Abstract
The ovary is a common site of metastasis. Differential diagnosis of ovarian carcinomas, including secondary tumors, remains a challenging task. Clinical decision-making depends on an accurate diagnosis of the type of ovarian cancer. This study was done to evaluate the pattern of metastatic tumors to the ovary and clinical details and to analyze the survival outcomes over a period of 5 years. Patients who had metastatic tumors to the ovary are identified from the electronic database from 1 January 2015 to 30 September 2019. Clinical details are collected from the electronic charts. Survival data is collected over the phone. The total number of ovarian cancers treated during the time period was 720, of which primary high-grade mucinous tumors contributed 9 (1.2%), and metastatic tumors to ovary 70 (10%). The highest levels of CEA were seen in carcinoma rectum, colon, and cholangiocarcinoma. CA 19-9 was very high in carcinoma gall bladder, pancreas, and cholangiocarcinoma. Common primaries were stomach (23%), gall bladder (13%), and colon (13%). Adenocarcinoma with signet ring cells was found in 29% of the patients. The median follow-up was 7 months (range 1 to 40 months). The median overall survival was 10 months after diagnosis (95% CI,7.9–12.0). There was no statistically significant difference in survival between patients who had peritoneal carcinomatosis with enlarged ovaries and those who had metastasis confined to ovaries (p value 0.360). A diagnosis of metastatic tumors to the ovary is associated with a very poor prognosis and the focus of treatment should be to improve the quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13193-020-01267-4.
Keywords: Metastatic, Ovary, Tumors
Introduction
In 1896, a German gynecologist and pathologist Friedrich Ernst Krukenberg described a new kind of primary ovarian cancer which he named “fibrosarcoma ovarii mucocellulare carcinomatodes.” The metastatic nature of this tumor was revealed 5 years later by Kraus, who first used the term “Krukenberg tumor.” Krukenberg tumors are secondary ovarian tumors histopathologically defined as carcinomas that have a significant component (arbitrarily defined as > 10% of the tumor) of mucin-filled signet-ring cells. However, this definition is not followed by all authors currently, and the designation Krukenberg tumor is sometimes applied loosely to all metastatic tumors to the ovary [1].
The proportion of malignant neoplasms of the ovary that are of metastatic origin varies according to the geographic region, ranging from 3 to 15% in western countries to 21–30% in eastern nations [2]. Sometimes an ovarian mass represents the first manifestation of disease from a clinically occult non-ovarian primary. Secondary tumors to the ovary commonly arise from the stomach, breast, gall bladder, gastrointestinal tract, pancreas, and rarely from other sites [3]. The correct diagnosis of secondary ovarian tumors may be challenging and they are frequently misdiagnosed as primary ovarian cancers, particularly in the case of mucinous adenocarcinomas. The distinction from the latter is essential, as it requires different treatments as well as the prognosis is also different.
The available data on secondary ovarian tumors is rather limited due to the relative heterogeneity of this group and the absence of any prospective trials. The rational workup, the role of cytoreductive surgery and adjuvant chemotherapy, and the prognosis are all ill-defined. Publications on this topic from India are sparse and mainly consist of case reports. In this study, we explore the clinicopathological characteristics, treatment, and outcomes of secondary tumors to the ovary.
Methods
Patients who had metastatic tumors to the ovary between 1 January 2015 to 30 September 2019 were identified from the electronic database. Demographic, clinical, and follow-up details were collected from the electronic medical record. Any patient presenting to the Gynecologic oncology outpatient department with features suggestive of ovarian cancer were evaluated for metastasis to the ovary. A good history and clinical examination, focusing on the possible primary sites like breast, gastrointestinal tract, and the lung, were undertaken. Serum CA-125 and CEA were done on all patients. Serum CA 19-9, stool occult blood, gastroduodenoscopy, colonoscopy, mammography, and image-guided biopsy were done at the clinician’s discretion or as indicated. The diagnosis was confirmed by biopsy, routine staining, and immunohistochemistry. In patients who were already diagnosed with an advanced primary tumor, radiological findings suggestive of a metastatic tumor to ovary were considered diagnostic.
Ethical clearance was obtained from the institutional review board of Christian Medical College, Vellore. The consent of the patients was not obtained given the retrospective nature of the study.
Statistical Analysis
Survival data were collected by telephonic contact of the patient or their family or by the latest hospital records. Survival time was calculated from the time of diagnosis of the metastatic tumor either by biopsy or by radiological methods onwards. Descriptive statistics, chi-squared tests, Kaplan Meier survival curves, and log-rank test were used for analysis. The data was analyzed using SPSS 20.0 (IBM Inc. Armonk, NY, USA).
Results
The total number of ovarian cancers (primary and metastatic) treated during the period was 720, of which there were 9 (1.2%) primary high-grade mucinous tumors, 40 (5.5%) primary low-grade mucinous tumors, and 70 (10%) metastatic tumors to the ovary.
The median age of the metastatic group was 45 years (range 17–72). The majority of patients had a performance status of ECOG 1 (57%) or ECOG 2 (18%) at the time of diagnosis. Forty-three patients (61%) came to the clinic with symptoms suggestive of gynecologic malignancy while twenty-seven (39%) patients were referred from other departments with suspected metastasis to ovaries (Table 1). The mean serum CA125 was 159.5 U/ml (range 7–1502). Serum CEA was elevated in 56 patients (80%) with highest levels seen in carcinoma rectum (mean: 636 ng/ml), cholangiocarcinoma (mean: 538), and carcinoma colon (mean: 141). Very high levels of serum CA19-9 were noted in carcinoma gall bladder (mean: 7992 U/ml), cholangiocarcinoma (mean: 5720 U/ml), and carcinoma pancreas (mean: 10620 U/ml) (Table 2). Metastasis to ovary was confirmed histologically in 96% of patients while in 4% of patients, the diagnosis was made radiologically.
Table 1.
Clinical details of patients with ovarian metastases (n = 70)
| Factor | Number (%) |
|---|---|
| Performance status (ECOG) | |
| 0 | 4 (8.9) |
| 1 | 26 (57.8) |
| 2 | 8 (17.8) |
| 3 |
7 (15.6) Missing (25) |
| Clinical presentation | |
| Directly to the gynecologic oncology clinic | 43 (61.4) |
| Referred from another department | 27 (38.6) |
| Diagnosis | |
| Carcinoma stomach | 16 (22.9) |
| Unknown primary | 11 (15.7) |
| Carcinoma gall bladder | 9 (12.9) |
| Carcinoma colon | 9 (12.9) |
| Carcinoma breast | 6 (8.6) |
| Carcinoma appendix | 4 (5.7) |
| Lower GIT unspecified | 4 (5.7) |
| Carcinoma rectum | 4 (5.7) |
| Carcinoma lung | 3 (4.3) |
| Carcinoma pancreas | 1 (1.4) |
| Cholangiocarcinoma | 1 (1.4) |
| Histology | |
| Adenocarcinoma | 35 (50.7) |
| Adenocarcinoma with signet ring cells | 20 (28.6) |
| Carcinoma of mammary origin | 6 (8.6) |
| GIST | 2 (2.9) |
| Adenocarcinoma with extracellular mucin | 3 (4.3) |
| Clear cell carcinoma | 1 (1.4) |
| Imaging | |
| Peritoneal carcinomatosis | 54 (76.8) |
| Isolated ovarian metastasis | 16 (23.2) |
| Interventions | |
| Laparoscopy and biopsy | 1 (1.4) |
| Laparoscopy and oophorectomy | 2 (2.9) |
| Laparotomy and cytoreduction | 12 (17.1) |
| Image-guided biopsy | 33 (47.1) |
| Gastrointestinal endoscopy and biopsy | 16 (22.9) |
| Endometrial biopsy | 1 (1.4) |
| Indication for surgery (n = 18) |
Diagnostic—2 (11%) Symptomatic—1 (5.6%) Ovarian function suppression for carcinoma breast—3 (16.7%) Suspected primary ovarian cancer—11 (61.1%) Metastasectomy—1 (5.6%) |
Table 2.
Tumor markers in carcinomas at various sites
| Serum marker | Mean/median (range) | p value |
|---|---|---|
| CA 125 (n = 68) U/ml | 159.5 (7.8–1502) | |
| CEA (n = 62) ng/ml | ||
| Breast | 3.4 (1.3–4.9) | |
| GIT | 71.0 (0.5–932.0) | 0.185 |
| Pancreaticobiliary | 211.9 (1.3–1250) | |
| Others | 54.2 (0.7–305.0) | |
| CA19-9 (n = 41) U/ml | ||
| Breast | 14.3 (13.5–15.1) | |
| GIT | 193.1 (2.5–1614) | 0.002 |
| Pancreaticobiliary | 6204.0 (10.8–20,000) | |
| Others | 73.4 (2.5–290) | |
Common primaries included stomach in 23%, gall bladder in 13%, and colon in 13%. Less common primaries were breast in 8%, lung 4%, appendix 5%, unspecified lower GI tract 5%, rectum 5%, pancreas 1%, bile duct 1%, GIST 3%, and unknown primary in 16%.
Adenocarcinoma with signet ring cells was found in 29% of the patients. On imaging, ovarian masses with peritoneal carcinomatosis were found in 77% of the patients and ovarian metastasis alone was found in 23% of the patients. Solid masses were found in 46%, solid and cystic in 54%, unilateral masses were found in 17% of the patients, bilateral in 83%, and ascites was present in 73% of patients.
Out of 70 patients, 18 patients underwent surgery (25%). Indications for surgery included diagnostic 3%, symptom control 1.5%, ovarian function suppression for metastatic breast cancer 4%, suspected primary ovarian malignancy 16%, and metastasectomy in 1.5%. Laparotomy and cytoreductive surgery were done in 17% of patients and laparoscopic bilateral salpingo-oophorectomy was done in 3% of patients. An image-guided biopsy was used for diagnosis in 47% of patients and gastrointestinal endoscopy and biopsy were done in 30% of the patients.
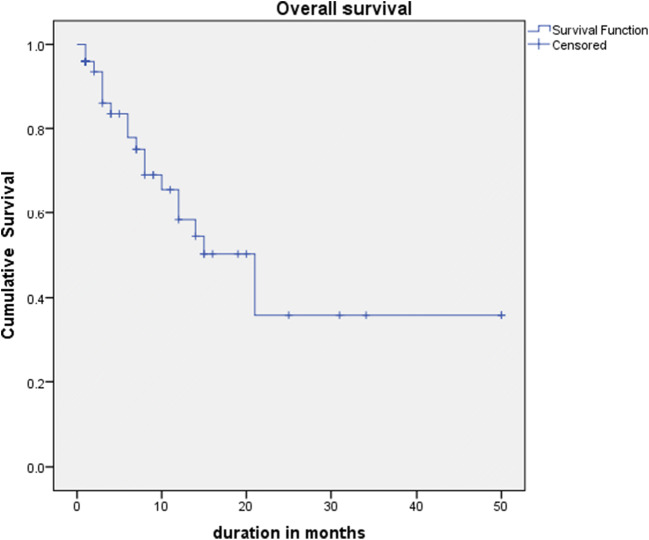
The median follow-up was 8 months (range 1 to 50 months). The median overall survival was 21 months after diagnosis (95% CI, 12.5–29.4) (Fig. 1). There was no statistically significant difference in survival between patients who had peritoneal carcinomatosis with enlarged ovaries and those who had metastasis confined to ovaries (p value 0.360) (Fig. 2). The difference in survival between different primary tumors was also not statistically significant (p value 0.085). Patients who presented with a good performance status had better survival compared to those with poor performance status(p value 0.003).
Fig. 1.
Median follow-up and median overall survival after diagnosis
Fig. 2.
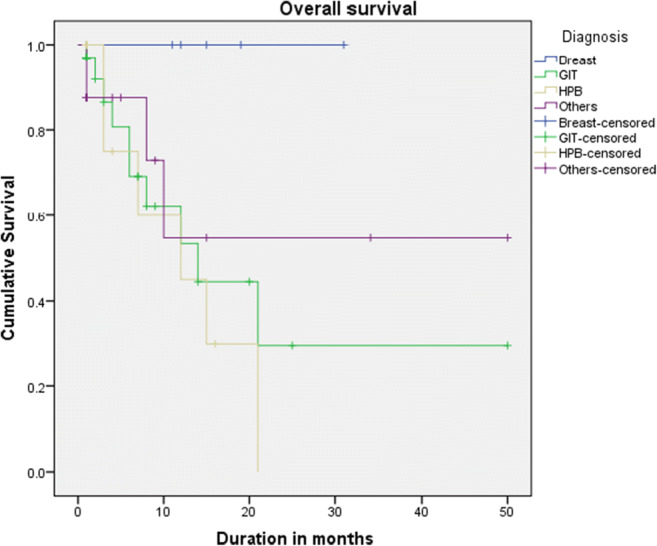
Comparison of overall survival with different primaries (p value: 0.171)
Discussion
Differentiating between primary ovarian tumors from metastatic tumors can be very difficult at times. It requires a detailed history taking and clinical examination, careful examination by pathologists along with the help of immunohistochemistry, tumor markers, and imaging studies.
Pathologic Examination
Gross features that favor metastases include small size (often < 10–12 cm), bilaterality, a nodular growth pattern, and presence of a tumor on the surface and/or in the superficial cortex of the ovary. In contrast, primary ovarian tumors are unilateral and large (> 10–12 cm). Sometimes, however, metastases can be large, unilateral, and cystic, simulating a primary ovarian neoplasm. Histological features that favor metastases include an infiltrative growth pattern with stromal desmoplasia, a nodular growth pattern, involvement of the ovarian surface and superficial cortex, and hilar and lymphovascular space involvement. On the contrary, primary ovarian tumors lack these features and have a confluent, glandular growth pattern. However, some metastatic carcinomas grow in confluent patterns. For endometrioid-like tumors, evidence of an adenofibromatous background, squamous differentiation, and endometriosis favor primary ovarian origin. The presence of signet-ring cells most of the time indicates metastatic carcinoma of the gastrointestinal tract or breast origin, with rare exceptions [2] (Supplementary Table 1). Besides, the presence of pseudomyxoma peritonei should always alert to the possibility of a primary from the appendix, rather than a primary ovarian tumor [4].
Immunohistochemistry
Nuclear PAX8 staining has been found in 99% of HGSOC and 71% of non-serous ovarian epithelial neoplasms and positivity strongly favors an ovarian primary [5]. WT1 is characteristic of the serous subtype and is rarely found in non-serous ovarian subtypes [6]. Mucinous ovarian intestinal-type tumors show a similar immunohistochemical profile as metastatic colorectal adenocarcinomas with positive CEA, CK20, and CDX2. However, CK7 is usually strongly positive in ovarian intestinal-type primaries with patchier CK20 and CDX2, whereas colorectal tumors are strongly and diffusely positive with CK20 and CDX2 and negative with CK7 [7]. Ovarian mucinous carcinoma is negative for ß-catenin and positive for MUC5AC, whereas colon carcinoma shows a reverse pattern [8]. The immunophenotypes of selected primary and secondary ovarian tumors are listed in Table 3 [9].
Table 3.
Immunohistochemistry
| Primary ovarian carcinoma | Positive | Negative |
|---|---|---|
| Serous | CK7, CA 125, PAX8, WT1 | CK20 |
| Mucinous | CK7, CK20, MUC5AC (VE), CEA, PAX8 | CA 125 (VE) |
| Endometrioid | CK7, CA 125, HAM54, ER, PR, PAX8 | CK20, CEA |
| Metastatic carcinoma | ||
| Colorectum | CK20, CEA. CDX2 | CK7 (VE in mucinous), CA 125, MUC5AC, HAM56 (VE) |
| Appendix | CK20, MUC5AC (VE), CEA | CK7 (VE), CA 125 |
| Stomach | CK7, CK20 (VE), MUC5AC | CA 125, HAM56 (VE) |
| Breast | GCDFP15, mammaglobin, GATA3, ER (VE), PR (VE) | Vimentin, WT1, CA 125 (VE) |
| Pancreas | CK7, CK20 (VE), MUC5AC, CEA, CA 19-9 | CA 125, HAM56, DPC4 (negative in ~5 0%) |
| Renal | Vimentin, AE1/AE3, CD10, RCC, PAX8 | CK7, CK20 |
| Cervix | p16, CEA, HPV infection | ER, PR |
VE variable expression
Tumor Markers
Elevated CA 125 can be found in 80% of women with primary epithelial ovarian cancer and 70% of those with metastatic to the ovary. A CA 125/CEA serum ratio greater than 25 was found to have a specificity of 100% and a sensitivity of 91% resulting in an overall test accuracy of 94% [10]. CA 19-9 can help differentiate a primary tumor from a pancreaticobiliary metastasis as the marker is usually expressed in the latter but not in ovarian tumors [11].
It has been reported that the majority of invasive mucinous tumors in the ovary are metastatic with only 2 to 3% of invasive mucinous carcinomas are truly primary ovarian in origin [10]. On analyzing our data, results are similar to other studies with high-grade primary mucinous tumors of the ovary contributing only 7.5% to all mucinous tumors of the ovary. The most common sites of primaries in our study patients were from the stomach, gall bladder, colon, and breast in descending order similar to other studies in the literature [12].
A study from India to evaluate the patterns of treatment and factors affecting outcomes in ovarian metastases of colorectal origins found that there was no significant difference in the median survival between patients treated with surgery plus chemotherapy (23 months) vs. those treated with chemotherapy alone (28 months). Age and presence of disease at other sites did not affect the outcomes. Non-signet ring cell histology showed better outcomes compared to signet ring cell histology (p = 0.02) [9].
Despite diagnostic uncertainty concerning the primary site of many metastatic mucinous carcinomas, the literature showed that the survival of women reclassified as having metastatic mucinous carcinomas compared with advanced primary ovarian mucinous carcinomas did not differ by much [13]. The tumors with better survival in our series were GIST with peritoneal and ovarian metastasis and metastatic ALK-positive lung cancer. Both these groups were treated with targeted therapies (Imatinib and crizotinib) and were alive at the time of reporting. All other metastatic tumors were associated with very poor prognosis and cytoreductive surgeries were not associated with any improvement in survival. Hence, the correct diagnosis of metastatic tumors to the ovary is very important as it can be mistaken for primary ovarian tumors and the patient can undergo unnecessary cytoreductive surgery. IHC was found to be very helpful in diagnosis along with clinical history and examination, imaging, tumor markers, and H&E staining. When patients are diagnosed with a metastatic tumor to the ovary and no targeted therapies are available, the aim of treatment should be palliative, and measures to improve the quality of life should be undertaken. In our study, we also found that the survival of those patients who presented with good performance status was better compared to those with poor performance status. However, the question of improving survival by aggressive treatment of such patients cannot be answered by our study.
As per departmental protocol, patients diagnosed with metastatic tumors were managed most often with palliative intent either with chemotherapy or best supportive care. Some patients underwent cytoreductive surgery with a mistaken diagnosis of primary ovarian malignancy. A few patients were operated for symptom relief because of a huge abdominal mass.
Conclusion
Metastatic tumors to the ovary should always be considered during the evaluation of an adnexal mass. Common primary sites include the gastrointestinal tract, gall bladder, and breast.
Evidence for the benefit of aggressive treatments like cytoreductive surgery or chemotherapy is lacking and such treatments when opted should be reserved for symptomatic relief. Differentiating between primary and metastatic tumors is difficult but should be done to prevent the unnecessary suffering of the patient. To conclude, a diagnosis of metastatic tumor to the ovary is associated with poor prognosis and a multidisciplinary team approach including a surgical oncologist, medical oncologist, and radiation oncologist, and palliative care specialist is needed for optimizing the outcome.
Supplementary Information
(DOCX 13 kb)
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ramesan C.K, Email: drrameshck82april@gmail.com.
Vinotha Thomas, Email: thomasvinotha@gmail.com.
Dhanya Susan Thomas, Email: dhanya.sus@gmail.com.
Sherin Daniel, Email: sherin1607@gmail.com.
Ajit Sebastian, Email: sebastian.ajit@gmail.com.
Anitha Thomas, Email: anithomas@cmcvellore.ac.in.
Rachel George Chandy, Email: rachelgchandy@gmail.com.
Abraham Peedicayil, Email: abraham@cmcvellore.ac.in.
References
- 1.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: Part I. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol. 2006;13(5):205–227. doi: 10.1097/01.pap.0000213038.85704.e4. [DOI] [PubMed] [Google Scholar]
- 2.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classifi cation of tumours of female reproductive organs. 4. Lyon: International Agency for Research on Cancer; 2014. p. 309. [Google Scholar]
- 3.Moore RG, Chung M, Granai CO, Gajewski W, Steinhoff MM. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol. 2004;93(1):87–91. doi: 10.1016/j.ygyno.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Kelemen LE, Köbel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol. 2011;12(11):1071–1080. doi: 10.1016/S1470-2045(11)70058-4. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira CR, Carvalho JP, Soares FA, Siqueira SAC, Carvalho FM. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: immunohistochemical evidence that they are secondary tumors. Int J Gynecol Cancer. 2008;18(1):59–65. doi: 10.1111/j.1525-1438.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 6.Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35(6):816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004;44(2):109–115. doi: 10.1111/j.1365-2559.2004.01787.x. [DOI] [PubMed] [Google Scholar]
- 8.Frumovitz M, Schmeler KM, Malpica A, Sood AK, Gershenson DM. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol. 2010;117(3):491–496. doi: 10.1016/j.ygyno.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubeček O, Laco J, Špaček J, Petera J, Kopecký J, Kubečková A, Filip S. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metastasis. 2017;34(5):295–307. doi: 10.1007/s10585-017-9856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer. 2011;117(3):554–562. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yedema CA, Kenemans P, Wobbes T, Thomas CM, Bon GG, Mulder C, et al. Use of serum tumor markers in the differential diagnosis between ovarian and colorectal adenocarcinomas. Tumour Biol J Int Soc Oncodev Biol Med. 1992;13(1–2):18–26. doi: 10.1159/000217748. [DOI] [PubMed] [Google Scholar]
- 12.Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor. Arch Pathol Lab Med. 2006;130:6. doi: 10.5858/2006-130-1725-AILAKT. [DOI] [PubMed] [Google Scholar]
- 13.Kammar PS, Engineer R, Patil PS, Ostwal V, Shylasree TS, Saklani AP. Ovarian metastases of colorectal origin: treatment patterns and factors affecting outcomes. Indian J Surg Oncol. 2017;8(4):519–526. doi: 10.1007/s13193-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)