Abstract
Purpose
Defocus blur imposed by positive lenses can induce hyperopia, whereas blur imposed by diffusers induces deprivation myopia. It is unclear whether the retina can distinguish between both conditions when the magnitude of blur is matched.
Methods
Ten emmetropic (average 0.0 ± 0.3 diopters [D]) and 10 subjects with myopia (−2.7 ± 0.9 D; 24 ± 4 years) watched a movie on a large screen (65 inches at 2 meters (m) distance. The movie was presented either unfiltered (“control”), with calculated low-pass filtering equivalent to a defocus of 2.5 D, or with binocular real optical defocus of +2.5 D. Spatial filtering was done in real-time by software written in Visual C++. Axial length was followed with the Lenstar LS-900 with autopositioning system.
Results
Watching unfiltered movies (“control”) caused no changes in axial length. In emmetropes, watching movies with calculated defocus caused axial eye elongation (+9.8 ± 7.6 µm) while watching movies with real positive defocus caused shorter eyes (−8.8 ± 9.2 µm; difference between both P < 0.0001). In addition, in myopes, calculated defocus caused longer eyes (+8.4 ± 9.0 µm, P = 0.001). Strikingly, myopic eyes became also longer with positive defocus (+9.1 ± 11.2 µm, P = 0.02). The difference between emmetropic and myopic eyes was highly significant (−8.8 ± 9.2 µm vs. +9.1 ± 11.2 µm, respectively, P = 0.001).
Conclusions
(1) In emmetropic human subjects, the retina is able to distinguish between real positive defocus and calculated defocus even when the modulation transfer function was matched, (2) in myopic eyes, the retina no longer distinguishes between both conditions because the eyes became longer in both cases. Results suggest that the retina in a myopic eye has reduced ability to detect positive defocus.
Keywords: sign of defocus, retina, human, eye growth
It is well known that low-pass filtered and low-contrast retinal image trigger exaggerated eye growth, resulting in “deprivation myopia” in a variety of animal models (chickens,1 guinea pigs,2 kestrels,3 mice,4 tree shrews,5 marmosets,6 rhesus monkeys,7 and in humans8). In addition, negative lenses placed in front of the eye generate myopia but, to date, it was not possible to clarify whether the underlying mechanism is different from the one that causes deprivation myopia (i.e. Thomson et al.9). Deprivation myopia represents an open loop condition for emmetropization while compensation of negative lenses occurs under conditions of a closed loop feedback system.10 In addition, the two mechanisms triggering myopia development, a third visually controlled growth mechanism operates in the eye, which acts with shorter time constants,11,12 is even more powerful,13 and strongly inhibits eye growth when myopic refractive errors are induced by positive lenses (guinea pigs,14 tree shrews,15 rhesus monkeys,16 and chickens17). This mechanism is so powerful that it can overwrite the growth stimulating effect of diffusers. Park and colleagues found in the chicken model that hyperopia can still be induced with positive lenses when the retinal image was degraded by Bangerter foils that were placed over the lenses.18 Detection of the sign of defocus by the retina is extremely stable in chickens: it operates successfully even when the image was constantly severely defocused. In chickens that were individually placed in the center of a drum where they had only one viewing distance, and had paralyzed accommodation, the retina could still trigger bidirectional growth changes of the eye.19 Because both, diffusers and negative lenses, as well as positive lenses act as low-pass filters on the retinal image, a key question is how the retina can extract the sign of defocus when image quality is poor.20 The question is almost as old as animal experimentation in myopia research but its significance for myopia development in humans is still not clear.
Read and colleagues21 were the first to show that miniature bidirectional changes in axial eye length can also be induced in young adult human subjects when they wear positive or negative lenses for short periods of time, such as 1 hour. Later, it was found that the measured changes in axial length were based on changes in choroidal thickness rather than on changes in size of the eyeball.22 However, such experiments can still not finally prove that the human retina distinguishes between positive and negative defocus. There may be other cues, such as how image sharpness changes with viewing distance and how defocus compares in the periphery and the center. Furthermore, lenses also cause changes in accommodation tonus and require re-alignment of vergence with accommodation. To learn more about sign of defocus detection in the human retina, we have controlled visual experience in more detail. Rather than applying diffusers, we have presented movies with calculated or real optical defocus. That deprivation myopia can be induced by low-pass filtered movies was already shown in chickens by Diether et al.23 More recently, Flitcroft et al.24 proposed that less energy at higher spatial frequencies, as they found in urban visual environment, may be a reason for the high prevalence of myopia in industrialized countries. To limit availability of other distance cues, subjects in our study watched movies in a dark room at defined distance of 2 m. Spatial frequency spectra in the movies were matched for real optical defocus and calculated low-pass filtering. Studies were done in emmetropic and myopic subjects and the amount of imposed optical defocus on the retina was individually controlled by different positive lenses that generated about +2.5 diopters (D) positive defocus on the retina in all subjects.
Rather than measuring changes in choroidal thickness in optical coherence tomography (OCT) images, as was often done in related studies,25,26 we used low coherence interferometry (the Lenstar LS 900 with automatic positioning feature). An advantage over OCT is that there is no need to manually or semi-automatically segment the choroid, a procedure that is prone to interobserver variability. A disadvantage is that one cannot determine whether the measured changes in axial length were due to changes in choroidal thickness or due to potential other changes in the eye. However, it is likely that shorter axial lengths result from choroidal thickening and longer axial lengths from choroidal thinning.25,27–29
Methods
Subjects
Twenty young adults (17 women) with an average age of 24 ± 4 years and with no known ocular pathologies other than moderate myopia (the highest myopia was −4.25 D) participated in the study. Ten subjects were emmetropic with an average refraction of 0.0 ± 0.3 D (range = −0.5 D to +0.5 D) and 10 were myopic with an average refraction of −2.7 ± 0.9 D (range = −1.5 D to −4.25 D). Prior to the experiment, noncycloplegic refractive states (spheres and cylinders) were determined by a commercial portable photorefractor (PlusOptix, Nürnberg, Germany) and converted into spherical equivalent. None of the emmetropic subjects needed a distance correction, while all myopic subjects had a habitual correction. None of the subjects exhibited astigmatism or anisometropia of more than 1 D. The project was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Swiss Research Ethics Committee (EKNZ, reference 2020-01576). Written informed consent was obtained from each subject prior to the experiments.
Experimental Protocol
The study protocol involved 60 minutes binocular watching of a movie on the large TV screen (LG OLED65C9, 65 inch, 4K, 2019, screen luminance ranging from 100–300 cd/m²) at 2 m distance in a dark room, on 2 separate days. The distance of 2 m was chosen to keep the accommodation effort minimal because we had previously found that watching movies at 30 cm from a regular computer screen may cause axial elongation (unpublished observation, and ref. 30). To exclude a potential impact of diurnal factors, the experiments were always performed in the morning between 9 and 11 AM. The movie watching period was divided into two parts. During the first 30 minutes, the subjects watched unfiltered movies with their habitual corrections and the measured axial length changes served as baseline for the subsequent test conditions. During test, subjects were asked to watch the same movie again, but this time with either +2.5 D optical defocus, or with calculated defocus, equivalent also to about 2.5 D (Fig. 1). Myopes watched control and low-pass filtered movies with their standard corrections. To impose optical defocus, emmetropic subjects wore spectacles with +3 D power, causing about +2.5 D relative defocus on the retina for a viewing distance of 2 m. Myopes with refractions between −2.5 and −3.25 D watched the movie without their habitual corrections. Myopes with less than −2.5 D of myopia were defocused with additional positive trial lenses. Myopes with more than −3.25 myopia were partially corrected with negative trial lenses to also experience +2.5 D of defocus on their retina (Table).
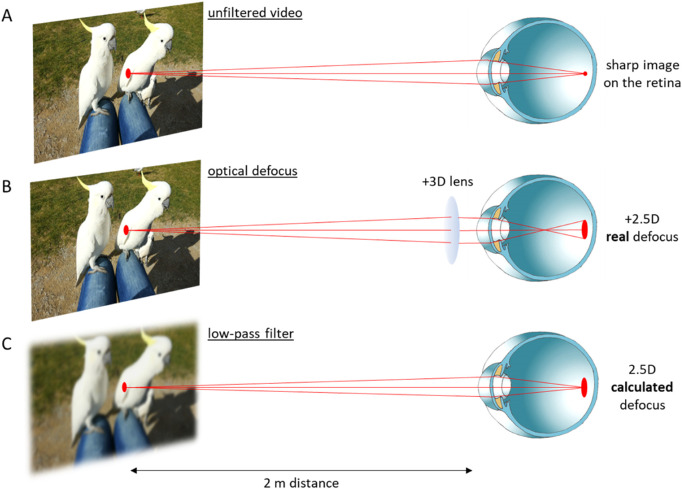
Figure 1.
Schematic illustration of the three experimental conditions. (A) An unfiltered movie presented at 2 meters distance represented the control condition. It was watched by the subjects with their habitual optical corrections if necessary. (B) Placing a +3 D lens in front of the eye caused +2.5 D relative defocus on the retina, assuming that subjects relaxed their accommodation to achieve the best possible focus. (C) The movie was low-pass filtered to create an equivalent level of blur in the retinal image, using the average pupil size of the subjects of 6.5 mm.
Table.
Imposed Optical Defocus in the Myopic Subjects
| Subject | Refraction | Addition Lens | Imposed Myopic Defocus |
|---|---|---|---|
| 1 | −1.5 | +2 | +3.5 |
| 2 | −2.5 | – | +2.5 |
| 3 | −2.75 | – | +2.75 |
| 4 | −3.25 | – | +3.25 |
| 5 | −3 | – | +3 |
| 6 | −4.25 | −1.25 | +3 |
| 7 | −2 | +1 | +3 |
| 8 | −4.25 | −1.25 | +3 |
| 9 | −1.5 | +2 | +3.5 |
| 10 | −2.5 | – | +2.5 |
To impose about +2.5 D of positive defocus on the retina, myopes with refractions between −2.5 and −3.25 D watched the movie without their habitual corrections. Subjects with lower or higher degrees of myopia watched the movie with additional trial lenses in front of their eyes to generate also about +2.5 D of defocus on their retina.
Real-Time Movie Filtering
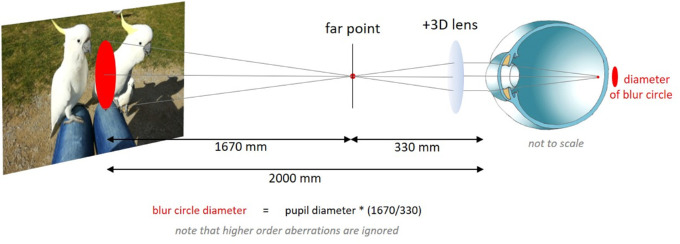
To filter movies in real-time, software was developed in Visual C++. The software read each pixel in each movie frame and averaged its value with the surrounding pixels in a circular area, simulating an aberration-free condition, that was calculated for a 6.5 mm pupil size and 2.5 D of defocus. Filtering also included the borders of the movie frame on the screen to exclude that higher spatial frequencies were generated. The circular area marked in red in Figure 2 illustrates how the diameters of the blur circle were determined by simple ray equation. It would not have been useful to implement the individual higher order aberration patterns of each subject into the calculated low pass filter because aberrations would then be added twice, once in the filtered movie and once in the eye.
Figure 2.
Illustration of the geometry of the experimental setup and the calculation of the blur circle generated with a +3 D lens. With a +3 D lens, an emmetropic subject was assumed to be focused at its far point to see the movie with the best possible focus. With a +3 D lens, the far point is at 330 mm distance and the diameter of the blur circle on the screen is 32.9 mm for a 6.5 mm pupil. Higher order aberrations and the small effects of vertex distance of the lens were ignored.
Subsequently, the averaged pixel values were written back to the video buffer and generated into the new movie frame. Because the movies were in red, green, and blue (RGB) format, the same filtering had to be done separately for each of the three channels. We did not include chromatic aberration in the filtering process (i.e. blue channel more low pass filtered than green and red) because chromatic aberration occurs in the eye and not in the visual environment. If movies were filtered according to the human chromatic aberration function, these effects would have been doubled. With a video format of 1280 × 720 pixels, low-pass filtering was possible at about 25 hertz (Hz) frame rate. To apply the frame rates for all conditions, even focused movies were run through the spatial filtering software, but pixels were repeatedly read at the same positions.
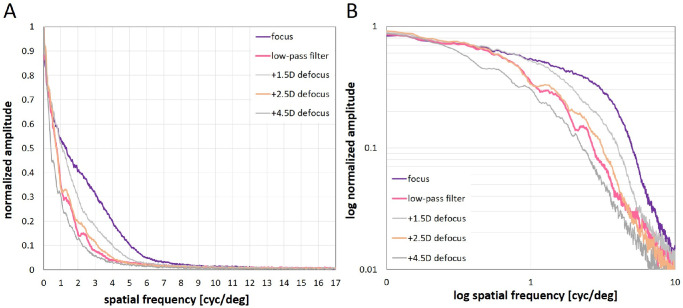
We used a monochrome camera (1920 × 1080 pixels resolution) to measure spatial frequency spectra for unfiltered, defocused, and low-pass filtered movies. Sample pictures for the three conditions were grabbed from 2 m distance with the camera with similar optical parameters as the human eye (16 mm focal length, f/# 2.6; human eye 16.7 mm focal length, f/# 2.6 for a 6.5 mm pupil). The camera's peak sensitivity was around 500 nm. Because the camera lens (Pentax B5014A, f/1.4, F = 50 mm C-mount objective) was corrected for longitudinal chromatic aberration, its effect on focus settings was negligible. The output of the camera was linearized by adjusting the camera driver settings to generate an almost linear relationship between pixel output and target luminance (DMK 33UX174, Imaging Source, Germany; driver settings: bright 0, gain 88, exp -9, gamma 100), as measured with a Minolta luminance meter (LS-100; Minolta Camera Co., LTD, Tokyo, Japan). A squared area of 1080 × 1080 pixels in the center of the frames was selected for Fourier analysis, which was done with publicly available software (ImageJ, National Institutes of Health [NIH], https://imagej.nih.gov/ij/), and its FFT function. Software was written in C++ for rotational averaging of the two-dimensional spatial frequency spectra to convert them into one-dimensional plots, as described by Flitcroft et al.24 Average pixel values obtained from rotational averaging were normalized so that the maximum brightness value was 1 and the lowest 0 (Fig. 3A). In Figure 3B, values were logarithmized as done by Flitcroft et al.24 To illustrate that our calculated low-pass filter generated a spatial frequency spectrum similar to the targeted defocus (+2.5 D), data of two further levels of defocus are also shown (+1.5 D and +4.5 D; see Fig. 3, gray lines). The linearity and correctness of the spatial frequency scale on the x-axis in Figure 3 was verified by measuring various sine wave gratings with known fundamental spatial frequencies. Different from descriptions by Flitcroft et al.,24 the resulting spatial frequency amplitudes showed no linear decline with increasing spatial frequencies between 1 and 10 cyc/deg (see Fig. 3B), possibly because of nonlinearities in the contrast distributions in the movies and/or nonlinearities in the screen showing the movies.
Figure 3.
Spatial frequency spectra of the movies, as determined by Fourier analysis and radial averaging in the two-dimensional spatial frequency amplitude plots obtained from ImageJ. (A) linear plots and (B) log-log plots of spatial frequency amplitudes plotted against spatial frequencies. Note that differences between movies in focus (purple curves), low pass filtered movies (pink), and defocused movies (orange and gray) were confined to the spatial frequency range between 1 and 10 cyc/deg. Note also that the calculated low pass filter (pink) generated a spectrum similar to the targeted defocus of +2.5 D (orange).
Measurements of Ocular Biometry
Axial length was measured before and immediately (less than 1 minute) after each movie watching episode, using a commercial low coherence interferometer, the Lenstar (Lenstar LS 900 with autopositioning system; Haag-Streit, Koeniz, Switzerland). Five repeated measurements were taken for each data point, which took about 2 to 3 minutes. Standard deviations of repeated measurements were below 10 µm in all subjects.
Statistics
Normal distribution of the data was confirmed with the Shapiro-Wilk test. Effects of the two different kinds of blur in each subject were compared using the paired Student's t-test, and differences between the emmetropic and myopic group with the unpaired Student's t-tests. The effects of low-pass filtering or imposed positive defocus over amount of developed refractive error were evaluated by Pearson's correlation coefficient. All statistical analyses were performed using a freely available software package for statistical computing, “R” (version R 4.0.1; R Core Team, R Foundation for Statistical Computing, Vienna, Austria). All measurements and data analyses were done for right eyes only.
Results
Effects of Watching Movies With Unfiltered, Calculated Low-Pass, and Real Optical Defocus on Axial Length
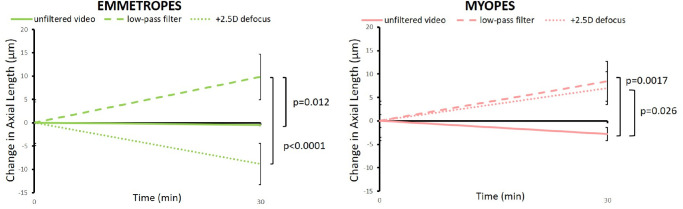
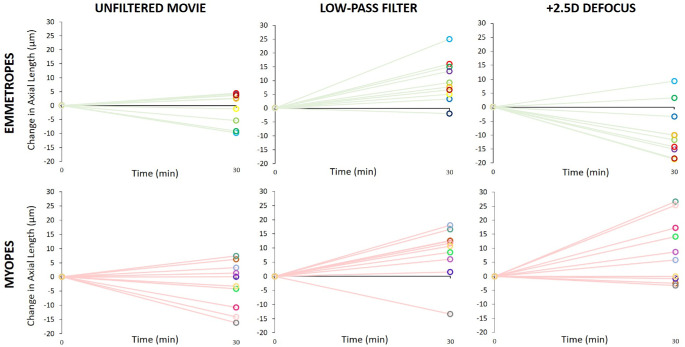
Watching unfiltered movies with no defocus did not elicit significant changes in axial length, neither in emmetropes nor in myopes (on average = −0.4 ± 5.6 µm and −3.0 ± 8.3 µm, respectively; Fig. 4). In emmetropes, watching movies with calculated low-pass filtering caused axial elongation (+9.8 ± 7.6 µm) while watching movies with real positive defocus caused shorter eyes (−8.8 ± 9.2 µm; P < 0.0001; Figs. 4, 5). In addition, in myopes, watching low-pass filtered movies caused significant axial elongation, compared to watching unfiltered movies (+8.4 ± 9.0 µm vs. −3.0 ± 8.3 µm, P = 0.0017; see Figs. 4, 5). However, in contrast to emmetropes, positive defocus did NOT reduce the length of their eyes but rather also caused axial elongation (positive defocus: +9.1 ± 11.2 µm vs. unfiltered movie: −3.0 ± 8.3 µm, P = 0.026; see Figs. 4, 5). Myopic eyes elongated similarly with low-pass filtered and with optically defocused movies. The difference in response to positive optical defocus between emmetropes and myopes was highly significant (−8.8 ± 9.2 µm vs. +9.1 ± 11.2 µm, P = 0.001; Fig. 6).
Figure 4.
Changes in axial length in emmetropes and myopes when they watched unfiltered or low-pass filtered movies, or movies with +3 D lenses in front of their eyes, causing +2.5 D of defocus at 2 m distance. Note that measurements were taken only at two time points and linear changes of axial length over time were assumed. With unfiltered (sharp) movies, the length of the eyes did not change in either group. However, the eyes became longer in emmetropes when movies were low-pass filtered and shorter when they were defocused by +3 D lens. In myopes, both low-pass filtering and positive defocus caused similar ocular elongation. Errors bars represent SEM.
Figure 5.
Individual data showing changes in axial length in emmetropes and myopes after watching unfiltered, low-pass filtered and +2.5 D defocused movies. Note that +2.5 D positive defocus had, on average, the opposite effects in emmetropic and myopic eyes (right) whereas the effects were similar with unfiltered and low-pass filtered movies (middle). Each color denotes an individual subject.
Figure 6.
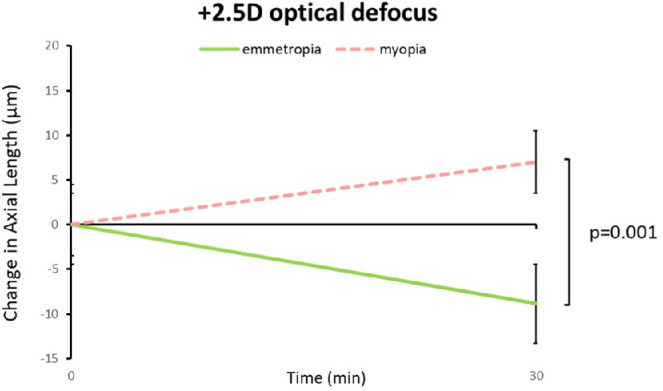
Comparison of the effects of positive defocus on axial length in emmetropic and myopic subjects. Eyes of myopic subjects elongated significantly when a movie was watched with +2.5 D defocus for 30 minutes, whereas eyes in emmetropic subjects become shorter. Error bars denote SEM.
Axial Length Changes as a Function of Individual Refractive Errors
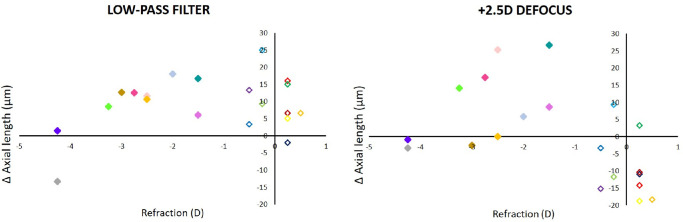
After the observed changes in axial length were analyzed as a function of the individual refractive errors, it became clear that subjects with higher myopia responded poorly to both low-pass filtering and positive defocus (Fig. 7). In 9 out of 10 emmetropic subjects, axial elongation was observed with low-pass filtered movies while eyes became shorter in 8 of 10 emmetropic subjects when movies were watched with +2.5 D of defocus (although this was not significant in all individual subjects). Because data on myopia progression in the subjects were not available (they were recruited by public announcements and could not tell about their progression), it could not be decided whether the variability of the effects were related to myopia progression rates.
Figure 7.
Changes in axial length induced by watching movies that were either low-pass filtered (left) or defocused with a similar spatial frequency spectrum (right) in subjects with different refractive errors (myopic = filled symbols, and emmetropic = open symbols). Note that emmetropic subjects developed shorter eyes when watching movies with positive defocus, whereas myopic subjects developed longer eyes. Two subjects with higher myopia (gray and dark blue symbols) were poor responders. Each color denotes an individual subject, as in Figure 5.
Discussion
Two main findings emerged from this study (1) the retina to be able to distinguish between calculated defocus and real positive defocus and (2) while most emmetropes (8 of 10) responded to positive defocus with eye shortening (likely due to choroidal thickening) and to calculated defocus with axial elongation (likely due to choroidal thinning; 9 of 10), myopic subjects displayed axial elongation (choroidal thinning) in both conditions (8 of 10, for both conditions). Low myopes showed the strongest effect, developing longer eyes both with positive defocus and calculated low-pass filtering. Two subjects with higher myopia were little responsive to either stimulus. The findings suggest that something has changed in the myopic retina: it can no longer respond to positive defocus. It remains unclear why one myopic subject unexpectedly responded to low-pass filtered movies with a shorter eye (see Figs. 5, 7, dark gray symbols). There were no peculiarities regarding refraction, ocular biometry, or age in this subject.
A more general issue is whether such short-term changes in axial length or choroidal thickness have predictive value for future refractive development.22,31 At least baseline choroidal thickness does not predict future myopia32 and it must be kept in mind that changes in choroidal thickness can also be dissociated from refractive development in experiments with chickens.33–35
Potential Mechanisms for Defocus Detection
The question of how the retina can distinguish calculated defocus from real defocus remains elusive and was the subject of extensive earlier discussions.36 Different from other studies where diffusers were placed in front of the eye to degrade the retinal image1–8 with unknown optical transfer function,37 we have matched the shapes of the low-pass filtering functions of real and calculated defocus (see Fig. 3). The spatial frequency analysis permitted to two interesting conclusions (1) as expected, the power spectra were similar for +2.5 D of real defocus and for 2.5 D of calculated defocus, and (2) differences between unfiltered movie and defocused or low-pass filtered movies were limited to the low spatial frequency range between 1 and about 10 cyc/deg, indicating that this spatial frequency range controlled the changes in axial length. A role of the lower spatial frequency range in emmetropization was also supported by previous experiments with Bangerter foils, which were found to reduce the contrast at spatial frequencies below 10 cyc/deg.24,38 Bangerter foils (<0.1 and “light perception”) are also known to induce deprivation myopia in chickens.38 Obviously, also the inhibitory effect of positive defocus on eye length is triggered by visual information in the spatial frequency range below 10 cyc/deg. The finding is in line with the idea that the periphery of the visual field, characterized by low visual acuity, drives emmetropization. Another open question is then how the peripheral retina can control axial length in the center, which was the variable that we measured with the Lenstar.
Role of Accommodation
It has previously been shown that also accommodation is driven by spatial frequencies below 10 cyc/deg.39 There is a striking difference in spatial frequencies needed to drive accommodation in emmetropes and myopes. Myopes needed around 9.33 ± 4.99 cyc/deg whereas emmetropes accommodated appropriately with only 2.75 ± 0.97 cyc/deg.39 However, the difference in the spatial frequency components needed by myopes and emmetropes for accommodation does not explain why they responded in opposite directions in the current study when positive defocus was imposed.
A small amount of accommodation of 0.5 D was probably elicited in emmetropes or corrected myopes when they watched movies at 2 m distance. It was already shown in 1998, that eyes become slightly longer during accommodation.30 However, we did not find any changes in eye length when unfiltered movies were watched, probably because the accommodation effort was too small. Finally, recent publications argue against a role of accommodation in emmetropization – accommodation appears to be largely ignored.40–43 In summary, we believe that our results are not explained by differences in accommodation.
Role of Higher Order Aberrations
Higher order aberrations were ignored in our calculations. The root mean square wavefront errors induced by higher order aberrations in eyes of young subjects with 5.7 mm pupil are equivalent to about 0.3 D,44,45 which is about 8 times lower than for the defocus that was imposed by positive lenses in the present study (+2.5 D). Furthermore, the different responses in myopes and emmetropes cannot be explained by differences in higher order aberrations because it has been shown that there are no differences in aberrations between refractive groups.46 It also seems unlikely that chromatic cues provided the sign of defocus information for the retina because the similar chromatic cues were available with real and calculated defocus. Moreover, chromatic aberration varies very little among human subjects.47
Speculations About the Underlying Mechanism for the Sign of Defocus Detection
So, what could it be? A remaining difference between optically defocused movies and calculated low-pass filtered movies is that light rays cross in front of the retina in the first case but not in the second. There is a chance that the retina distinguishes between these two conditions by involving the Stiles Crawford effect. In the first case, optical defocus would cause less stimulation of cones because light rays reach the cone outer segments obliquely while they are aligned with the outer segments in case of calculated low-pass. The expected differences in detected brightness would be small. The hypothesis would also exclude a role for the rods because they have no Stiles Crawford effect. It may be difficult to exclude that rods play a role because peripheral retina has much more rods than cones but still plays a major role in emmetropization48,49 but has much more rods than cones. Nevertheless, the idea of a role of the Stiles Crawford effect in emmetropization was previously raised by Carmichael and Vohnson50 and more recently by Collins et al.51 Another possible cue to determine the sign of defocus could be retinal image magnification, which increases with positive lenses (about 7% with our camera). The question was studied previously by Curry et al., who used afocal lenses that changed magnification of the retinal image, but not focus. However, they found no effect on eye growth.52 Another hypothesis is that eye movements are necessary for the detection of the sign of defocus detection by the retina. It is well known that responses of retinal ganglion cells become much more specific when the retinal image is in motion (first shown by Greschner et al. 200253). Bitzer and Schaeffel have found that the ZENK protein in the retina can no longer respond to the sign of imposed defocus when chickens were anesthetized, having their eyes opened by lid clamps but with no head or eye movements.54 These findings suggest that (fixational) eye movements may be important for the detection of the sign of defocus. However, it does not seem very likely that the small differences in eye movements that could exist between emmetropes and low myopes can explain the effects. Only high myopes have been shown to have reduced fixation stability.55
Why Were the Differences Between Myopes and Emmetropes not Described Before?
There are a few published studies with a similar design. Read et al.21 had investigated whether the imposed defocus can induce short-term changes in axial length in emmetropic and myopic young adult subjects. Although they found bidirectional changes in axial length with positive versus negative lenses, no differences were measured between myopes and emmetropes. However, their data were differently analyzed. In our study, data of the emmetropic and the myopic group were separately analyzed and the average differences between both groups determined. In the study by Read et al.,21 data from all subjects were averaged and refractive errors were treated as a continuous variable. A lack of an effect of myopic defocus on axial length was also found by Wang et al.56 in myopic children (range = −1.5 to −6.6 D).
Potential Effects of Diurnal Changes in Ocular Biometry
It has been previously found in humans that axial length of the eye, as well as choroidal thickness, vary over a day.57 Although there is evidence for a thicker choroid in the morning, the changes are slow. It can be assumed that the 30-minute time window was too short to be confounded by diurnal choroidal thickness drifts. In addition, differences between myopes and emmetropes were measured at the same time on each day. We always used the same presentation sequence (first movie in focus, then low-pass filtered or defocused), which could have influenced the attention of the observers but differences between myopes and emmetropes could not be explained by such factors.
Conclusions and Implications of the Study
It has been widely speculated that short-term choroidal thickness changes may be an index of future myopia progression or treatment efficacy. Our study expands on this speculation, suggesting that future myopia progression can be predicted in each individual subject from responses of the choroids to imposed positive defocus. Perhaps, it may even be possible that the future success of an optical intervention to slow myopia progression (multifocal optics and Defocus Incorporate Multiple Segments [DIMS] type lenses) can be predicted.
Acknowledgments
The authors thank all the subjects who have participated in this study, and Juan Tabernero (University of Murcia, Spain) and Thorsten Strasser (University of Tuebingen, Germany) for their comments on the low-pass filtering software.
Supported by funding from the Institute of Molecular and Clinical Ophthalmology Basel (IOB), Switzerland.
Disclosure: B. Swiatczak, None; F. Schaeffel, None
References
- 1. Wallman J, Turkel J, Trachtman J.. Extreme myopia produced by modest change in early visual experience. Science. 1978; 201: 1249–1251. [DOI] [PubMed] [Google Scholar]
- 2. Howlett MH, McFadden SA.. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res. 2006; 46: 267–283. [DOI] [PubMed] [Google Scholar]
- 3. Andison ME, Sivak JG, Bird DM.. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol A. 1992; 170: 565–574. [DOI] [PubMed] [Google Scholar]
- 4. Pardue MT, Stone RA, Iuvone PM.. Investigating mechanisms of myopia in mice. Exp Eye Res. 2013; 114: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norton TT. Experimental myopia in tree shrews. Ciba Found Symp. 1990; 155: 178–194; discussion 194-179. [PubMed] [Google Scholar]
- 6. Troilo D, Nickla DL, Wildsoet CF.. Form deprivation myopia in mature common marmosets (Callithrix jacchus). Invest Ophthalmol Vis Sci. 2000; 41: 2043–2049. [PubMed] [Google Scholar]
- 7. Smith EL 3rd, Bradley DV, Fernandes A, Boothe RG.. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999; 76: 428–432. [DOI] [PubMed] [Google Scholar]
- 8. Meyer C, Muller M.. [Form deprivation myopia caused by keratitis scrophulosa]. Ophthalmologe. 1996; 93: 361–366. [PubMed] [Google Scholar]
- 9. Thomson K, Karouta C, Ashby R.. Form-deprivation and lens-induced myopia are similarly affected by pharmacological manipulation of the dopaminergic system in chicks. Invest Ophthalmol Vis Sci. 2020; 61: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schaeffel F, Howland HC.. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991; 31: 717–734. [DOI] [PubMed] [Google Scholar]
- 11. Zhu X, Winawer JA, Wallman J.. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003; 44: 2818–2827. [DOI] [PubMed] [Google Scholar]
- 12. Zhu X, Wallman J.. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallman J, Winawer J.. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 14. Howlett MH, McFadden SA.. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 15. Metlapally S, McBrien NA.. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008; 8:1 1–12. [DOI] [PubMed] [Google Scholar]
- 16. Smith EL 3rd, Hung LF.. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999; 39: 1415–1435. [DOI] [PubMed] [Google Scholar]
- 17. Schaeffel F, Glasser A, Howland HC.. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988; 28: 639–657. [DOI] [PubMed] [Google Scholar]
- 18. Park TW, Winawer J, Wallman J.. Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vision Res. 2003; 43: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 19. Schaeffel F, Diether S.. The growing eye: an autofocus system that works on very poor images. Vision Res. 1999; 39: 1585–1589. [DOI] [PubMed] [Google Scholar]
- 20. Troilo D, Smith EL 3rd, Nickla DL, et al.. IMI - report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci. 2019; 60: M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Read SA, Collins MJ, Sander BP.. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010; 51: 6262–6269. [DOI] [PubMed] [Google Scholar]
- 22. Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ.. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 3103–3112. [DOI] [PubMed] [Google Scholar]
- 23. Diether S, Gekeler F, Schaeffel F.. Changes in contrast sensitivity induced by defocus and their possible relations to emmetropization in the chicken. Invest Ophthalmol Vis Sci. 2001; 42: 3072–3079. [PubMed] [Google Scholar]
- 24. Flitcroft DI, Harb EN, Wildsoet CF.. The spatial frequency content of urban and indoor environments as a potential risk factor for myopia development. Invest Ophthalmol Vis Sci. 2020; 61: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chakraborty R, Read SA, Collins MJ.. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012; 103: 47–54. [DOI] [PubMed] [Google Scholar]
- 26. Sander BP, Collins MJ, Read SA.. Short-term effect of low-dose atropine and hyperopic defocus on choroidal thickness and axial length in young myopic adults. J Ophthalmol. 2019; 2019: 4782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prousali E, Dastiridou A, Ziakas N, Androudi S, Mataftsi A.. Choroidal thickness and ocular growth in childhood [published ahead of print July 4, 2020]. Surv Ophthalmol, 10.1016/j.survophthal.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 28. Xiong F, Tu J, Mao T, Yu L, Lin N, Liao H.. Subfoveal choroidal thickness in myopia: an OCT-based study in young Chinese patients. J Ophthalmol. 2020; 2020: 5896016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiang ST, Turnbull PRK, Phillips JR.. Additive effect of atropine eye drops and short-term retinal defocus on choroidal thickness in children with myopia. Sci Rep. 2020; 10: 18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF.. Eye elongation during accommodation in humans: differences between emmetropes and myopes. Invest Ophthalmol Vis Sci. 1998; 39: 2140–2147. [PubMed] [Google Scholar]
- 31. Fontaine M, Gaucher D, Sauer A, Speeg-Schatz C.. Choroidal thickness and ametropia in children: a longitudinal study. Eur J Ophthalmol. 2017; 27: 730–734. [DOI] [PubMed] [Google Scholar]
- 32. Hansen MH, Kessel L, Li XQ, Skovgaard AM, Larsen M, Munch IC.. Axial length change and its relationship with baseline choroidal thickness - a five-year longitudinal study in Danish adolescents: the CCC2000 eye study. BMC Ophthalmol. 2020; 20: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006; 192: 399–407. [DOI] [PubMed] [Google Scholar]
- 34. Nickla DL, Totonelly K.. Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom. 2015; 98: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guggenheim JA, Chen YP, Yip E, et al.. Pre-treatment choroidal thickness is not predictive of susceptibility to form-deprivation myopia in chickens. Ophthalmic Physiol Opt. 2011; 31: 516–528. [DOI] [PubMed] [Google Scholar]
- 36. Schaeffel F, Wildsoet C.. Can the retina alone detect the sign of defocus? Ophthalmic Physiol Opt. 2013; 33: 362–367. [DOI] [PubMed] [Google Scholar]
- 37. Perez GM, Archer SM, Artal P.. Optical characterization of Bangerter foils. Invest Ophthalmol Vis Sci. 2010; 51: 609–613. [DOI] [PubMed] [Google Scholar]
- 38. Tran N, Chiu S, Tian Y, Wildsoet CF.. The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Res. 2008; 48: 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanz Diez P, Ohlendorf A, Schaeffel F, Wahl S.. Effect of spatial filtering on accommodation. Vision Res. 2019; 164: 62–68. [DOI] [PubMed] [Google Scholar]
- 40. Aleman A, Schaeffel F.. Lag of accommodation does not predict changes in eye growth in chickens. Vision Res. 2018; 149: 77–85. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Drobe B, Zhang C, et al.. Accommodation is unrelated to myopia progression in Chinese myopic children. Sci Rep. 2020; 10: 12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mutti DO, Mitchell GL, Hayes JR, et al.. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006; 47: 837–846. [DOI] [PubMed] [Google Scholar]
- 43. Berntsen DA, Sinnott LT, Mutti DO, Zadnik K, Group CS.. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res. 2011; 51: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Artal P. Optics of the eye and its impact in vision: a tutorial. Adv Opt Photon. 2014; 6: 340–367. [Google Scholar]
- 45. Guirao A, Porter J, Williams DR, Cox IG.. Calculated impact of higher-order monochromatic aberrations on retinal image quality in a population of human eyes. J Opt Soc Am A Opt Image Sci Vis. 2002; 19: 1–9. [DOI] [PubMed] [Google Scholar]
- 46. Llorente L, Barbero S, Cano D, Dorronsoro C, Marcos S. Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. J Vis. 2004; 4: 288–298. [DOI] [PubMed] [Google Scholar]
- 47. Marcos S, Burns SA, Moreno-Barriusop E, Navarro R.. A new approach to the study of ocular chromatic aberrations. Vision Res. 1999; 39: 4309–4323. [DOI] [PubMed] [Google Scholar]
- 48. Smith EL 3rd, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF.. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005; 46: 3965–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith EL 3rd, Ramamirtham R, Qiao-Grider Y, et al.. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carmichael Martins A, Vohnsen B. Analysing the impact of myopia on the Stiles-Crawford effect of the first kind using a digital micromirror device. Ophthalmic Physiol Opt. 2018; 38: 273–280. [DOI] [PubMed] [Google Scholar]
- 51. Collins MJ, Yi F, Davis BA, Hoseini-Yazdi SH, McNeill H.. Rapid changes in the Stiles Crawford Function in response to plus defocus. Invest Ophthalmol Vis Sci. 2020; 61: 548–548. [Google Scholar]
- 52. Curry TA, Sivak JG, Callender MG, Irving EL.. Afocal magnification does not influence chick eye development. Optom Vis Sci. 1999; 76: 316–319. [DOI] [PubMed] [Google Scholar]
- 53. Greschner M, Bongard M, Rujan P, Ammermuller J.. Retinal ganglion cell synchronization by fixational eye movements improves feature estimation. Nat Neurosci. 2002; 5: 341–347. [DOI] [PubMed] [Google Scholar]
- 54. Bitzer M, Schaeffel F.. ZENK expression of retinal glucagon amacrine cells in chicks: the effect of defocus presented in vivo, in vitro and under anesthesia. Vision Res. 2006; 46: 848–859. [DOI] [PubMed] [Google Scholar]
- 55. Zhu X, He W, Zhang K, Zhang Y, Fan Q, Lu Y.. Fixation characteristics in highly myopic eyes: the Shanghai High Myopia Study. Sci Rep. 2019; 9: 6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang D, Chun RK, Liu M, et al.. Optical defocus rapidly changes choroidal thickness in schoolchildren. PLoS One. 2016; 11: e0161535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chakraborty R, Read SA, Collins MJ.. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011; 52: 5121–5129. [DOI] [PubMed] [Google Scholar]