Abstract
Purpose of review:
In this narrative review, we describe general aspects, histological alterations, treatment, and implications of Fabry disease (FD) nephropathy. This information should be used to guide physicians and patients in a shared decision-making process.
Source of information:
Original peer-reviewed articles, review articles, and opinion pieces were identified from PubMed and Google Scholar databases. Only sources in English were accessed.
Methods:
We performed a focused narrative review assessing the main aspects of FD nephropathy. The literature was critically analyzed from a theoretical and contextual perspective, and thematic analysis was performed.
Key findings:
FD nephropathy is related to the progressive accumulation of GL3, which occurs in all types of renal cells. It is more prominent in podocytes, which seem to play an important role in the pathogenesis of this nephropathy. A precise detection of renal disorders is of fundamental importance because the specific treatment of FD is usually delayed, making reversibility unlikely and leading to a worse prognosis.
Limitations:
As no formal tool was applied to assess the quality of the included studies, selection bias may have occurred. Nonetheless, we have attempted to provide a comprehensive review on the topic using current studies from experts in FD and extensive review of the literature.
Keywords: Fabry, renal failure, end-stage renal disease, chronic kidney disease, kidney disease
Abrégé
Objet de la revue:
Dans cette revue narrative, nous discutons des aspects généraux, des modifications histologiques, du traitement et des implications de la néphropathie liée à la Maladie de Fabry. Des informations qui serviront à guider les médecins et les patients dans un processus commun de prise de décision.
Sources:
Les originaux d’articles évalués par les pairs, d’articles-synthèses et d’articles d’opinion ont été répertoriés dans les bases de données Pubmed et Google Scholar. Seuls les articles en anglais ont été consultés.
Méthodologie:
Nous avons procédé à une revue narrative ciblée examinant les principaux aspects de la néphropathie liés à la maladie de Fabry. La documentation a fait l’objet d’une critique rigoureuse du point de vue théorique et contextuel, et une analyse thématique a été effectuée.
Principaux résultats:
La néphropathie liée à la maladie de Fabry est associée à l’accumulation progressive de GL3, qui se produit dans tous les types de cellules rénales. Elle est plus présente dans les podocytes, qui semblent jouer un rôle important dans la pathogenèse de la néphropathie. Un dépistage précis des troubles rénaux est d’une importance capitale puisque le traitement spécifique de la maladie de Fabry est généralement retardé, ce qui rend la réversibilité peu probable et conduit à un pronostic plus défavorable.
Limites:
Des biais de sélection pourraient s’être introduits puisqu’aucun outil formel n’a été utilisé pour évaluer les études incluses. Nous avons néanmoins tenté de procéder à un examen complet du sujet grâce aux études actuelles menées par des experts de la maladie de Fabry et à une revue approfondie de la documentation.
Why is This Review Important?
Although rare, Fabry disease (FD) is multisystemic and can affect the kidneys, progressively leading to end-stage kidney disease (ESKD), which results in a worse prognosis. Comprehensive assessment of FD, especially in the field of nephrology, is of paramount importance for early diagnosis and selection of the adequate therapeutic management, both essential to improving outcomes.
What are the Key Messages?
FD nephropathy still often goes unnoticed and, consequently, underdiagnosed. Therefore, better awareness of FD is fundamental for changing this scenario. Renal biopsy can be an important auxiliary tool for the diagnosis of FD, especially in the presence of variants of uncertain significance (VUS), and for defining the start of therapy, as well as for evaluating its effectiveness. Specific therapy should be evaluated on a case-by-case basis considering the patient’s age, gender, and the genetic variant. Early diagnosis and treatment, when indicated, are associated with better outcomes.
Definition and General Aspects of FD
FD is an inborn error of metabolism with X-linked inheritance due to variants of the GLA gene that encodes the lysosomal enzyme α-galactosidase A (α-GAL). The markedly reduced or absent activity of the enzyme results in progressive accumulation of glycosphingolipids, mainly globotriaosylceramide (GL3) and its metabolite globotriaosylsphingosine (lyso-GL3). The involvement of target organs can lead to renal, cardiac, and cerebrovascular complications.1-3
The GLA gene is located on the X chromosome, at position Xq22. Although more than 1 000 different variants of the gene have already been described, not all result in FD, as many of them are benign ones or polymorphisms without clinical implications.2,4
Each variant tends to be specific to each family, which partially justifies the marked variability in residual enzyme activity and, consequently, the difference in clinical presentation.3,5,6
The estimated prevalence of the disease is approximately 1:40 000 to 1:117 000 males.1,7 Studies of FD screening in newborns reveal the possibility of a higher prevalence, but many of the identified variants are those without great clinical significance.8,9
The prevalence of FD in the high-risk population varies possibly due to different types of screening. For patients on dialysis, the prevalence ranges from 0.20% to 0.99% in males and 0.05% to 0.33% in females.10-14 Among patients with heart disease, the prevalence ranges from 0.21% to 2.99% in men and 0.28% to 1.68% in women.15-20 For individuals with cerebrovascular disease, including stroke, the prevalence ranges from 0.17% to 2.3% in males and from 2.5% to 4.7% in females.21-25 A recent reanalysis of 63 studies that screened 51 363 patients (33 943 males) excluded benign/likely benign variants to provide more accurate gender-specific and phenotype-specific results. The prevalence estimates found were 0.21% males and 0.15% females in 36 820 hemodialysis screenees; 0.94% males and 0.90% females in 5491 cardiac screenees; and 0.13% males and 0.14% females in 5978 stroke screenees.26
As for the disease diagnosis, the first step aims to establish the activity of the α-GAL enzyme. The classic phenotype is found in males with less than 1% enzyme activity, and it is caused by different types of rearrangements, splicing defects, and missense or nonsense variants. On the other hand, male patients with more than 1% enzyme activity may have missense or splicing variants and show a “late-onset” phenotype.1,2 In female patients, even in those with symptoms, the enzyme activity may be within the normal range. Approximately 33% to 50% of GLA-mutation confirmed heterozygotes have normal or only slightly decreased α-GAL activity.27,28 This is due to the fact that the defective gene can be compensated by a functional copy of the equivalent gene on the other X chromosome. For this reason, this test is not recommended for diagnosis in women.27,28 A screening strategy in women that improved accuracy in the detection of the disease combined the enzyme activity with lyso-GL3 levels.29
The confirmatory test is the genetic analysis. This is reserved for women with clinical or laboratory suspicion and for men with low enzyme activity.29,30 It is recommended that the family members of index patients undergo direct genetic analysis. Family pedigree is of great value because often allows the diagnosis at earlier stages. On average, for each index patient, another 5 family members are diagnosed; however, in some studies, this number is much higher.31,32
Two clinical presentations are described. Patients with the classic phenotype may have their first symptoms during childhood or adolescence. The initial manifestations are acroparesthesia, severe pain crises known as “Fabry crises,” and gastrointestinal symptoms. Other symptoms may appear later as a result of the progression of systemic deposits. Angiokeratomas are described as dark red, nonpruritic papular dermatological lesions. The involvement of sweat glands can lead to hypohidrosis or anhidrosis, which causes intolerance to temperature changes. Cornea verticillata is a nonpathognomonic finding of FD, caused by the deposits of GL3 in the corneal tissue, without compromising visual acuity. Hearing loss is a common finding. Cognitive deficit can occur as a result of multiple micro brain infarctions.1,33 With increasing age and the progression of GL3 deposits, FD causes lesions in target organs, which can culminate around the fourth decade of life in heart failure, stroke, and chronic kidney disease (CKD). These combined factors reduce life expectancy by an average of 25 years for men and 10 years for women.34
The other form of clinical presentation includes the late-onset phenotype, in which the classic signs and symptoms are absent or appear in a milder form.33,35 The cardiac variant manifests itself most commonly through concentric left ventricular hypertrophy, around the fifth decade of life. Cardiac involvement may also present as a differential diagnosis of arrhythmias, hypertrophic obstructive cardiomyopathy, or idiopathic cardiomegaly.34 The renal variant shows signs of renal impairment, described in a specific section of this article. The progressive decline in renal function is evident between the ages of 40 and 50 years, leading to ESKD.33,36
The spectrum of clinical presentation in women is broad. The phenotypic heterogeneity is due, in part, to the lyonization phenomenon, responsible for the random inactivation of the X chromosome. The residual enzyme activity is consequently quite variable. Although some women manifest a classic clinical presentation, women can be asymptomatic and generally show milder signs and symptoms, with a slower evolution.37,38 Rare cases of homozygous females are described, with a clinical picture similar to the classic phenotype in males.39 Although FD is potentially detected in a greater number of women because it is X chromosome linked, a study revealed that 88% of FD patients who initiated dialysis were males, suggesting a more insidious clinical characteristic in women.40
Regarding the treatment of FD, before the advent of enzyme replacement therapy (ERT), the approach aimed only at supportive measures to minimize the most prominent symptoms. Lifestyle changes are recommended.2,3 Other traditional measures consist in drugs that can delay the involvement of target organs, such as angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptors blockers (ARB), which, in addition to optimizing blood pressure (BP) control, have been shown to decrease proteinuria and kidney disease progression.41
Specific treatment for FD is carried out with ERT or migalastat. There are currently 2 enzymes available: agalsidase alfa (Replagal) and agalsidase beta (Fabrazyme).42,43 Both proteins are structurally and functionally similar and are administered intravenously every 15 days.
The other specific treatment, migalastat (Galafold), is a drug that belongs to the chaperone class, to be used in patients with “amenable” variants, which constitute a type of “missense” mutation, and also in some small deletions. It acts by selectively and reversibly binding with the α-GAL mutant forms, which facilitates its adequate transport to lysosomes, where the dissociation of this binding then occurs, resulting in an increase in enzymatic function. It is administered orally, at a dose of 123 mg every other day, with good tissue distribution and, unlike ERT, it crosses the blood-brain barrier.44-46 The drug is limited to adult patients, as it has not yet been approved for use in patients younger than 16 years. Migalastat is substantially eliminated by the kidneys and is not recommended for use in patients with glomerular filtration rate (GFR) <30 mL/min/1.73 m2 or ESKD requiring dialysis. No dose adjustment is required when GFR >30 mL/min/1.73 m2.47
The goal of ERT and migalastat is to reverse pathological changes in FD, prevent the development of disease, and slow the progression of multiple organ dysfunction in older patients. As for the nephropathy, therapy is aimed at preventing the development of albuminuria, stabilizing already manifested albuminuria, preventing or slowing the progression of massive proteinuria, stabilizing renal function, and preventing progression to ESKD.48
There are several additional therapies under development.49,50 The substrate reduction therapy (SRT) aims to limit the formation of metabolites that cannot be degraded. In patients with residual enzyme activity, SRT may be sufficient to reduce the substrate to a level compatible with the remaining enzyme activity. In patients with minimal or no residual enzyme activity, SRT may not be sufficient as a single therapy.49 The most studied drug is lucerastat, which works by inhibiting glucosylceramide synthase, thus inhibiting GL3 production.51,52 Pegunigalsidase-α, the pegylated dimerized version of agalsidase, would provide a longer half-life and an apparent low immunogenicity.53 Another option is the moss-derived enzyme, which has a higher affinity for mannose receptors and seems to have access to renal tissue.54 Gene therapy and gene editing are also under development and may represent a promising safe alternative.55,56
Nephropathy in FD
General Aspects
The renal involvement is multifactorial. Progressive GL3 accumulation leads to histological damage, the release of inflammatory mediators, the increase in oxidative stress, and the positive regulation of the adhesion molecule expression.48 Moreover, an increase in cytokines such as transforming growth factor-β and apoptosis have been described.57 Podocyte involvement was associated with an increase in autophagosomes and also a decrease in mammalian target of rapamycin and protein kinase B signaling cascades, which might predispose to further cell damage.58 Lyso-GL3 may have an important role, promoting the proliferation of vascular smooth muscle cells and the release of glomerular injury mediators.59 Angiotensin II also contributes to nephropathy through vasoconstriction, as well as the spread of inflammation and fibrosis.60
GL3 accumulation occurs in all types of kidney cells. The deposits increase with disease progression and, in addition to podocytes, they can be found in the epithelium of Bowman’s capsule, in the mesangial and endothelial cells, in the smooth muscle cells of the arteries and arterioles, interstitial cells, and in the distal tubule. The proximal tubule is less affected due to the constant renewal of its cells.61,62
Clinical and Laboratory Findings
Patients with FD generally have lower systemic BP values, mainly as a consequence of the effects of autonomic dysfunction on cardiac and vascular function, although other factors may be involved.48 High BP becomes more prevalent as CKD progresses.63
The presence of renal cysts, mainly parapelvic ones, has been reported. The pathogenesis of cysts is unknown.59,61 Although uncommon, hematuria can also occur.48
Glomerular manifestations are similar to those of diabetic nephropathy, with hyperfiltration in the early stages, albuminuria, proteinuria, and progressive decrease in GFR.61 Glomerular hyperfiltration is relatively frequent in young patients.64 One of the treatment guidelines even considers hyperfiltration (GFR >135 mL/min/1.73 m2) as 1 of the criteria for the indication of specific therapy.65
Tubular manifestations are less frequent and include distal renal tubular acidosis and isosthenuria. Fanconi syndrome has also been described, but it is rare.61 Tubular damage and dysfunction may be accompanied by the excretion of tubular lesion markers, such as α1-microglobulin and retinol-binding protein.66
Podocytes seem to play an important role in the pathogenesis of FD nephropathy. When injured, the result is glomerulosclerosis, indicative of irreversible damage to the glomeruli.67-69 Endothelial cells contribute to the development of fibrosis, through luminal obstruction and ischemia.70 The mesangial cells seem to play a less important role in the pathophysiology of nephropathy.62
As the disease progresses, endothelial cells, and especially podocytes, become hypertrophic with foamy vacuoles. Irreversible ischemic changes in the renal microvasculature result in glomerulosclerosis, thickening of the capillary wall, tubular atrophy, interstitial fibrosis, and arterial and arteriolar sclerosis.71 Gradual evolution to renal replacement therapy usually occurs between the fourth and fifth decades of life, mainly in untreated male classic cases.61
As for the natural history of FD nephropathy, the rate of GFR decrease in untreated patients varies substantially. In a study of male classic patients, the decline in GFR was approximately 12.2 mL/min/y, with rapid progression to ESKD.72 Another study showed GFR decline rates for men with GFR ≥60 mL/min/1.73 m2 and GFR <60 mL/min/1.73 m2 of 3.0 and 6.8 mL/min/1.73 m2/y, respectively. In women, the GFR decline was 0.9 and 2.1 mL/min/1.73 m2/y, respectively. Factors such as baseline proteinuria, reduced baseline GFR, and male gender have been associated with a faster disease progression.34 In patients with late-onset variants or in female patients, the GFR decline is generally slower and less predictable.38,73
Signs indicative of early and progressive kidney damage include microalbuminuria and proteinuria, which may already be present in the second decade of life. However, potentially irreversible histological changes can be observed in the renal biopsy from children before the onset of albuminuria.74,75 In addition, a report of early disease status in asymptomatic classic boys revealed histological evidence of GL3 accumulation, and cell and vascular injury in renal tissue prior to the onset of microalbuminuria and the development of clinically significant renal events.76
Therefore, although albuminuria and proteinuria are the most often used markers, they have a low sensitivity to identify incipient nephropathy.74 In addition, proteinuria may not be evident in patients with advanced kidney disease and may not correlate with the GFR decline.59
The discovery of alternative markers of renal impairment is extremely important. One study compared albuminuria with other urinary biomarkers of glomerular and tubular dysfunction for the identification of incipient FD nephropathy. There was a significant increase in all biomarkers, even in the subgroup of patients with no evidence of nephropathy, overcoming the limitations of albuminuria as a sensitive marker of early renal dysfunction. These biomarkers might improve the management of kidney disease, providing criteria for starting the specific therapy and defining new early stages of FD nephropathy.77
Urine microscopy can be clinically useful in diagnosing and assessing disease progression. It is a noninvasive and inexpensive technique that aims to identify 3 basic findings in FD: “Maltese cross” particles, “urinary mulberry cells,” and podocyturia.48,65 There are, however, some limitations associated with these tests, as the diagnostic value is not well established in late-onset phenotypes, and these findings are not pathognomonic for FD.59
Vacuolated epithelial cells filled with glycosphingolipids have the appearance of a “Maltese cross,” visualized through polarized light microscopy.78 The “urinary mulberry cells” have a lamellar appearance, with a characteristic image of the mulberry body. They are distal tubular epithelial cells, in which GL3 has accumulated and can be detected before kidney damage can be identified.71,78
The loss of podocytes in the urine correlates with continuous glomerular injury, and its quantification provides robust evidence of podocyte damage.69 This has been suggested as a potential diagnostic tool and a way to guide treatment strategies in FD nephropathy. In some studies, even in patients with normoalbuminuria or normoproteinuria, the number of podocytes in the urine was significantly higher when compared with that in healthy individuals, suggesting that this biomarker of clinically silent kidney damage may precede proteinuria.71 Podocyturia was inversely related to GFR in male patients and is correlated with clinical severity in FD nephropathy, which may have a potential prognostic value.67,68
Individuals on ERT have lower podocyturia, higher proteinuria, and worse renal function, which may indicate that therapy was currently started at an advanced stage.76,79
In women, due to the random X chromosome inactivation and the consequent phenomenon of mosaicism, podocytes show a heterogeneous involvement. The relative number of podocytes without the FD phenotype increases with age in these patients, representing a disproportionate loss of cells with the FD phenotype over time.80
Although efforts have been made, an ideal biomarker has not yet been identified. Despite the decrease in GL3 urinary levels during therapy, it is not an ideal biomarker because most of the late-onset FD males and FD females do not exhibit high plasma GL3 levels, and its baseline value has no correlation with the biochemical parameters of renal function and disease severity.81,82
Conversely, the measurement of lyso-GL3 levels has been used as a reliable diagnostic tool to mainly discern classic FD from individuals without the disease. However, some studies suggest that lyso-GL3 levels may also be applicable in patients with the nonclassic phenotype and female patients. In cases of VUS without phenotypic or biochemical characteristics of classic FD, increased values of lyso-GL3 >1.3 nmol/L corroborate with the diagnosis. Normal levels of lyso-GL3 do not exclude FD in women but make the diagnosis highly unlikely in men.83-85 Lyso-GL3 also shows a better correlation with the response to therapy.85
Through metabolomic approaches, new analogs of lyso-GL3 have been discovered, which may play a role in the pathogenesis and be useful for diagnosis and monitoring.86,87 In plasma, unlike what is seen in urine, lyso-GL3 is significantly more abundant than its analogs.88 Levels of urinary analogs have been reduced after ERT in men, supporting their potential use in therapy monitoring.86 In a more recent study, the concentration of urinary lyso-GL3 together with its analogs proved to be a promising diagnostic biomarker for patients with classic and late-onset FD.89
The clinical and laboratorial manifestations of FD nephropathy are summarized in Table 1.
Table 1.
Clinical and Laboratory Manifestations of FD Nephropathy.
| Systemic BP | Lower systemic BP |
|---|---|
| Ultrasound findings | Renal cysts, mainly parapelvic ones |
| Urine microscopy | Hematuria, “Maltese cross,” “urinary mulberry cells,” podocyturia |
| Glomerular manifestations | Hyperfiltration in the early stages, albuminuria, proteinuria, generally non-nephrotic, progressive decrease in GFR |
| Tubular manifestations | Distal renal tubular acidosis, isosthenuria, rarely Fanconi syndrome |
| Podocyte involvement | Podocyturia, proteinuria, glomerulosclerosis |
| Biomarkers | GL3 and lyso-GL3 |
Note. FD = Fabry disease; BP = blood pressure; GFR = glomerular filtration rate.
Histological Findings
Renal biopsy can be useful in all patients with any level of proteinuria or renal dysfunction, as it assesses the degree of glomerulosclerosis and interstitial damage, which are markers of chronicity with high prognostic significance.48 In patients with minimal proteinuria and normal kidney function, the biopsy can also determine whether there are deposits of GL3 and quantify them, especially in podocytes.75 Kidney biopsy also assumes greater importance when clinical and laboratory markers of renal impairment are absent in women. In such cases, the presence of histological signs of chronicity, together with significant renal deposits, may serve as an indication to start specific therapy.
Indications for renal biopsy also include cases with atypical presentations and the need to rule out other nephropathies or overlapping diseases.90-93 In patients who are already on ERT, biopsy serves mainly to assess the response to treatment, when there is suspicion or confirmed presence of antibodies against the enzyme.48 Renal biopsy indication must be supported by convincing arguments to avoid unnecessary risks.74,94 The indications for kidney biopsy in FD nephropathy are summarized in Table 2.
Table 2.
Indications for Kidney Biopsy in FD Nephropathy.
| To assess the degree of glomerulosclerosis and interstitial damage. |
| In patients with 30- to 300-mg/g albumin-to-creatinine ratio and normal kidney function, the biopsy can determine whether there are GL3 deposits and quantify them. |
| In women without evidence of FD nephropathy, the presence of significant renal deposits may serve as an indicator for the start of specific therapy. |
| Cases of atypical presentations. |
| Cases in which it is necessary to rule out other nephropathies or overlapping diseases. |
| Cases with suspected or confirmed presence of antibodies against the enzyme to evaluate the response to ERT. |
Note. FD = Fabry disease; ERT = enzyme replacement therapy.
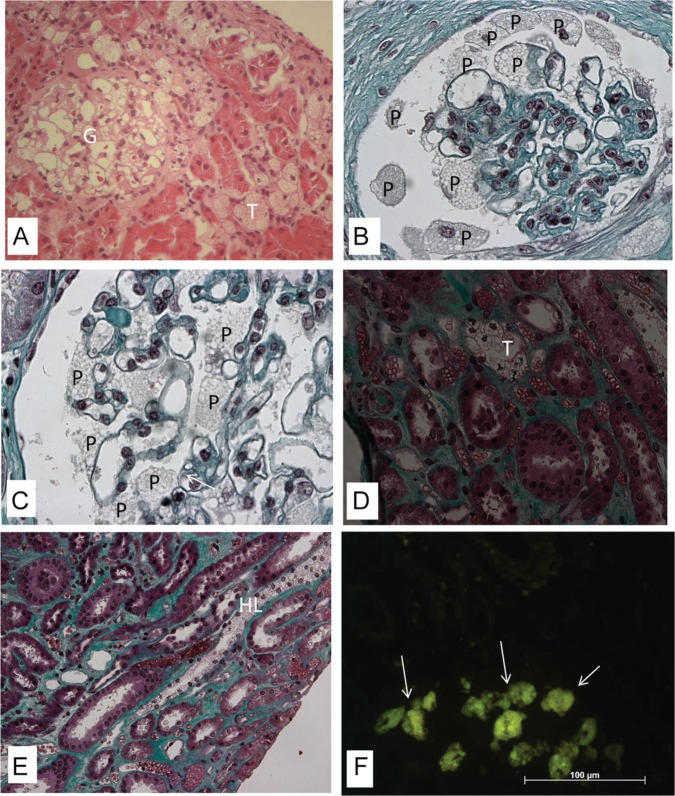
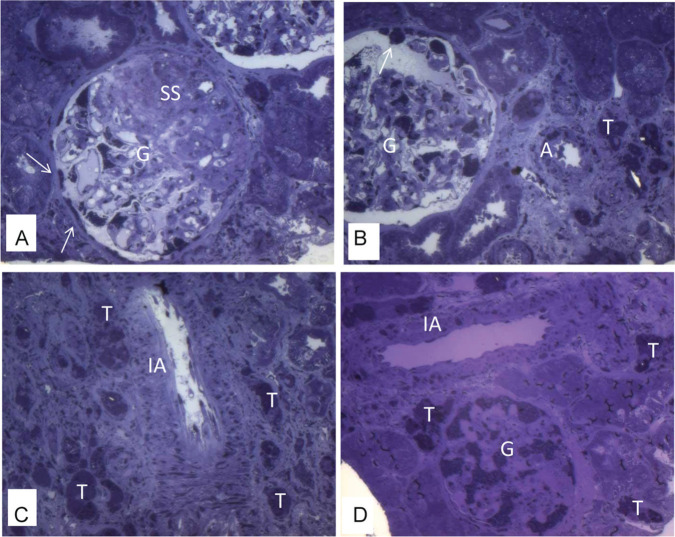
Light microscopy can show vacuolation in different cells (Figure 1), such as podocytes (Figure 1A-C), mesangial, and endothelial cells. Cytoplasmic vacuolation results from deposits of lipids such as GL3, which, when embedded in paraffin, dissolve during processing. Vacuolation can also be present in the epithelial cells of the distal tubules (Figure 1D), in the loops of Henle (Figure 1E) and in the collecting ducts. The involvement of proximal tubular cells is uncommon. In frozen material, GL3 deposits show natural fluorescence (Figure 1F) and are birefringent, producing a characteristic “Maltese cross” pattern under polarized light. In this type of material, deposits can be visualized with lipid dyes, such as Sudan. In material included in resin, GL3 deposits are preserved and thus well evidenced by the toluidine blue dye (Figure 2).36,65 Vascular lesions include deposits in smooth muscle and endothelial cells (Figure 2), sometimes associated with hyaline deposits in arteries and arterioles. As the disease progresses, glomerulosclerosis, both segmental (Figure 2A) and global, interstitial fibrosis, tubular atrophy, and thickening of the vascular walls occur.36
Figure 1.
Kidney biopsy specimen under light microscopy (A-E—embedded in paraffin): The vacuoles seen in the cytoplasm of different cells: especially podocytes (P) in the glomerulus (G), distal tubular cells (T), and Henle loops (HL). (A) Hematoxylin and eosin (magnification: 20× objective; 2.0× optovar). (B-E) Masson’s trichrome (magnification: 160×). (F) Yellowish green natural fluorescence of GL3 in the glomerular and tubular cells (arrows) in frozen section under fluorescence microscopy (magnification: 40× objective; 1.25× optovar).
Figure 2.
Kidney biopsy specimen under light microscopy—osmicated, epoxy-embedded tissue, stained with toluidine blue. There are GL3 deposits stained in dense, dark blue granules in the cytoplasm of different cells: especially in enlarged podocytes in glomerulus (G) and parietal epithelial cells (Bowman capsule; arrows), tubular cells (T), endothelial cells and smooth muscle cells in arteriole (A), and in interlobular arteria (IA). There is segmental sclerosis (SS) in glomerulus (A-D, magnification: 20× objective; 2.0× optovar).
Under immunofluorescence microscopy, deposits characteristic of immunocomplexes are not found, but nonspecific findings such as IgM or C3 may occur in areas of sclerosis.36,61
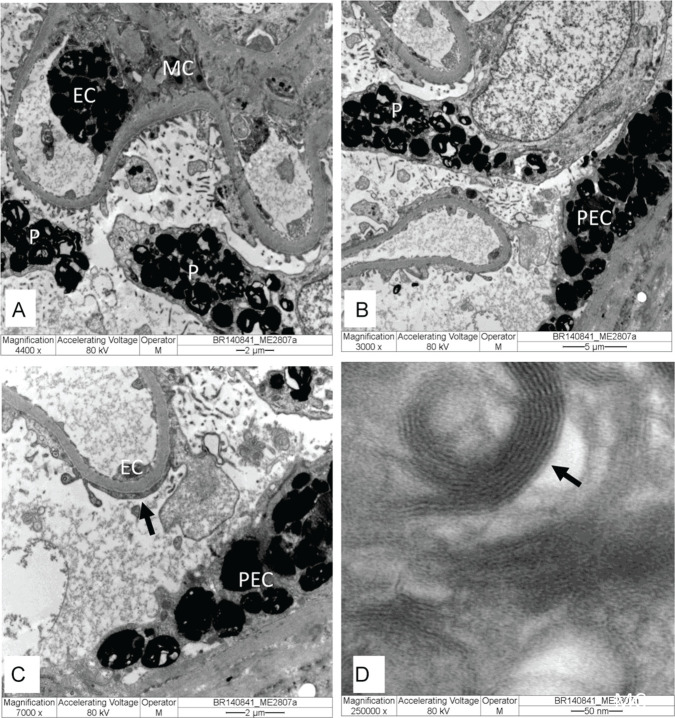
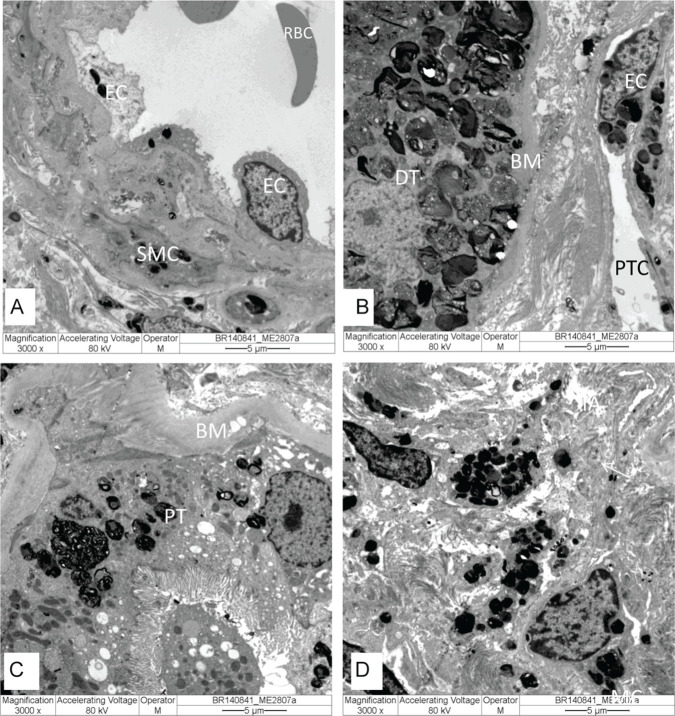
Electronic microscopy (EM) is considered the only tool available to reliably confirm or exclude FD nephropathy.71 When a genetic variant is present but the diagnosis of FD is uncertain, renal biopsy with EM analysis should be performed to confirm the diagnosis.95 GL3 deposits are observed inside lysosomes of different cell types (Figures 3 and 4) and can take forms known as “myelin figure,” “onion skin,” or “zebra bodies.” Under high magnification, GL3 deposits consist of electron-dense, multilamellated concentric layers, with a periodicity of 3.5 to 5 nm (Figure 3D).
Figure 3.
Kidney biopsy specimen under electron microscopy: (A-C) Glomerulus with electron-dense GL3 deposits in endothelial cells (EC), mesangial cells (MC), parietal epithelial cells (PEC), and podocytes (P), with effacement foot process (arrow). (D) In high magnification, GL3 deposits consist of electron-dense multilamellated concentric layers, with periodicity 3.5 to 5 nm (arrow). Original magnification: (A) = 4.400×, (B) = 3.000×, (C) = 7.000×, and (D) = 250.000×.
Figure 4.
Kidney biopsy specimen under electron microscopy with electron-dense GL3 deposits in lysosomes in different cells: (A) arteriole in endothelial cells (EC) and smooth muscle cells (SMC); (B) distal tubule (DT) and in endothelial cells (EC) of peritubular capillary (PTC); (C) proximal tubule (PT); and (D) cells in the interstitial compartment. Original magnification: (A-D) = 3.000×.
Note. RBC = red blood cell; BM = basal membrane.
Podocytes show the largest amount of deposits because they are terminal differentiation cells. The effacement of foot processes is also present.36,61 The deposits shown under EM are not pathognomonic in FD. The main differential histological diagnoses involve other nephropathies that present with foamy podocytes, such as toxicity by α-GAL enzyme inhibitors (chloroquine, hydroxychloroquine, amiodarone, among others) and other renal lipidoses (gangliosidosis GM1, Hurler’s syndrome, Niemann-Pick’s disease, Farber’s disease). In these lipidoses, the morphology and location of deposits differ from those found in FD, and none of them have prominent myelinated bodies disseminated in the different types of compartments and renal cells. Silica-induced nephropathy can also disclose images similar to FD, but other specific changes coexist.36,96 The histological findings of FD nephropathy are summarized in Table 3.
Table 3.
Histological Findings in FD Nephropathy.
| Vacuolations in podocytes, mesangial, endothelial, and epithelial cells of the distal tubules; loops of Henle; and collecting ducts. Involvement of proximal tubular cells is uncommon. |
| Vascular lesions include deposits in smooth muscle cells and endothelial cells, sometimes associated with hyaline deposits in arteries and arterioles. |
| Segmental or global glomerulosclerosis, interstitial fibrosis, tubular atrophy, and thickening of the vascular walls. |
| Immunofluorescence microscopy: deposits characteristic of immunocomplexes are not found, but nonspecific findings such as IgM or C3 may be identified in areas of sclerosis. |
| Electronic microscopy: GL3 deposits are observed inside lysosomes of different cell types and can take forms known as “myelin figure,” “onion skin,” or “zebra bodies.” |
| Effacement of foot processes. |
Note. FD = Fabry disease.
A standardized grading system for analyzing renal changes through renal biopsy was developed and validated to determine whether baseline histological information may be related to the rate of disease progression or response to therapy.75 Findings of renal impairment, such as GL3 deposits, glomerulosclerosis, tubular atrophy, and interstitial fibrosis, were observed even in the early stages of the disease. Histological evidence of renal involvement precedes the clinical and laboratory signs of early nephropathy. Therefore, the absence of the typical signs and symptoms of CKD does not exclude renal involvement due to FD, thus emphasizing the importance of the renal biopsy role.75
In that same study, clinical disease was more attenuated in women, with a lower degree of global sclerosis and fewer inclusions in podocytes, tubules, and vessels. Arterial hyalinosis was the single most prevalent pathological finding in females, which may be related to the higher mean age of the assessed patients.75
Uniform analyses of histological changes can help determine the prognosis, quantify the response to treatment, and facilitate the investigation of pathological mechanisms that lead to progressive renal dysfunction (Table 3).62
Therapeutic Aspects of FD Nephropathy
Regarding the therapy of FD nephropathy, in addition to the possibility of using ERT or migalastat, attaining adequate control of proteinuria by using ACEi or ARB is extremely important. The therapeutic goal is to achieve albuminuria levels <30 mg/g of urine albumin-to-creatinine ratio (ACR) if baseline values are between 30 and 300 mg/g, or an ACR <300 mg/g if baseline values are >300 mg/g. A careful dosage is necessary to avoid adverse effects, which include hypotension and hyperkalemia.97,98 The additional cardioprotective action of these drugs further justifies their use.99 For BP control, the targets are systolic BP ≤130 mm Hg and diastolic BP ≤80 mm Hg, preferably using ACEi or ARB.41
As for the dialysis modalities, the choice of method depends on individual preferences. The outcomes of kidney transplantation, both regarding graft and patient survival, are similar to those of transplanted patients from other causes. Long-term graft survival seems to be negatively influenced by cardiovascular involvement.100,101 Clinical recurrence of post-transplant FD nephropathy has been reported, even with signs of histological recurrence of the disease, but with no impact on long-term graft survival. One study encompassed results from renal graft biopsies, evidencing a few typical lamellar inclusions, presumably originating from invading host macrophages and vascular endothelial cells.100
Regarding the results of specific therapies, studies have suggested that kidney disease progression is attenuated in patients who start ERT at a younger age and preserved kidney function at baseline, giving support in favor of an early intervention.102-104 Some patients do not achieve the therapeutic goal of stabilizing or improving the GFR due to a more advanced level of tissue damage when therapy is started. Patients with similar levels of albuminuria may respond differently, depending on the extent of kidney damage present before treatment.105-107 Adults are at risk for progressive loss of GFR despite ERT, particularly if ACR ≥1000 mg/g.60 One study showed that, in men after 10 years of ERT with agalsidase alfa, proteinuria increased proportionally more than in those with altered values when the therapy was initiated.108
A prospective study evaluated 57 patients (30 males) and 6 adolescents, showing that renal function decreased in men (−3.4 mL/min/1.73 m2/y) even with ERT, but followed the natural course in women (−0.8 mL/min/1.73 m2/y). Long-term ERT combined with optimal supportive treatment did not prevent nephropathy progression, but the longer treatment duration may decrease the risk of developing additional complications. ERT in advanced disease seems to have few benefits.109
The benefit of a higher cumulative dose of agalsidase was reported in a 10-year follow-up study with serial renal biopsies in male classic patients with stable microalbuminuria and normal GFR. The elimination of GL3 deposits in podocytes and the reduction of plasma lyso-GL3 correlated with the cumulative effect of the offered enzyme dose.110
In a study of serial renal biopsies in children, both at the beginning of ERT and 5 years later, total clearance of endothelial, glomerular, and mesangial inclusions was evidenced, and some patients showed a substantial clearance of podocyte inclusions, which correlated with the cumulative dose.111 Limited elimination of arterial and arteriolar GL3 raises concerns about the long-term vascular effects of the current therapy.110
In a prospective observational study, a group of patients who received the standard dose of agalsidase beta was switched to agalsidase alfa, due to problems with the worldwide supply of the drug. After this period, they returned to their previous treatment with agalsidase beta, and dose-dependent benefits were observed such as GFR decline and levels of lyso-GL3.112
A multicenter, retrospective cohort study with 387 patients on ERT showed the decrease in plasma lyso-GL3 was more robust with agalsidase beta treatment, specifically in men with the classic phenotype, but there was no difference regarding the decline in GFR between the 2 groups.113
The migalastat effectiveness has been investigated in 2 main clinical trials. In the Formoterol and Corticosteroids Establishing Therapy study, the primary objective was to assess the effect on kidney involvement. After 6 months, the patients showed a reduction of >50% of peritubular interstitial capillary inclusions and a significant reduction of podocyte inclusions. Moreover, the results suggest that treatment improved renal function in patients in all proteinuria levels.114 The 18-month randomized and active-controlled ATTRACT study aimed to assess the effects on renal function in patients previously treated with ERT. Migalastat and ERT had similar effects on renal function.115 The efficacy in the Japanese patient population was similar to that of the overall ATTRACT population. Migalastat treatment increased α-GAL activity and stabilized renal function. Plasma lyso-GL3 levels remained low and stable.116 In another study, migalastat treatment lasting 6 months demonstrated effective GL3 podocyte clearance.117
Additional data proved the efficacy of migalastat in stabilizing GFR, reducing plasma lyso-GL3 and GL3 renal inclusions, regardless of disease severity. The amount of podocyte deposits was the only assessed item that showed stability after the follow-up period, in the subgroup with the classic phenotype.118
Concerning the indication of specific treatment in relation to renal manifestations, the recommendation for male classic patients, for either symptomatic or asymptomatic, is that therapy should be considered and is appropriate at any age of presentation. For symptomatic females, the signs or symptoms suggesting major organ involvement and evidence of renal impairment would warrant the start of therapy. For asymptomatic females, it should be considered whether there is laboratory, histological, or imaging evidence of renal injury, represented by GFR <90 mL/min/1.73 m2, persistent ACR >30 mg/g, podocyte foot process effacement or glomerulosclerosis on renal biopsy, and moderate or severe GL3 inclusions. In males and females with late-onset mutation or VUS, therapy should be considered when there is laboratory, histological, or imaging evidence of renal injury, even in the absence of typical symptoms. The abnormalities should be attributable to FD; this may require histological assessment or biochemical evidence of GL3 accumulation.97
Histological evidence of GL3 accumulation and cell and vascular injury is already present in renal tissues at very early stages, before the onset of albuminuria and the clinically significant renal events, suggesting that the early start of the therapy may offer better potential long-term results.119 One study associated podocyte injury with the need for therapeutic intervention before critical podocyte loss occurs.120
In the pediatric population, specific therapy should be considered in boys or girls at any age when signs and symptoms suggest major organ involvement. In these cases, renal disease would be defined by GFR decline, albuminuria or proteinuria, cellular GL3 accumulation, or evidence of tissue damage. For asymptomatic boys with a pathogenic GLA variant, a family history of disease severity in males, undetectable α-GAL activity, and plasma lyso-GL3 >20 nmol/L, ERT should be considered. There are currently no data supporting ERT start in asymptomatic girls, but in case of skewing in favor of the mutant GLA allele expression in the X chromosome inactivation testing, ERT should be considered. Patients with the nonclassic or attenuated or late-onset variants, or those identified through family history or newborn screening programs should be monitored closely and treated only when symptoms or signs emerge. ERT is suggested when there is biopsy evidence,121,122 although it should be noted that ERT is permitted only in children aged at least 7 or 8 years.42,43
In cases of kidney transplantation, therapy is not recommended for nephropathy, but can be continued for nonrenal indications.123 The treatment can include lipid-lowering and antiplatelet agents, mainly to prevent cardiovascular and cerebrovascular events.74 Other measures include dietary salt restriction and vitamin D replacement, when levels are deficient.97,98 Some studies also suggest the use of paricalcitol, due to its potential antiproteinuric effect.124
For the best follow-up of patients, GFR should be performed annually in patients with low risk of progression to CKD, every 6 months if there is moderate risk and every 3 months if there is high risk. As for albuminuria or proteinuria, it should be measured annually for patients at low risk, every 6 months if there is moderate risk and every 3 months if there is high risk.97
Conclusion
Unfortunately, specific treatment for FD is usually delayed until proteinuria or renal dysfunction occurs, when the reversibility of the damage is difficult to achieve, and the prognosis is poor. For this reason, implementing strategies for early detection of nephropathy is mandatory to prevent devastating results in the long term. The growing knowledge of the mechanisms involved in FD nephropathy needs to be translated into the development of both new drugs and therapeutic concepts to support and complement the current therapy for the disease.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: The authors have consented publication of this article.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: José A. Moura-Neto  https://orcid.org/0000-0003-1339-3731
https://orcid.org/0000-0003-1339-3731
References
- 1. Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A Deficiency: Fabry Disease. The Online Metabolic and Molecular Bases of Inherited Disease. New York. McGraw Hill; 2014:1-64. [Google Scholar]
- 2. Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiffmann R. Fabry disease. Handb Clin Neurol. 2015;132:231-248. [DOI] [PubMed] [Google Scholar]
- 4. The human gene mutation database. http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GLA. Accessed in May 2020.
- 5. Curiati MA, Aranda CS, Kyosen SO, et al. The challenge of diagnosis and indication for treatment in Fabry disease. J Inborn Errors Metab Screen. 2017;5:1-7. [Google Scholar]
- 6. Varela P, Mastroianni Kirsztajn G, Ferrer H, et al. Functional characterization and pharmacological evaluation of a novel GLA missense mutation found in a severely affected Fabry disease family. Nephron. 2020;144(3):147-155. [DOI] [PubMed] [Google Scholar]
- 7. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249-254. [DOI] [PubMed] [Google Scholar]
- 8. Sawada T, Kido J, Yoshida S, et al. Newborn screening for Fabry disease in the western region of Japan. Mol Genet Metab Rep. 2020;22:100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colon C, Ortolano S, Melcon-Crespo C, et al. Newborn screening for Fabry disease in the north-west of Spain. Eur J Pediatr. 2017;176(8):1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merta M, Reiterova J, Ledvinova J, et al. A nationwide blood spot screening study for Fabry disease in the Czech Republic haemodialysis patient population. Nephrol Dial Transplant. 2007;22(1):179-186. [DOI] [PubMed] [Google Scholar]
- 11. Linthorst GE, Hollak CE, Korevaar JC, Van Manen JG, Aerts JM, Boeschoten EW. Alpha-galactosidase A deficiency in Dutch patients on dialysis: a critical appraisal of screening for Fabry disease. Nephrol Dial Transplant. 2003;18(8):1581-1584. [DOI] [PubMed] [Google Scholar]
- 12. Kotanko P, Kramar R, Devrnja D, et al. Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol. 2004;15(5):1323-1329. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka M, Ohashi T, Kobayashi M, et al. Identification of Fabry’s disease by the screening of alpha-galactosidase A activity in male and female hemodialysis patients. Clin Nephrol. 2005;64(4):281-287. [DOI] [PubMed] [Google Scholar]
- 14. Terryn W, Poppe B, Wuyts B, et al. Two-tier approach for the detection of alpha-galactosidase A deficiency in a predominantly female haemodialysis population. Nephrol Dial Transplant. 2008;23(1):294-300. [DOI] [PubMed] [Google Scholar]
- 15. Elliott P, Baker R, Pasquale F, et al. Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry Disease survey. Heart. 2011;97(23):1957-1960. [DOI] [PubMed] [Google Scholar]
- 16. Mawatari K, Yasukawa H, Oba T, et al. Screening for Fabry disease in patients with left ventricular hypertrophy. Int J Cardiol. 2013;167(3):1059-1061. [DOI] [PubMed] [Google Scholar]
- 17. Kubo T, Ochi Y, Baba Y, et al. Prevalence and clinical features of Fabry disease in Japanese male patients with diagnosis of hypertrophic cardiomyopathy. J Cardiol. 2017;69(1):302-307. [DOI] [PubMed] [Google Scholar]
- 18. Maron MS, Xin W, Sims KB, et al. Identification of Fabry disease in a tertiary referral cohort of patients with hypertrophic cardiomyopathy. Am J Med. 2018;131(2):200.e1-200.e8. [DOI] [PubMed] [Google Scholar]
- 19. Terryn W, Deschoenmakere G, De Keyser J, et al. Prevalence of Fabry disease in a predominantly hypertensive population with left ventricular hypertrophy. Int J Cardiol. 2013;167(6):2555-2560. [DOI] [PubMed] [Google Scholar]
- 20. Sadasivan C, Chow JTY, Sheng B, et al. Screening for Fabry disease in patients with unexplained left ventricular hypertrophy. PLoS ONE. 2020;15(9):e0239675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baptista MV, Ferreira S, Pinho-E-Melo T, Carvalho M, Cruz VT, et al. PORTuguese Young STROKE InvestigatorsMutations of the GLA gene in young patients with stroke: the PORTYSTROKE study–screening genetic conditions in Portuguese young stroke patients. Stroke. 2010;41(3):431-436. [DOI] [PubMed] [Google Scholar]
- 22. Wozniak MA, Kittner SJ, Tuhrim S, et al. Frequency of unrecognized Fabry disease among young European-American and African-American men with first ischemic stroke. Stroke. 2010;41(1):78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubuc V, Moore DF, Gioia LC, Saposnik G, Selchen D, Lanthier S. Prevalence of Fabry disease in young patients with cryptogenic ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(8):1288-1292. [DOI] [PubMed] [Google Scholar]
- 24. Romani I, Borsini W, Nencini P, et al. De novo diagnosis of Fabry disease among Italian adults with acute ischemic stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2015;24(11):2588-2595. [DOI] [PubMed] [Google Scholar]
- 25. Lanthier S, Saposnik G, Lebovic G, et al. Prevalence of Fabry disease and outcomes in young Canadian patients with cryptogenic ischemic cerebrovascular events. Stroke. 2017;48(7):1766-1772. [DOI] [PubMed] [Google Scholar]
- 26. Doheny D, Srinivasan R, Pagant S, Chen B, Yasuda M, Desnick RJ. Fabry disease: prevalence of affected males and heterozygotes with pathogenic GLA mutations identified by screening renal, cardiac and stroke clinics, 1995-2017. J Med Genet. 2018;55(4):261-268. [DOI] [PubMed] [Google Scholar]
- 27. Linthorst GE, Vedder AC, Aerts JM, Hollak CE. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin Chim Acta. 2005;353(1-2):201-203. [DOI] [PubMed] [Google Scholar]
- 28. Echevarria L, Benistan K, Toussaint A, et al. X-chromosome inactivation in female patients with Fabry disease. Clin Genet. 2016;89(1):44-54. [DOI] [PubMed] [Google Scholar]
- 29. Balendran S, Oliva P, Sansen S, et al. Diagnostic strategy for females suspected of Fabry disease. Clin Genet. 2020;97(4):655-660. [DOI] [PubMed] [Google Scholar]
- 30. Germain DP, Benistan K, Angelova L. X-linked inheritance and its implication in the diagnosis and management of female patients in Fabry disease. Rev Med Interne. 2010;31(suppl 2):S209-S213. [DOI] [PubMed] [Google Scholar]
- 31. Laney DA, Fernhoff PM. Diagnosis of Fabry disease via analysis of family history. J Genet Couns. 2008;17(1):79-83. [DOI] [PubMed] [Google Scholar]
- 32. Silva CA, Barreto FC, Dos Reis MA, Moura Junior JA, Cruz CM. Targeted screening of Fabry disease in male hemodialysis patients in Brazil highlights importance of family screening. Nephron. 2016;134(4):221-230. [DOI] [PubMed] [Google Scholar]
- 33. Arends M, Wanner C, Hughes D, et al. Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol. 2017;28(5):1631-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiffmann R, Warnock DG, Banikazemi M, et al. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24(7):2102-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333(5):288-293. [DOI] [PubMed] [Google Scholar]
- 36. Del Pino M, Andrés A, Bernabéu AÁ, et al. Fabry nephropathy: an evidence-based narrative review. Kidney Blood Press Res. 2018;43(2):406-421. [DOI] [PubMed] [Google Scholar]
- 37. Veloso VSP, Ataides TL, Canziani MEF, et al. A novel missense GLA mutation (p.G35V) detected in hemodialysis screening leads to severe systemic manifestations of Fabry disease in men and women. Nephron. 2018;138(2):147-156. [DOI] [PubMed] [Google Scholar]
- 38. Pan X, Ouyang Y, Wang Z, et al. Genotype: a crucial but not unique factor affecting the clinical phenotypes in Fabry disease. PLoS ONE. 2016;11(8):e0161330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodríguez-Marí A, Coll MJ, Chabás A. Molecular analysis in Fabry disease in Spain: fifteen novel GLA mutations and identification of a homozygous female. Hum Mutat. 2003;22(3):258. [DOI] [PubMed] [Google Scholar]
- 40. Thadhani R, Wolf M, West ML, et al. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002;61(1):249-255. [DOI] [PubMed] [Google Scholar]
- 41. Jain G, Warnock DG. Blood pressure, proteinuria and nephropathy in Fabry disease. Nephron Clin Pract. 2011;118(1):c43-8. [DOI] [PubMed] [Google Scholar]
- 42. Fabrazyme monograph. https://www.drugs.com/monograph/fabrazyme.html. Accessed May 18, 2020.
- 43. Replagal monograph. https://www.ema.europa.eu/en/documents/product-information/replagal-epar-product-information_en.pdf. Accessed May 18, 2020.
- 44. Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290(4):C1076-C1082. [DOI] [PubMed] [Google Scholar]
- 45. Germain DP, Giugliani R, Hughes DA, et al. Safety and pharmacodynamic effects of a pharmacological chaperone on α-galactosidase A activity and globotriaosylceramide clearance in Fabry disease: report from two phase 2 clinical studies. Orphanet J Rare Dis. 2012;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Germain DP, Fan JQ. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther. 2009;47(suppl 1):S111-S117. [PubMed] [Google Scholar]
- 47. Migalastat monograph. https://www.drugs.com/monograph/migalastat-hydrochloride.html. Accessed May 20, 2020.
- 48. Waldek S, Feriozzi S. Fabry nephropathy: a review—how can we optimize the management of Fabry nephropathy? BMC Nephrol. 2014;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Veen SJ, Hollak CEM, van Kuilenburg ABP, Langeveld M. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020;43(5):908-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feriozzi S, Hughes DA. New drugs for the treatment of Anderson-Fabry disease [published online ahead of print March 20, 2020]. J Nephrol. doi: 10.1007/s40620-020-00721-4. [DOI] [PubMed] [Google Scholar]
- 51. Welford RWD, Mühlemann A, Garzotti M, et al. Glucosylceramide synthase inhibition with lucerastat lowers globotriaosylceramide and lysosome staining in cultured fibroblasts from Fabry patients with different mutation types. Hum Mol Genet. 2018;27(19):3392-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guérard N, Oder D, Nordbeck P, et al. Lucerastat, an iminosugar for substrate reduction therapy: tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin Pharmacol Ther. 2018;103(4):703-711. [DOI] [PubMed] [Google Scholar]
- 53. Schiffmann R, Goker-Alpan O, Holida M, et al. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: a 1-year Phase 1/2 clinical trial. J Inherit Metab Dis. 2019;42(3):534-544. [DOI] [PubMed] [Google Scholar]
- 54. Hennermann JB, Arash-Kaps L, Fekete G, Schaaf A, Busch A, Frischmuth T. Pharmacokinetics, pharmacodynamics, and safety of moss-aGalactosidase A in patients with Fabry disease. J Inherit Metab Dis. 2019;42(3):527-533. [DOI] [PubMed] [Google Scholar]
- 55. Tuttolomondo A, Simonetta I, Pinto A. Gene therapy of Anderson-Fabry disease. Curr Gene Ther. 2019;19(1):3-5. [DOI] [PubMed] [Google Scholar]
- 56. Felis A, Whitlow M, Kraus A, Warnock DG, Wallace E. Current and investigational therapeutics for Fabry disease. Kidney Int Rep. 2019;5(4):407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kantola IM. Renal involvement in Fabry disease. Nephrol Dial Transplant. 2019;34(9):1435-1437. [DOI] [PubMed] [Google Scholar]
- 58. Germain DP, Charrow J, Desnick RJ, et al. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52(5):353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warnock DG, Thomas CP, Vujkovac B, et al. Antiproteinuric therapy and Fabry nephropathy: factors associated with preserved kidney function during agalsidase-beta therapy. J Med Genet. 2015;52(12):860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Madsen CV, Granqvist H, Petersen JH, et al. Age-related renal function decline in Fabry disease patients on enzyme replacement therapy: a longitudinal cohort study. Nephrol Dial Transplant. 2019;34(9):1525-1533. [DOI] [PubMed] [Google Scholar]
- 61. Warnock DG, Ortiz A, Mauer M, et al. Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant. 2012;27(3):1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rozenfeld PA, de Los Angeles Bolla M, Quieto P, et al. Pathogenesis of Fabry nephropathy: the pathways leading to fibrosis. Mol Genet Metab. 2020;129(2):132-141. [DOI] [PubMed] [Google Scholar]
- 63. Liebau MC, Braun F, Höpker K, et al. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS ONE. 2013;8(5):e63506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Riccio E, Sabbatini M, Capuano I, Pisani A. Early biomarkers of Fabry nephropathy: a review of the literature. Nephron. 2019;143(4):274-281. [DOI] [PubMed] [Google Scholar]
- 65. Abensur H, Reis MA. Renal involvement in Fabry disease. J Bras Nefrol. 2016;38(2):245-254. [DOI] [PubMed] [Google Scholar]
- 66. Eikrem Skrunes ØR, Tøndel C, Leh S, Houge G, Svarstad E, Marti HP. Pathomechanisms of renal Fabry disease. Cell Tissue Res. 2017;369(1):53-62. [DOI] [PubMed] [Google Scholar]
- 67. Kleinert J, Dehout F, Schwarting A, et al. Prevalence of uncontrolled hypertension in patients with Fabry disease. Am J Hypertens. 2006;19(8):782-787. [DOI] [PubMed] [Google Scholar]
- 68. Riccio E, Sabbatini M, Bruzzese D, et al. Glomerular hyperfiltration: an early marker of nephropathy in Fabry disease. Nephron. 2019;141(1):10-17. [DOI] [PubMed] [Google Scholar]
- 69. Sirrs S, Bichet DG, Iwanochko RM, et al. Canadian Fabry disease treatment guidelines 2018. https://garrod.ca/wp-content/uploads/2020/02/Canadian-Fabry-Treatment-Guidelines-2019-final.pdf. Accessed May 10, 2020.
- 70. Prabakaran T, Birn H, Bibby BM, et al. Long-term enzyme replacement therapy is associated with reduced proteinuria and preserved proximal tubular function in women with Fabry disease. Nephrol Dial Transplant. 2014;29(3):619-625. [DOI] [PubMed] [Google Scholar]
- 71. Fall B, Scott CR, Mauer M, et al. Urinary podocyte loss is increased in patients with Fabry disease and correlates with clinical severity of Fabry nephropathy. PLoS ONE. 2016;11(12):e0168346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pereira EM, Silva AS, Labilloy A, Monte Neto JT, Monte SJ. Podocyturia in Fabry disease. J Bras Nefrol. 2016;38(1):49-53. [DOI] [PubMed] [Google Scholar]
- 73. Sanchez-Niño MD, Perez-Gomez MV, Valiño-Rivas L, Torra R, Ortiz A. Podocyturia: why it may have added value in rare diseases. Clin Kidney J. 2018;12(1):49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weidemann F, Sanchez-Niño MD, Politei J, et al. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Colpart P, Félix S. Fabry nephropathy. Arch Pathol Lab Med. 2017;141(8):1127-1131. [DOI] [PubMed] [Google Scholar]
- 76. Wijburg FA, Bénichou B, Bichet DG, et al. Characterization of early disease status in treatment-naive male paediatric patients with Fabry disease enrolled in a randomized clinical trial. PLoS ONE. 2015;10(5):e0124987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schiffmann R, Hughes DA, Linthorst GE, et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91(2):284-293. [DOI] [PubMed] [Google Scholar]
- 78. Fogo AB, Bostad L, Svarstad E, et al. Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant. 2010;25(7):2168-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aguiar P, Azevedo O, Pinto R, et al. New biomarkers defining a novel early stage of Fabry nephropathy: a diagnostic test study. Mol Genet Metab. 2017;121(2):162-169. [DOI] [PubMed] [Google Scholar]
- 80. Shimohata H, Ogawa Y, Maruyama H, Hirayama K, Kobayashi M. A renal variant of Fabry disease diagnosed by the presence of urinary mulberry cells. Intern Med. 2016;55(23):3475-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Trimarchi H, Canzonieri R, Costales-Collaguazo C, et al. Early decrease in the podocalyxin to synaptopodin ratio in urinary Fabry podocytes. Clin Kidney J. 2019;12(1):53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trimarchi H, Canzonieri R, Schiel A, et al. Podocyturia is significantly elevated in untreated vs treated Fabry adult patients. J Nephrol. 2016;29(6):791-797. [DOI] [PubMed] [Google Scholar]
- 83. Mauer M, Glynn E, Svarstad E, et al. Mosaicism of podocyte involvement is related to podocyte injury in females with Fabry disease. PLoS ONE. 2014;9(11):e112188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moura AP, Hammerschmidt T, Deon M, Giugliani R, Vargas CR. Investigation of correlation of urinary globotriaosylceramide (Gb3) levels with markers of renal function in patients with Fabry disease. Clin Chim Acta. 2018;478:62-67. [DOI] [PubMed] [Google Scholar]
- 85. Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci USA. 2008;105(8):2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ. Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet. 2015;52(4):262-268. [DOI] [PubMed] [Google Scholar]
- 87. Maruyama H, Miyata K, Mikame M, et al. Effectiveness of plasma lyso-Gb3 as a biomarker for selecting high-risk patients with Fabry disease from multispecialty clinics for genetic analysis. Genet Med. 2019;21(1):44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nowak A, Mechtler TP, Desnick RJ, Kasper DC. Plasma LysoGb3: a useful biomarker for the diagnosis and treatment of Fabry disease heterozygotes. Mol Genet Metab. 2017;120(1-2):57-61. [DOI] [PubMed] [Google Scholar]
- 89. Lavoie P, Boutin M, Auray-Blais C. Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for Fabry disease by tandem mass spectrometry. Anal Chem. 2013;85(3):1743-1752. [DOI] [PubMed] [Google Scholar]
- 90. Dupont FO, Gagnon R, Boutin M, Auray-Blais C. A metabolomic study reveals novel plasma lyso-Gb3 analogs as Fabry disease biomarkers. Curr Med Chem. 2013;20(2):280-288. [DOI] [PubMed] [Google Scholar]
- 91. Boutin M, Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb₃-related analogues in Fabry disease. Anal Chem. 2014;86(7):3476-3483. [DOI] [PubMed] [Google Scholar]
- 92. Alharbi FJ, Baig S, Rambhatla SB, et al. The clinical utility of total concentration of urinary globotriaosylsphingosine plus its analogues in the diagnosis of Fabry disease. Clin Chim Acta. 2020;500:120-127. [DOI] [PubMed] [Google Scholar]
- 93. Svarstad E, Marti HP. The changing landscape of Fabry disease. Clin J Am Soc Nephrol. 2020;15(4):569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang N, Wang X, Xu F, Zeng C, Wang J, Liu Z. Clinical and pathological characteristics of Fabry disease combined with IgA nephropathy in Chinese patients. Clin Nephrol. 2017;87(4):188-195. [DOI] [PubMed] [Google Scholar]
- 95. Maixnerová D, Tesař V, Ryšavá R, et al. The coincidence of IgA nephropathy and Fabry disease. BMC Nephrol. 2013;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou W, Ni Z, Zhang M. Hemizygous Fabry disease associated with membranous nephropathy: a rare case report. Clin Nephrol. 2018;90(3):227-231. [DOI] [PubMed] [Google Scholar]
- 97. Najafian B, Fogo AB, Lusco MA, Alpers CE. AJKD Atlas of renal pathology: Fabry nephropathy. Am J Kidney Dis. 2015;66(5):e35-e36. [DOI] [PubMed] [Google Scholar]
- 98. van der Tol L, Svarstad E, Ortiz A, et al. Chronic kidney disease and an uncertain diagnosis of Fabry disease: approach to a correct diagnosis. Mol Genet Metab. 2015;114(2):242-247. [DOI] [PubMed] [Google Scholar]
- 99. de Menezes Neves PDM, Machado JR, Custódio FB, et al. Ultrastructural deposits appearing as “zebra bodies” in renal biopsy: Fabry disease?- comparative case reports. BMC Nephrol. 2017;18(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123(4):416-427. [DOI] [PubMed] [Google Scholar]
- 101. Ortiz A, Cianciaruso B, Cizmarik M, et al. End-stage renal disease in patients with Fabry disease: natural history data from the Fabry Registry. Nephrol Dial Transplant. 2010;25(3):769-775. [DOI] [PubMed] [Google Scholar]
- 102. Ortiz A, Sanchez-Niño MD. Enzyme replacement therapy dose and Fabry nephropathy. Nephrol Dial Transplant. 2018;33(8):1284-1289. [DOI] [PubMed] [Google Scholar]
- 103. Ersözlü S, Desnick RJ, Huynh-Do U, et al. Long-term outcomes of kidney transplantation in Fabry disease. Transplantation. 2018;102(11):1924-1933. [DOI] [PubMed] [Google Scholar]
- 104. Capelli I, Aiello V, Gasperoni L, et al. Kidney transplant in Fabry disease: a revision of the literature. Medicina. 2020;56(6):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Parini R, Pintos-Morell G, Hennermann JB, et al. Analysis of renal and cardiac outcomes in male participants in the Fabry Outcome Survey starting agalsidase alfa enzyme replacement therapy before and after 18 years of age. Drug Des Devel Ther. 2020;14:2149-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Weidemann F, Niemann M, Störk S, et al. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med. 2013;274(4):331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ramaswami U, Beck M, Hughes D, et al. Cardio-renal outcomes with long-term agalsidase alfa enzyme replacement therapy: a 10-year Fabry Outcome Survey (FOS) analysis. Drug Des Devel Ther. 2019;13:3705-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kampmann C, Perrin A, Beck M. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis. 2015;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rombach SM, Smid BE, Bouwman MG, Linthorst GE, Dijkgraaf MG, Hollak CE. Long term enzyme replacement therapy for Fabry disease: effectiveness on kidney, heart and brain. Orphanet J Rare Dis. 2013;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Skrunes R, Tøndel C, Leh S, et al. Long-term dose-dependent agalsidase effects on kidney histology in Fabry disease. Clin J Am Soc Nephrol. 2017;12(9):1470-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tøndel C, Bostad L, Larsen KK, et al. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24(1):137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Krämer J, Lenders M, Canaan-Kühl S, et al. Fabry disease under enzyme replacement therapy-new insights in efficacy of different dosages. Nephrol Dial Transplant. 2018;33(8):1362-1372. [DOI] [PubMed] [Google Scholar]
- 113. Arends M, Biegstraaten M, Wanner C, et al. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: an international cohort study. J Med Genet. 2018;55(5):351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375(6):545-555. [DOI] [PubMed] [Google Scholar]
- 115. Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54(4):288-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Narita I, Ohashi T, Sakai N, et al. Efficacy and safety of migalastat in a Japanese population: a subgroup analysis of the ATTRACT study. Clin Exp Nephrol. 2020;24(2):157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mauer M, Sokolovskiy A, Barth JA, et al. Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment. J Med Genet. 2017;54(11):781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Germain DP, Nicholls K, Giugliani R, et al. Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet Med. 2019;21(9):1987-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Branton M, Schiffmann R, Kopp JB. Natural history and treatment of renal involvement in Fabry disease. J Am Soc Nephrol. 2002;13(suppl 2):S139-S143. [PubMed] [Google Scholar]
- 120. Germain DP, Fouilhoux A, Decramer S, et al. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin Genet. 2019;96(2):107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Najafian B, Tøndel C, Svarstad E, Gubler MC, Oliveira JP, Mauer M. Accumulation of globotriaosylceramide in podocytes in Fabry nephropathy is associated with progressive podocyte loss. J Am Soc Nephrol. 2020;31(4):865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hopkin RJ, Jefferies JL, Laney DA, et al. The management and treatment of children with Fabry disease: a United States-based perspective. Mol Genet Metab. 2016;117(2):104-113. [DOI] [PubMed] [Google Scholar]
- 123. Terryn W, Cochat P, Froissart R, et al. Fabry nephropathy: indications for screening and guidance for diagnosis and treatment by the European Renal Best Practice. Nephrol Dial Transplant. 2013;28(3):505-517. [DOI] [PubMed] [Google Scholar]
- 124. Sanchez-Niño MD, Sanz AB, Carrasco S, et al. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 2011;26(6):1797-1802. [DOI] [PubMed] [Google Scholar]