Abstract
Purpose
Recent evidence suggests that the smaller retinal vessels are significantly involved in the regulation of retinal blood flow and that this regulation may differ among the macular area and the retinal periphery. An alternative to studying blood flow regulation in smaller retinal vessels that are difficult to resolve is to assess the metabolic consequences of changes in the microcirculation using oximetry.
Methods
In 20 normal persons aged (mean ± SD, range) 30.1 ± 3.8 (24–37) years, the oxygen saturation and diameter of retinal arterioles and venules to the macular area and the retinal periphery were studied before and during an increase in the arterial blood pressure induced by isometric exercise.
Results
The isometric exercise increased the mean arterial blood pressure by (mean ± SEM) 10.0 ± 1.1 mm Hg but induced no significant changes in the diameter of the arterioles (P = 0.83). The isometric exercise had no significant effect on the oxygen saturation in the arterioles supplying the macular area and the retinal periphery (P > 0.42 for both comparisons). However, there was a significant increase in the oxygen saturation in venules draining the retinal periphery to reduce the oxygen extraction from (mean ± SEM) 36.0% ± 2.3% to 30.6% ± 2.1% (P = 0.002) but no significant change in the preexisting low oxygen extraction in the macular area that changed from (mean ± SEM) 18.2% ± 3.0% to 16.2% ± 1.9% (P = 0.37).
Conclusions
Minor changes in the arterial blood pressure can induce changes in retinal rheology with significant regional variation. The finding may help explain regional variations in manifestations of retinal vascular disease such as hyperpermeability in the macular area and capillary occlusion in the retinal periphery.
Keywords: retinal oximetry, shunting, regional variation, vascular disease
Retinal blood flow is autoregulated, which has hitherto mainly been studied in the larger resistance vessels.1,2 However, evidence suggests that the smaller vessels also play a significant role in this regulation.3–6 In the macular area, vessel diameters and flow have been studied in perifoveal capillaries,7,8 whereas less evidence has been obtained about the extrafoveal microcirculation.9,10 Smaller retinal vessels outside the macular area are difficult to study, but the relevance of also investigating blood flow in this zone is testified by observations of differences in diameter regulation,11 oxygen saturation,12 and ischemic conditioning13 in vessels supplying the macular area and the retinal periphery.
An alternative to studying blood flow in the smaller retinal vessels that are difficult to resolve is to evaluate the metabolic consequences of changes in the microcirculation using oximetry.14 With this approach, it has been shown that the oxygen saturation is higher in venules draining the macular area than the retinal periphery12 and that retinal neovascularizations15 and microvascular abnormalities16 in diabetic retinopathy act as arteriovenous (A-V) shunts that can bypass areas of capillary occlusion. However, the effects of normal physiologic regulation of retinal blood flow on oxygen saturation in different parts of the retina have not been studied in detail.
Therefore, changes in oxygen saturation of retinal vessels supplying the macular area and the retinal periphery were studied in 20 normal persons before and during a moderate increase in the arterial blood pressure induced by isometric exercise.
Materials and Methods
Participants
Twenty normal persons (11 men and 9 women) aged (mean ± SD, range) 30.1 ± 3.8 (24–37) years with no previous or current systemic or ocular disease were studied. The persons were recruited by announcement among the staff and students at the Department of Ophthalmology, Aarhus University Hospital. The study was a part of an investigation of regional differences in retinal blood flow17 and had been approved by the regional ethics committee.
Ophthalmologic Examination
The test persons were subjected to an ophthalmologic examination including measurement of best-corrected visual acuity and slit-lamp examination. The pupils were dilated using tropicamide 1% (Alcon, Copenhagen, Denmark) and phenylephrine 10% (Amgros I/S, Copenhagen, Denmark) eye drops, and for the documentation of normal retinal morphology, two fundus photographs (Canon CF 60Z, Amstelveen, the Netherlands) with a 60-degree field-of-view photograph were acquired with one field centered on the macular area and another field centered on the optic disk.
Isometric Exercise
Before the examination, the test person was seated at rest for at least 5 minutes, the cuff of an automatic blood pressure monitor (705IT; Omron Healthcare, Kyoto, Japan) was placed on the upper left arm, and blood pressure was measured three times. Subsequently, the person was asked to lift a 2-kg hand weight in the straight right arm. After 30 seconds, blood pressure was measured, and the arm was lowered to relax for 5 minutes. This procedure of lifting the hand weight, measurement of blood pressure, and relaxation was subsequently repeated twice. The average of each of the three values was calculated to represent the arterial blood pressure before and during exercise.
Retinal Oximetry
Retinal oximetry was performed using a dual-wavelength oximeter (model T1; Oxymap, Reykavik, Iceland). The principle of the technique has been described in detail previously.18 Basically, a fundus photograph (Topcon TRC-50DX; Topcon, Tokyo, Japan) is recorded at two different wavelengths, which is used to calculate the oxygen saturation in retinal vessels with a diameter larger than approximately 60 µm.19,20
The procedures described in Jørgensen and Bek12 were followed. The test person was seated at rest in front of the oximeter for at least 20 minutes, and five oximetry images were recorded in the right eye, following a sequence that included images centered on the optic disk and the upper temporal vascular arcade.12
After 5 minutes at rest, the procedure of lifting the hand weight with the right arm was repeated, and after 30 seconds of lifting, the acquisition of the sequence of oximetry recordings was repeated, so that the photograph centered on the upper temporal arcade was captured within 5 seconds.
Data Analysis
Figure 1 shows examples of the selection of vascular segments for extracting retinal oxygen saturations. The peripapillar oxygen saturations and vessel diameters (Fig. 1A) were calculated as the average of the saturations measured in the four arterial and venous vascular arcades in segments located between two circles centered on the optic disk. The inner of these circles had a diameter 30 pixels larger than the disk and the outer circle a diameter three times that of the optic disk.21 The oxygen saturations and diameters from the upper temporal vascular arcade (Fig. 1B) were collected from macular and peripheral branches with a diameter of at least 8 pixels and at least one-third the diameter of the vascular arcade proximal from the branching.12 Starting from the branching point, the longest unbranched segment not exceeding a length of 50 pixels was marked. The distance of the branching point from the optic disk along the vessel was measured in pixels using the inbuilt analysis software in the oximeter. This resulted in the identification of 20 macular (ma) and 20 peripheral (pa) arterioles, as well as 17 macular (mv) and 15 peripheral (pv) venules that fulfilled the inclusion criteria. For each identified branch, the proximal (x) segments on the vascular arcade were marked similarly 50 pixels backward from the branching point.
Figure 1.
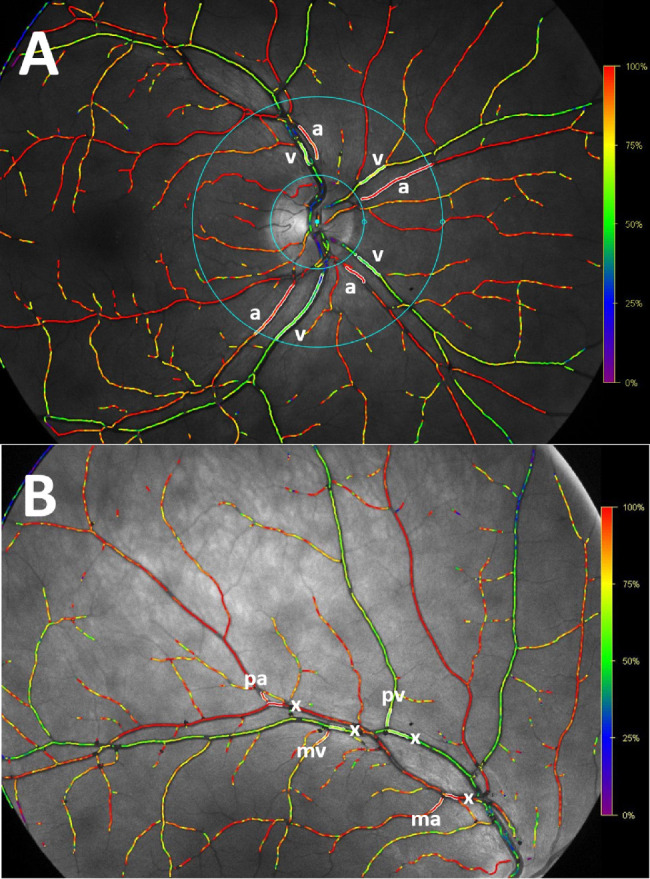
Fundus photographs from the oximeter with color coding of the retinal oxygen saturation ranging from high (red) to low (blue) saturations. The black segments represent areas where the software does not identify the vessel borders, such as areas with low contrast or at vessel crossings. The vascular segments marked in white represent those used to extract the oxygen saturations in (A) a photograph centered on the optic disk. The peripapillar saturations were extracted from the highlighted segments of arterioles (a) and venules (v) located between the two rings concentrical with the optic disk. (B) A photograph centered on the upper temporal vascular arcade. Example shows where the oxygen saturations were collected from segments highlighted (x) proximal from the branchings of a macular arteriole (ma), macular venule (mv), peripheral arteriole (pa), and peripheral venule (pv).
The retinal oxygen saturation was calculated from the intensity of light reflected from the vessels (I) and the surrounding retina (Io) at 570 nm and 600 nm as described before.19,20 The optical density was calculated as OD = log(Io/I) at each of the two wavelengths, which was subsequently used to calculate the optical density ratio ODR = OD600/OD570. The oxygen saturation was calculated as SatO2 = a*ODR + b, with a = –1.28 and b = 1.24 (Oxymap Analyzer version 2.5.2) that provided values without corrections tied to the vessel diameter.22
There was no significant difference between the distances from the optic disk of the arteriolar and the venular branchings toward respectively the macular area and the retinal periphery (P > 0.15 for all comparisons) and no significant difference between the saturation in arterioles and venules proximal from the branchings toward the macular area and the retinal periphery (P > 0.09 for all comparisons).
In the output data, the Oxymap Analyzer software had automatically converted all pixel values of vessel diameters to approximate distances at the retinal plane.23 This disqualified a comparison of vessel diameters among individuals because of the individually varying magnification in the optics of the eye but had no influence on the calculations of percentage changes in vessel diameters at the retinal plane from the same person.
Statistics
STATA (version 16.0; StataCorp, College Station, TX, USA) was used for the statistical analyses. Normal distribution was ensured by probability plots.
Unpaired t-test was used to test differences in the distance from the optic disk to the branching of arterioles and venules toward the macular area and the retinal periphery, as well as differences in oxygen saturation and diameter between macular and peripheral arterioles and venules. For the presentation in column diagrams, the most proximal of the segments on each vascular arcade, related to either the macular or the peripheral branch, was used to represent that vessel.
Paired t-test was used to test for changes in diameters and oxygen saturation of the same vessels induced by isometric exercise.
In the presentation of results, variations are indicated with SD for descriptive parameters and as SEM for values used for comparisons among groups.
Results
The isometric exercise increased the mean arterial blood pressure significantly (P < 0.0001) by (mean ± SEM) 10.0 ± 1.1 mm Hg (from 89.4 ± 1.9 to 99.4 ± 1.7 mm Hg) without inducing significant changes in the diameter of the temporal arterioles at the optic disk, from (mean ± SEM) 126.6 ± 2.4 µm to 126.4 ± 2.6 µm (P = 0.83), which might suggest an activation of pressure autoregulation. Isometric exercise induced no significant (P = 0.67) change in the peripapillar oxygen saturation in arterioles (from 99.1% ± 0.7% to 98.9% ± 0.6%) but significantly (P = 0.0004) increased saturation in the corresponding venules (from 61.6% ± 1.2 % to 70.5% ± 2.5%).
Figure 2 shows the oxygen saturations proximally from and in the macular and peripheral branches from the upper temporal vascular arcades. It appears that isometric exercise induced (1) no significant change in oxygen saturation in arterioles proximal from the branchings (from 98.2% ± 0.8% to 98.7% ± 0.9%, P = 0.67), in peripheral (from 102.6% ± 0.9% to 101.8% ± 0.9%, P = 0.42) or in macular (from 98.6% ± 1.8% to 99.5 ± 1.2%, P = 0.57) arterioles and (2) a significant increase in the oxygen saturation in venules proximal from the branching (from 60.1% ± 2.0% to 64.5% ± 1.7%, P = 0.004) and in peripheral venular branches (from 66.9% ± 2.5% to 71.8% ± 1.9%, P = 0.003) but no significant increase in the macular venules (from 80.0% ± 1.8% to 82.5% ± 1.5%, although this difference was borderline significant [P = 0.05]). Similarly, (3) the A-V saturation difference decreased significantly in the vessels proximal from the branchings (from 37.2% ± 1.8% to 33.9% ± 2.0%, P = 0.04) and in peripheral (from 36.0% ± 2.3% to 30.6% ± 2.1%, P = 0.002) but not in macular (from 18.2% ± 3.0% to 16.2% ± 1.9%, P = 0.37) vessels. The increase in the oxygen saturation in peripheral venules during isometric exercise was so prominent that it could be observed directly in the color coding of the vessels (Fig. 3).
Figure 2.
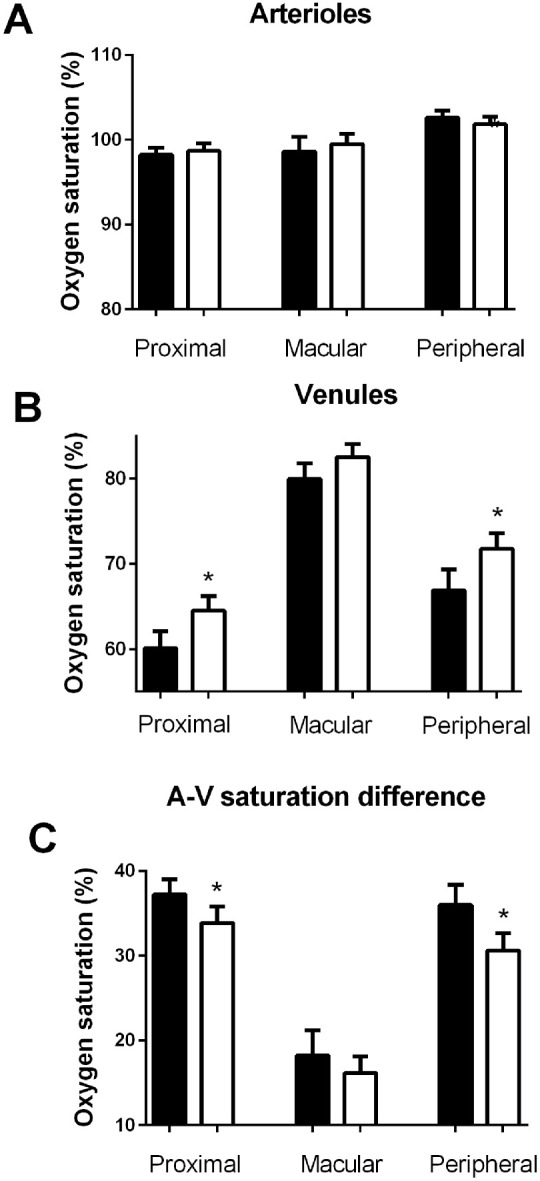
The oxygen saturations proximal from and in macular and peripheral branches from the upper temporal vascular arcade vessels before (solid bars) and during (open bars) an increase in the arterial blood pressure induced by isometric exercise in (A) arterioles, (B) venules, and (C) arteriovenous saturation difference. *P < 0.05.
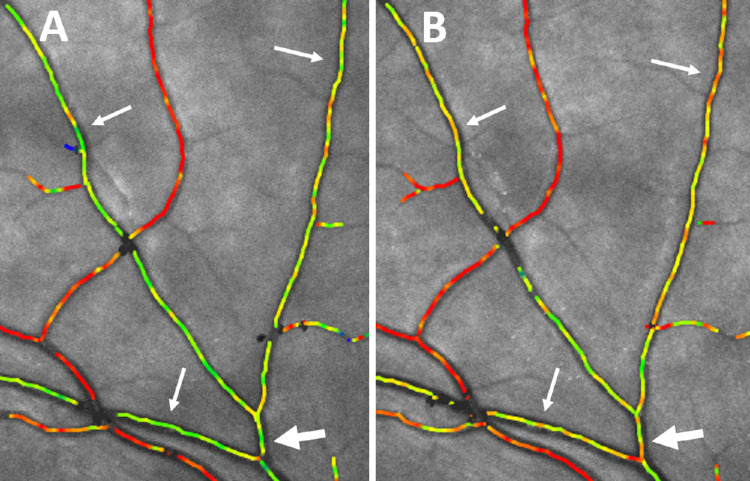
Figure 3.
High magnification of oximetry images centered on the upper temporal arcades. (A) Before isometric exercise. The arrows point to venular segments draining the retinal periphery marked in green. The large arrow points to the segment with a diameter approximately 100 µm from which the saturation was measured to be 66%. (B) The same area 30 seconds after starting isometric exercise that increased the mean arterial blood pressure by 11.8 mm Hg. The color of the venules pointed to by the arrows can be seen to have become more yellowish, and the oxygen saturation pointed at with the large arrow had increased to 73%.
At baseline, the diameter of the peripheral venules (mean ± SEM, 128.5 ± 9.1 µm) was significantly larger (P = 0.03) than that of the macular venules (mean ± SEM, 105.1 ± 5.3 µm), whereas there was no significant difference in the diameter among the studied peripheral (mean ± SEM, 105.5 ± 4.9 µm) and macular (mean ± SEM, 114.3 ± 5.9 µm) arterioles (P = 0.26).
Figure 4 shows that isometric exercise induced no significant changes in the diameters of arterioles and venules proximal from the branchings or in the macular and peripheral branches (P > 0.10 for all comparisons). The changes in oxygen saturations and diameters from the vessel segments distal from the branchings were similar to those of the peripheral vessels (not shown).
Figure 4.
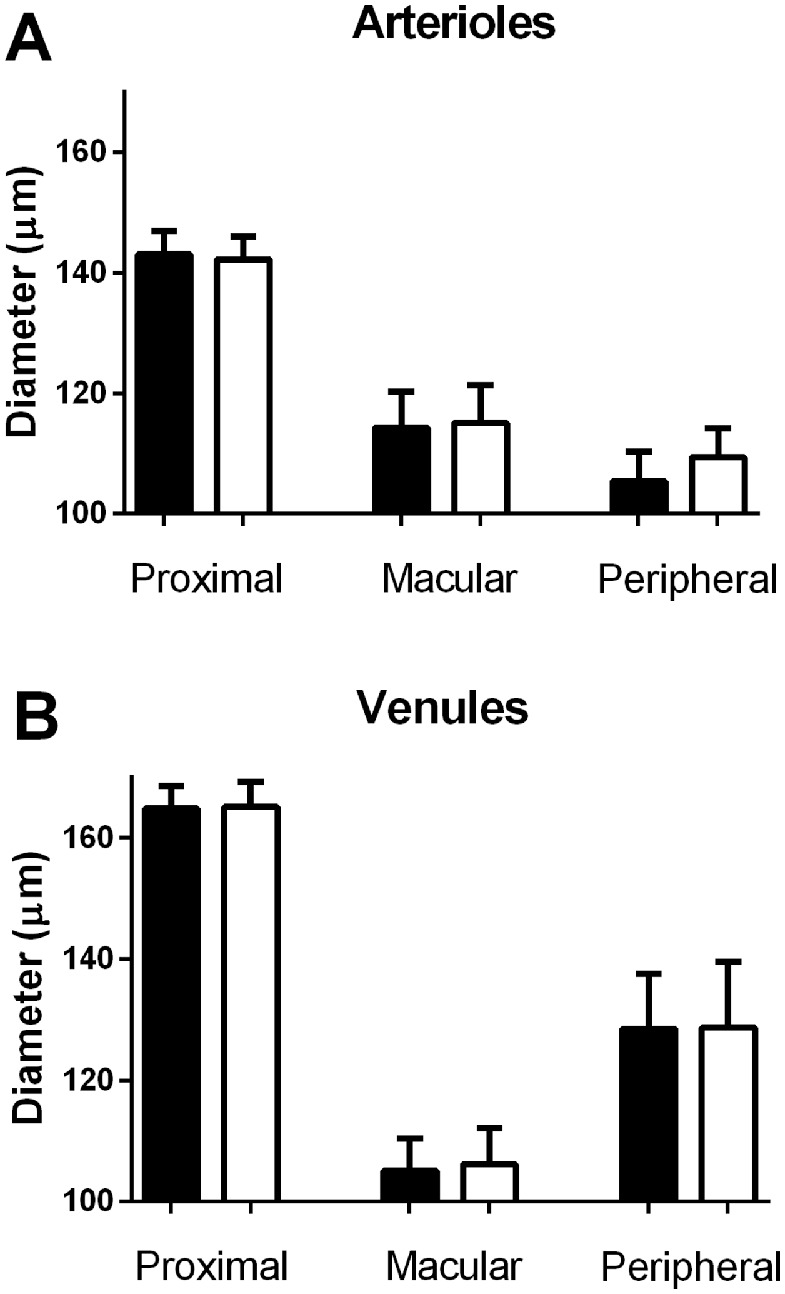
The vessel diameters proximal from and in macular and peripheral branches from the upper temporal vascular arcade vessels before (solid bars) and during (open bars) an increase in the arterial blood pressure induced by isometric exercise in (A) arterioles and (B) venules.
Discussion
The purpose of the present study was to investigate whether an increase in the arterial blood pressure induced by isometric exercise would affect oxygen extraction differently in the macular area and the retinal periphery. The peripapillar oxygen saturations and vessel diameters were similar to those previously observed in normal persons,12,17,24 and there were no significant differences in the distance from the optic disk to the branchings toward the macular area and the retinal periphery that might have caused differences in diffusion loss in these vessels. The observed lower oxygen saturation in the larger venules draining macular and peripheral branches might potentially be a consequence of differences in the linear velocity of the blood that has been shown to be inversely related to the oxygen saturation measured by dual-wavelength oximetry.17 This affects the calibration of the oximeter and can explain observations of apparent arteriolar oxygen saturations higher than 100%.18 However, when the blood volume in the microcirculation is constant, changes in blood flow with its derived effects on oxygen saturation will be similar in arterioles and venules. This implies that the A-V saturation difference is robust to the effect of the velocity of the blood on measured oxygen saturations.17 Therefore, since an increase in the oxygen saturation in venules draining the retinal periphery was accompanied by lower A-V saturation differences, these changes can be assumed to have been real.
The purpose of acquiring oximetry images shortly after the initiation of isometric exercise was to ensure that the test person could hold the head steady for the image quality to become sufficiently high. With this approach, it was not considered realistic to increase blood pressure by the around 15 to 20 mm Hg, which would induce contraction of retinal arterioles as a part of normal pressure autoregulation.25–27 The technique used to record the vessel diameters had a coefficient of variation of a few percent,23 suggesting that the observed lack of a diameter response was real and that the increase in the arterial blood pressure had been below a threshold that should be exceeded to induce contraction of the arterioles. A similar threshold has been found in the cerebral circulation28 and implies that minor fluctuations in the arterial blood pressure are transmitted to the precapillary arterioles that regulate recruitment and intermittency of blood flow in the microvascular units.29 A prominent role of these smaller vessels for regulating retinal blood flow is consistent with observations in vitro5,6,30–32 and with vivo findings that increased metabolism, resulting in a 3% to 4% dilatation of the larger retinal arterioles, elicits an approximately 10 times higher increase in retinal blood flow.4,33
In the present study, the increased arterial blood pressure can be expected to have been transmitted to the macular microcirculation since no adaptive changes were observed in diameter and oxygen saturation in the larger vessels to this area. The impact of the increased arterial blood pressure on the microcirculation can be expected to be considerably higher in conditions in which retinal autoregulation is impaired and the larger retinal arterioles are dilated.34–36 This may explain why hyperpermeability, exudates, and edema are predominantly observed in the macular area in retinal vascular disease.37–39
The increased arterial blood pressure induced adaptive changes in the peripheral microcirculation, as evidenced by an increase in the oxygen saturation of the venules and a reduction in the A-V saturation difference in this area. This might be the result of an increased capillary blood flow in the retinal periphery so that the oxygen supply had exceeded the consumption. However, a maintenance of this hyperperfusion would require a reduction in pressure autoregulation in the smaller retinal arterioles that normally protect the capillary network from the impact of increased blood pressure. Additionally, an increase in the capillary blood flow would require a reduction in metabolic autoregulation that is activated when oxygen supply exceeds consumption.24 Therefore, it is also possible that the increased blood flow may have been diverted through vascular shunts to protect the capillary bed from exposure to the increased intravascular pressure. A circumvention of the capillary bed in the retinal periphery might be hypothesized to accelerate if the opening of vascular shunts should induce a steal phenomenon from the smaller precapillary arterioles, leaving at a right angle from the larger feeder vessels.40–42 This might explain the increased oxygen saturation in the larger venules with increasing severity of diabetic retinopathy.43,44 The need for an increased shunting capacity to circumvent the capillary bed might also potentially contribute to the development of intraretinal microvascular abnormalities (intra-retinal microvascular abnormalities (IRMA) vessels)16 and neovascularizations.15 A transformation of the functional changes in capillary blood flow into irreversible capillary damage might explain postmortem observations of glial cells that have invaded the lumen to obliterate the capillaries in retinal vascular disease.45–47
The observed increase in the arteriovenous saturation difference from rest to exercise in the peripheral venules corresponds to a (30.6–36.0)/100 = 17.6% reduction in the delivery of oxygen and therefore a similar increase in the equivalents of blood volume in which oxygen has become unavailable for diffusion to the retinal tissue. Similarly, an increase in the systemic blood pressure from 89 mm Hg at rest to 99 mm Hg during isometric exercise represents an increase of 11.1%. If this can be translated to a corresponding change in the driving pressure in the vessels supplying the retinal periphery, retinal blood flow can be expected to have increased similarly in this area. These two estimates are in the same order of magnitude and may therefore reflect the approximate extent of the rheological changes in the retinal periphery induced by the increased arterial blood pressure. The exact magnitude as well as the identification of the vessels that might be responsible for a possible physiologic shunting remains to be determined but might involve the larger vessels irradiating from the vascular arcades to the retinal periphery.38 The larger diameter of the venular branches draining the retinal periphery than those draining the macular area observed in the present study might potentially be related to differences in the shunting capacity among these regions.
On the basis of the present findings, it might be hypothesized that the larger variation in the oxygen saturation measured by oximetry in retinal venules than in arterioles20,22 could be a result of variations in physiologic shunting secondary to a lack of stabilization of the arterial blood pressure. Therefore, the reliability of retinal oximetry might be improved by ensuring that recordings are carried out when the patient is at rest and the arterial blood pressure stable. The result also suggests that there is a need for investigating the influence of chronic changes in the blood pressure such as arterial hypertension on saturation values obtained by retinal oximetry.
Altogether, the findings indicate that minor changes in the arterial blood pressure can induce significant changes in retinal rheology in normal persons with different manifestations in the macular area and the retinal periphery. The finding may potentially explain how an increased blood pressure in diabetic patients, aggravated by impaired autoregulation, may lead to hyperpermeability in the macular area but capillary occlusion in the retinal periphery. Future studies should aim at studying vascular shunting in the retinal periphery and regional differences in the response to increased arterial blood pressure in retinal vascular disease.
Acknowledgments
Supported by Synoptikfonden, the Danish Eye Research Foundation, and the Toyota Foundation.
Disclosure: T. Bek, None; S.K. Jeppesen, None
References
- 1. Jeppesen P, Gregersen P, Bek T.. Age-dependent decrease in the myogenic response of retinal arterioles as studied with the Retinal Vessel Analyzer (RVA). Graefes Arch Clin Exp Ophthalmol. 2004; 242: 914–919. [DOI] [PubMed] [Google Scholar]
- 2. Seidel G, Aschinger G, Singer C, et al. Estimating retinal blood flow velocities by optical coherence tomography. JAMA Opthalmol. 2016; 134(10): 1104–1110. [DOI] [PubMed] [Google Scholar]
- 3. Puro DG. Retinovascular physiology and pathophysiology: new experimental approach/new insights. Prog Retin Eye Res . 2012; 31(3): 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aschinger GC, Schmetterer L, Fondi K, et al. Effect of diffuse luminance flicker light stimulation on total retinal blood flow assessed with dual-beam bidirectional Doppler OCT. Invest Ophthalmol Vis Sci . 2017; 58: 1167–1187. [DOI] [PubMed] [Google Scholar]
- 5. Skov Jensen P, Jeppesen P, Bek T. Differential diameter responses in macular and peripheral retinal arterioles may contribute to the regional distribution of diabetic retinopathy lesions. Graefes Arch Clin Exp Ophthalmol. 2011; 249(3): 407–412. [DOI] [PubMed] [Google Scholar]
- 6. Skov Jensen P, Aalkjaer C, Bek T.. Differential effects of nitric oxide and cyclo-oxygenase inhibition on the diameter of porcine retinal vessels with different caliber during hypoxia ex vivo. Exp Eye Res . 2017; 160: 38–44. [DOI] [PubMed] [Google Scholar]
- 7. Palkovits S, Fuchsjäger-Mayrl G, Kautzky-Willer A, et al. Retinal white blood cell flux and systemic blood pressure in patients with type 1 diabetes. Graefes Arch Clin Exp Ophthalmol. 2013; 251(6): 1475–1478. [DOI] [PubMed] [Google Scholar]
- 8. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G.. Optical coherence tomography angiography. Prog Retin Eye Res . 2018:64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garhofer G, Bek T, Boehm AG, et al. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010; 88(7): 717–722. [DOI] [PubMed] [Google Scholar]
- 10. Puchner S, Schmidl D, Ginner L, et al. Changes in retinal blood flow in response to an experimental increase in IOP in healthy participants as assessed with Doppler optical coherence tomography. Invest Ophthalmol Vis Sci . 2020; 61(2): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skov Jensen P, Aalkjaer C, Bek T.. The vasodilating effect of glucose differs among vessels at different branching level in the porcine retina ex vivo. Exp Eye Res . 2019; 179: 150–156. [DOI] [PubMed] [Google Scholar]
- 12. Jørgensen CM, Bek T.. Lack of differences in the regional variation of oxygen saturation in larger retinal vessels in diabetic maculopathy and proliferative diabetic retinopathy. Br J Ophthalmol. 2017; 101(6): 752–757. [DOI] [PubMed] [Google Scholar]
- 13. El Dabagh Y, Petersen L, Pedersen M, Bek T.. Reduced baseline diameter and contraction of peripheral retinal arterioles after remote ischemia in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2019; 257(10): 2095–2101. [DOI] [PubMed] [Google Scholar]
- 14. Stefánsson E, Olafsdottir OB, Eliasdottir TS, et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog Retin Eye Res . 2019; 70: 1–22. [DOI] [PubMed] [Google Scholar]
- 15. Bek T. Arterial oxygen saturation in neovascularizations in proliferative diabetic retinopathy. Retina . 2018; 38(12): 2301–2308. [DOI] [PubMed] [Google Scholar]
- 16. Petersen L, Bek T.. The oxygen saturation in vascular abnormalities depends on the extent of arteriovenous shunting in diabetic retinopathy. Invest Ophthalmol Vis Sci . 2019; 60(12): 3762–3767. [DOI] [PubMed] [Google Scholar]
- 17. Jeppesen SK, Bek T.. The retinal oxygen saturation measured by dual wavelength oximetry in larger retinal vessels is influenced by the linear velocity of the bloood. Curr Eye Res . 2019; 44(1): 46–52. [DOI] [PubMed] [Google Scholar]
- 18. Pálsson O, Geirsdottir A, Hardarson SH, Olafsdottir OB, Kristjánsdottir JV, Stefánsson E.. Retinal oximetry images must be standardized: a methodological analysis. Invest Ophthalmol Vis Sci . 2012; 53(4): 1729–1733. [DOI] [PubMed] [Google Scholar]
- 19. Beach JM, Schwenzer KJ, Srinivas S, Kim D, Tiedeman JS.. Oximetry of retinal vessels by dual-wavelength imaging: calibration and influence of pigmentation. J Appl Physiol . 1999; 86: 748–758. [DOI] [PubMed] [Google Scholar]
- 20. Hardarson SH, Harris A, Karlsson RA, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci . 2006; 47(11): 5011–5016. [DOI] [PubMed] [Google Scholar]
- 21. Jørgensen C, Bek T.. Increasing oxygen saturation in larger retinal vessels after photocoagulation for diabetic retinopathy. Invest Ophthalmol Vis Sci . 2014; 55(8): 5365–5369. [DOI] [PubMed] [Google Scholar]
- 22. Geirsdóttir A, Pálsson O, Hardarson SH, Ólafsdóttir OB, Kristjánsdottir JV, Stefánsson E.. Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci . 2012; 53(9): 5433–5442. [DOI] [PubMed] [Google Scholar]
- 23. Blondal R, Sturludóttir MK, Hardarson SH, Halldórsson GH, Stefánsson E.. Reliability of vessel diameter measurements with a retinal oximeter. Graefes Arch Clin Exp Ophthalmol. 2011; 249(9): 1311–1317. [DOI] [PubMed] [Google Scholar]
- 24. Werkmeister R, Schmidl E, Aschinger G, et al. Retinal oxygen extraction in humans. Sci Rep . 2015; 5: 15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jeppesen P, Sanye-Hajari J, Bek T.. Increased blood pressure induces a diameter response of retinal arterioles that increases with decreasing arteriolar diameter. Invest Ophthalmol Vis Sci . 2007; 48(1): 328–331. [DOI] [PubMed] [Google Scholar]
- 26. Bek T, Hajari J, Jeppesen P.. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008; 246(5): 763–769. [DOI] [PubMed] [Google Scholar]
- 27. Tilma KK, Bek T.. Dilatation of retinal arterioles induced by topical dorzolamide for one week is impaired in patients with type 1 diabetes and mild retinopathy. Ophthalmologica. 2020; 243(3): 236–242. [DOI] [PubMed] [Google Scholar]
- 28. Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol . 1998; 274(1, pt 2): H233–H241. [DOI] [PubMed] [Google Scholar]
- 29. Bek T. Diabetic retinopathy caused by disturbances in retinal vasomotion: a new hypothesis. Acta Ophthalmol. 1999; 77(4): 376–380. [DOI] [PubMed] [Google Scholar]
- 30. Jeppesen P, Aalkjaer C, Bek T.. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci . 2002; 43(6): 1891–1896. [PubMed] [Google Scholar]
- 31. Bek T. Translational research in retinal vascular disease: an approach. Acta Ophthalmol . 2019; 97(5): 441–450. [DOI] [PubMed] [Google Scholar]
- 32. Ernst C, Jensen PS, Aalkjaer C, Bek T.. Differential effects of intra- and extravascular ATP on the diameter of porcine vessels at different branching level ex vivo. Invest Ophthalmol Vis Sci . 2020; 61(12): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharifizad M, Witkowskal KJ, Aschinger GC, Sapeta S, Rauch A, Schmidl D.. Factors determining flicker-induced retinal vasodilatation in healthy subjects. Invest Ophthalmol Vis Sci . 2016; 57: 3306–3312. [DOI] [PubMed] [Google Scholar]
- 34. Kohner EM, Patel V, Rassam SM.. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes . 1995; 44: 603–607. [DOI] [PubMed] [Google Scholar]
- 35. Kristinsson JK, Gottfredsdóttir MS, Stefánsson E.. Retinal vessel dilatation and elongation precedes diabetic macular oedema. Br J Ophthalmol. 1997; 81(4): 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frederiksen CA, Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Bek T.. The blood pressure induced diameter response of retinal arterioles decreases with increasing diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol. 2006; 244: 1255–1261. [DOI] [PubMed] [Google Scholar]
- 37. Bek T. Regional morphology and pathophysiology of retinal vascular disease. Prog Retin Eye Res . 2013; 36: 247–259. [DOI] [PubMed] [Google Scholar]
- 38. Ometto G, Assheton P, Calivá F, et al. Spatial distribution of early red lesions is a risk factor for development of vision-threatening diabetic retinopathy. Diabetologia. 2017; 60(12): 2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bek T, Jørgensen CM.. The systemic blood pressure and oxygen saturation in retinal arterioles predict the effect of intravitreal anti-VEGF treatment on diabetic maculopathy. Invest Ophthalmol Vis Sci . 2016; 57(13): 5429–5434. [DOI] [PubMed] [Google Scholar]
- 40. Bek T, Jensen PK.. Three-dimensional structure of human retinal vessels studied by vascular casting. Acta Ophthalmol. 1993; 71(4): 506–513. [DOI] [PubMed] [Google Scholar]
- 41. Bek T. Inner retinal ischaemia: current understanding and needs for further investigations. Acta Ophthalmol. 2009; 87: 362–367. [DOI] [PubMed] [Google Scholar]
- 42. Stefánsson E, Chan YK, Bek T, Hardarson SH, Wong D, Wilson DI.. Laws of physics help explain capillary non-perfusion in diabetic retinopathy. Eye . 2018; 32: 210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bek T, Stefánsson E, Hardarson SH, Retinal oxygen saturation is an independent risk factor for the severity of diabetic retinopathy. Br J Ophthalmol. 2019; 103(8): 1167–1172. [DOI] [PubMed] [Google Scholar]
- 44. Bek T. Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep . 2017; 17(10): 82. [DOI] [PubMed] [Google Scholar]
- 45. Bek T. Glial cell involvement in vascular occlusion of diabetic retinopathy. Acta Ophthalmol. 1997; 75(3): 239–243. [DOI] [PubMed] [Google Scholar]
- 46. Bek T. Immunohistochemical characterization of retinal glial cell changes in areas of vascular occlusion secondary to diabetic retinopathy. Acta Ophthalmol. 1997; 75(4): 388–392. [DOI] [PubMed] [Google Scholar]
- 47. Bek T. Ocular changes in heredo-oto-ophthalmo-encephalopathy. Br J Ophthalmol 2000; 84(11): 1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]