Abstract
Background
The aim of this study to determine maternal adiponectin and leptin levels as biomarkers of pre-eclampsia and compare adiponectin and leptin ratio.
Materials and Methods
This is a prospective study. The enrolled women were divided into two groups: first, study group (n = 60) comprised of women diagnosed with pre-eclampsia and second, control group (n = 60) comprised of age- and gestation-matched normotensive and nonproteinuric women.
Main Outcome Measures
Maternal serum adiponectin and leptin levels and their ratio were compared in pre-eclamptic (study group) and normotensive (control group) women.
Results
Adiponectin levels were insignificantly higher in study group than control group. Leptin levels in study group were significantly higher than control group (p < 0.001). Adiponectin/leptin ratio was significantly lower in study group than controls (p < 0.0001). Sensitivity, specificity, positive predictive value and negative predictive value of serum leptin and serum adiponectin/leptin ratio as a biomarker of pre-eclampsia were 90%, 88.3%, 88.5%, 89.8% and 68.3%, 90%, 87.2%, 74%, respectively. Serum leptin levels and serum adiponectin/leptin ratio had cut-off point as 23.3 ng/ml and < 0.153, respectively. Accuracy of both serum leptin and adiponectin/leptin ratio was significant (p < 0.0001).
Conclusion
Maternal leptin-level estimation should be integrated into the investigations for pre-eclampsia, and a cut-off level of > 23.3 ng/ml should be used as a biomarker for diagnosis. Adiponectin–leptin ratio should be considered as a biomarker for PE and should be determined in all cases of pre-eclampsia, and a cut-off of < 0.153 should be used for diagnosis.
Keywords: Leptin, Adiponectin, Adiponectin and leptin ratio, Pre-eclampsia, Biomarkers
Introduction
Pre-eclampsia syndrome is a pregnancy-specific disorder characterised by onset of hypertension (BP ≥ 140/90 mmHg) with proteinuria or other organ involvement, which may include thrombocytopenia, impaired liver function, new development of renal dysfunction, new onset of cerebral or visual disturbances or pulmonary oedema after 20 weeks of gestation in a previously normotensive, nonproteinuric pregnant woman [1]. It affects 2–8% of pregnancies and is associated with considerable feto-maternal morbidity and mortality [2]. The exact aetiopathogenesis of pre-eclampsia (PE) remains elusive so far, although various theories have been put forward. However, the final common pathway of all these theories is endothelial cell dysfunction, inflammation and angiogenesis [2–4]. Conditions associated with insulin resistance (IR) like polycystic ovarian syndrome (PCOS), diabetes, obesity and hyperinsulinemia also play a role in development of PE [4].
Two bioactive adipokines, namely adiponectin and leptin, have been hypothesised to be involved in the development of PE, as they are involved in endothelial cell dysfunction, inflammation, angiogenesis, blood pressure regulation and IR [2]. These proteins are secreted almost exclusively by adipocytes [2]. These adipokines have also been shown to play a role in normal and abnormal pregnancy. It is speculated that there is dysregulation of adipokines in PE and that they may have pathophysiological and prognostic significance in this condition [5]. Alterations in adiponectin–leptin (A/L) ratio have also been evaluated for the prediction and diagnosis of PE [2, 6]. If they have such a role, maternal levels of these adipokines may differ in normal pregnancy and pregnancies complicated by PE [2]. Thus, they may be potential biomarkers and may have a future role in the diagnosis and prediction of PE and also for therapeutic strategies for its management [2].
Leptin is a 16 kDa protein, known as satiety hormone since it reduces appetite and enhances metabolism [7]. The levels of leptin protein and messenger RNA are increased significantly in placentae of pre-eclamptic women [5]. Increased levels of leptin have been reported in PE even before the clinical appearance of the disease [5, 8, 9].
Adiponectin is a 30 KDa protein which is produced almost exclusively by adipocytes [4] and circulates at relatively high concentration (µg/ml) [10]. A significant increase in adiponectin in PE has been reported by a number of studies [2, 11, 12].
The adiponectin–leptin (A/L) ratio is fast emerging as a marker of adipose tissue dysfunction. It correlates negatively with BMI and markers of low-grade chronic inflammation. Recently, some studies have evaluated this ratio as a biomarker for PE [2, 6].
Materials and Methods
This is a prospective study, which was conducted in the Department of Obstetrics and Gynaecology in V.M.M.C and Safdarjung Hospital, New Delhi.
In this study, women having age above 18 years and BMI > 18.5 and < 23 were included. Women having multiple pregnancies, pre-gestational and gestational hypertension, diabetes and renal disease were excluded.
The enrolled women were divided into two groups: first, study group (n = 60) comprised of women diagnosed with pre-eclampsia and second, control group (n = 60) comprised of age- and gestation-matched normotensive and non proteinuric women.
Algorithm
A detailed history was elicited. Height (m), weight (kg), BMI (kg/m2) and blood pressure of each participant were taken; 5 ml of venous blood samples were collected from each for adiponectin and leptin (ng/ml); 5 ml of urine was collected for urine protein determination. All were managed till delivery. At each visit, women were specifically asked for appearance of new symptoms or worsening of previous status. Maternal serum adiponectin and leptin levels and their ratio were compared in pre-eclamptic (study group) and normotensive (control group) women.
Statistical Analysis
Data entry was done on Microsoft Excel spreadsheet, and data analysis was done using the statistical software SPSS version 21. The data were summarised and presented in the tables and appropriate diagrams. The qualitative data were summarised as proportions and quantitative data as mean with confidence interval. Qualitative data were analysed using Chi-square/Fisher exact test, while quantitative data were analysed by T test. The p value of less than 0.05 was taken as statistically significant.
Observation and Result
The present study aimed to determine maternal adiponectin and leptin levels in pre-eclamptic and normotensive pregnant women and to compare adiponectin and leptin ratio in these two groups. Permission was taken from the ethical committee of the institution.
Adiponectin levels were higher in study group than control group. However, the difference was not statistically significant. Leptin levels in study group were significantly higher than control group (p < 0.001). A/L ratio was significantly lower in study group than controls (p < 0.0001)). Sensitivity, specificity, positive predictive value and negative predictive value of serum leptin and serum adiponectin/leptin ratio as a biomarker of pre-eclampsia were 90%, 88.3%, 88.5%, 89.8% and 68.3%, 90%, 87.2%, 74%, respectively. Serum leptin levels and serum adiponectin/leptin ratio had cut-off point as 23.3 ng/ml and < 0.153, respectively. Accuracy of both serum leptin and adiponectin/leptin ratio was significant (p < 0.0001). These are shown in Tables 1, 2, 3 and 4 and Figs. 1 and 2.
Table 1.
Socio-demographic profile of study group (PE) and control group (normotensive women)
| S. no | Parameters | Study group (n = 60) | Control group (n = 60) | p value | ||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |||
| 1 | Age (years) | |||||
| 18–23 | 14 | 23.33 | 13 | 21.66 | 0.666 | |
| 24–29 | 27 | 45 | 28 | 46.66 | ||
| 30–36 | 19 | 31.66 | 19 | 31.66 | ||
| 2 | Parity | |||||
| Primi | 36 | 60 | 33 | 55 | 0.580 | |
| Multi | 24 | 40 | 27 | 45 | ||
| 3 | Educational status | |||||
| Illiterate | 28 | 46.67 | 27 | 45 | 0.113 | |
| Primary | 14 | 23.33 | 18 | 30 | ||
| Secondary | 16 | 26.67 | 8 | 13.33 | ||
| Graduate | 2 | 3.33 | 7 | 11.67 | ||
| 4 | Occupational status | |||||
| Homemaker | 50 | 83.33 | 49 | 81.67 | 0.289 | |
| Professional | 8 | 13.33 | 11 | 18.33 | ||
| Working | 2 | 3.33 | 0 | 0.00 | ||
| 5 | Residence | |||||
| Rural | 45 | 75.00 | 23 | 38.33 | 0.021 | |
| Urban | 15 | 25.00 | 37 | 61.67 | ||
| 6 | BMI (kg/m2) | |||||
| 18.5–22.9 | 44 | 73.33 | 45 | 75 | > 0.05 | |
| 23–24.9 | 13 | 21.66 | 12 | 20 | ||
| > 25 | 3 | 5 | 3 | 5 | ||
Table 2.
Systolic and diastolic BP, adiponectin, leptin and adiponectin–leptin ratio in study and control group
| Parameter | Study group (n = 60) | Control group (n = 60) | p value |
|---|---|---|---|
| SBP (mm hg) | 148.77 ± 9.01 | 109.18 ± 6.32 | < 0.0001 |
| DBP | 98.45 ± 8.35 | 72.8 ± 8.51 | < 0.0001 |
| Adiponectin (µg/ml)* | 9.62 ± 14.86 | 8.56 ± 6.93 | 0.089 |
| Leptin (ng/ml)* | 67.31 ± 43.95 | 12.54 ± 16.26 | < 0.0001 |
| Adiponectin–leptin ratio | 0.22 ± 0.37 | 9.80 ± 38.82 | < 0.0001 |
*Values are expressed in mean ± SD
Table 3.
Adiponectin and leptin levels and A/L ratio in mild and severe pre-eclampsia
| Parameter | Mild (n = 49) | Severe (n = 11) | p value |
|---|---|---|---|
| S.Adiponectin(µg/ml)* | 4.84 ± 5.62 | 30.88 ± 23.27 | < 0.0001 |
| S.Leptin(ng/ml)* | 66.28 ± 44.56 | 71.91 ± 42.83 | 0.547 |
| A/L ratio | 0.13 ± 0.23 | 0.61 ± 0.6 | 0.0001 |
Table 4.
Association of adiponectin with leptin, BMI, SBP and DBP in study and control group
| Parameter | Study group (n = 60) | Control group (n = 60) | ||
|---|---|---|---|---|
| R | p value | R | p value | |
| Adiponectin and leptin | 0.194 | 0.138 | − 0.125 | 0.340 |
| Adiponectin and BMI | − 0.234 | 0.0714 | 0.181 | 0.165 |
| Adiponectin and SBP | 0.428 | 0.0006 | − 0.055 | 0.678 |
| Adiponectin and DBP | 0.28 | 0.0303 | − 0.131 | 0.317 |
| Leptin and BMI | 0.014 | 0.9157 | − 0.191 | 0.1443 |
| Leptin and SBP | 0.024 | 0.8537 | 0.002 | 0.9886 |
| Leptin and DBP | 0.044 | 0.7368 | 0.143 | 0.2752 |
| Adiponectin–leptin ratio and BMI | − 0.265 | 0.0409 | 0.248 | 0.0558 |
| Adiponectin–leptin ratio and SBP | 0.357 | 0.0052 | − 0.017 | 0.8968 |
| Adiponectin–leptin ratio and DBP | 0.23 | 0.0767 | − 0.176 | 0.1774 |
Fig. 1.
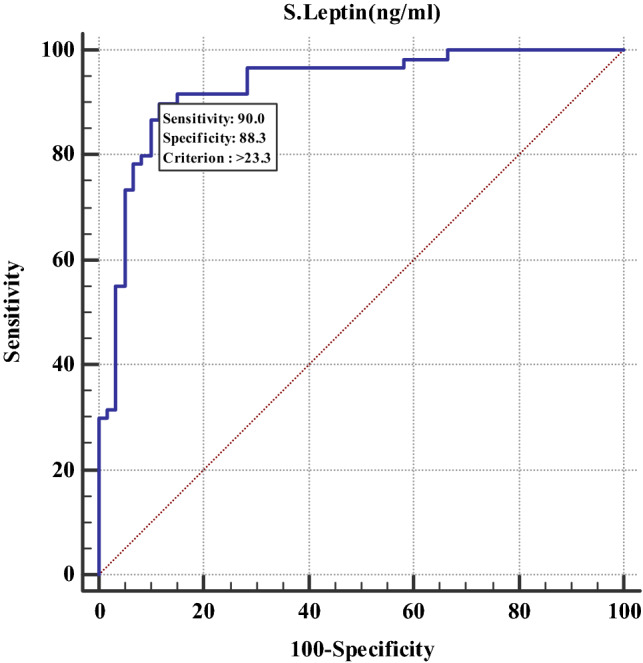
Receiver operating characteristic (ROC) curve of serum leptin as a biomarker of pre-eclampsia
Fig. 2.
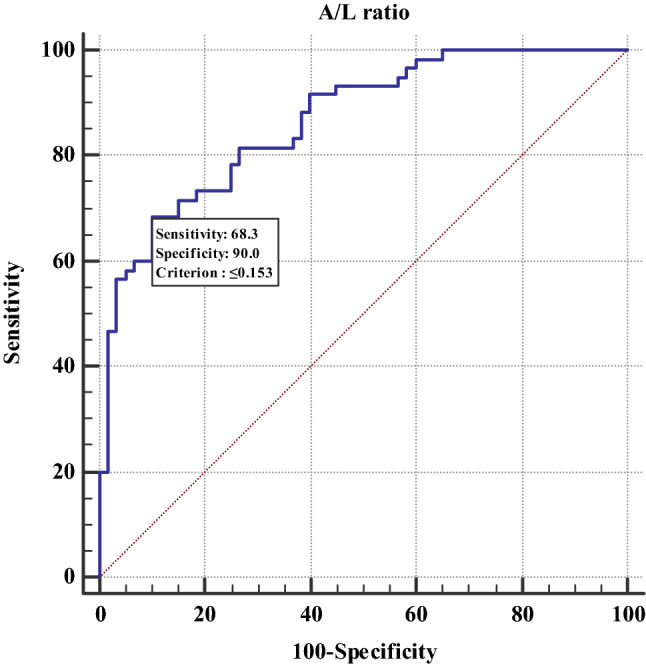
ROC curve of A/L ratio as a biomarker of PE
Discussion
Demographic Study
The present study observed that in study group the mean maternal age was 27 ± 3.91 years with most (45%) of women in age group 24–29 years. It was comparable with control group with mean maternal age of 26.93 ± 4.36 years with most (46.66%) of women in age group 24–29 years. There were no significant differences in parity, educational status, occupational status and BMI in two groups. However, the proportion of rural women was significantly higher in study group than controls (p = 0.021) (Table 1).
BP in Study and Control Group
Women in study group had significantly higher systolic and diastolic BP at first visit, than control group, with p value < 0.0001 for each. Mean SBP and DBP in study group were 148.77 ± 9.01 and 98.45 ± 8.35, while in control group, they were 109.18 ± 6.32 and 72.8 ± 8.51, respectively. Two studies, one from Iranian and another from Ghanaian population, have reported similar observations [2, 11] (Table 2).
Adiponectin
This study observed slightly higher adiponectin levels in study group than control group. The mean level in study group was 9.62 ± 14.86 µg/ml (levels ranging from 0.13 to 84.2 µg/ml), while the mean level in control group was 8.56 ± 6.93 ug/ml (levels ranging from 0.8 to 27.5 µg/ml. However, the difference was not statistically significant (p = 0.089).
On further categorisation, in study group, 93.33% (55 out of 60) of the subjects had adiponectin level < 30 ug/ml, while 5% (3 out of 60) had levels between 30 and 60 ug/ml and only 1.7% (1 out of 60) had a level of 84.2 ug/ml.
Fondjo LA et al., Khosrowbeygi A et al., Abd-Aaleem et al. and Aloush M K et al. from Ghanaian, Iranian and Egyptian (two studies) populations, respectively, also reported elevated levels in pre-eclamptic women than controls; however, the differences in the studies were statistically significant (p < 0.05 in all). However, Mazaki-Tovi S et al. and Ouyang Y et al. reported significantly lower adiponectin levels in pre-eclamptic women as compared to normotensive [13].
It is established that pre-eclampsia syndrome is associated with insulin resistance, exaggerated systemic inflammatory response and an anti-angiogenic state. As adiponectin has anti-diabetic, anti-inflammatory and angiogenic properties, the above-mentioned factors, all of which are presented in PE, are associated with reduced adiponectin levels [10].
In PE, there are higher levels of angiotensin II which lead to hypertension. Adiponectin inhibits production of angiotensin II; hence, hyperadiponectinemia is probably a protective feedback mechanism to reduce angiotensin II production and to control hypertension. It is also suggested that increase in adiponectin may protect against proteinuria associated with PE. Besides, adiponectin due to its anti-inflammatory effect may help to restore the integrity of endothelium in PE. The authors suggest that as a whole, increased levels of adiponectin assist in antagonising the pathological worsening of PE [2].
Leptin
In this study, significantly higher levels of leptin were observed in study group (pre-eclamptic) than control group (normotensive). The mean leptin levels in study group were of 67.31 ± 43.95 ng/ml (ranging from 3.5 to 197.2 ng/ml), while the mean leptin levels in control group were 12.54 ± 16.26 ng/ml (ranging from 0.07 to 78.7 ng/ml). The difference in levels in two groups was statistically significant (p value < 0.0001).
On further categorisation, in the PE group, 51.67% (31 out of 60) had levels < 50 ng/ml, 26.67% (16 out of 60) had levels between 50 and 100 ng/ml, and 13.33% (8 out of 60) had levels between 100 and 150 ng/ml. In 8.33% (5 out of 60) women, levels were > 150 ng/ml. In the normotensive control group, 95% (57 out of 60) had levels < 50 ng/ml, while only 3.33% (2 out of 60) had levels > 50 ng/ml.
The significant increase in leptin levels in study group observed in this study is in concordance with Indian study [14] and studies from Ghana, Pakistan, Iran and China [1, 15]. On the contrary, the American study did not find any significant difference in leptin levels in the two groups [4].
The increased levels of leptin in pre-eclampsia may be due to a number of reasons. Even during normal pregnancy, the placenta secretes leptin, which is similar in size and immunoreactivity to that derived from adipose tissue, contributing to increased levels [16]. Elevation in levels may also be due to placental stress caused by hypoxia, present in pre-eclamptic placenta [17]. As leptin has a strong angiogenic effect, its levels may be elevated in PE to increase placental blood supply by neovascularisation. In addition, leptin regulates nutrient transport across the placenta, indicating that increased leptin levels in PE may be a compensatory response to enhance nutrient transport to the underperfused placenta of PE [18].
Receiver operating characteristic (ROC) curve analysis shows that a cut-off leptin level of 23.3 ng/ml can be used to detect the presence of PE, with a sensitivity of 90%, specificity of 88.3% (p < 0.0001), positive predictive value of 88.5% and negative predictive value of 89.8%.
Mild and Severe Pre-eclampsia
The present study observed that in study group, women with severe PE had significantly higher mean levels of adiponectin, i.e. 30.88 ± 23.27 ug/ml, as compared to mild PE, i.e. 4.84 ± 5.62 ug/ml (p < 0.0001). However, leptin levels among the mild and severe PE, i.e. 66.28 ± 44.56 and 71.91 ± 42 ng/ml, respectively, were comparable (Table 3).
An Egyptian study by Aloush [19] reported significantly higher values of adiponectin in severe PE as compared to mild PE like this study. Regarding leptin levels, observations of the present study are similar to those of Molvarec A et al. who reported no significant difference in leptin levels between women with mild and severe PE [20].
It is suggested that increased adiponectin in severe PE may be a compensatory mechanism for the pro-atherogenic and anti-angiogenic milieu of severe PE [21]. It may help in improving insulin sensitivity and cardiovascular health in pre-eclamptic women because of its pro-angiogenic, anti-atherogenic and anti-inflammatory actions in the endothelium [22]. The observations of the present study suggest that hyperadiponectinemia may therefore be a marker of severe pre-eclampsia.
Adiponectin/Leptin (A/L) Ratio in Study and Control Group
This study observed that the adiponectin–leptin ratio (A/L ratio) was significantly lower in study group than control group, i.e. 0.142 ± 12.40 and 0.682 ± 1.98, respectively, p < 0.0001. In contrast, Ghanaian study reported significantly higher A/L ratio in pre-eclamptic women as compared to normotensive controls. A study from Denmark did not observe any association of A/L ratio with development of PE; however, adiponectin levels in this study were estimated in the first trimester [5].
The significantly lower A/L ratio in study group observed in the present study suggests that there is dysregulation of adipokines in PE. The decreased A/L ratio in pre-eclamptic group probably reflects the increased insulin resistance and inflammation known to be present in PE. Hence, alteration in A/L ratio in pregnancy may be a biomarker of PE and associated adipose tissue dysfunction, and its estimation could be a part of the investigations for the diagnosis of PE.
ROC analysis showed that a cut-off A/L ratio of ≤ 0.153 can be used to predict PE with a sensitivity of 68.3%, specificity of 90% (p < 0.0001) with positive predictive value of 87.2, and negative predictive value of 74.
Adiponectin–Leptin (A/L) Ratio in Mild and Severe Pre-eclampsia
This study observed significantly increased A/L ratio in severe PE than mild PE, i.e. 0.61 ± 0.6 and 0.13 ± 0.23, respectively (p = 0.0001). This is because adiponectin levels were significantly higher in the severe PE group as compared to mild PE (p < 0.0001), while leptin levels in the two groups were comparable. The Iranian study reported significant increase in L/A ratio in severe PE as compared to mild (p = 0.03) [11].
Adiponectin and Leptin
In this study, in control group, adiponectin correlated inversely with leptin, whereas it had a positive correlation with leptin in pre-eclamptic study group. However, the association was not statistically significant in both the groups (p = 0.340, p = 0.138, respectively).
BMI and Systolic and Diastolic BP
In control group, adiponectin had significant positive correlation with BMI, while in study group, adiponectin had significant negative correlation with BMI. In study group, leptin had an insignificant linear correlation with BMI, whereas, in control group, it had insignificant negative correlation (p > 0.04 in both) (Table 4).
In study group, adiponectin had significant positive correlation with systolic and diastolic BP, p = 0.0006, p = 0.0303, respectively. In control group, it insignificantly correlated inversely with systolic and diastolic BP. This study observed that in both the preeclamptic and control groups, leptin had an insignificant linear correlation with SBP and DBP. The Ghanaian study also reported statistically significant positive correlation between adiponectin and SBP and DBP in both groups [2]. Kharb et al. also reported a positive correlation with SBP in both groups; however, it was significant only in the pre-eclamptic group; diastolic BP negatively correlated with leptin levels in the normotensive group, but the association was reversed in the pre-eclamptic women [14].
Adiponectin–Leptin Ratio and BMI and SBP and DBP
In study group, A/L ratio had significant negative correlation with BMI (p = 0.041), whereas in the control group, it had insignificant positive correlation (p = 0.056) (Table 4). The Ghanaian study has reported significant inverse relationship between A/L ratio and BMI in both the groups [2].
The present study observed that in the study group, A/L ratio had positive correlation with systolic and diastolic BP; however, only the association with SBP was significant (p = 0.005). In the control group, A/L ratio had insignificant negative correlation with the SBP and DBP.
The Ghanaian study has reported significant inverse correlation of A/L ratio with systolic and diastolic BP in both the groups.
Strength
As there is paucity of Indian data on this subject, the present study is an attempt to present observations in Indian population.
Limitations
Sample size was relatively small. Only one time estimation of maternal adiponectin and leptin was done, at the time of recruitment of subjects.
Conclusion
Significant elevation of maternal leptin levels was observed in pre-eclampsia. Severe pre-eclampsia is associated with significantly elevated adiponectin levels as compared to mild. Adiponectin–leptin ratio was significantly lower in pre-eclamptic, and it was significantly increased in severe pre-eclampsia as compared to mild.
Recommendation
Maternal leptin-level estimation and adiponectin–leptin ratio should be integrated into the investigations for pre-eclampsia, and a cut-off level of > 23.3 ng/ml and < 0.153, respectively, should be used as a biomarker for diagnosis.
Dr. Sheen Rao
has done M.B.B.S from V.M.M.C and Safdarjung Hospital and is currently doing postgraduate studies in Obstetrics and Gynaecology at V.M.M.C and Safdarjung Hospital, New Delhi. The above study is her thesis work.
Funding
No funding received.
Compliance with Ethical Standards
Compliance with Ethical Standards-Yes.
Conflict of interest
Authors have no conflict of interest.
Ethical Approval
Ethical approval was taken from V.M.M.C and Safdarjung Hospital institutional ethical committee.
Informed Consent
Informed written consent was obtained from all individual participants included in the study.
Footnotes
Sheen Rao is a Junior Resident, Department of Obstetrics and Gynaecology, V.M.M.C and Safdarjung Hospital, New Delhi; Anju Kumari, M.S. (OBS and GYNAE) is a Senior Resident, Department of Obstetrics and Gynaecology, V.M.M.C and Safdarjung Hospital, New Delhi, India; Manjula Sharma is a Professor and Consultant, Department of Obstetrics and Gynaecology, V.M.M.C and Safdarjung Hospital, New Delhi, India; and B. C. Kabi is a Director Professor, Department of Biochemistry, V.M.M.C and Safdarjung Hospital, New Delhi, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pritchard JA, MacDonald PC, Gant NF. Williams obstetrics. New York: Appleton-Century-Crofts; 1980. [Google Scholar]
- 2.Fondjo LA, Gyamfi EA, Owiredu WKBA, Turpin CA, Mante DA, Anto EO. Maternal serum adiponectin, leptin and adiponectin leptin ratio as possible biomarkers of pre-eclampsia. Edorium J Gynecol Obstet. 2016;2:41–47. [Google Scholar]
- 3.Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of pre-eclampsia. J Clin Med Res. 2014;6(1):1–7. doi: 10.5897/JCMR11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalamaga M, Srinivas SK, Elovitz MA, Chamberland J, Mantzoros CS. Serum adiponectin and leptin in relation to risk for pre-eclampsia: results from a large case control study. Metabolism. 2011;60(11):1539–1544. doi: 10.1016/j.metabol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miehle K, Stepant H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol. 2012;76:2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 6.Thagaard IN, Hedley PL, Holm JC, Lange T, Larsen T, Krebs L, Christiansen M. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertension. 2019;15:78–83. doi: 10.1016/j.preghy.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ghantous CM, Azrak Z, Hanache S, Abou-kheir W, Zeidan A. Role of leptin and adiponectin in cardiovascular system. Int J Endocrinol. 2015 doi: 10.1155/2015/534320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samolis S, Papastefanou I, Panagopoulos P, et al. Relation between first trimester maternal serum leptin levels and body mass index in normotensive and pre-eclamptic pregnancies—role of leptin as a marker of pre-eclampsia: a prospective case–control study. Gynecol Endocrinol. 2010;26:338–343. doi: 10.3109/09513590903511463. [DOI] [PubMed] [Google Scholar]
- 9.Ning Y, Williams MA, Muy-Rivera M, et al. relationship of maternal plasma leptin and risk of pre-eclampsia: a prospective study. J Maternal-Fetal Neonatal Med. 2004;15:186–192. doi: 10.1080/14767050410001668293. [DOI] [PubMed] [Google Scholar]
- 10.Nien JK, Mazaki-Tovi S, Romero R, et al. Adiponectin in severe pre-eclampsia. J Perinat Med. 2007;35(6):503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosrowbeygi A, Ahmadvand H. Maternal Serum levels of adiponectin in pre-eclampsia. J Ayub Med Coll Abbotabad. 2009;21(3):79–82. [PubMed] [Google Scholar]
- 12.Ramsay JE, Jamieson N, Greer IA, Sattar N, et al. Paradoxical elevation in adiponectin concentration in women with pre-eclampsia. Hypertension. 2003;42:891–894. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 13.Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Maternal serum adiponectin multimers in pre-ecampsia. J Perinat Med. 2009;37(4):349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharb S, Panjeta P, Ghalaut VS, et al. Maternal factors affecting serum leptin levels in pre-eclampsia and normotensive pregnant women and outcome of pregnancy. J Pregnancy Child Health. 2016;3:1. [Google Scholar]
- 15.Mumtaz F, Memon AR, Yousfani S, Tahir SM, Khushk I, Memon M, Memon A. Role of serum leptin level as a marker of severity of pre eclampsia. J Ayub Med Coll Abbottabad. 2008;20(1):13–15. [PubMed] [Google Scholar]
- 16.Pérez-Pérez A, Toro A, Vilariño-García T, Maymó J, Guadix P, Dueñas JL, Fernández-Sánchez M, Varone C, Sánchez-Margalet V. Leptin action in normal and pathological pregnancies. J Cell Mol Med. 2018;22(2):716–727. doi: 10.1111/jcmm.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrelias G, Makris GM, Papanota AM, et al. Serum inhibin and leptin: risk factors for pre-eclampsia? Clin Chim Acta. 2016;463:84–87. doi: 10.1016/j.cca.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Davis E, Polheimer J, Yong HE, et al. Epethelial-mesenchymal transition during extravillous trophoblast differentiation. Cell Adhes Migr. 2016;10:310–321. doi: 10.1080/19336918.2016.1170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, Davis JK. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10(4 Suppl):294–302. [PubMed] [Google Scholar]
- 20.Molvarec A, Szarka A, Walentin S, Bekő G, Karádi I, Prohászka Z, Rigó J. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9(1):124. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Gómez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 22.Megahed MA, Dawoud SM, El-Sarha AI, Fayed MA, Ahmed MI. Maternal serum level of adiponectin and macroscopic changes of placentae in preeclampsia. Am J Biomed Sci. 2018;10(2):72–81. doi: 10.5099/aj180200072. [DOI] [Google Scholar]