) Quantification of expression levels of CD11c, CD86 and MHC-Ⅰ on the surface of DCs. e) Representative flow cytometric analysis images of T cells, and f-i) corresponding quantification of CD3+, CD4+ and CD8+ T cells. (The letters in red represent low, medium, and high concentrations of CTX, L: 50 mg kg−1, M: 100 mg kg−1, H: 200 mg kg−1). *P < 0.05, **P < 0.01, ***P < 0.001, ****p < 0.0001.
3.4. The PLEL based combined treatment strategy has a significant advantage over the single-agent treatment
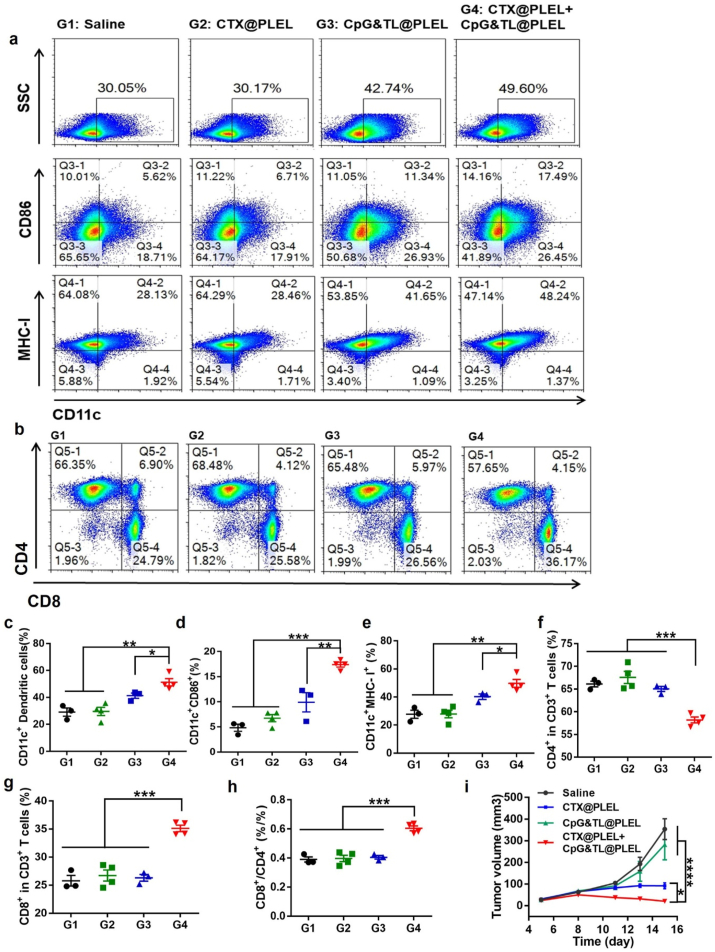
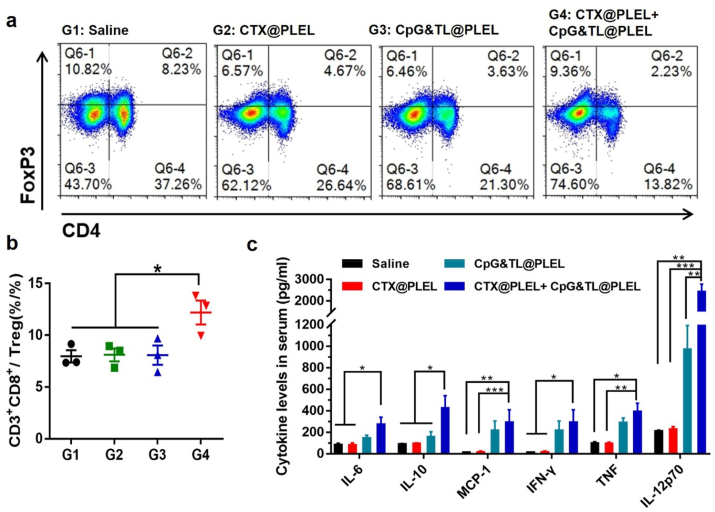
Peripheral lymphoid organs, including lymph nodes and spleen, are important sites for immune cells to present antigens and trigger immune responses, where the proliferation and activation of immune cells indicate the strength of subsequent anti-tumor immune responses [47]. After extracting lymph nodes from mice in different treatment groups, we performed flow cytometry and found that under the condition of medium concentration of CTX, compared with the single CTX@PLEL and the single CpG&TL@PLEL group, the combined treatment showed the highest proportion of CD11c+, CD11c + CD86+, and CD11c + MHC-I + DCs (Fig. 5a, c-e), which implied that PLEL based combination therapy significantly promoted DCs maturation and activation. Besides, this combination strategy simultaneously down-regulated CD4+ T cells (Fig. 5f), up-regulated CD8+ T cells (Fig. 5g), obviously increased the ratio of CD8+/CD4+ (Fig. 5h), promoting the transition of T cell subsets to Th1 type. These suggested that our combined treatment strategy elicited a powerful cytotoxic T lymphocyte (CTL) anti-tumor immune response. In addition, the tumor growth curve also verified the significant inhibitory effect of the combined treatment strategy on CT26 tumors (Fig. 5i). Also, after combined treatment, the ratio of CD4+CD25+FoxP3+Treg inhibitory T lymphocytes in mouse spleen was remarkably reduced (Fig. 6a), and the ratio of CTL/Treg cells (Fig. 6b) and anti-tumor related cytokines IL12p70, IFN-γ and TNF were significantly increased (Fig. 6c). A similar trend was observed in blood immune cells (Fig. S4). The PLEL hydrogel-based combination therapy dramatically increased the proportion of CD45+ total immune cells in the blood and upregulated the proportion of CD8+/CD4+T lymphocytes. All of these further verified that our PLEL based-combined treatment strategy caused a strong anti-tumor cellular immune response.
Fig. 5.
CTL anti-tumor immune response caused by combination therapy. Representative flow cytometric analysis images of DCs a) and T cells b). c-e) Quantification of surface markers on DCs. f-h) Quantification of surface markers on T cells. i) Tumor growth curves over time.
Fig. 6.
Suppressor Treg cells of spleen and serum cytokine analysis. a) Representative flow cytometric analysis images of Treg cells. b) Quantification of the CTL to Treg cells ratio. c) Serum cytokine levels of mice in each group (n = 3–4).
3.5. Anti-tumor immune memory response after CTX@PLEL + CpG&TL@PLEL treatment to prevent tumor recurrence
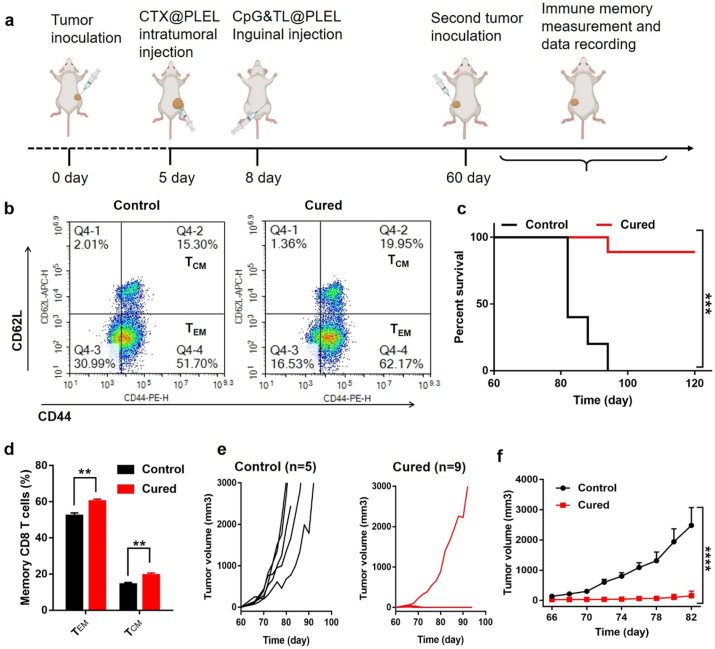
Tumor recurrence is one of the important reasons for the failure of many treatments. When tumor cells are reproduced, the memory tumor-specific T cells in the body can recognize tumor antigens again and quickly trigger an anti-tumor immune response to kill the tumor. Therefore, the formation of anti-tumor immune memory is the key to preventing tumor recurrence. For this reason, we investigated the anti-tumor immune memory response mediated by the PLEL-based hydrogel combination treatment. As shown in Fig. 7a, two months after treatment, the mice whose tumors completely regressed were again inoculated with the same number of CT26 cells. After extracting the spleens of the control and the cured mice for flow cytometry analysis, we observed that the CD3+CD8+CD44HiCD62LLo (effector memory T cells, TEM) and CD3+CD8+CD44HiCD62LHi (central memory T cells, TCM) of the cured mice increased by about 10% and 5% respectively (Fig. 7b, d), suggesting that the combined treatment strategy triggered a powerful immune memory response. Moreover, we found that compared with the control group, the re-inoculated tumor growth of the cured mice was significantly inhibited (Fig. 7e and f). Besides, all the cured mice survived for 40 days, and nearly 90% of the cured mice survived for at least 60 days, while the control mice were all sacrificed within 40 days (Fig. 7c). These results meant that the CTX@PLEL + CpG&TL@PLEL strategy could not only inhibit the progression of primary tumors and prolong the survival time of mice but also effectively restrain tumor recurrence.
Fig. 7.
Anti-tumor immune memory response after CTX@PLEL + CpG&TL@PLEL treatment. a) Schematic diagram of evaluating immune memory response mediated by PLEL-based combination therapy strategy. b) Representative flow cytometric analysis images and d) percentage of effector and central memory T cells (n = 4). e, f) Tumor growth and c) Survival rate curves of control (n = 5) and the cured mice (n = 9). The cured: CT26 tumor-bearing mice survived for 60 days after CTX@PLEL + CpG&TL@PLEL treatment.
3.6. CTX@PLEL + CpG&TL@PLEL combined therapy induces immune responses that treat distant tumors
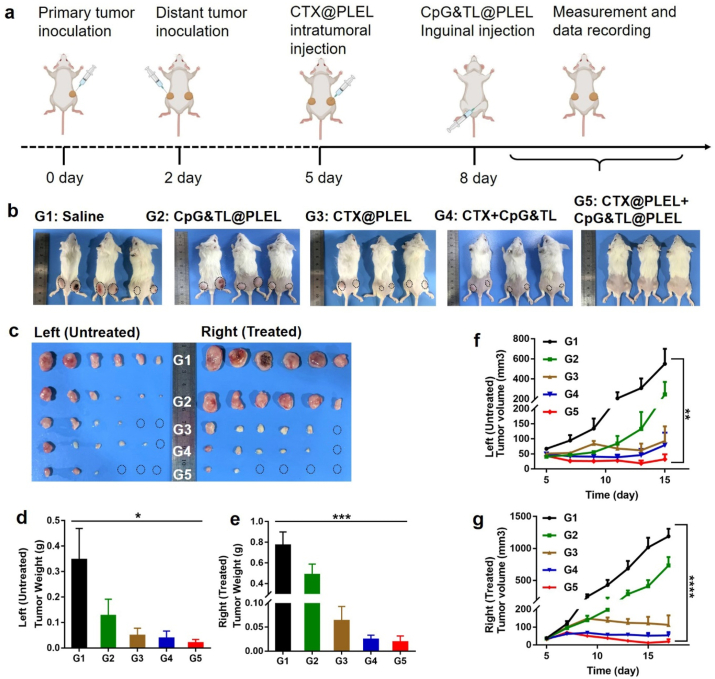
In clinical practice, some early tumors are difficult to be found or cannot be surgically removed, systemic anti-tumor immunity can effectively inhibit the growth of these tumors to improve the cure rate. Therefore, a distant tumor model was established to evaluate the inhibitory effect of the combined treatment system on untreated tumors. As is shown in Fig. 8a, the distant tumor on the left was established 2 days after the establishment of the tumor on the right, and local CTX treatment for the tumor on the right was started 3 days later, and then bilateral inguinal vaccine was administered 3 days later. In the saline and the single hydrogel vaccine group, the tumors at the local injection site and the distant site all grew gradually while there is a suppressed trend in the CTX@PLEL, CTX + CpG&TL, and CTX@PLEL + CpG&TL@PLEL groups (Fig. 8f and g). However, only in the CTX@PLEL + CpG&TL@PLEL group, the tumors at the treatment site and the distant end showed a significant regression trend, and the complete regression rate was 66.67% and 50%, respectively (Fig. 8b and c). The treated tumors were significantly suppressed in group 5 compared to group 2, while the distant tumors of the two groups were both well suppressed, possibly because the hydrogel vaccine alone could also produce a certain degree of systemic anti-tumor immunity. Besides, the weight and volume of tumors on the treated and untreated sides were also minimal in the CTX@PLEL + CpG&TL@PLEL group (Fig. 8d–g). This revealed that the PLEL-based combination therapy strategy produced a stronger systemic anti-tumor immune response than other groups. Namely, this local administration strategy activates the systemic immune system to kill distant untreated tumors that are undetected or cannot be removed surgically in the clinic.
Fig. 8.
The PLEL-based combined therapy for treating distant tumors. a) Schematic illustration to evaluate the inhibition efficacy to distant tumors generated by PLEL-based combination therapy strategy. b) Representative photographs of mice. c) Photographs of tumors in each group. d) Untreated and e) Treated tumor weight of mice. f) Untreated and g) Treated tumor growth curves of mice.
3.7. Safety evaluation of PLEL-based combined therapy
By comparing the weight changes of mice in different concentrations of CTX before and after loaded with PLEL hydrogel in Fig. 3, it can be seen that our PLEL hydrogel significantly reduced the toxicity of the drug. Moreover, compared with the group not using hydrogel (CTX + CpG&TL), the treatment effect of the CTX@PLEL + CpG&TL@PLEL group was improved and the toxicity was reduced (Fig. S5). Besides, H&E staining and detection of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatinine (CREA), urea (UREA), and uric acid (UA) levels showed that the liver, kidney, and other major organs of the mice in each treatment group existed no obvious damage (Figs. S6–9). These results meant that the PLEL-based combination therapy strategy is safe and has the potential to be promoted to the clinic.
4. Discussion and conclusion
Many studies have shown that CTX activate the immune response by inducing immunogenic cell death (ICD) of the tumor cells, and we observed similar results in this work. The ICD caused by CTX injected at the tumor site provided an initial personalized antigen for immunotherapy, which caused the proliferation and activation of T lymphocytes (Fig. 1, Fig. 2) [25,48]. However, effective immunogenicity requires sufficient tumor cells killed by high dose CTX to produce a large number of antigens [49]. A higher concentration of CTX has greater side effects and has limited antitumor effects due to its rapid clearance. Facts have proved that our PLEL hydrogel has overcome these limitations very well. After the CTX-containing PLEL preparation was injected into the tumor site, it formed a gel at body temperature and released CTX continuously and slowly, avoiding the rapid clearance of CTX and reducing systemic toxicity (Fig. 3). As shown in Fig. 3c, more than 60% of CTX was released on day 1 and the release rate was the highest. According to our previous studies and related literatures [50,51], the possible mechanism of PLEL releasing CTX is mainly diffusion, and drug release due to hydrogel degradation may occur in the later stage. Moreover, the tumor site formed a source for the release of personalized tumor-related antigens, and anti-tumor immunity is primed. The dendritic cells after identifying and ingesting the personalized tumor antigens in the first step were further recruited and activated by the subsequent PLEL hydrogel tumor lysates vaccine. Then the activated dendritic cells play a key role in the early stage of the immune cycle, which in turn induces the subsequent powerful CTL anti-tumor immunity (Fig. 4, Fig. 5, Fig. 6). The PLEL-assisted tumor lysate vaccine not only continued the advantages of tumor lysate that is cheap, safe, and contains all tumor antigens but also avoided the rapid degradation of antigens and adjuvants in vivo. At the same time, it formed a local repository of antigens and adjuvants, slowly releasing danger signals to sustainedly activate antigen-presenting cells. Thus, our PLEL hydrogel played a key role in the overall combination therapy strategy, further demonstrating the great potential of PLEL as a good drug carrier.
In clinical research, rhythmic chemotherapy combined with ICB required multiple injections of chemotherapeutics or ICB [52], while our PLEL-assisted combination therapy strategy only required one injection of CTX and hydrogel vaccines, respectively, to achieve a good tumor inhibition effect and even generate a lasting immune memory response against recurrent tumors (Fig. 7). This strategy used CTX to generate personalized tumor antigens to initiate the immune response then used tumor lysates vaccine containing tumor-associated antigens (TAAs) and neoantigens to enhance the immune response, coupled with PLEL hydrogel to form effective tumor suppression, improve survival rate and reduce the recurrence rate. Fig. 3c showed that more than 80% of CTX was released on day 3, meaning that most CTX was released to kill tumor cells to produce sufficient antigens, at which point hydrogel vaccine injection could further promote anti-tumor immunity. For this reason, we chose vaccination three days later. Of course, in future research, it is possible to screen for optimal injection intervals to find that which induces the strongest immune response. Unlike ICB-induced non-tumor-specific immune responses, this tumor-vaccine-based chemotherapeutic immunotherapy triggered tumor-specific immune effects, similar to the personalized cancer vaccine whose preparation process has been simplified. Moreover, unlike traditional DC vaccines [53], the immune adjuvant used in this system was essential to greatly enhance the tumor-specific immune response based on dendritic cells. In a word, it could serve as a powerful cancer immunotherapy strategy worthy of promotion, providing an important reference for clinical chemotherapy and immunotherapy combination.
Targeted vaccines against patient-specific TAAs, namely neoantigens, were a promising strategy for generating patient-specific strong immune responses [54]. However, this strategy required sampling, sequencing, predicting immunogenic mutations, and synthesizing antigens from the patient's tumor before treatment to produce a personalized vaccine for the patient, which had caused huge financial expenditure and technical complexity [55]. In our work, the antigens generated after ICD caused by CTX was directly derived from the tumor-bearing mice. Moreover, the tumor lysates used were prepared from tumor cells consistent with the established tumor type, and clinically tumor lysates could also be prepared from the patient's own surgically removed tumor, thus avoiding the complexity of vaccine preparation and reducing unnecessary financial costs. Additionally, this treatment strategy could also be applied to other tumors besides CT26, such as 4T1 breast cancer, and be combined with other immunotherapies such as ICB, and the specific regimen needs further research.
In short, we designed a simple PLEL hydrogel-based treatment strategy for the combination of chemotherapy and cancer vaccine. A mixture of PLEL hydrogel and CTX was injected into the tumor site to cause ICD to prime immunotherapy, and then the tumor lysates, adjuvant CpG, and PLEL were simply mixed and injected into the groins to further boost immunotherapy. We demonstrated its effective tumor suppression function and ability to cause a cytotoxic T lymphocyte (CTL) mediated immune response. This immunotherapy strategy based on PLEL hydrogel had the characteristics of simple and easy-to-operate, and at the same time, it could cause a long-lasting immune memory response to prevent tumor recurrence and distant tumor growth, which was beneficial to promote the commercial production of cancer vaccines and clinical combination applications of chemotherapy and cancer vaccines.
CRediT authorship contribution statement
Fan Yang: Conceptualization, Methodology, Investigation, Software, Validation, Formal analysis, Writing - original draft. Kun Shi: Investigation, Funding acquisition, Formal analysis. Ying Hao: Investigation, Funding acquisition, Software, Formal analysis. Yanpeng Jia: Software, Formal analysis. Qingya Liu: Investigation. Yu Chen: Investigation. Meng Pan: Investigation. Liping Yuan: Investigation. Yongyang Yu: Conceptualization, Writing - review & editing, Supervision. Zhiyong Qian: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31930067, 31525009, 31800797 and 31771096), the National Key Research and Development Program of China (2017YFC1103502), China Postdoctoral Science Foundation (2018M631094 and 2019M653410), and 1·3·5project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18002).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.03.003.
Contributor Information
Yongyang Yu, Email: yuyongyang@hotmail.com.
Zhiyong Qian, Email: zhiyongqian@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.da Silva J.L., Dos Santos A.L.S., Nunes N.C.C., de Moraes Lino da Silva F., Ferreira C.G.M., de Melo A.C. Cancer immunotherapy: the art of targeting the tumor immune microenvironment, Cancer Chemother. Pharmacol. 2019;84(2):227–240. doi: 10.1007/s00280-019-03894-3. [DOI] [PubMed] [Google Scholar]
- 2.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z.F., Lin H., Li C., Wu Z.M. Recent progress of fully synthetic carbohydrate-based vaccine using TLR agonist as build-in adjuvant. Chin. Chem. Lett. 2018;29(1):19–26. [Google Scholar]
- 4.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16(4):489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei C., Dong X., Liang J., Zhang Y., Zhu D.W., Kong D.L., Lv F. Real-time imaging tracking of a dual fluorescent vaccine delivery system based on ovalbumin loaded zinc phthalocyanine-incorporated copolymer nanoparticles. J. Biomed. Nanotechnol. 2019;15(1):100–112. doi: 10.1166/jbn.2019.2669. [DOI] [PubMed] [Google Scholar]
- 6.Fan Q., Ma Q.L., Bai J.Y., Xu J.L., Fei Z.Y., Dong Z.L., Maruyama A., Leong K.W., Liu Z., Wang C. An implantable blood clot-based immune niche for enhanced cancer vaccination. Science advances. 2020;6(39):12. doi: 10.1126/sciadv.abb4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small E.J., Schellhammer P.F., Higano C.S., Redfern C.H., Nemunaitis J.J., Valone F.H., Verjee S.S., Jones L.A., Hershberg R.M. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 8.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018;18(3):168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Harari A., Graciotti M., Bassani-Sternberg M., Kandalaft L.E. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat. Rev. Drug Discov. 2020;19(9):635–652. doi: 10.1038/s41573-020-0074-8. [DOI] [PubMed] [Google Scholar]
- 11.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., Zhang W., Luoma A., Giobbie-Hurder A., Peter L., Chen C., Olive O., Carter T.A., Li S., Lieb D.J., Eisenhaure T., Gjini E., Stevens J., Lane W.J., Javeri I., Nellaiappan K., Salazar A.M., Daley H., Seaman M., Buchbinder E.I., Yoon C.H., Harden M., Lennon N., Gabriel S., Rodig S.J., Barouch D.H., Aster J.C., Getz G., Wucherpfennig K., Neuberg D., Ritz J., Lander E.S., Fritsch E.F., Hacohen N., Wu C.J. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W., Lee K.-W., Srivastava R.M., Kuo F., Krishna C., Chowell D., Makarov V., Hoen D., Dalin M.G., Wexler L., Ghossein R., Katabi N., Nadeem Z., Cohen M.A., Tian S.K., Robine N., Arora K., Geiger H., Agius P., Bouvier N., Huberman K., Vanness K., Havel J.J., Sims J.S., Samstein R.M., Mandal R., Tepe J., Ganly I., Ho A.L., Riaz N., Wong R.J., Shukla N., Chan T.A., Morris L.G.T. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat. Med. 2019;25(5):767–775. doi: 10.1038/s41591-019-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., Oliveira G., Giobbie-Hurder A., Felt K., Gjini E., Shukla S.A., Hu Z., Li L., Le P.M., Allesøe R.L., Richman A.R., Kowalczyk M.S., Abdelrahman S., Geduldig J.E., Charbonneau S., Pelton K., Iorgulescu J.B., Elagina L., Zhang W., Olive O., McCluskey C., Olsen L.R., Stevens J., Lane W.J., Salazar A.M., Daley H., Wen P.Y., Chiocca E.A., Harden M., Lennon N.J., Gabriel S., Getz G., Lander E.S., Regev A., Ritz J., Neuberg D., Rodig S.J., Ligon K.L., Suvà M.L., Wucherpfennig K.W., Hacohen N., Fritsch E.F., Livak K.J., Ott P.A., Wu C.J., Reardon D.A. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldous A.R., Dong J.Z. Personalized neoantigen vaccines: a new approach to cancer immunotherapy, Bioorg. Med. Chem. 2018;26(10):2842–2849. doi: 10.1016/j.bmc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Boudousquie C., Boand V., Lingre E., Dutoit L., Balint K., Danilo M., Harari A., Gannon P.O., Kandalaft L.E. Development and optimization of a GMP-compliant manufacturing process for a personalized tumor lysate dendritic cell vaccine. Vaccines. 2020;8(1):1–20. doi: 10.3390/vaccines8010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X., Liang X., Chen Q., Miao Q., Chen X., Zhang X., Mei L. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13(3):2956–2968. doi: 10.1021/acsnano.8b07371. [DOI] [PubMed] [Google Scholar]
- 17.Joshi V.B., Geary S.M., Gross B.P., Wongrakpanich A., Norian L.A., Salem A.K. Tumor lysate-loaded biodegradable microparticles as cancer vaccines. Expet Rev. Vaccine. 2014;13(1):9–15. doi: 10.1586/14760584.2014.851606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J., Waxman D.J. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Canc. Lett. 2018;419:210–221. doi: 10.1016/j.canlet.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aikins M.E., Xu C., Moon J.J. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc. Chem. Res. 2020;53(10):2094–2105. doi: 10.1021/acs.accounts.0c00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J., Wang W.Q., Pei Q., Lord M.S., Yu H.J. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol. Sin. 2020;41(7):986–994. doi: 10.1038/s41401-020-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou B., Zhou L., Wang H., Saeed M., Wang D., Xu Z., Li Y., Yu H. Engineering stimuli‐activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv. Mater. 2020;32(12):1907210. doi: 10.1002/adma.201907210. [DOI] [PubMed] [Google Scholar]
- 22.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kareva I., Waxman D.J., Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Canc. Lett. 2015;358(2):100–106. doi: 10.1016/j.canlet.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., Nam G.H., Kim G.B., Kim Y.K., Kim I.S. Intrinsic cancer vaccination. Adv. Drug Deliv. Rev. 2019;151–152:2–22. doi: 10.1016/j.addr.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Zhou J., Hu L., Han X., Zou X., Chen Q., Chen Y., Liu Z., Wang C. In situ formed fibrin scaffold with cyclophosphamide to synergize with immune checkpoint blockade for inhibition of cancer recurrence after surgery. Adv. Funct. Mater. 2019;30(7):1906922. [Google Scholar]
- 26.He C., Tang Z., Tian H., Chen X. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv. Drug Deliv. Rev. 2016;98:64–76. doi: 10.1016/j.addr.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Jiang J., Chan R., Ji Y., Lu J., Liao Y.P., Okene M., Lin J., Lin P., Chang C.H., Wang X., Tang I., Zheng E., Qiu W., Wainberg Z.A., Nel A.E., Meng H. Improved efficacy and reduced toxicity using a custom-designed irinotecan-delivering silicasome for orthotopic colon cancer. ACS Nano. 2019;13(1):38–53. doi: 10.1021/acsnano.8b06164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipazzi P., Pilla L., Mariani L., Patuzzo R., Castelli C., Camisaschi C., Maurichi A., Cova A., Rigamonti G., Giardino F., Di Florio A., Asioli M., Frati P., Sovena G., Squarcina P., Maio M., Danielli R., Chiarion-Sileni V., Villa A., Lombardo C., Tragni G., Santinami M., Parmiani G., Rivoltini L. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin. Canc. Res. 2012;18(23):6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 29.Marr L.A., Gilham D.E., Campbell J.D., Fraser A.R. Immunology in the clinic review series; focus on cancer: double trouble for tumours: bi-functional and redirected T cells as effective cancer immunotherapies. Clin. Exp. Immunol. 2012;167(2):216–225. doi: 10.1111/j.1365-2249.2011.04517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q., Chen M., Liu Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019;48(22):5506–5526. doi: 10.1039/c9cs00271e. [DOI] [PubMed] [Google Scholar]
- 31.Saeed M., Gao J., Shi Y., Lammers T., Yu H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics. 2019;9(26):7981–8000. doi: 10.7150/thno.37568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao Y., Liang C., Tao H., Du Y., Wu D., Dong Z., Jin Q., Chen G., Xu J., Xiao Z., Chen Q., Wang C., Chen J., Liu Z. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Science advances. 2020;6(10) doi: 10.1126/sciadv.aaz4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adu-Berchie K., Mooney D.J. Biomaterials as local niches for immunomodulation. Acc. Chem. Res. 2020;53(9):1749–1760. doi: 10.1021/acs.accounts.0c00341. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Najibi A.J., Sobral M.C., Seo B.R., Lee J.Y., Wu D., Li A.W., Verbeke C.S., Mooney D.J. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 2020;11(1):5696. doi: 10.1038/s41467-020-19540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C., Liu Y., Wang H., Chen G., Wu X., Ren J., Zhang H., Zhao Y. Multifunctional chitosan inverse opal particles for wound healing. ACS Nano. 2018;12(10):10493–10500. doi: 10.1021/acsnano.8b06237. [DOI] [PubMed] [Google Scholar]
- 36.Shi K., Wang Y.L., Qu Y., Liao J.F., Chu B.Y., Zhang H.P., Luo F., Qian Z.Y. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016;6:19077. doi: 10.1038/srep19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worbs T., Hammerschmidt S.I., Förster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17(1):30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 38.Yang F., Shi K., Jia Y., Hao Y., Peng J., Yuan L., Chen Y., Pan M., Qian Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Applied Materials Today. 2020;19:100608. [Google Scholar]
- 39.Zhao H., Zhao B., Wu L., Xiao H., Ding K., Zheng C., Song Q., Sun L., Wang L., Zhang Z. Amplified cancer immunotherapy of a surface-engineered antigenic microparticle vaccine by synergistically modulating tumor microenvironment. ACS Nano. 2019;13(11):12553–12566. doi: 10.1021/acsnano.9b03288. [DOI] [PubMed] [Google Scholar]
- 40.Yang X.M., Lai C.H., Liu A.Q., Hou X.Q., Tang Z.R., Mo F.Z., Yin S.H., Lu X.L. Anti-tumor activity of mannose-CpG-oligodeoxynucleotides-conjugated and hepatoma lysate-loaded nanoliposomes for targeting dendritic cells in vivo. J. Biomed. Nanotechnol. 2019;15(5):1018–1032. doi: 10.1166/jbn.2019.2755. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z.X., Xu L.F., He N.Y., Huang F.Y., Xu T.F., Li L., Zhang Y.W., Zhang L.M. Cy5.5-MSA-G250 nanoparticles (CMGNPs) induce M1 polarity of RAW264. 7 macrophage cells via TLR4-dependent manner. Chin. Chem. Lett. 2019;30(6):1320–1324. [Google Scholar]
- 42.Molenkamp B.G., Sluijter B.J., van Leeuwen P.A., Santegoets S.J., Meijer S., Wijnands P.G., Haanen J.B., van den Eertwegh A.J., Scheper R.J., de Gruijl T.D. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin. Canc. Res. 2008;14(14):4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 43.Liu J.F., Wang J., Zhu Q.Q., Yu C.Q., Yin J.R., Zheng L.P., Li A. Mannosylated PEGylated-polyethyleneimine as efficient CpG oligodeoxynucleotide carriers for efficient dendritic cell targeting delivery and activation. J. Biomed. Nanotechnol. 2019;15(7):1454–1467. doi: 10.1166/jbn.2019.2790. [DOI] [PubMed] [Google Scholar]
- 44.Shi K., Xue B., Jia Y., Yuan L., Han R., Yang F., Peng J., Qian Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Research. 2019;12(6):1389–1399. [Google Scholar]
- 45.Zhang H., Cong Y., Osi A.R., Zhou Y., Huang F., Zaccaria R.P., Chen J., Wang R., Fu J. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv. Funct. Mater. 2020;30(13):1910573. [Google Scholar]
- 46.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020;17(4):251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 48.Wu J., Waxman D.J. Metronomic cyclophosphamide schedule-dependence of innate immune cell recruitment and tumor regression in an implanted glioma model. Canc. Lett. 2014;353(2):272–280. doi: 10.1016/j.canlet.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanyi J.L., Bobisse S., Ophir E., Tuyaerts S., Roberti A., Genolet R., Baumgartner P., Stevenson B.J., Iseli C., Dangaj D., Czerniecki B., Semilietof A., Racle J., Michel A., Xenarios I., Chiang C., Monos D.S., Torigian D.A., Nisenbaum H.L., Michielin O., June C.H., Levine B.L., Powell D.J., Jr., Gfeller D., Mick R., Dafni U., Zoete V., Harari A., Coukos G., Kandalaft L.E. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018;10(436) doi: 10.1126/scitranslmed.aao5931. eaao5931. [DOI] [PubMed] [Google Scholar]
- 50.Shukla S., Favata J., Srivastava V., Shahbazmohamadi S., Tripathi A., Shukla A. Effect of polymer and ion concentration on mechanical and drug release behavior of gellan hydrogels using factorial design. J. Polym. Sci. 2020;58(10):1365–1379. [Google Scholar]
- 51.Rankin L.A., Lee B., Mineart K.P. Effect of network connectivity on the mechanical and transport properties of block copolymer gels. J. Polym. Sci. 2020;59(1):34–42. [Google Scholar]
- 52.Voorwerk L., Slagter M., Horlings H.M., Sikorska K., van de Vijver K.K., de Maaker M., Nederlof I., Kluin R.J.C., Warren S., Ong S., Wiersma T.G., Russell N.S., Lalezari F., Schouten P.C., Bakker N.A.M., Ketelaars S.L.C., Peters D., Lange C.A.H., van Werkhoven E., van Tinteren H., Mandjes I.A.M., Kemper I., Onderwater S., Chalabi M., Wilgenhof S., Haanen J., Salgado R., de Visser K.E., Sonke G.S., Wessels L.F.A., Linn S.C., Schumacher T.N., Blank C.U., Kok M. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 53.Chang A.E., Redman B.G., Whitfield J.R., Nickoloff B.J., Braun T.M., Lee P.P., Geiger J.D., Mulé J.J. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin. Canc. Res. 2002;8(4):1021–1032. [PubMed] [Google Scholar]
- 54.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 55.Bräunlein E., Krackhardt A.M. Identification and characterization of neoantigens as well As respective immune responses in cancer patients. Front. Immunol. 2017;8:1702. doi: 10.3389/fimmu.2017.01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.