Abstract
BACKGROUND:
Cigarette smoking is a frequent habit across blood donors (approx. 13% of the donor population), that could compound biologic factors and exacerbate oxidant stress to stored red blood cells (RBCs).
STUDY DESIGN AND METHODS:
As part of the REDS-III RBC-Omics (Recipient Epidemiology Donor Evaluation Study III Red Blood Cell-Omics) study, a total of 599 samples were sterilely drawn from RBC units stored under blood bank conditions at Storage Days 10, 23, and 42 days, before testing for hemolysis parameters and metabolomics. Quantitative measurements of nicotine and its metabolites cotinine and cotinine oxide were performed against deuterium-labeled internal standards.
RESULTS:
Donors whose blood cotinine levels exceeded 10 ng/mL (14% of the tested donors) were characterized by higher levels of early glycolytic intermediates, pentose phosphate pathway metabolites, and pyruvate-to-lactate ratios, all markers of increased basal oxidant stress. Consistently, increased glutathionylation of oxidized triose sugars and lipid aldehydes was observed in RBCs donated by nicotine-exposed donors, which were also characterized by increased fatty acid desaturation, purine salvage, and methionine oxidation and consumption via pathways involved in oxidative stress-triggered protein damage-repair mechanisms.
CONCLUSION:
RBCs from donors with high levels of nicotine exposure are characterized by increases in basal oxidant stress and decreases in osmotic hemolysis. These findings indicate the need for future clinical studies aimed at addressing the impact of smoking and other sources of nicotine (e.g., nicotine patches, snuff, vaping, secondhand tobacco smoke) on RBC storage quality and transfusion efficacy.
Red blood cell (RBC) storage in the blood bank promotes the accumulation of a series of biochemical and morphological changes to the erythrocyte collectively referred to as the storage lesion.1 As storage progresses, the combined effect of refrigerated storage temperatures and the progressive acidification of the intracellular pH to negatively impacts the activity of key enzymes regulating RBC energy2 and ion pump homeostasis.3,4 These events ultimately result in the depression of energy metabolism—that is, glycolysis in RBCs, a phenomenon that is observed during storage in all currently licensed storage additives, including SAGM4-7; additive solutions 1,8 3,9,10 and 511; and PAGGSM,12,13—and is in part ameliorated by alkaline storage additives.12,14-16 Depression of energy metabolism promotes the consumption of high-energy phosphate compounds adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG). These metabolites are critical mediators of hemoglobin affinity for oxygen. As such, ATP and DPG consumption promotes increases in hemoglobin oxygen saturation, a phenomenon that is paralleled by the generation of reactive oxygen species.4,17 While RBCs are equipped with several systems to prevent oxidant damage or recycle/repair oxidatively damaged components,18 these systems become progressively inefficient as a function of storage duration.1
Over the past decade, the use of omics technologies has contributed substantially not only to qualitatively characterize the storage lesion, but also to identify and quantify metabolic markers of the biologic storage age of the RBCs that vary as a function of processing strategies and donor biology.19-23 As a result, the concept of the “metabolic age” of the unit was introduced to distinguish between the impact of storage on RBC biology from the chronological age of the unit, that is, days elapsed since time of donation.24,25 Metabolic markers of the storage lesion have been extensively associated with US Food and Drug Administration markers of storage quality like end-of-storage hemolysis26 and posttransfusion recovery in healthy autologous volunteers in humans.27 Studies in rodent models have further highlighted genetic underpinnings of the metabolic storage lesion and its impact on the capacity of stored RBCs to circulate at 24 hours from transfusion in mice.27-30 For example, ATP breakdown and oxidation promotes the accumulation of hypoxanthine, a positive and negative predictor of hemolysis and posttransfusion recoveries in mice and humans, respectively.12,27,31 Strain-specific heterogeneity in the activity of enzymes critical to RBC iron redox homeostasis (e.g., the ferroreductase STEAP3) can also drive the heterogeneity with respect to the degree of lipid peroxidation in stored mouse RBCs, another parameter that is negatively associated with the capacity of transfused RBCs to circulate upon transfusion.30 In other words, in keeping with recent reassuring evidence from randomized clinical trials,32-35 the age of blood per se may be an inaccurate predictor of the quality of stored blood, whose storability is indeed affected by several variables such as donor-related factors. One representative example of such factors is the impact of common enzymopathies on RBC antioxidant capacity. Several antioxidant systems in RBCs rely on a critical reducing equivalent, the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) to directly recycle oxidized small-molecule antioxidants (e.g., glutathione) or enzymes with antioxidant activity (e.g., peroxiredoxins; thioredoxins; flavin reductases or, indirectly, glutathione peroxidases; glutaredoxins; and the ascorbate system).18 Generation of NADPH in the mature RBC, which is devoid of mitochondria and thus of the NADPH-biosynthetic pathways therein, is reliant on glucose oxidation through the pentose phosphate pathway (PPP). However, mutations of glucose 6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the PPP, are common in humans as a result of positive evolutionary selection owing to their protective role against malaria.36 As such, G6PD deficiency affects approximately 400 million people worldwide, including approximately 10% of the African American donor population in some metropolitan areas.37 Of note, RBCs from G6PD-deficient donors are characterized by increased oxidation of glucose via glycolysis as well as by an impaired redox metabolism,38,39 which in part explains the lower capacity of stored RBCs from G6PD-deficient donors to circulate upon transfusion to sickle cell recipients.40
Increases in basal levels of oxidant stress to RBCs may also be attributable to environmental variables. For example, cigarette smoking has been previously reported to increase the basal levels of oxidant stress to RBCs.41-45 Increases of oxidant stress markers at the time of donation and during storage have been reported in smokers, including increases in carboxyhemoglobin levels, increased depletion of clusterin, accumulation of potentially toxic metal contaminants of cigarettes, and increased hematocrit and hemoglobin count as a marker of hypoxia in this population.43-46 Smokers are routinely accepted as blood donors and in one recent study comprised approximately 13% of the donor population.42 However, despite preliminary evidence of a potential increase in oxidant stress markers in RBCs donated by smokers, the impact of cigarette smoking on RBC metabolism during storage in the blood bank is not known. By the same token, it is not known whether increases in the basal levels of oxidant stress as a function of smoking may increase the RBC susceptibility to hemolyze following additional insults, like refrigerated storage alone, gamma irradiation, or other oxidative and osmotic insults in donors and recipients.
Over the past 5 years, large-scale studies like the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health Recipient Epidemiology and Donor Evaluation Study (REDS-III) have been designed to further our understanding of the impact of donor and recipient biology on storage quality and transfusion outcomes.47 The REDS-III RBC Omics study involved four blood centers across the United States, where 13,758 healthy donor volunteers of different ages, sexes, and ethnicities were enrolled to determine interdonor heterogeneity in end-of-storage hemolysis, either spontaneous or following oxidative or osmotic insults.23,48 These studies highlighted a significant impact of donor sex (and testosterone levels49), age, and ethnicity on the hemolytic propensity of stored RBCs,23,47 a variable that was substantially consistent within the same donor at multiple subsequent donations.48 Recently, preliminary metabolomics analyses were performed on a sub-set of REDS III donors who were asked to return for additional studies (recalled donors) to highlight a significant impact of processing strategies (e.g., storage additives)21 and donor sex, age, ethnicity, and G6PD status (D’Alessandro et al. and Page et al., unpublished data). These donors, along with the entire Omics cohort were also administered a questionnaire to report on their self-reported cigarette smoking habits. However, no direct assessment was performed to assess the impact of nicotine exposure (such as tobacco smoking, nicotine patches, snuff, vaping, secondhand tobacco smoke) on the metabolic age of stored RBCs from these donors. As such, as part of the RBC-Omics study efforts toward personalized transfusion medicine, in the present study we performed quantitative measurement of nicotine and its metabolites (cotinine and cotinine oxide) in the REDS-III RBC Omics recalled donor population. Metabolomics analyses were performed on these donors and correlated to the levels of cotinine and cotinine oxide, as well as to smoking group identification on the basis of questionnaire responses. We confirm and expand on the existing literature suggesting a negative impact of nicotine-exposure on the basal redox status of the RBCs, by documenting that RBCs from nicotine-exposed donors are characterized by increases in the levels of oxidized and glutathionylated sugar and lipid aldehydes, increased oxidation and consumption of methionine, basal activation of the PPP, and increased markers of methemoglobinemia (pyruvate-to-lactate ratios). Notably, we also document a disagreement between the smoking group characterization based on self-reported questionnaires and direct measurements of nicotine metabolites in RBCs, an observation that may affect the design of future clinical studies addressing the impact of donor smoking habits and other nicotine exposures on stored blood quality and transfusion outcomes.
MATERIALS AND METHODS
REDS-III RBC-Omics study participants and samples
Donor selection and recruitment for the RBC-Omics study were previously described in detail.23,48,50 Donors were enrolled at the four participating REDS-III US blood centers. Overall, 97% (13,403) of the whole blood donations provided by 13,758 donors aged 18+ who provided informed consent were evaluable for hemolysis parameters. Donors in the 5th and 95th percentiles of the distributions for the oxidative, osmotic, or spontaneous hemolysis measurements were recalled and asked to donate whole blood that was subsequently processed to generate a leukoreduced RBC unit. These units were sampled at Storage Days 10, 23, and 42, as described.21 Blood collection, sample processing, and other aspects of the screening and recall phases of the RBC Omics Study, including results of pilot studies to optimize processing and storage of samples for metabolomics analyses, are detailed in prior publications.47,48 At enrollment, donors were asked to self-report their cigarette smoking behavior, on a scale from 0 to 7 as follows: 0: zero per day; 1: more than zero, but less than one cigarette per day; 2: 1 cigarette per day; 3: 2 to 5 cigarettes per day; 4: 6 to 15 cigarettes per day (about one-half pack); 5: 16 to 25 cigarettes per day (about one pack); 6: 26 to 35 cigarettes per day (about 1.5 packs); 7: more than 35 cigarettes per day (about 2 packs or more).
Sample processing and metabolite extraction
An isotopically labeled internal standard mixture including a mix of 13C15N-labeled amino acid standards (2.5 μM) was prepared in methanol. A volume of 100 μL of frozen RBC aliquots was mixed with water and the mixture of isotopically labeled internal standards (1:1:1, v/v/v). The samples were extracted with methanol (final concentration of 80% methanol). After incubation at −20°C for 1 hour, the supernatants were separated by centrifugation and stored at −80°C until analysis.51 Samples were vortexed and insoluble material pelleted as described.9,52 A 20-μL aliquot of each extract was treated with 2 μL of a mixture of deuterium (D3)-labeled internal standards for cotinine (DLM-1819-0.01, Cambridge Isotope Laboratories), nicotine (DLM-1818-PK, Cambridge Isotope Laboratories), and cotinine oxide (sc-219705, Santa Cruz Bio-technology) so that the final concentrations of the standards were 0.2 μM, then dried and resuspended in 22 μL of 0.1% formic acid in water. Retention times, extraction recovery efficiency, linearity, and reproducibility of measurements were validated in a pilot study on units kindly donated by Vitalant Research Institute Denver for research purposes. Cotinine measurements greater than 10 ng/mL (>0.057 uM) were considered consistent with active smoking or other nicotine exposure (patches, vaping), as previously described.42,53
Ultra-high-pressure liquid chromatography–mass spectrometry metabolomics
Analyses were performed with a ultra-high-pressure liquid chromatography (UHPLC; Vanquish, Thermo Fisher) coupled online to a mass spectrometer (Q Exactive, Thermo Fisher). Samples were analyzed with use of a 3-minute isocratic condition54 or a 5, 9, or 17-minute gradient as described.9,28,55,56 For the analysis of nicotine and related metabolites, samples were analyzed with a 6-minute C18 gradient and mass spectrometry (MS) positive mode acquisition as described.54 MS acquisition, data analysis, and elaboration were performed as described. Solvents were supplemented with 0.1% formic acid for positive mode runs and 1 mM ammonium acetate for negative mode runs. MS acquisition, data analysis, and elaboration was performed as described.9,54 Additional analyses, including untargeted analyses were performed with computer software (Compound Discoverer 2.0, (Thermo Fisher). Graphs and statistical analyses (either t test or repeated measures analysis of variance [ANOVA]) were prepared with computer software (Prism 8.0, GraphPad Software, Inc.).
RESULTS
Cotinine measurements confirm literature data on nicotine-exposure incidence across healthy blood donors
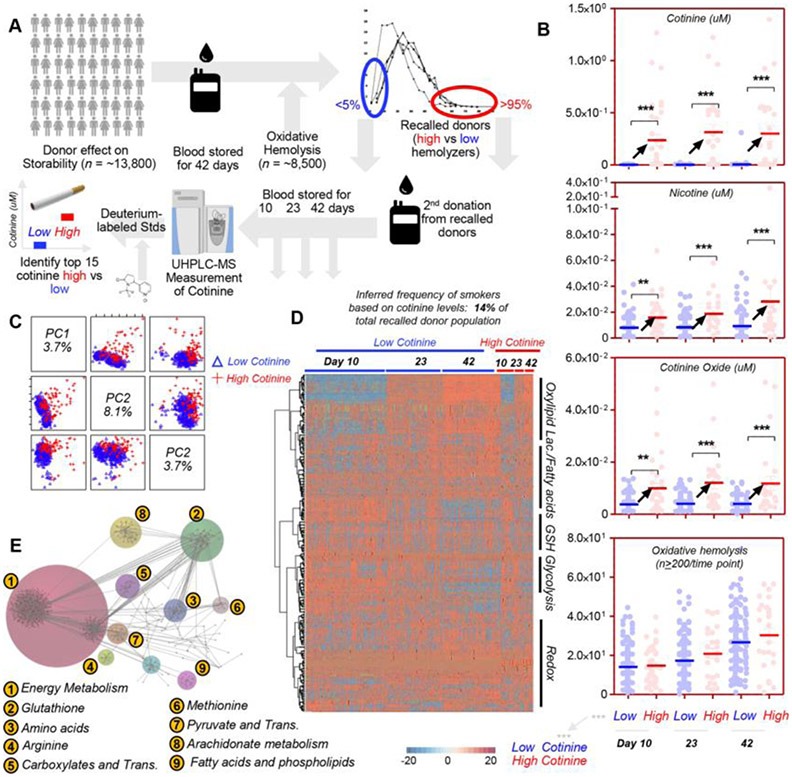
Within the framework of the REDS-III Omics study, 13,800 donors were recruited to donate a unit of blood in four different blood centers across the United States. Units were stored for 42 days until the end of their shelf life, when they were tested for the RBC propensity to hemolyze either spontaneously or following oxidant or osmotic insults. Donors below the 5th or above the 95th percentile for each type of hemolysis (spontaneous, oxidative, or osmotic) from the REDS III original cohort were asked to donate a second unit of blood, which was sampled at Storage Days 10, 23, and 42 for metabolomics analyses (n = 250 at Day 10; Fig. 1A). Following preliminary evidence of a potential negative impact of smoking habits on the storage lesion of RBCs,42-44 we performed measurements of nicotine and its primary metabolites cotinine and cotinine N-oxide against deuterium-labeled internal standards to identify nicotine-exposed blood donors based on cotinine levels greater than 10 ng/mL42,53 (Fig. 1B). Despite the select nature of the recalled donor population, which was enriched for donors showing the highest and lowest hemolysis parameters as determined in the first phase of the REDS III Omics study, approximately 14% of donors in the recalled population were identified as nicotine-exposed (cotinine >10 ng/mL) (Fig. 1B), strikingly consistent with a previous report (13%) in which the same cotinine threshold was adopted to identify smokers.42 Measurements of these metabolites, along with additional relative and absolute quantitative measurements of a total of 385 variables, in the nicotine-exposed and non–nicotine-exposed population are reported in Table S1, available as supporting information in the online version of this paper. Unsupervised principal component analysis (Fig. 1C) and hierarchical clustering analysis (Fig. 1D) showed limited, albeit appreciable metabolic differences between nicotine-exposed and non–nicotine-exposed donors as a function of storage duration. Specifically, we generated a pathway analysis of the significant metabolites by ANOVA (two-way, repeated measures) through Omicsnet (Fig. 1D). This analysis revealed a significant overall impact of nicotine exposure on metabolites involved in energy homeostasis, glutathione and methionine metabolism, fatty acid level and oxidation, carboxylic acid metabolism, and transamination pathways (Fig. 1E). A vectorial version of this panel with enhanced read-ability of the color code of the heat map is provided as Fig. S1, available as supporting information in the online version of this paper.
Fig. 1.
Metabolomics analysis of recalled REDS III donors ranked by cotinine levels, as measured by UHPLC–MS. The 5th and 95th percentile lowest and highest hemolyzers from the REDS III original cohort (n = 13,800) were asked to donate a second unit of blood, which was sampled at Storage Days 10, 23, and 42 for metabolomics analyses (A). Measurements of nicotine and its metabolites cotinine and cotinine oxide were obtained against deuterium-labeled internal standards (B). Cotinine levels were used to discriminate between smokers (or other nicotine exposures resulting in cotinine levels higher than 10 ng/mL – group indicated as “high” and represented as red dots in panel B) and nonsmokers (“low,” blue dots). In the same panel, cotinine, nicotine, cotinine oxide, and oxidative hemolysis measurements are shown for the subjects with low/blue versus high/red cotinine (B). Unsupervised principal component analysis (C), hierarchical clustering analysis (D), and pathway analyses (E) were performed on subjects characterized by low (blue) vs high (red) cotinine levels at Storage Days 10, 23, and 42, highlighting differences in energy and redox metabolism between the two groups at the freshest available storage time point (here Day 10) and at the end of storage. In E, since the color code may be too difficult to appreciate, numbers have been added to highlight the pathways that are most significantly affected between subjects with high and low levels of cotinine.
Metabolic impact of nicotine exposure in the 15 donors with the highest levels of cotinine versus the 15 donors with the lowest levels of cotinine measured
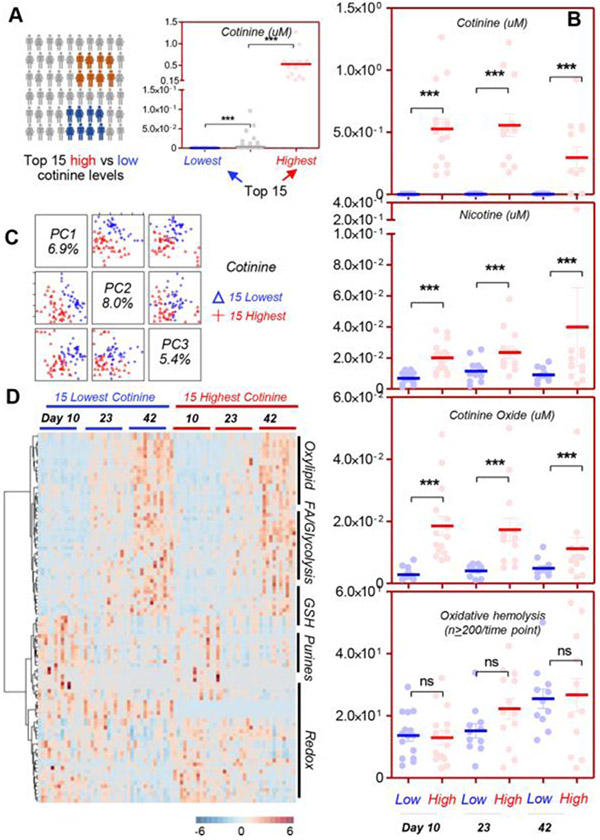
While cotinine measurements above the 10 ng/mL threshold are indicative of active nicotine exposure such as tobacco smoking, a significant heterogeneity in cotinine measurements was noted across donors who did not self-report cigarette smoking, possibly as a result of secondhand exposure to smoking or other sources of nicotine exposure in this population. To address this issue, we hypothesized that the potential metabolic impact of nicotine exposure would be better studied when comparing the donors with the lowest versus the highest levels of cotinine at the earliest time point available in this study, that is, Day 10. Of note, the highest-cotinine group had three clear outliers showing approximately 1-μM cotinine levels, consistent with their self-reported habit of smoking more than 35 cigarettes/day (Fig. 2A). Highest-cotinine donors (red) versus lowest-cotinine donors (blue) were confirmed to have significantly (p < 0.0001 ANOVA) higher levels of nicotine, cotinine, and cotinine oxide throughout storage (Storage Days 10, 23, and 42). However, the propensity of RBCs to hemolyze following oxidative injury was nonsignificantly increased in the highest-cotinine donors at each tested time point (Fig. 2B). Despite these limited differences, unsupervised principal component and hierarchical clustering analyses highlighted more evident changes between highest-cotinine donors and lowest-cotinine donors (Fig. 2C, D), which warranted further investigation in a pathway-specific fashion.
Fig. 2.
Comparison of the metabolic phenotypes of the 15 subjects with the highest (red) and lowest (blue) levels of cotinine in the recalled REDS III donor population. A subgroup of the REDS III recalled donor population was identified on the basis of the highest (red) or lowest (blue) cotinine measurements at Day 10 (A) (n = 15 per group, n = 220 for the rest of the population with intermediate cotinine measurements in light gray). Highlights of the measurements of nicotine and its metabolites cotinine and cotinine oxide were obtained against deuterium-labeled internal standards in the 15 subjects with the highest or lowest cotinine levels at Day 10, along with the propensity of RBCs to hemolyze following oxidative insult with 2,2’-Azobis(2-amidinopropane) dihydrochloride (AAPH) (B). Unsupervised principal component analysis (C), hierarchical clustering analysis (D), and pathway analyses (E) were performed on the 15 subjects with the lowest levels of cotinine (indicating minimal exposure to smoke or other source of nicotine exposure [blue]) versus highest levels of cotinine (red) at Storage Days 10, 23, and 42, highlighting differences in energy and redox metabolism between the two groups at the freshest available storage time point (here Day 10) and at the end of storage.
Impact of nicotine exposure on stored RBC energy and redox metabolism
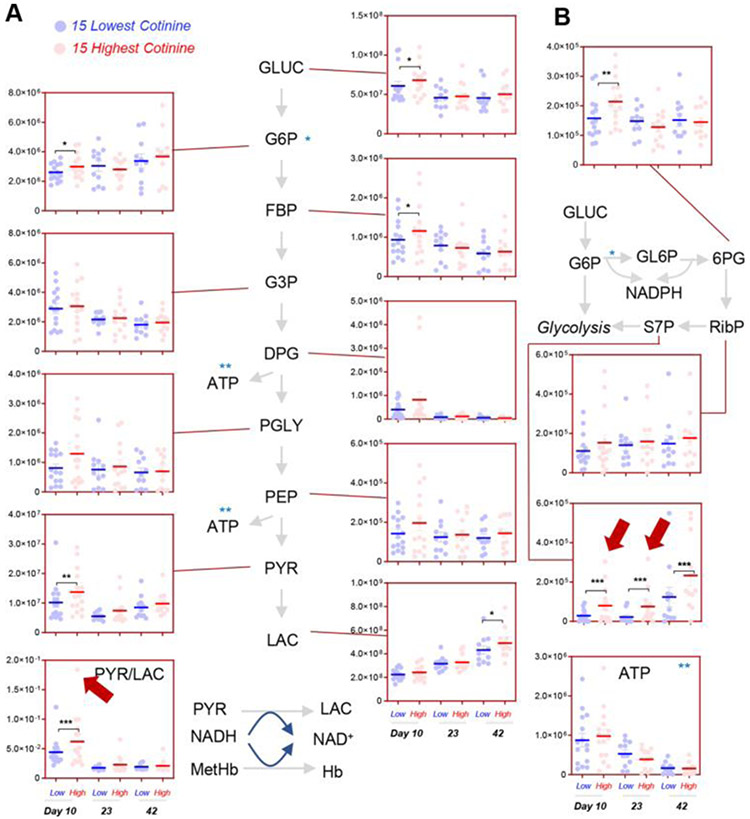
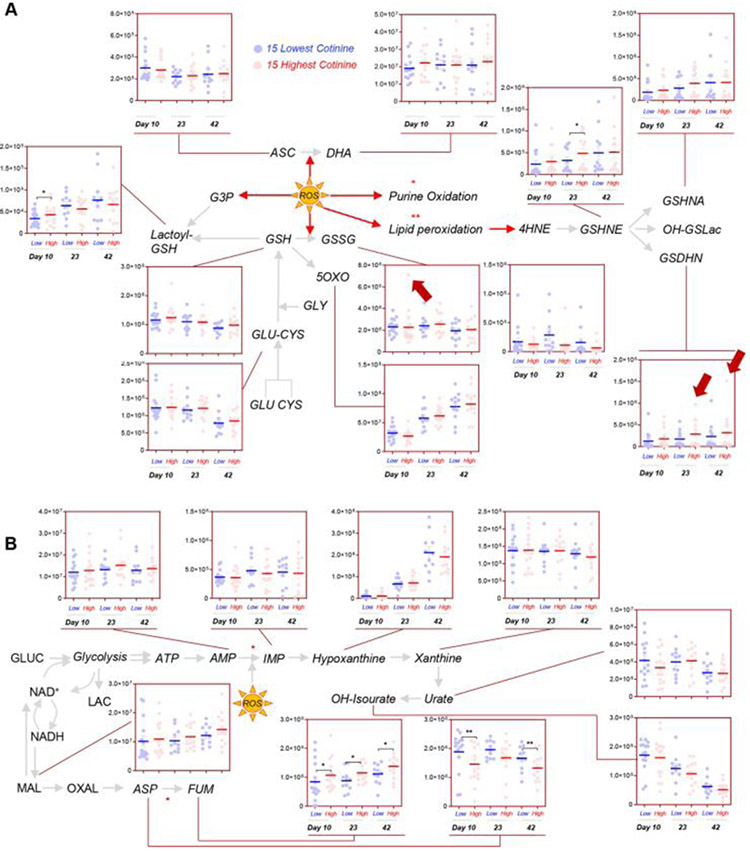
Significantly higher levels of early glycolytic intermediates, including glucose, glucose 6-phosphate (hexose phosphate isobars), and fructose bisphosphate were observed in Day 10 RBCs from donors with a cotinine level greater than 10 ng/mL (henceforth referred to as nicotine exposed) in comparison to donors with a cotinine level of 10 ng/mL or less (henceforth referred to as non–nicotine exposed) (Fig. 3A). Higher levels of other triose phosphate compounds were measured in nicotine-exposed donors, though below the significance threshold (p < 0.05 repeated measure ANOVA). However, significantly higher levels of pyruvate and pyruvate/lactate ratios were observed in RBCs from nicotine-exposed donors, with the highest levels of the ratio observed in the nicotine-exposed donors with the highest cotinine levels. These results are consistent with increased hemoglobin oxidation in stored RBCs from smokers reported in prior studies.43,44 In addition, these results are suggestive of altered glucose oxidation and recapitulate prior observation in donors suffering from G6PD deficiency—a condition that constrains the generation of critical NADPH-reducing equivalents through the PPP.39 Of note, significantly higher levels of oxidative phase intermediates of the PPP (e.g., 6-phosphogluconate) were observed in Day 10 RBCs from nicotine-exposed donors, while non–oxidative phase metabolites (e.g., sedoheptulose phosphate) were significant higher in nicotine-exposed donors at all the time points tested in this study (Fig. 3B). Despite these observations, no significant decreases in the levels of reduced glutathione were noted in nicotine-exposed donors (Fig. 4A). However, increased glutathionylation of oxidized triose sugars (glyoxylate pathway, active in RBCs57) at Storage Day 10, and of lipid aldehydes resulting from oxidative stress at Storage Days 23 and 42 (especially 4-hydroxynonenal derivatives, such as 4-hydroxynonenal glutathione conjugate and glutathionyl-1,4-dihydroxynonene58; Fig. 4A) were observed. On the other hand, we did not observe any significant increase in purine oxidation products in nicotine-exposed donors (Fig. 4B), which could be explained in part by an overactivation of purine salvage reactions leading to the consumption of asparate and the generation of fumarate (Fig. 4B), as previously described.27 Finally, minor albeit nonsignificant decreases in the antioxidant urate59 were noted in the nicotine-exposed group at Storage Day 10 (Fig. 4B).
Fig. 3.
The effect of nicotine exposure on RBC glycolysis (A) and PPP (B) in the 15 subjects with the highest (red) and lowest (blue) levels of cotinine in the recalled REDS III donor population. Red arrows highlight the subjects with the highest levels of cotinine when the subject is an outlier for a given metabolite measurement.
Fig. 4.
The effect of nicotine exposure on RBC glutathione/ascorbate metabolism (A) and purine oxidation (B) in the 15 subjects with the highest (red) and lowest (blue) levels of cotinine in the recalled REDS III donor population. Red arrows highlight the subjects with the highest levels of cotinine when the subject is an outlier for a given metabolite measurement.
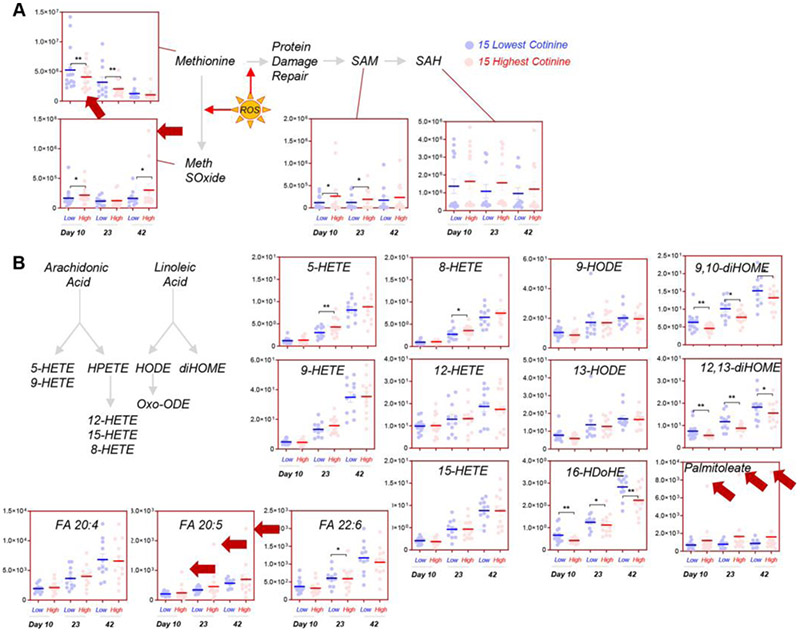
Consistent with a higher degree of oxidation at earlier storage time points, nicotine-exposed donors were characterized by higher methionine consumption and accumulation of methionine sulfoxide (direct oxidation product) and S-adenosyl-methionine (Fig. 5A), involved in damage repair mechanism of oxidized proteins.60 Only some arachidonic acid oxidation products were increased in nicotine-exposed donors (including 5- and 8-hydroxyeicosatetraenoic acid [HETE]; Fig. 5B). On the other hand, desaturation products of linoleic acid and docosahexaenoic acid were significantly lower through the tested time points (9,10- and 12,13-dihomogammalinolenic acid and 16-hydroxy-docosahexaenoic acid; Fig. 5B). In contrast, palmitoleic acid (fatty acid 16:1 n-7, desaturation product of palmitate) and eicosapentaenoic acid were both highest in the top nicotine-exposed donors (highest levels of cotinine; Fig. 5B).
Fig. 5.
The effect of nicotine exposure on RBC methionine (A) and lipid oxidation metabolism (B) in the 15 subjects with the highest (red) and lowest (blue) levels of cotinine in the recalled REDS III donor population. Red arrows highlight the subjects with the highest levels of cotinine when the subject is an outlier for a given metabolite measurement.
Metabolic correlates to nicotine exposure based on cotinine levels
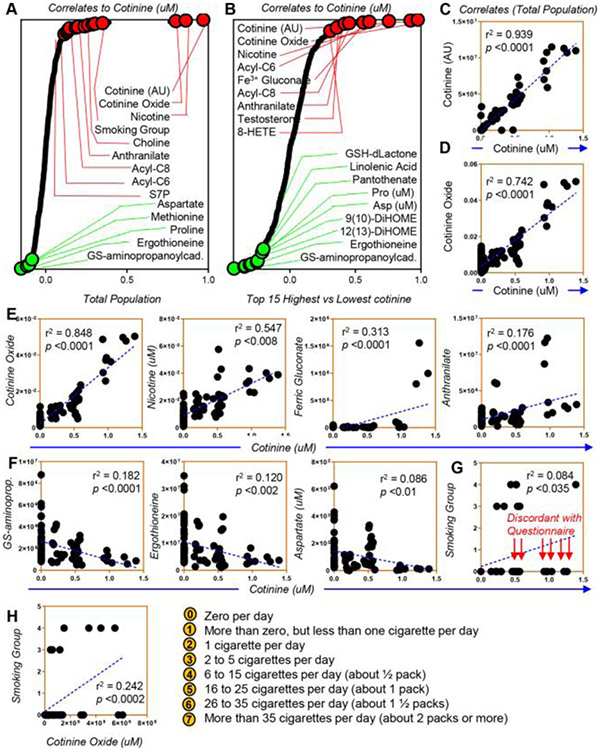
In light of the observations above, we performed correlative analyses (Spearman) of metabolic measurements to cotinine levels (reported extensively in tabulated form in Table S1, available as supporting information in the online version of this paper). Notably, relative and absolute quantitative measurements of cotinine in both the total population (Fig. 6A) and the 15 donors with the highest cotinine levels (Fig. 6B) correlated significantly among each other (p < 0.0001, r2 = 0.939; Fig. 6C), supportive of the reliability of the relative quantitative measurements when no stable isotope-labeled internal standards were available for the determination of absolute concentrations for some of the metabolites investigated in this study. As expected, significant positive correlations were observed between cotinine levels and nicotine or cotinine oxide (Fig. 6D, E), and between cotinine and oxidative stress markers such as ferric gluconate and anthranilate (tryptophan metabolite; Fig. 6E), acylcarnitines (C6 and C8) and sedoheptulose phosphate (Fig. 6A). On the other hand, negative correlations were observed between cotinine levels and antioxidant metabolites (glutathionylaminopropylcadaverine, the dietary antioxidant ergothioneine and aspartate; Fig. 6F), as well as amino acids methionine and proline (Fig. 6A). Some of these correlations, though significant, appear to follow a nonlinear, bimodal distribution. For example, increases in ferric gluconate are observed only in donors with cotinine levels >1 μM (Fig. 6E).
Fig. 6.
Metabolic correlates to cotinine levels in the REDS III recalled donor population (A) and in the 15 subjects with the highest and lowest levels of cotinine (B). In C, correlation between relative and absolute cotinine levels (arbitrary units—[AU] vs. concentration—μM against stable isotope-labeled internal standards) in the REDS III RBC Omics recalled donor population. In D and E, positive correlates to cotinine measurements, including cotinine oxide in the whole population (D) or the 15 subjects with extreme highest or lowest cotinine measurements (E), nicotine, ferric gluconate, and anthranilate. In F, top negative correlates to cotinine. In G and H, correlations between cotinine and cotinine oxide and the smoking group defined by the self-reported questionnaire.
Correlation of cotinine levels with self-reported cigarette smoking
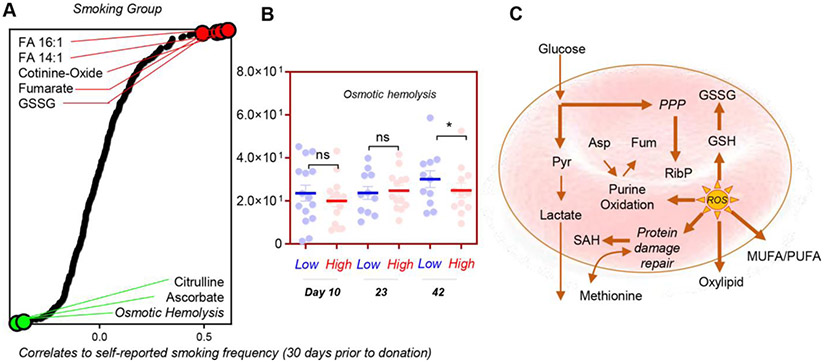
A total of 211 of 250 subjects provided a yes/no response to their smoking habit. A total of 54% of the samples (209/599 samples) and 60% of Day 10 samples (150/250 subjects) provided a response about their average smoking for the past 30 days in the questionnaire. Yet cotinine levels fared significantly well (p = 1.0157 e-20) as a predictor of smoking behavior, with an area under the curve of 0.938 (confidence interval, 87.9%−98.2%), with a 90% sensitivity and 80% specificity for cotinine measurements; of note, the threshold for smokers was here calculated to be 1.53 ng/mL, while 7 subjects who self-reported as nonsmokers had cotinine levels greater than 25 ng/mL at Storage Day 10 (Fig. S2, available as supporting information in the online version of this paper). In this study, data on self-reported cigarette smoking were not available for 39 of 250 subjects, and 7 of the subjects who reported not to smoke had cotinine levels above the threshold for smoking, suggestive of either other nicotine exposure (patches, tobacco chewing, vaping) or possibly underreporting of a potentially stigmatizing behavior. Of note, a significantly (p < 0.035) low correlation was noted between the self-reported cigarette smoking data (yes/no) and direct measurements of cotinine or cotinine oxide (Fig. 6G, H). While limitations of such a correlation between a continuous and noncontinuous variable are acknowledged, it is relevant to note that direct quantitative measurement of cotinine is a more comprehensive and reliable measure of nicotine exposure than ascertainment through a self-reported questionnaire that does not comprehensively measure all sources of nicotine exposure and relies on blood donor recall and willingness to answer potentially sensitive questions. Despite such limitations, we performed a correlative analysis between self-reported cigarette-smoking groups and metabolic measurements, highlighting indeed some of the positive and negative correlates that had already emerged from the pathway analysis above (palmitoleic acid and another fatty acid desaturation product, tetradecenoic acid; Fig. 7A). Of note, the self-reported cigarette-smoking group had an inverse correlation with osmotic hemolysis (a marker of RBC capacity to cope with osmotic stress), ascorbate levels (the antioxidant vitamin C), and citrulline, which is generated by the redox-sensitive nitric oxide synthase (which could be present and active in the mature erythrocyte61). A breakdown of osmotic hemolysis as a function of cotinine levels indicated a trend toward decrease osmotic hemolysis propensity (significant at Day 42) in the highest-cotinine-level group (as compared to the lowest-cotinine-level group) (Fig. 7B). Despite these observations, this basic correlation analysis does not take into account the potential effect of other covariates. As such, a more formal breakdown of the regression estimates for oxidative and osmotic hemolysis in the REDS-III RBC Omics recall donor population is provided in Table S2, available as supporting information in the online version of this paper. Overall, these results indicate that nicotine-exposure promotes a significant basal increase in oxidative hemolysis and decrease in osmotic fragility (Table S2, available as supporting information in the online version of this paper, and Fig. 7B). These phenotypes were accompanied by changes in the main antioxidant metabolic pathways, including glutathionylation of oxidized sugars and lipid aldehydes, consumption of sulfur amino acids, activation of the PPP, increased pyruvate-to-lactate ratios, and fatty acid desaturation (Fig. 7C).
Fig. 7.
Metabolic correlates to the questionnaire-determined smoking group in the REDS III recalled donor population (A), highlighting a lower osmotic hemolysis in smokers at the end of the storage period (B). A schematic overview of the metabolic pathways affected by nicotine exposure in this study (C).
DISCUSSION
In the present study, we provide a comprehensive metabolomics overview of the effect of nicotine exposure (as measured by cotinine levels) on RBCs during storage. Self-reported cigarette-smoking data were also available on a proportion of samples tested but, as expected, incompletely ascertained nicotine exposure as identified by cotinine measurements (>10 ng/mL for nicotine exposure including smoking, as previously described).42,53 In the present study, the questionnaire did not capture information related to noncigarette sources of exposure to nicotine, such as snuff, tobacco chewing, vaping, nicotine patches, or secondhand smoking exposure, which at least in part can explain the inconsistences between the results from the questionnaire and direct cotinine measurements.
Interestingly, in the present study, we analyzed RBCs from donors enrolled in the REDS-III RBC Omics study who were recalled to donate a second unit of blood since they ranked below the 5th or above the 95th percentile of the original study donors with respect to the propensity of their RBCs to hemolyze spontaneously (after cold storage) or following oxidant or osmotic insult. As such, we would have expected that if smoking promoted oxidant stress to RBCs as noted in prior studies on RBCs and white blood cells (WBCs),41-43,45,46,62 the nicotine-exposed donor population would be enriched owing to a skewed oxidative hemolysis propensity. In the present study, cotinine measurements were consistent with a frequency of approximately 14% of nicotine exposure among donors (of 250 subjects in the recalled donor population whose blood was available for testing), strikingly comparable to a previously reported estimate of 13%.42 A tentative explanation for this observation stems from the appreciation that RBCs from nicotine-exposed (cotinine >10 ng/mL) donors were characterized by a trend toward a higher oxidative hemolysis propensity and lower osmotic hemolysis propensity in comparison to non–nicotine-exposed donors (cotinine ≤10 ng/mL), balancing out any potential bias for enrichment of this population inadvertently introduced by the study design (which did not originally consider nicotine exposure as a variable). No significant bias with respect to donor sex and age was noted in the nicotine-exposed versus non–nicotine-exposed population in this study, suggesting that other known biologic confounders23 are not influencing the present analysis on the impact of nicotine exposure on stored RBC metabolism.
Consistent with previous studies reporting higher levels of carboxyhemoglobin in smoking donors,43 in the present study, we identified a series of markers of oxidant stress in the RBCs of nicotine-exposed donors that are suggestive of increased basal and storage-dependent levels of oxidant stress in this group. Such markers were more evident in those donors showing cotinine levels significantly higher than the median of the nicotine-exposed population (approx. 1 μM), donors who also happened to self-reportedly smoke more than 35 cigarettes/day. This observation is consistent with previous reports that significant markers of oxidant stress are measurable only in those donors smoking more than 20 cigarettes/day, even though abstinence for approximately 12 hours before donation can reduce carboxyhemoglobin levels in this group.44 This consideration is corroborated by the low modules of quadratic correlations between some correlates and cotinine levels. Indeed, metabolites like ferric gluconate seemed to follow a bimodal distribution, showing significant increases only when cotinine levels greater than 1 μM (self-reported as heaviest smokers). It is in these “chain-smoking” subjects that markers of lipid peroxidation (especially arachidonic acid, but not linolenic and docosahexaenoic acid derivatives) were significantly higher than in the rest of the population. Of note, these metabolites (especially HETEs) have been mechanistically associated with poor posttransfusion recoveries in murine models of blood storage and transfusion.30 Similar increases in fatty acid desaturation were noted in the donors with the highest cotinine levels. Though the impact of dietary factors on fatty acid (and fatty acid oxidation) profiles across these donors cannot be ruled out, it is interesting to speculate about a potential impact of desaturating enzymes as contributors to reducing equivalent homeostasis in the stored RBCs from nicotine-exposed donors, in like fashion to previous reports in cancer cells63 with metabolic phenotypes mimicking those of the mitochondria-devoid mature erythrocyte.64
In this view, it is worthwhile to note that acidosis and excess lactate accumulation in the closed system of a blood bag could fuel desaturase activity to recycle nicotinamide adenine dinucleotide (NADH) and keep glycolysis going. Consistently, basal levels of glycolytic intermediates (hexoses and trioses) were higher in the nicotine-exposed group, where pyruvate-to-lactate ratios—a proxy marker of NAD/NADH ratio as per the law of mass action—phenocopy trends observed in G6PD-deficient donors with overactivation of methemoglobin reductase.39 Consistently, overactivation of glycolysis (especially higher, albeit not significant basal levels of ATP and DPG) are consistent with a previously reported hypoxic-like signature with respect to hematologic variables of hematocrit, mean cell hemoglobin concentration and WBC count in smokers,46 suggesting that a less functional (carboxy)hemoglobin could trigger a hypoxic response in the subject.
Of note, nicotine exposure impacted a series of metabolic pathways consistent with increased levels of oxidant stress in this group. Such pathways included increased activation of the NADPH-generating PPP and consumption of methionine, a marker of oxidative hemolysis and a substrate for the synthesis of S-adenoyslmethionine as part of a mechanisms of isoaspartyl damage repair following oxidant injury to aspartyl and asparaginyl residues.60 As this pathway is up regulated in G6PD deficiency,65 and the frequency of both smoking42 and G6PD deficiency37 approximate 13% in the general and African American donor population, respectively, it would be interesting to investigate whether nicotine-exposed donors who are also G6PD deficient would be more exposed to the pro-oxidant effects of smoking on RBCs. While genotyping data were available in the tested cohort, none of the subjects enrolled in this study who were characterized by polymorphisms consistent with G6PD deficiency (African American or Mediterranean variants) also ranked among the donors with the top 15 cotinine measurements.
Increased levels of glutathionylation of triose and oxidized lipid aldehydes were observed in RBCs from nicotine-exposed donors, despite comparable levels of reduced glutathione between the two groups during storage. In the absence of tracing experiments, these results are suggestive that glutathione synthesis in nicotine-exposed donors may be constitutively more active (as a compensatory mechanism to cope with basal oxidant stress), or that other antioxidants are being consumed more in this group. Consistently, the levels of the antioxidants ascorbate, ergothioneine, and methionine were among the top negative correlates to self-reported cigarette-smoking frequency and cotinine levels, respectively, with methionine sulfoxide accumulating significantly in the nicotine-exposed group. Future studies could consider exploring the predictable benefits of dietary antioxidants (vitamin C, ergothioneine, taurine) in nicotine-exposed blood donors.
Finally, no significant increases in ATP-breakdown and purine oxidation products were observed in the nicotine-exposed group. However, significant consumption of aspartate and accumulation of fumarate is suggestive of a compensatory increase in purine salvage reactions in this group.27 Alternatively, these results are consistent with the overactivation of cytosolic isoforms of enzymes involved in carboxylic acid metabolism, an alternative mechanism through which RBCs could control the homeostasis of reducing equivalents in the face of hypoxia or oxidant stress.66
Overall, in the present study we report the increase of several markers of oxidant stress in the stored RBCs from nicotine-exposed donors, which accounted for approximately 14% of the total donor population in this study. The results are consistent with the basal activation in RBCs donated by nicotine-exposed donors of a series of pathways usually involved in oxidative damage repair in the mature erythrocyte, including but not limited to the PPP, methemoglobin reductase (as gleaned indirectly by pyruvate-to-lactate ratios), methionine oxidation and consumption in protein damage-repair mechanisms, consumption of dietary antioxidants (e.g., ascorbate and ergothioneine), glutathionylation of oxidized sugars and lipid aldehydes, fatty acid desaturation, and lipid oxidation. In the light of these preliminary observations, future studies could further explore the potential cross-talk between common enzymopathies (e.g., G6PD deficiency) or environmental factors (e.g., dietary antioxidants or storage additives with different effect on the RBC capacity to cope with oxidant insults21 resulting from storage or processing, like gamma irradiation; Roubinian et al., unpublished data) with active nicotine exposure and the impact this could have on storage quality and transfusion outcomes. Such studies will have to consider technical caveats, such as how best to ascertain nicotine exposure and its source(s) through direct analytical measurements.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was funded by the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and -00009I, as well as funds from the National Institute of General and Medical Sciences (RM1GM131968 to ADA), NHLBI R01HL146442 (ADA) and R01HL148151 (ADA, JCZ); the Boettcher Webb-Waring Investigator Award (ADA); and a Shared Instrument grant by the National Institutes of Health (S10OD021641). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to express their gratitude Dr. Simone Glynn of NHLBI for her support throughout this study, the RBC-Omics research staff at all participating blood centers and testing labs for their contribution to this project, and to all blood donors who agreed to participate in this study.
ABBREVIATIONS:
- ATP
adenosine triphopshate
- DPG
2,3-diphosphoglycerate
- G6PD
glucose 6-phosphate dehydrogenase
- HETE
hydroxyeicosatetraenoic acid
- MS
mass spectrometry
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NHLBI
National Heart, Lung, and Blood Institute
- PPP
pentose phosphate pathway
- UHPLC
ultra-high-pressure liquid chromatography
Appendix
RBC-OMICS STUDY GROUP MEMBERS
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), Red Blood Cell (RBC)-Omics Study, is the responsibility of the following persons: Hubs: A. E. Mast, J. L. Gottschall, W. Bialkowski, Anderson, J. Miller, A. Hall, Z. Udee, and V. Johnson, BloodCenter of Wisconsin, Milwaukee, WI; D. J. Triulzi, J. E. Kiss, and P. A. D’Andrea, The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA; E. L. Murphy and A. M. Guiltinan, University of California, San Francisco, San Francisco, CA; R. G. Cable, B. R. Spencer, and S. T. Johnson, American Red Cross Blood Services, Farmington, CT; data coordinating center: D. J. Brambilla, M. T. Sullivan, S. M. Endres, G. P. Page, Y. Guo, N. Haywood, D. Ringer, and B. C. Siege, RTI International, Rockville, MD; central and testing laboratories: M. P. Busch, M. C. Lanteri, M. Stone, and S. Keating, Blood Systems Research Institute, San Francisco, CA; T. Kanias and M. Gladwin, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA; steering committee chairman: S. H. Kleinman, University of British Columbia, Victoria, BC, Canada; National Heart, Lung, and Blood Institute, National Institutes of Health: S. A. Glynn, K. B. Malkin, and A. M. Cristman.
Footnotes
CONFLICT OF INTEREST
DS, XF, JAR, TK, GPP, LD, NR, MS, SK, and MB have disclosed no conflicts of interest. Though unrelated to the contents of this manuscripts, the authors declare that AD and TN are founders of Omix Technologies Inc and Altis Biosciencens LLC. JCZ serves as a consultant for Rubius Therapeutics.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Appendix S1: Supplementary Information.
REFERENCES
- 1.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus 2019;17:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurkovich JT, Zielinski DC, Yang L, et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J Biol Chem 2017;292:19556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatto C, Milanick M. Red blood cell Na pump: insights from species differences. Blood Cells Mol Dis 2009;42:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Alessandro A, D’Amici GM, Vaglio S, et al. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica 2012;97:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gevi F, D’Alessandro A, Rinalducci S, et al. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics 2012;76:168–80. [DOI] [PubMed] [Google Scholar]
- 6.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion 2016;56:852–62. [DOI] [PubMed] [Google Scholar]
- 7.Pertinhez TA, Casali E, Lindner L, et al. Biochemical assessment of red blood cells during storage by 1H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus 2014;12:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev 2014; 28:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017;57:325–36. [DOI] [PubMed] [Google Scholar]
- 10.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion 2015;55:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessandro A, Hansen KC, Silliman CC, et al. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang 2014;108:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion 2018;58:1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfsson Ó, Sigurjonsson ÓE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang 2017;112:326–35. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Nemkov T, Hansen KC, et al. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion 2015;55:2955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Korte D New additive solutions for red cells. ISBT Sci Ser 2016;11(S1):165–70. [Google Scholar]
- 16.Hess JR. An update on solutions for red cell storage. Vox Sang 2006;91:13–9. [DOI] [PubMed] [Google Scholar]
- 17.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016;128:e32–42. [DOI] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Hansen KC, Eisenmesser EZ, et al. Protect, repair, destroy or sacrifice: a role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paglia G, D’Alessandro A, Rolfsson Ó, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016;128:e43–50. [DOI] [PubMed] [Google Scholar]
- 20.D’alessandro A, Nemkov T, Reisz J, et al. Omics markers of the red cell storage lesion and metabolic linkage. Blood Transfus 2017;15:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored Red Blood Cell metabolism as much as storage time: lessons from REDS III – Omics. Transfusion 2018;59:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanias T, Busch MP, National Heart, Lung, and Blood Institute Recipient Epidemiology Donor Evaluation Study III (REDS-III) Programme. Diversity in a blood bag: application of omics technologies to inform precision Transfusion Medicine. Blood Transfus 2019;5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Alessandro A, Zimring JC, Busch M. Chronological storage age and metabolic age of stored red blood cells: are they the same? Transfusion 2019;59:1620–1623. [DOI] [PubMed] [Google Scholar]
- 25.Koch CG, Duncan AI, Figueroa P, et al. Real age: red blood cell aging during storage. Ann Thorac Surg 2019;107:973–980. [cited 2018 Oct 23]. Available from: http://www.sciencedirect.com/science/article/pii/S0003497518314954. [DOI] [PubMed] [Google Scholar]
- 26.Van’t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion 2015; 55:1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018;103:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu X, Felcyn JR, Odem-Davis K, et al. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion 2016;56:2560–70. [DOI] [PubMed] [Google Scholar]
- 29.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016;101:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howie HL, Hay AM, de Wolski K, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv 2019;3:2272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casali E, Berni P, Spisni A, et al. Hypoxanthine: a new paradigm to interpret the origin of transfusion toxicity. Blood Transfus 2016;14:555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015;372:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA 2012;308:1443–51. [DOI] [PubMed] [Google Scholar]
- 34.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med 2016;375:1937–45. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410–8. [DOI] [PubMed] [Google Scholar]
- 36.Nkhoma ET, Poole C, Vannappagari V, et al. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis 2009;42:267–78. [DOI] [PubMed] [Google Scholar]
- 37.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang 2013;105:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisz JA, Tzounakas VL, Nemkov T, et al. Metabolic linkage and correlations to storage capacity in erythrocytes from glucose 6-phosphate dehydrogenase-deficient donors. Front Med 2018;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med 2016;96:152–65. [DOI] [PubMed] [Google Scholar]
- 40.Sagiv E, Fasano RM, Luban NLC, et al. Glucose-6-phosphate-dehydrogenase deficient red blood cell units are associated with decreased posttransfusion red blood cell survival in children with sickle cell disease. Am J Hematol 2018;93:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuk T, Očić T, Jukić I. Influence of cigarette smoking on haemoglobin concentration - do we need a different approach to blood donor selection? Transfus Med 2019;29(Suppl 1):70–1. [DOI] [PubMed] [Google Scholar]
- 42.DeSimone RA, Hayden JA, Mazur CA, et al. Red blood cells donated by smokers: a pilot investigation of recipient transfusion outcomes. Transfusion 2019;59:2537–43. [DOI] [PubMed] [Google Scholar]
- 43.Boehm R, Cohen C, Pulcinelli R, et al. Toxic elements in packed red blood cells from smoker donors: a risk for paediatric transfusion? Vox Sang 2019;14:808–815. [DOI] [PubMed] [Google Scholar]
- 44.Boehm RE, Arbo BD, Leal D, et al. Smoking fewer than 20 cigarettes per day and remaining abstinent for more than 12 hours reduces carboxyhemoglobin levels in packed red blood cells for transfusion. PLoS One 2018;13:e0204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Apolipoprotein J/clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence. PLoS One 2011;6:e26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malenica M, Prnjavorac B, Bego T, et al. Effect of cigarette smoking on haematological parameters in healthy population. Med Arch 2017;71:132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion 2019;59:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016;56:2571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone M, Keating SM, Kanias T, et al. Piloting and implementation of quality assessment and quality control procedures in RBC-Omics: a large multi-center study of red blood cell hemolysis during storage. Transfusion 2019;59:57–66. [DOI] [PubMed] [Google Scholar]
- 51.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018;58:2978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemkov T, Hansen KC, Dumont LJ, et al. Metabolomics in transfusion medicine. Transfusion 2016;56:980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57:79–115. [DOI] [PubMed] [Google Scholar]
- 54.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 2017;31:663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reisz JA, Zheng C, D’Alessandro A, et al. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. Methods Mol Biol 1978;2019:121–35. [DOI] [PubMed] [Google Scholar]
- 56.Nemkov T, Reisz JA, Gehrke S, et al. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol Biol 1978;2019:13–26. [DOI] [PubMed] [Google Scholar]
- 57.Knight J, Wood KD, Lange JN, et al. Oxalate formation from glyoxal in erythrocytes. Urology 2016;88:226.e11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frohnert BI, Long EK, Hahn WS, et al. Glutathionylated lipid aldehydes are products of adipocyte oxidative stress and activators of macrophage inflammation. Diabetes 2014;63:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzounakas VL, Karadimas DG, Anastasiadi AT, et al. Donorspecific individuality of red blood cell performance during storage is partly a function of serum uric acid levels. Transfusion 2018;58:34–40. [DOI] [PubMed] [Google Scholar]
- 60.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018;58:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006;107:2943–51. [DOI] [PubMed] [Google Scholar]
- 62.Piperakis SM, Visvardis EE, Sagnou M, et al. Effects of smoking and aging on oxidative DNA damage of human lymphocytes. Carcinogenesis 1998;19:695–8. [DOI] [PubMed] [Google Scholar]
- 63.Kim W, Deik A, Gonzalez C, et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. Cell Metab 2019;29:856–870.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghashghaeinia M, Köberle M, Mrowietz U, et al. Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes. Cell Cycle 2019;18:1316–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ingrosso D, Cimmino A, D’Angelo S, et al. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes. FEBS J 2002;269:2032–9. [DOI] [PubMed] [Google Scholar]
- 66.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med 2017;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.