Abstract
The existence of latent tuberculosis infection (LTBI) is one of the main obstacles hindering eradication of tuberculosis (TB). To better understand molecular mechanisms and explore biomarkers for the pathogen during LTBI, we cultured strains of Mycobacterium tuberculosis (Mtb) under stress conditions, mimicking those in the host granuloma intracellular environment, to induce entry into the non-replicating persistence stage. The stresses included hypoxia, low pH (5.0), iron deprivation (100 μM of 2, 2’˗dipyridyl) and nutrient starvation (10% M7H9 medium). Three Mtb strains were studied: two clinical isolates (drug-susceptible Beijing (BJ) and multidrug-resistant Beijing (MDR-BJ) strains) and the reference laboratory strain, H37Rv. We investigated the proteomics profiles of these strains cultured in stressful conditions and then validated the findings by transcriptional analysis. NarJ (respiratory nitrate reductase delta chain) was significantly up-regulated at the protein level and the mRNA level in all three Mtb strains. The narJ gene is a member of the narGHJI operon encoding all nitrate reductase subunits, which play a role in nitrate metabolism during the adaptation of Mtb to stressful intracellular environments and the subsequent establishment of latent TB. The identification of up-regulated mRNAs and proteins of Mtb under stress conditions could assist development of biomarkers, drug targets and vaccine antigens.
Keywords: Mycobacterium tuberculosis, Proteomics, Multiple stresses, Beijing, narJ
Highlights
-
•
The proteomics profiles between BJ and MDR-BJ strains of M. tuberculosis (Mtb) cultured in vitro in stressful conditions.
-
•
NarJ is a common protein and significantly up-regulated protein of BJ and MDR-BJ Mtb strains.
-
•
The unique proteins found on BJ and MDR-BJ Mtb strain were of “Rv3764c/tcrY” and “Rv1356c and Rv1420/uvrC”, respectively.
1. Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a major global health problem and one of the leading causes of death worldwide [[1], [2], [3]]. Currently, the major obstacle to control and eradication of TB is the ability of Mtb to persist as a life-long infection in humans in a dormant or non-replicating state (NRP) [3,4]. This condition is asymptomatic and is known as latent TB infection (LTBI) [4]. In a small proportion of LTBI cases (5–10%), the pathogen might be reactivated, leading to active TB [1]. Humans are the only reservoir of this pathogen, which needs to be considered for TB control [5].
Of particular concern are strains belonging to the East Asian or Beijing (BJ) lineages of Mtb. These have caused numerous outbreaks worldwide [6]. The hyper-virulent BJ lineage has been emerging throughout the world associated with disease outbreaks and antibiotic resistance [7,8]. Multidrug resistant BJ strains (MDR-BJ) show resistance to several current anti-tuberculosis drugs.
Metabolic processes play important roles in the pathogenesis of Mtb [9]. However, the metabolic processes that occur on entering dormancy, leading to survival and drug resistance in the host, are poorly understood [9]. Multiple-stress conditions within the host, such as oxygen depletion and immune responses, induce mycobacteria to enter a dormancy stage during which they are phenotypically drug resistant. Study of gene regulation and protein expression of Mtb may clarify the processes by which Mtb achieves dormancy. This adaptation may produce more virulent phenotypes of Mtb that can survive within granulomas of host [4,10].
Mycobacterium tuberculosis H37Rv is the most studied strain of TB in research laboratories [11]. However, a laboratory strain might not exhibit the same virulence properties as clinical strains [4]. To better understand the NRP stage of Mtb, in vitro models have been designed to mimic intracellular stress conditions. In this study, we aimed to mimic multiple stresses in vitro by combining conditions of hypoxia, acidic pH, iron deprivation and nutrient starvation. These conditions induce Mtb to enter the NRP stage. We investigated the protein expression patterns (using proteomic approaches and mRNA quantitative analysis) of Mtb reference strain H37Rv, and two clinical strains, BJ and MDR-BJ, cultured in this way.
2. Materials and methods
2.1. Bacterial strains, media and growth conditions
Mycobacterium tuberculosis H37Rv laboratory strain (NCBI:txid1773) and two clinical isolates, drug-susceptible Beijing (BJ) (NCBI:txid634955) and multidrug-resistant Beijing (MDR-BJ), were used. The strains used in this study cannot be linked to patient information, a condition approved by the Khon Kaen University Ethics Committee (No. HE621448). Three strains of Mtb were cultured in 20 ml of Middlebrook 7H9 (M7H9) medium (Sigma) and supplemented with 0.2% of glycerol and 10% of BBL™ Middlebrook OADC Enrichment (BD, US). Bacterial cells were grown for 7 days with shaking at 37 °C. The number of culturable cells was estimated by plate count technique.
To mimic the multiple stress conditions, these media were prepared as follows: M7H9 medium with supplements was diluted to 10% with sterile water. The iron chelator was added to 100 μM, methylene blue was added to 1.5 μg/ml, 36% HCl was added to adjust the pH to 5.0. Log-phase Mtb cells, 105 CFU/ml were then inoculated into 20 ml of multiple stress media with parafilm-sealed test tubes, which were incubated at 37 °C without shaking. The decolorization of methylene blue was used to indicate oxygen depletion (3–4 weeks), which causes Mtb cells to enter the non-replicating/dormant stage after 4 weeks. Log-phase Mtb cells, 105 CFU/ml were then inoculated into 20 ml of control (M7H9 media) tubes were not sealed and incubated at 37 °C with shaking for 4 weeks.
2.2. Protein preparation, in-gel digestion and LC˗MS/MS
Bacterial cell pellets were harvested by centrifugation after 4 weeks of incubation. Protein was then isolated from the cells by using lysis buffer (0.5 M Na2HPO4, 5 M NaCl, 1 M imidazole, 100 mg/ml lysozyme, 1X protease inhibitor cocktail (Amresco, USA), 1 M dithiothreitol) with 0.1 mm zirconia/silica beads (BioSpec Products, Inc.). Protein concentrations were determined by the Bradford protein assay, using Bradford reagent (Bio-Rad Laboratories, Inc.). To separate proteins, 5 μg of total proteins of each sample were electrophoresed through 12.5% SDS-PAGE. Gels were then cut, and in-gel digestion was performed.
To investigate the protein expression patterns of Mtb, liquid chromatography tandem mass spectrometry (LC˗MS/MS) analysis and associated bioinformatics analysis were performed as previously described [12]. The detailed methods of this part are shown in supplementary materials and methods.
2.3. RNA extraction and cDNA synthesis
Bacterial cells were harvested by centrifugation after 4 weeks of incubation. RNA was isolated using Trizol reagent (Invitrogen, USA) with silica beads, according to the manufacturer's instructions. Five hundred nanograms of RNA was treated with DNase I (Invitrogen, USA), to remove genomic DNA, according to the manufacturer's instructions. cDNA was then synthesized using SuperScript III Reverse Transcriptase (Invitrogen, USA) according to the manufacturer's instructions.
2.4. Quantitative real time PCR (qRT-PCR)
Mycobacterium tuberculosis genes that were differentially expressed between the active stage and NRP stage, including narJ, tcrY, uvrC, Rv1356c and Rv3134c, were selected for further study. The primer sequences used in this study are listed in Table 1. The expression of Mtb genes was examined by qRT-PCR. The PCR reaction was performed on a real-time PCR instrument (Applied Biosystems QuantStudio 6 Flex Real-Time PCR System) using SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratory, Inc., USA). The relative expression of genes was examined by 2-ΔΔCt. The 16S rRNA gene was used as an internal control.
Table 1.
Primer pairs used to amplify cDNA.
| Gene | Direction/position of primer | Sequence |
|---|---|---|
| 16S rRNAa | Forward, 469-491 | 5′-TTGACGGTAGGTGGAGAAGAAGC-3′ |
| Reverse, 909-888 | 5′-CCTTTGAGTTTTAGCCTTGCGG-3′ | |
| narJ | Forward, 439-512 | 5′-CCTATGAGTACACCGTGGCG-3′ |
| Reverse, 603-584 | 5′-GGGACGGTCAAGGTAAACGG-3′ | |
| tcrY | Forward, 796-815 | 5′-CATGAACTGCGAACTCCCCT-3′ |
| Reverse, 930-911 | 5′-GACGAGACGTGTTATCCGCT-3′ | |
| uvrC | Forward, 114-133 | 5′-CGAGTCATCTACGTCGGCAA-3′ |
| Reverse, 308-289 | 5′-GAATCGCGGATCGAACTCCT-3′ | |
| Rv1356c | Forward, 531-550 | 5′-CCTACCGCTAAGCATGTCCC-3′ |
| Reverse, 643-624 | 5′-CCGGGATTCTCGCTGCTATT-3′ | |
| Rv3134c | Forward, 472-491 | 5′-GAGGTGGACAATGGTGTGGT-3′ |
| Reverse, 610-591 | 5′-TTCGACGTCATCGGGTGTTT-3′ |
The 16S rRNA gene was used as a housekeeping gene to normalize the data. Primers from Haile et al. (2002) [13].
2.5. Construction of protein-protein interaction networks
STRING (search tool for the retrieval of interacting genes/proteins) software was used to construct interaction networks of protein. Predicted functional partners of protein by STRING version 11.0 (https://string-db.org/) with high confidence interaction score (0.700).
2.6. Statistical analysis
GraphPad prism 5.0 was used for all data analysis. All experiments were done in triplicate and values are expressed as mean ± SD. Student's t-test was used to analyze the difference among two groups. Statistically significant differences between groups are indicated by *p < 0.05; **p < 0.01; ***p < 0.001.
3. Results
3.1. Proteins detected only during the NRP stage or the active stage
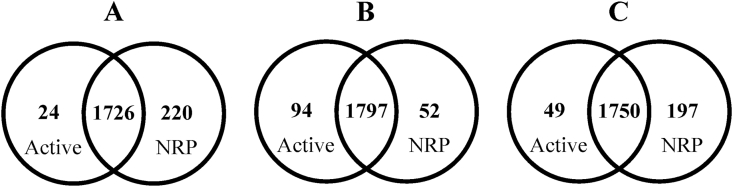
To identify proteins found only in Mtb cultures under multiple stress conditions (i.e. during the NRP stage), proteomic profiles of the NRP and active stages were compared. During the NRP stage of H37Rv, BJ and MDR-BJ strains, 1,946, 1849 and 1947 proteins were detected, respectively. Of these, 220, 52 and 197 proteins were found only in the NRP stage of H37Rv, BJ and MDR-BJ strains, respectively (Fig. 1A˗C). The details and complete lists of these proteins and their relative quantities are given in Table S1.
Fig. 1.
Venn diagram showing the number of proteins present in Mtb strains during the NRP stage and the active stage, analyzed by LC-MS/MS. The strains are Mtb H37Rv (A), BJ (B) and MDR-BJ (C).
The same analyses identified 1,750, 1891 and 1799 proteins expressed by H37Rv, BJ and MDR-BJ strains, respectively, during growth in culture. There were 24, 94 and 49 proteins unique to the active stage of H37Rv, BJ and MDR-BJ strains, respectively (Fig. 1A˗C).
3.2. Common unique proteins in NRP stage
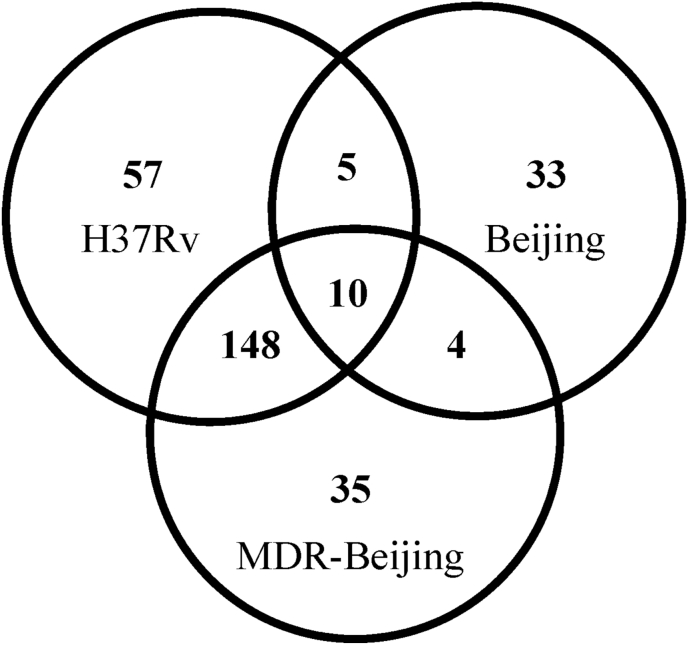
Ten proteins were found only in the NRP stage in all three Mtb strains (Fig. 2). Further analysis revealed only one protein, narJ, that was characterized in the reference strains, whereas another 9 proteins were classified as non-characterized proteins. Fifty-seven, 33 and 35 proteins were unique to H37Rv, BJ and MDR-BJ strains, respectively, during the NRP stage. But further analysis found only 1, 1 and 2 proteins, respectively, that were well-characterized in database of the M. tuberculosis H37Rv reference strain and significantly up-regulated. These proteins were Rv3134c (in the H37Rv strain), tcrY (BJ strain) and Rv1356c and uvrC (both in the MDR-BJ strain). The details and complete list of proteins in the three Mtb strains, and their relative quantities are given in Table 2 and Table S2.
Fig. 2.
Venn diagram showing the number of unique proteins present in Mtb strains during the NRP stage. LC-MS/MS analysis of Mtb cells induced to enter the NRP stage by in vitro multiple stressors condition. The strains are Mtb H37Rv, BJ and MDR-BJ.
Table 2.
The relative intensities of unique proteins in three Mtb strains during the NRP stage induced by culture in conditions of multiple stresses.
| GI no. | ORF no. | Gene symbol | Gene description | Log 2 fold | SD | P-value |
|---|---|---|---|---|---|---|
| M. tuberculosis H37Rv strain | ||||||
| gi|15,610,270 | Rv3134c | – | Universal stress protein family protein | 16.986 | 0.090 | 0.004 |
| M. tuberculosis Beijing strain | ||||||
| gi|499,188,641 | Rv3764c | tcrY | Possible two component sensor kinase | 13.579 | 0.664 | 3.97E-04 |
| MDR M. tuberculosis Beijing strain | ||||||
| gi|15,608,496 | Rv1356c | – | Hypothetical protein | 12.741 | 1.639 | 0.003 |
| gi|15,608,558 | Rv1420 | uvrC | Probable excinuclease ABC (subunit C-nuclease) UvrC | 14.889 | 0.819 | 5.04E-04 |
3.3. Validation of proteomic analysis by quantitative real-time PCR
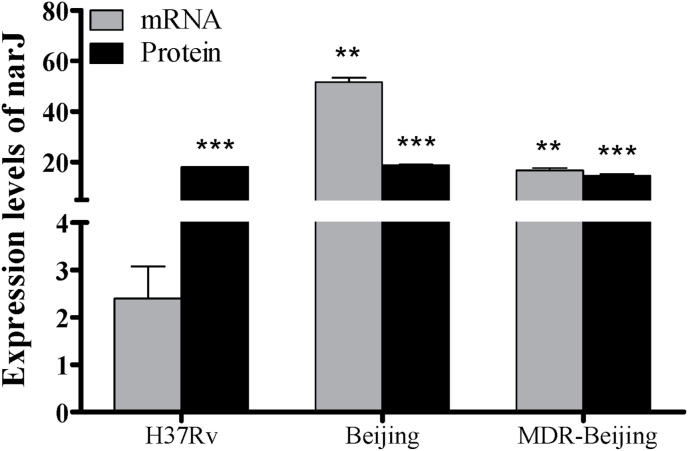
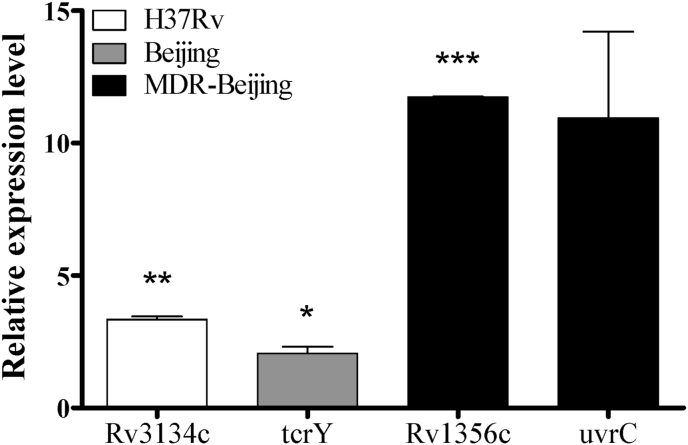
The shared and strain-specific proteins found by LC-MS/MS analysis to be over-expressed during the NRP stage in the three different Mtb strains were then further validated by measuring mRNA expression levels using qRT-PCR. The relative mRNA expression level of narJ was up-regulated in all strains. The fold-changes of expression level in H37Rv, BJ and MDR-BJ strains were 2.397 (p > 0.05), 51.536 (p < 0.01) and 16.661 (p < 0.01), respectively. These results were correlated with relative intensities (log2-fold) of proteins analyzed by LC-MS/MS. The intensity (log2-fold) of narJ in H37Rv, BJ and MDR-BJ strains were 17.953 (p < 0.001), 18.730 (p < 0.001) and 14.614 (p < 0.001), respectively (Fig. 3). In addition, the relative mRNA expression levels of Rv3134c, tcrY, Rv1356c and uvrC were significant up-regulated, with fold-changes of 3.344, 2.057, 11.735 and 10.941, respectively (Fig. 4). The results of qRT-PCR for all proteins confirmed the results of the LC-MS/MS.
Fig. 3.
Quantitation of narJ mRNA and protein expression in three Mtb strains. The relative expression levels (fold change) of narJ mRNA were determined by qRT-PCR. The data presented are means of expression fold-change normalized by the expression level of 16S rRNA. The relative intensity level (peptide intensity, log2-fold) of narJ protein was determined by LC-MS/MS. *: p < 0.05; **: p < 0.01; ***: p < 0.001 (T-test comparing the multiple stresses culture versus the control culture of each strain).
Fig. 4.
The relative expression levels of individual mRNA encoding strain-specific proteins in H37Rv (white), Beijing (gray) and MDR-Beijing (black) through quantitative RT-PCR. The expression levels were determined following culture for 4 weeks in conditions of multiple stressors, compared to the expression levels in control growth conditions. The data presented are means of expression fold-change normalized by the expression level of 16S rRNA. *: p < 0.05; **: p < 0.01; ***: p < 0.001 (T-test comparing the multiple stresses culture versus the control culture of each strain).
4. Discussion
There have been few comparisons of proteomics profiles of BJ and MDR-BJ strains in the same experiment. These strains are very similar, differing only by several mutations associated with drug resistance. However, our proteomic profiles revealed a large number of strain-specific proteins—38 (33 + 5) in BJ and 183 (35 + 148) in MDR-BJ (Fig. 2). This suggests that the multidrug-resistant phenotype might be associated with a large number of proteins.
The narJ protein was found only in the NRP stage of BJ and MDR-BJ. This protein is a subunit of nitrate reductase enzyme which bacteria use nitrate as a final electron acceptor instead of oxygen [14]. Nitrate reductase activity occurs at a low level during an aerobic growth of Mtb and significantly increases during the NRP stage under hypoxia [14]. Nitrate reductase activity of Mtb is also correlated with the virulence and is also associated with its growth under anaerobic conditions [15]. NarJ is a promising biomarker for the dormancy stage, especially in the BJ strain.
STRING analysis of narJ, the respiratory nitrate reductase delta chain, revealed the protein association networks involving this gene, including proteins encoded in narGHIJ gene clusters. The narG/Rv1161, narH/Rv1162 and narI/Rv1164 genes encoded respiratory nitrate reductase alpha, beta and gamma chains, respectively. These proteins interact with each other at the same time and place. The other proteins shown in Fig. S1 with high and significant score of association with narJ included narX/Rv1736c, nitrate reductase, narK2/Rv1737c, nitrate/nitrite transporter, narU/Rv0267, integral membrane nitrite extrusion protein (nitrite facilitator), nirD/Rv0253, nitrite reductase [NAD(P)H] small subunit, typA/Rv1165, GTP-binding translation elongation factor TypA (tyrosine phosphorylated protein A) (GTP-binding protein) and moeA1/Rv0994, molybdopterin biosynthesis protein. Network nodes represent proteins; spliced isoforms or post-translational modifications are collapsed, i.e. each node represents all the proteins produced by a single protein-coding gene locus. Therefore, Mtb nitrate respiration is also the genes encoded narGHJI operon and is also associated with narK 1, narK2, narK 3, narL, narX, and narU [15].
The narGHJI operon encodes nitrate reductase [14]. To survive in a stress condition, narK2/Rv1737c is up-regulated during anaerobic conditions [15], which allows the transport of nitrate into and nitrite out of the cell [16]. These transportations may generate the ATP, which necessary to Mtb survival in the absence of oxygen as a terminal electron acceptor [16]. Respiratory reduction of nitrate through narGHJI operon which could provide energy for the latent stage survival of the Mtb [16].
Nitrate respiration within phagosomes in macrophages is due to the anaerobic environment there [14]. The composition of nitrate reductase operon family in mycobacterial: the narG and narH bind to the plasma membrane of phagosome via the interaction between a hydrophobic patch of narH and narI which are bound to the cell membrane [15]. NarJ is a specific ligand that recognizes and binds to narG, and forms a complex with narGH to facilitate narJ, [4Fe–4S] cluster, and molybdenum cofactor successively. NarJ dissociates from the complex before interacting with membrane-binding narI. Nitrate passes through the cell wall into the cytoplasm via transmembrane protein narK2. It is reduced via narG and nitrite is released outside the macrophage via an unknown exporter [15]. Therefore, nitrate can be used as an alternative nitrogen source when the availability of other nitrogen sources is limited [16]. Further studies are needed concerning the availability and capability for utilization of other nitrogen sources during the latent infection stage. Such studies would provide a deeper insight into the possible link between dormancy and nitrogen assimilation and assist in identifying future drug targets.
Rv3134c, found only in H37Rv strain during the NRP stage, was annotated as a universal stress protein which could play an important role in adaptation to hypoxia and its up-regulation by at least 10-fold [17]. Moreover, its participating in the phosphorelay in the two-component regulatory system devRS [17]. A member of the dormancy Rv3132c/3133c/3134c regulon, it was induced in response to hypoxia, a strain lacking Rv3134c gene does not induce most genes of the dormancy regulon, the hypoxic regulation of hspX was eliminated. These results suggest a possible role for Rv3132c/3133c/3134c in latent stage of Mtb [17,18]. The regulon might give insights into the dormant or NRP stage of Mtb infection [17,18]. Rv3134c was significantly correlated with the expected six proteins including devR/Rv3133c, devS/Rv3132c, dosT/Rv2027c, Rv0079, hspX/Rv2031c and Rv1738. The network of direct interconnections between Rv3134c and other proteins (STRING version 11.0) with a high confidence score (0.700) is shown in Fig. S2.
TcrY, a protein seen only in the BJ strain during the NRP stage, was annotated as a two-component sensor kinase. This is associated with a two-component regulatory signal-transduction system, tcrYX, which activates phosphorylation [19,20]. Two component signal transduction systems, its process the signals resulting from the stress environment which developed by bacterium [20]. M. tuberculosis H37Rv cells lacking the tcrYX regulon show an increased virulence with significantly shorter survival times in SCID mice [19,20]. The network of direct high-confidence scoring (0.700) interconnections between TcrY and other proteins, as analyzed by STRING version 11.0, is shown in Fig. S3.
MDR-BJ strain-specific proteins during the NRP stage were Rv1356c and uvrC. These were annotated as a hypothetical protein and excinuclease ABC (subunit C - nuclease), respectively. Rv1356c is significantly up-regulated after 24 h under nutrient starvation in the H37Rv strain [21]. UvrC is a DNA-repair enzyme that catalyzes the excision of UV-damaged nucleotide segments producing oligomers having the modified base(s) [22]. In fact, Mtb is exposed to a variety of environmental, endogenous physical and chemical stresses that could produce genotoxic damage, but it possesses an efficient system to counteract the harmful effects of DNA-damaging assaults [22]. The STRING database in combination between the Rv1356c and uvrC with other proteins (STRING analysis version 11.0, with high confidence scores (0.700)) (Fig. S4). In the right-hand group, we found that the Rv1356c was associated with Rv1353c, Rv1354c and moeY/Rv1355c which is cya/Rv1625, a central protein interconnecting the two groups with a high confidence score. In the left-hand group, there are three connectors (rpoB/Rv0667, DNA-directed RNA polymerase (beta chain) RpoB (transcriptase beta chain) (RNA polymerase beta subunit), pykA/Rv1617, probable pyruvate kinase PykA and ndkA/Rv2445c, probable nucleoside diphosphate kinase NdkA (NDK) (NDP kinase) (nucleoside-2-P kinase)). However, we want to focus on rpoB, a protein involved in resistance to first-line drugs (rifampicin) [23]. RpoB connects with gyrA/Rv0006, DNA gyrase (subunit A) GyrA (DNA topoisomerase (ATP-hydrolysing)) (DNA topoisomerase II) (type II DNA topoisomerase) and gyrB/Rv0005, DNA gyrase (subunit B) GyrB (DNA topoisomerase (ATP-hydrolysing)) (DNA topoisomerase II) (type II DNA topoisomerase), all associated with resistance to second-line drugs (fluoroquinolones) [23], and then gyrAB associated between uvrA and uvrB, Uvr ABC system proteins with uvrC also known as excinuclease ABC.
Strains of the M. tuberculosis Beijing lineage are globally distributed and are often associated with severely pathogenic and virulent drug-resistant TB. The ability of Mtb to enter the NRP stage renders it even more resistant to drugs and allows it to persist in the host without causing symptoms. To deal with the problem of LTBI, understanding of the molecular mechanisms used by the pathogen is necessary. Strain-specific proteins identified in this study, particularly narJ, could be used in the search for new therapeutic targets to prevent and control TB, especially LTBI.
Author statement
Bhanubong Saiboonjan: Methodology, Validation, Investigation, Conceptualization, and Writing – review & editing. Sittiruk Roytrakul: Funding acquisition and Resources. Arunnee Sangka: Supervision and Project administration. Viraphong Lulitanond: Supervision and Funding acquisition. Kiatichai Faksri: Supervision and Investigation. Wises Namwat: Supervision, Conceptualization, Investigation, and Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Invitation research grant (Grant Number IN63102), Faculty of Medicine; Research and Diagnostic Center for Emerging Infectious Diseases (RCEID), Khon Kaen University; and National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Thailand. We would like to acknowledge Prof. David Blair, for editing the MS via Publication Clinic Khon Kaen University, Thailand.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100960.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hauck F., Neese B.H., Panchal A.S. Identification and management of latent tuberculosis infection. Am. Fam. Physician. 2009;79:879–886. [PubMed] [Google Scholar]
- 2.Gopinath V., Raghunandanan S., Gomez R.L. Profiling the proteome of Mycobacterium tuberculosis during dormancy and reactivation. Mol. Cell. Proteomics. 2015;14:2160–2176. doi: 10.1074/mcp.M115.051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chee C.B.E., Reves R., Zhang Y. Latent tuberculosis infection: opportunities and challenges. Respirology. 2018;23:893–900. doi: 10.1111/resp.13346. [DOI] [PubMed] [Google Scholar]
- 4.Devasundaram S., Gopalan A., Das S.D. Proteomics analysis of three different strains of Mycobacterium tuberculosis under in vitro hypoxia and evaluation of hypoxia associated antigen's specific memory T cells in healthy household contacts. Front. Microbiol. 2016;7:1275. doi: 10.3389/fmicb.2016.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demissie A., Leyten E.M.S., Abebe M. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2006;13:179–186. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson R., Warren R., Strauss O. An outbreak of drug-resistant tuberculosis caused by a Beijing strain in the western Cape, South Africa. Int. J. Tubercul. Lung Dis. 2006;10:1412–1414. [PubMed] [Google Scholar]
- 7.Kato-Maeda M., Kim E.Y., Flores L. Differences among sublineages of the East-Asian lineage of Mycobacterium tuberculosis in genotypic clustering. Int. J. Tubercul. Lung Dis. 2010;14:538–544. [PMC free article] [PubMed] [Google Scholar]
- 8.Luo T., Comas I., Luo D. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis beijing family with han Chinese. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:8136–8141. doi: 10.1073/pnas.1424063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deb C., Lee C.M., Dubey V.S. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PloS One. 2009;4 doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.H., Karakousis P.C., Bishai W.R. Roles of SigB and SigF in the Mycobacterium tuberculosis sigma factor network. J. Bacteriol. 2008;190:699–707. doi: 10.1128/JB.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camus J.C., Pryor M.J., Médigue C. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148:2967–2973. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 12.Kaewseekhao B., Naranbhai V., Roytrakul S. Comparative proteomics of activated THP-1 cells infected with Mycobacterium tuberculosis identifies putative clearance biomarkers for tuberculosis treatment. PloS One. 2015;10 doi: 10.1371/journal.pone.0134168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haile Y., Bjune G., Wiker H.G. Expression of the mceA, esat-6 and hspX genes in Mycobacterium tuberculosis and their responses to aerobic conditions and to restricted oxygen supply. Microbiology. 2002;148:3881–3886. doi: 10.1099/00221287-148-12-3881. [DOI] [PubMed] [Google Scholar]
- 14.Sohaskey C.D., Wayne L.G. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J. Bacteriol. 2003;185:7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Q., Abdalla A.E., Xie J. Phylogenomics of Mycobacterium nitrate reductase operon. Curr. Microbiol. 2015;71:121–128. doi: 10.1007/s00284-015-0838-2. [DOI] [PubMed] [Google Scholar]
- 16.Khan A., Sarkar D. Nitrate reduction pathways in mycobacteria and their implications during latency. Microbiology. 2012;158:301–307. doi: 10.1099/mic.0.054759-0. [DOI] [PubMed] [Google Scholar]
- 17.Sherman D.R., Voskuil M., Schnappinger D. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voskuil M.I., Schnappinger D., Visconti K.C. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parish T., Smith D.A., Kendall S. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect. Immun. 2003;71:1134–1140. doi: 10.1128/IAI.71.3.1134-1140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya M., Biswas A., Das A.K. Interaction analysis of TcrX/Y two component system from Mycobacterium tuberculosis. Biochimie. 2010;92:263–272. doi: 10.1016/j.biochi.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Betts J.C., Lukey P.T., Robb L.C. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 22.Lahiri S., Rizzi M., Rossi F. Mycobacterium tuberculosis UvrB forms dimers in solution and interacts with UvrA in the absence of ligands. Proteins Struct. Funct. Bioinforma. 2018;86:98–109. doi: 10.1002/prot.25412. [DOI] [PubMed] [Google Scholar]
- 23.Smith T., Wolff K.A., Nguyen L. Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr. Top. Microbiol. Immunol. 2013;374:53–80. doi: 10.1007/82-2012-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.