Abstract
The Breast Imaging Reporting and Data System (BI-RADS) is a comprehensive guideline to systematize breast imaging reporting, and as per its recommendations, any lesion with likelihoods of malignancy greater than 2% is deemed as suspicious and tissue diagnosis is recommended. The aim of the study is to determine the positive predictive value (PPV) of BI-RADS categories 4a, 4b, and 4c for malignancy and association of mammographic morphological features of BI-RADS 4 subgroups with malignant outcomes. We retrospectively reviewed all the patients undergoing mammography with BI-RADS score of 4 followed by biopsy from May 2019 to April 2020. The predictive values of BI-RADS 4 subcategories and morphological features with malignancy are performed taking histopathology report as the gold standard. The PPV of BI-RADS subcategories 4a, 4b, and 4c for malignancies were 34, 89, and 97%, respectively. BI-RADS 4c patients tend to be older (50.2 ± 12.2 vs. 44.6 ± 10.3 years) with larger mass (44 ± 16 vs. 32.9 ± 16.8 mm) at presentation than 4a. Postmenopausal state (P = 0.03) and older age (P = 0.019) were significantly associated with malignancy. There is no meaningful difference observed in the predictability of BI-RADS category 4c lesions among different breast density patterns. The overall higher PPV for BI-RADS 4a and 4b reflects subjectivity in subcategory assignments of BI-RADS 4. In patients, less than 40 years with the BI-RADS 4a category on mammograms may undergo supplementary imaging with MRI which may downscale the lesion classification in turn reducing unnecessary biopsy and surgery.
Keywords: BI-RADS 4, Malignancy, Mammography, Morphology, Positive predictive value
Introduction
The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) is a comprehensive quality assurance tool intended to systematize mammography reporting, reduce confusion concerning breast imaging interpretation and its plausible management recommendations, and also help in monitoring results of mammography [1]. These risk-stratifying categories (0–6) are aimed to explicitly communicate the likelihood of malignancy to referring clinicians and guide management recommendations. The practice and outcomes of BI-RADS assessment categories are key measures of radiologist performance, because of its variability and outcomes below the desirable benchmarks that substantially influence patient care [2, 3]. Mammograms assigned a BI-RADS assessment category 4 have been defined as those with findings that do not have the typical characteristics of malignancy but are adequately suspicious to warrant the recommendation for biopsy [1, 4]. Category 4 comprises a broad range of the likelihood of malignancy ranging from more than 2 to less than 95%. Details of BI-RADS 4 subdivisions are shown in Table 1.
Table 1.
BI-RADS final assessment scoring system for category 4 lesion (derived from BI-RADS atlas fifth edition)
| Category | Definition | Likelihood of malignancy (%) |
|---|---|---|
| 4a | Low suspicion for malignancy—biopsy advised | (> 2 to < 10) |
| 4b | Moderate suspicion for malignancy requiring biopsy | (> 10 to < 50) |
| 4c | High suspicion for malignancy requiring biopsy | (> 50 to < 95) |
Indication of biopsy for a suspicious mammographic finding is multifactorial and the BI-RADS score endures the single most significant variable in prognosticating cancer diagnosis. Additional determinants like patient preference and the anxiety associated with nonintervention may drive a patient to undergo a biopsy despite the likelihood of cancer are low.
Nevertheless, advantages from urgent tissue diagnosis ought been debated in the subgroup of BI-RADS 4a concerning potential harms such as overdiagnosis of indolent lesions, the additional expense of testing, potential radiation, and anxiety associated with falsely positive screening/diagnostic mammography and erroneous sense of security from falsely negative mammograms [5–7]. Given the low cancer risk among category 4a, some researchers advocate for different approaches to stratify and manage patients who fall within this category. Flowers et al. recommend reclassifying BI-RADS 4a as low-risk lesions that can be clinically evaluated and followed preferably than immediately sampled via biopsy [8].
To the best of our knowledge, limited studies are available in India showing predictive accuracy of BI-RADS 4 subgroups and their mammographic morphological characteristics, so the usefulness of BI-RADS 4 predictability and its management recommendation need to be assessed in the Indian setting.
In the present study, we endeavor to ascertain the PPV of BI-RADS subcategories 4a, 4b, and 4c concerning breast cancer outcome; define the individual mammographic morphology in subcategories and its association and predictive implications amidst malignancy; and evaluate the potential application of those demographic and mammographic morphological variables to define a low-risk group that can be utilized besides BI-RADS scoring in low suspicion cases guiding whether an individual would be a more suitable candidate for active surveillance rather than undergoing urgent biopsy.
Materials and methods
A record-based analytical study was conducted in the department of radiodiagnosis after institutional review board approved the research for data acquisition and analysis. We retrospectively reviewed all patients who underwent mammography followed by biopsy over 1 year from May 2019 to April 2020. By thorough review of case records, mammograms, histopathological, operative records, and reporting database of our hospital, we have shortlisted 280 patient’s data with BI-RADS 4 assessment score. Out of which 34 patients’ records were dropped due to the insufficiency of the follow-up records, nondiagnostic quality of mammograms, and nonavailable histopathological results. The sampling method for case assortment was purposive that is of a non-probability type which means we have categorically incorporated case records with BI-RADS 4 evaluation score and known pathological result. Subsequently, 246 (N) patients’ records with an overall BI-RADS score of 4 had been finalized for analysis in the existing study. Figure 1 illustrates the inclusion and exclusion criteria.
Fig. 1.
Details of the Inclusion and Exclusion criteria for the study.
Senographe Pristina (GE Medical System SCS, France) digital mammography and Philips HD7 ultrasound systems were employed for imaging purposes. All mammograms were evaluated by a set of two 5-megapixel (5MP BARCO) medical-grade monitors in the department. Mammography machines utilized for imaging were US FDA-approved with regular quality assessment done by our physicist with image phantom. Board-certified technologists and radiologists with experience in breast imaging were involved in image acquisition and interpretation. Mediolateral oblique (MLO) and craniocaudal (CC) views were considered standard for all patients, but additional views like exaggerated view and compression views were also used as and when required. All patients whose mammography was performed in our department during the study period using BI-RADS fifth edition mammographic morphological lexicons and assessment category are only included for analysis. Morphological characterization of mammography was done regarding breast density, mass shape, size, margin, location, mass density, calcifications, asymmetry, satellite lesions, skin thickening, lymph node, and architectural distortion. BI-RADS fifth edition classification assigns mammographic breast density into 4 categories: (a) entirely fatty, (b) scattered areas of fibro glandular density, (c) heterogeneously dense, and (d) extremely dense. The shape of the mass lesion was further defined as round, oval, and irregular, and the mass margin classified into well-circumscribed, microlobulated, obscured, indistinct, and spiculated. BI-RADS final assessment scoring 4 was further divided into 4a, 4b, and 4c based on the degree of suspiciousness of mass lesion based on most suspicious mammographic morphology and whenever required by concomitant usage of ultrasound findings (Fig. 2). Ultrasound was utilized concurrently along with mammography in 95 patients out of a total of 246 patients in whom the breasts happened to be extremely dense and in cases with clinical-radiological discordances to arrive at a conclusive diagnosis, and its findings were reported on initial mammography report. In all these patients (n = 246), histopathological records were retrieved which comprise of core needle (205), ultrasound-guided (10), and postsurgical (31) biopsies. The outcome of histopathology was categorized as positive and negative for malignancy which was treated as the outcome of the existing study for predictive analysis. The clinical and demographic attributes of patients like age, family history, menopause, lump, discharge, pain, and skin changes were also noted. All these data were inscribed into a predefined proforma by the researchers in Microsoft Excel format. The data were twice cross-checked for any duplicate or missing data by other independent researchers. The P values were calculated using logistic regression and chi-square analysis. The PPV of the individual morphological variable and BI-RADS sub-scoring had been evaluated for malignancy outcome. Statistical analysis was accomplished using SPSS v.20. For value to be significant, P value < 0.05 had been considered at a 95% confidence limit and appropriate degrees of freedom.
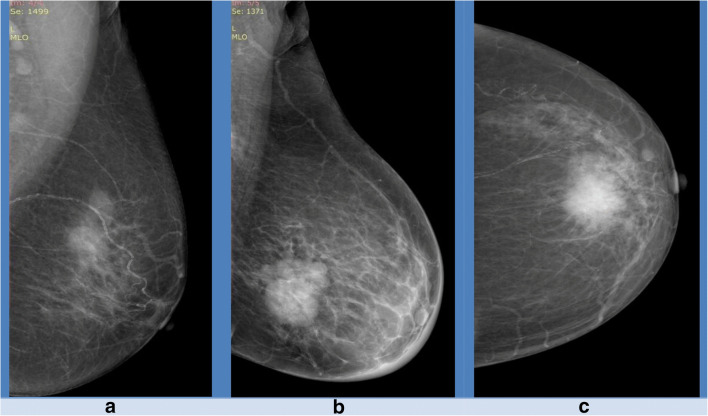
Fig. 2.
Demonstrating imaging features in BI-RADS 4 subcategories (4a, 4b, and 4c). a Irregular mass with obscured margin (4a). b Irregular mass with microlobulation (4b). c irregular mass with microlobulation with irregular mass with spiculations (4c)
Results
A total of 246 women with BI-RADS 4 score were subclassified into 4a (72, 29.4%), 4b (110, 44.8%), and 4c (64, 26.8%) with 184 (74.8%) malignant and 62 (25.2%) benign outcomes. In the malignant cohort (n = 184), 24, 98, and 62 patients had BI-RADS scores of 4a, 4b, and 4c, respectively. A statistically significant difference in the incidence of breast cancer diagnosis was noted between BI-RADS 4a and 4b subgroups. Details of BI-RADS 4 subdivisions and their outcome are presented in Table 2.
Table 2.
BI-RADS 4 subgroups and its outcomes (malignant)
| BI-RADS score | PPV (%) | Odds ratio (OR) | 95% CI | P value |
|---|---|---|---|---|
| BI-RADS 4a | 34 | 0.044 | 0.015 to 0.123 | < 0.0001 |
| BI-RADS 4b | 89 | 4.75 | 1.78 to 12.66 | 0.0018 |
| BI-RADS 4c | 97 | 15.246 | 1.98 to 117 | 0.0088 |
PPV = positive predictive value, OR = odds ratio, CI = confidence interval (BI-RADS 4a = 72, 4b = 110, and 4c = 64, odds ratio calculated using univariate logistic regression, total cases (n) = 246)
Our analysis yielded cancer predictive probability (PPV) of 34, 89, and 97% for 4a, 4b, and 4c, respectively. Infiltrating ductal carcinoma (IDC) was the commonest pathological subtype (170 out of 184) of malignancy. The median age of the study population was 47.2 + 11 years. Women in the BI-RADS 4c subgroup were older (mean age 50.2 + 12.2 years) than women with BI-RADS 4a (mean age 44.7 ± 10.3 years) and 4b (mean age 47.2 + 10.4 years) subgroups, respectively. None of the patients had malignant outcomes with BI-RADS 4a score and age less than 40 years. Out of 102 postmenopausal patients, 86 were malignant. Postmenopausal status (P = 0.03) and older age (P = 0.019) were significantly associated with malignancy outcomes. The age group distribution of the BI-RADS 4 subclassification score and its outcome are given in Table 3.
Table 3.
Age group distribution among BI-RADS 4 subgroups and its outcome
| Age group | BI-RADS 4a/malignant | BI-RADS 4b/malignant | BI-RADS 4c/malignant | Total/malignant |
|---|---|---|---|---|
| < 40 years | 24/0 | 34/30 | 16/16 | 74/46 |
| 41–50 | 32/12 | 42/40 | 16/16 | 90/68 |
| 51–60 | 12/10 | 26/20 | 20/20 | 58/50 |
| 61–70 | 4/2 | 6/6 | 8/8 | 18/16 |
| > 70 | 0/0 | 2/2 | 4/2 | 6/4 |
| Total | 72/24 | 110/98 | 64/62 | 246/184 |
The clinical characteristics of the study population at presentation and its pathological outcomes are given in Table 4.
Table 4.
Clinical presentations and histopathological outcomes
| Clinical presentations | Total | Malignant |
|---|---|---|
| Lump | 246 | 184 |
| Pain | 10 | 2 |
| Discharge | 6 | 2 |
| Skin changes | 64 | 50 |
The average size of the malignant mass was (39.5 ± 16.3 mm) and benign mass was (37 ± 21.2 mm). The average size of the mass in BI-RADS 4c (44 ± 16 mm) was more compared to the rest of BI-RADS 4a (32 ± 9.7 mm) and BI-RADS 4b (39 ± 17 mm). Mass size exhibits no correlation with malignancy outcome (P = 0.49). There is no meaningful difference perceived in the predictability of BI-RADS category 4c lesions among various breast density patterns. An incongruous observation like the similar predictive value of overall BI-RADS 4 lesion with breast density d and b (75 and 74%). Breast density distribution with its BI-RADS score and the pathological outcome are given in Table 5.
Table 5.
Breast density division among BI-RADS subgroups and its histopathological outcomes
| Breast density category | Malignancy/total in BI-RADS 4a (%) | Malignancy/total in BI-RADS 4b (%) | Malignancy/total in BI-RADS 4c (%) | Malignancy/total in BI-RADS 4 (%) |
|---|---|---|---|---|
| A | 4/6 (67) | 20/20 (100) | 18/20 (90) | 42/46 (91.3) |
| B | 10/26 (38.5) | 40/48 (83.3) | 18/18 (100) | 68/92 (74) |
| C | 6/30 (20) | 32/36 (88.9) | 18/18 (100) | 56/84 (66.7) |
| D | 4/10 (40) | 6/6 (100) | 8/8 (100) | 18/24 (75) |
| Total | 24/72 (33) | 98/110 (89) | 62/64 (97) | 184/246 (75) |
Microlobulated margin (25%) is showing less predictive value than a well-circumscribed margin (50%) with BI-RADS 4a score. The corresponding frequency, PPV, and association (P values) for carcinoma as a function of BI-RADS morphological descriptors in BI-RADS 4 subgroups are shown in Tables 6, 7 and 8.
Table 6.
Details of mammographic shape and margin, its positive predictive values, and P value
| Mammographic morphological features | BI-RADS category | ||||
|---|---|---|---|---|---|
| BI-RADS 4a (PPV) | BI-RADS 4b (PPV) | BI-RADS 4c (PPV) | BI-RADS 4 (PPV) | P value | |
| Mass shape | |||||
| • Round | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| • Oval | 34 (29.3) | 22 (91) | 0 (0) | 56 (53.6) | 0.0032# |
| • Irregular | 38 (36.8) | 88 (86.4) | 64 (96.9) | 190 (80.0) | 0.0096 |
| Mass margin | |||||
| • Well-circumscribed | 8 (50) | 2 (0) | 0 (0) | 10 (40.0) | 0.009## |
| • Microlobulated | 8 (25) | 24 (91.7) | 28 (100) | 60 (86.7) | 0.015 |
| • Masked | 24 (33.3) | 28 (85.7) | 14 (85.7) | 66 (66.7) | 0.075 |
| • Indistinct | 32 (31.2) | 46 (91.3) | 16 (100) | 94 (72.3) | 0.312 |
| • Spiculated | 0 (0) | 10 (100) | 6 (100) | 16 (100) | 0.209 |
Calculated by chi-square analysis on 2 × 2 table, n = 246
#,##oval shape and well-circumscribed margin showed P value less than 0.05, but on phi coefficient calculation, it showed value of −0.2654 and −0.165, respectively, s/o inverse associations
Table 7.
Additional mammographic observations, its positive predictive value, and P value
| BI-RADS category | |||||
|---|---|---|---|---|---|
| BI-RADS 4a (PPV) | BI-RADS 4b (PPV) | BI-RADS 4c (PPV) | BI-RADS 4 (PPV) | P value | |
| Additional mammographic observations | |||||
| • Skin changes | 18 (22.2) | 42 (85.7) | 28 (100) | 88 (77.3) | 0.504 |
| • Architectural distortion | 12 (33.3) | 28 (92.8) | 22 (100) | 62 (83.9) | 0.057 |
| • Axillary adenopathy | 12 (50) | 28 (85.7) | 26 (100) | 66 (84.9) | 0.028 |
| • Microcalcifications | 10 (0) | 34 (100) | 26 (100) | 70 (85.7) | 0.013 |
| • Satellite nodules | 0 (0) | 6 (100) | 10 (100) | 16 (100) | 0.209 |
| Mass density | |||||
| • Hyperdense | 28 (35.7) | 62 (90.3) | 40 (95.0) | 130 (80.0) | 0.001 |
Calculated by chi-square analysis on 2 × 2 table, n = 246
Table 8.
Factors predicting the presence of malignancy in category 4 mammographic lesions (using multivariate and univariate logistic regression), n = 246
| Factor(mammography) | Odds ratio | 95% CI | P value |
|---|---|---|---|
|
Breast density (density D = 1) • A • B • C • D |
3.5 0.94 0.66 1 |
0.88–13.9 0.33–2.6 0.23–1.8 - |
0.075 0.91 0.44 - |
|
Shape • Round# • Oval • Irregular |
- 0.2697 3.7074 |
- 0.14–0.51 1.96–7.02 |
- 0.0001 0.0001 |
|
Margin • Well-circumscribed • Microlobulated • Masked • Indistinct • Spiculated |
0.2074 2.6591 0.5714 0.8117 12.2404 |
0.056–0.76 1.18–5.97 0.31–1.06 0.45–1.46 0.72–207 |
0.0172 0.0106 0.0773 0.4857 0.0826 |
#round shape had zero observation
Discussion
Understanding BI-RADS category 4 utilization patterns and results is particularly important, as this category accounts for the majority of tissue diagnosis recommendations after mammography. Besides that, category 4 subdivisions are encouraged in practice because of their potential benefits, but limited information is available about their utilization and effects in clinical practice.
BI-RADS 4 category lesions have been subdivided into three subgroups based on degree suspicion of malignancy of most concerned morphological characteristics among the observations but subcategorization is subjective based on clinical judgment and preference of radiologist, and no objective principles have been established for this. In the present study, PPV for BI-RADS 4a, 4b, and 4c were 34, 89, and 97%, respectively. For BI-RADS 4a and 4b, it is considerably more compared to the likelihood of malignancy rate of BI-RADS category as per fifth edition BI-RADS atlas (Table 1). Plausible reasons for such an inconsistency comprise isolated distinctive observations subsequent to sample size, patient disposition, and selection bias. Other causes explaining such discrepancies may be nonrigid BI-RADS recommendations leading to improper categorizations in BI-RADS 4 subgroups, like some of the lesions categorized as BI-RADS 4a may belong to BI-RADS 4b or 4c. Comparable variation in observation was also cited in the past by Lazarus et al 2006 (PPV for 4a = 5.6%, 4b = 50%, and 4c = 33.3%) and Leblebici 2014 (4a = 6%, 4b = 15%, and 4c = 53%) [9, 10]. Although both BI-RADS 4a and 4b category undergo biopsy as per BI-RADS recommendation, intercategory differentiation of BI-RADS 4 is also essential as subclasses render further graded risk stratification and prioritization which expedites informed patient counseling and management strategy including establishing patients and healthcare provider expectations for outcomes. Lesion characterization essentially is more or less experience-based requiring visual eyeballing, but if rigid and explicit morphological criteria for distinguishing various BI-RADS subgroups are laid, it will be helpful for radiologists and surgeons in the early part of their training. Moreover, objective/unbiased morphological criteria might help in computer-assisted reporting and development of robust artificial intelligence in mammography.
In our study, the overall median age of the patient was 47.2 ± 11 years. BI-RADS 4a patients tend to be younger than BI-RADS 4b and 4c which was similar to earlier observations [11]. A notable difference in PPV was observed in less than 40 year age group where all BI-RADS 4a assessment category lesions were benign emphasizing reduced predictability (0 out of 24) of mammography in the aforementioned age group. We had a follow-up record available in 15 patients out of 24 patients in this group with MRI (magnetic resonance imaging) which downstage the BI-RADS 4a scoring to BI-RADS 2 or 3. So additional noninvasive imaging follows up with breast MRI might be utilized as a supplement in young patients with BI-RADS 4a assessment which may reduce unnecessary biopsy and surgery by 92% as quoted by Strobel K et al. in 2015 [12].
Attaining menopause as such does not cause cancer, but the risk of breast cancer development increases as a woman age because she is exposed to estrogen for a perpetual duration. So, women who have been through natural menopause are twice more likely to develop cancer [13]. In postmenopausal ladies, incidences of malignancy in 4a, 4b, and 4c lesions were 66.7, 87, and 93.7%, respectively. Postmenopausal status showed a significant association (P = 0.03) with malignancy outcomes in our study with similar results cited by Leblebici et al. [10].
Breast density is a major factor determining the sensitivity and specificity of breast screening and diagnosis. A dense breast obscures the subtle finding which makes the detection of early breast cancer more difficult resulting in lower sensitivity and higher rate of interval cancer detection [14]. In the present study, BI-RADS 4a showed the greatest variation in diagnostic accuracy with 67% detection rate in category a breast density compared to 20% in category c and 40% in category d breast density, respectively. BI-RADS 4c category does not show a significant change in diagnostic accuracy along with all breast density combinations.
Presurgical evaluation of breast tumor size is essential for choosing relevant treatment strategies, especially with the advent of neoadjuvant therapy and minimal radical surgeries. Both breast ultrasound and X-ray mammography tend to underestimate the breast tumor size, while clinical assessment often overestimates it [15]. In our study, the mean size of the BI-RADS 4c is greater than BI-RADS 4a and BI-RADS 4b with similar findings in an earlier study by Cholatip et al. Larger average tumor size in our study was observed compared to median tumor size of 2.23 cm in earlier studies which possibly represents delayed detection due to lack of population-based screening programs in our country and the selection of solely palpable breast lesions in the existing research [16].
Radiologically, round to oval masses with internal fat content (hypodense) and the well-defined margin are often associated with benign breast lesions. Isodensity of mass with lobulated obscured and indistinct margins are categorized as suspicious. Highly suspicious lesions tend to be mostly higher in density, irregular shape, spiculated, and indistinct margins [17]. Nevertheless, around 10% of malignant lesions may show overlapping or benign characteristics such as round, oval shape, and well-defined margin. Seldom spiculations in the mass margin and neighboring parenchymal changes may be too subtle to demonstrate. These scenarios may lead to potentially malignant breast lesions being overlooked or misinterpreted, with wrong interpretation accounting for 52% of errors in mammography [18]. In the present study, irregular and lobulated oval shape with obscured or masked margin was the commonest pattern in BI-RADS 4a. But an irregular shape with microlobulation or spiculations was common for BI-RADS 4c lesions. Isodensity of the mass with surrounding parenchyma common in BI-RADS 4a with reduced PPV of 22.7% in isodense mass subgroup opposed to PPV of 35.7% in the hyperdense mass subgroup of BI-RADS 4a. Spiculated margin, microcalcifications, architectural distortions, and satellite nodules were common descriptors in BI-RADS 4b and 4c subcategories. Microlobulated margin (P = 0.01) and axillary adenopathy (P = 0.02) showed meaningful statistical association with malignant outcomes with similar results cited in earlier observation [17, 18].
Microcalcifications in the breast result from calcium oxalate and calcium phosphate deposition in the parenchyma or ducts. Calcium oxalate is generated by apocrine cells which is seen more often with benign breast conditions but can also be seen less commonly with malignancy. Calcium phosphate is more frequently correlated with malignant breast lesions than calcium oxalate [19]. Mammographic detection of microcalcifications with characteristic shape and location is crucial to the diagnosis of breast carcinoma. Nevertheless, mammography can demonstrate breast microcalcifications in only 30–50% of breast cancers. In the present study, diffuse and segmental microcalcifications were present in 10 cases in BI-RADS 4a without any malignant outcome (PPV = 0). Pleomorphic and fine linear branching patterns of microcalcification were seen in 60 cases in BI-RADS 4b and 4c with all lesions being malignant (PPV = 100%). Overall suspicious microcalcification showed meaningful association with malignancy outcomes (P = 0.01) as quoted by earlier studies [20].
The shortcoming of our research was its retrospective design and the sampling technique which was purposive with selection bias that is the incorporation of only diagnostic mammography and palpable lesions. Other limitations were its smaller sample size, lack of follow-up treatment, and postsurgical outcomes.
The quality of mammography reporting differs greatly across the institutes, such that the predictive value of an abnormal mammogram may be three times higher in academic centers than in community-based practices [21]. Other determinants that could have contributed to higher predictability and sensitivity of mammography in the present study may be due to the inclusion of only diagnostic cases (no screening mammography) which results in overall higher predictability of mammography.
Conclusion
Our study is a retrospective record-based research to examine the outcome and predictive values of the BI-RADS 4 subcategories and individual morphological characters; notwithstanding possible limitations, we conclude with the following:
1. Digital mammography offered excellent predictability among BI-RADS 4 subcategories in Indian subjects.
2. BI-RADS 4a patients tend to be younger with smaller mass sizes at the presentation compared to BI-RADS 4b and 4c.
3. Young patients (less than 40 years) with the BI-RADS 4a category on mammograms may undergo supplementary noninvasive imaging with MRI which may downscale the lesion category in turn reducing unnecessary biopsy and surgery.
4. BI-RADS 4 categorization is subjective with diverse predictability among researchers, but with the progress of knowledge, objective criteria definition is required to diminish inter-observer disparity and improve the predictive significance of mammography.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suvendu Kumar Mohapatra, Email: drsuvendumohapatra@gmail.com.
Abhisek Mishra, Email: drabhisekmishra@gmail.com.
Tapan Kumar Sahoo, Email: drtapankumars8@gmail.com.
Rashmita Binod Nayak, Email: rashmitabnayak@gmail.com.
Prafulla Kumar Das, Email: pk.das12365@gmail.com.
Bhagyalaxmi Nayak, Email: blnayak2260@gmail.com.
References
- 1.D’Orsi CJ, Sickles EA, Mendelson EB et al (2013) ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA
- 2.Inappropriate use of “probably benign” assessment category in screening mammograms national quality strategy domain: efficiency and cost reduction. https://www.acr.org/%20~/ media/ACR/Documents/P4P/2016%20PQRS/DX/2016_PQRS_Measure_146_11_17_2015.pdf. Updated November 17, 2015. Accessed January 14, 2017.
- 3.Elezaby M, Li G, Bhargavan-Chatfield M, Burnside ES, DeMartini WB. ACR BI-RADS assessment category 4 subdivisions in diagnostic mammography: utilization and outcomes in the National Mammography Database. Radiology. 2018;287(2):416–422. doi: 10.1148/radiol.2017170770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Orsi CJ, Mendelson EB, Ikeda DM. Breast imaging reporting and data system: ACR BI-RADS—breast imaging atlas. American College of Radiology: Reston, Va; 2003. [Google Scholar]
- 5.Puliti D, Duffy SW, Miccinesi G, de Koning H, Lynge E, Zappa M, Paci E. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19(1 suppl):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481–492. doi: 10.7326/0003-4819-155-8-201110180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monticciolo DL, Helvie MA, Hendrick RE. Current issues in the overdiagnosis and overtreatment of breast cancer. Am J Roentgenol. 2018;210(2):285–291. doi: 10.2214/AJR.17.18629. [DOI] [PubMed] [Google Scholar]
- 8.Flowers CI, O’Donoghue C, Moore D, Goss A, Kim D, Kim JH, Elias SG, Fridland J, Esserman LJ. Reducing false-positive biopsies: a pilot study to reduce benign biopsy rates for BI-RADS 4A/B assessments through testing risk stratification and new thresholds for intervention. Breast Cancer Res Treat. 2013;139:769–777. doi: 10.1007/s10549-013-2576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006;239(2):385–391. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 10.Leblebici İM, Bozkurt S, Eren TT, Ozemir IA, Sagiroglu J, Alimoglu O. Comparison of clinicopathological findings among patients whose mammography results were classified as category 4 subgroups of the BI-RADs. Northern Clin Istanb. 2014;1(1):1–5. doi: 10.14744/nci.2014.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaiwerawattana A, Thanasitthichai S, Boonlikit S, Apiwanich C, Worawattanakul S, Intakawin A, Rakiad S, Thongkham K. Clinical outcome of breast cancer BI-RADS 4 lesions during 2003-2008 in the National Cancer Institute Thailand. Asian Pac J Cancer Prev. 2012;13(8):4063–4066. doi: 10.7314/APJCP.2012.13.8.4063. [DOI] [PubMed] [Google Scholar]
- 12.Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 lesions detected with screening mammography and screening US: utility of MR imaging. Radiology. 2015;274(2):343–351. doi: 10.1148/radiol.14140645. [DOI] [PubMed] [Google Scholar]
- 13.Cooper K (1998) Springhouse: Springhouse Corp. Pathophysiology Made Incredibly Easy.
- 14.Winkel RR, von Euler-Chelpin M, Nielsen M, Petersen K, Lillholm M, Nielsen MB, Lynge E, Uldall WY, Vejborg I. Mammographic density and structural features can individually and jointly contribute to breast cancer risk assessment in mammography screening: a case–control study. BMC Cancer. 2016;16(1):414. doi: 10.1186/s12885-016-2450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch AM, Kessels AG, Beets GL, Rupa JD, Koster D, van Engelshoven JM, von Meyenfeldt MF. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol. 2003;48(3):285–292. doi: 10.1016/S0720-048X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 16.Wiratkapun C, Bunyapaiboonsri W, Wibulpolprasert B, Lertsithichai P. Biopsy rate and positive predictive value for breast cancer in BI-RADS category 4 breast lesions. Med J Med Assoc Thai. 2010;93(7):830. [PubMed] [Google Scholar]
- 17.Popli MB. Pictorial essay: Mammographic features of breast cancer. Indian J Radiol Imaging. 2001;11(4):175. [Google Scholar]
- 18.Bird RE, Wallace TW, Yankaskas BC. Analysis of cancers missed at screening mammography. Radiology. 1992;184(3):613–617. doi: 10.1148/radiology.184.3.1509041. [DOI] [PubMed] [Google Scholar]
- 19.Winston JS, Yeh IT, Evers K, Friedman AK. Calcium oxalate is associated with benign breast tissue: can we avoid biopsy? Am J Clin Pathol. 1993;100(5):488–492. doi: 10.1093/ajcp/100.5.488. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors. J Mammary Gland Biol Neoplasia. 2005;10(2):181–187. doi: 10.1007/s10911-005-5400-6. [DOI] [PubMed] [Google Scholar]
- 21.Retsky M, Demicheli R, Hrushesky W. Breast cancer screening for women aged 40–49 years: screening may not be the benign process usually thought. J Natl Cancer Inst. 2001;93(20):1572. doi: 10.1093/jnci/93.20.1572. [DOI] [PubMed] [Google Scholar]