Highlights
-
•
Maltreatment and brain development related to risky decision making were studied.
-
•
Neglect predicted slower increases in risk-related insula and dACC activation.
-
•
Abuse predicted steeper decreases in control-related fronto-parietal activation.
-
•
Abuse predicted accelerated development of risk- and control-related brain regions.
-
•
Abuse and neglect had differential effects on adolescent brain development.
Keywords: Child maltreatment, Abuse, Neglect, Risk processing, Cognitive control, Functional neuroimaging
Abstract
The profound effects of child maltreatment on brain functioning have been documented. Yet, little is known about whether distinct maltreatment experiences are differentially related to underlying neural processes of risky decision making: valuation and control. Using conditional growth curve modeling, we compared a cumulative approach versus a dimensional approach (relative effects of abuse and neglect) to examine the link between child maltreatment and brain development. The sample included 167 adolescents (13–14 years at Time 1, 53 % male), assessed annually four times. Risk processing was assessed by blood-oxygen-level-dependent responses (BOLD) during a lottery choice task, and cognitive control by BOLD responses during the Multi-Source Interference Task. Cumulative maltreatment effects on insula and dorsolateral anterior cingulate cortex (dACC) activation during risk processing were not significant. However, neglect (but not abuse) was associated with slower developmental increases in insula and dACC activation. In contrast, cumulative maltreatment effects on fronto-parietal activation during cognitive control were significant, and abuse (but not neglect) was associated with steeper developmental decreases in fronto-parietal activation. The results suggest neglect effects on detrimental neurodevelopment of the valuation system and abuse effects on accelerated neurodevelopment of the control system, highlighting differential effects of distinct neglect versus abuse adverse experiences on neurodevelopment.
Adolescence is characterized by an increase in risk taking behaviors. Current neuroscience work views risk taking in adolescence to be derived in part from distinct developmental trajectories of two neural systems (Casey et al., 2008): one underlying the assessment of value and risk associated with appetitive/aversive stimuli (i.e., the valuation system), and a second system exerting control over the pursuit or avoidance of risky options (i.e., the control system). Prior neuroimaging research has linked maltreatment and brain functioning underlying risky decision making by showing that maltreated individuals exhibit blunted reward-related brain activation (Hanson et al., 2015) and impaired regulation-related brain activation (Lim et al., 2015). However, the ways in which different maltreatment experiences (abuse and neglect) may be related to the development of two underlying neural processes of risky decision making (the valuation and the control systems) during adolescence is not clearly understood.
There are two predominant perspectives in the neuroscience literature that address how childhood adversity affects the developing brain. One perspective rooted in stress physiology emphasizes the similarities of childhood adversity effects, arguing that because disruptions in physiological stress responses are the main consequence of various adverse experiences (e.g., poverty, parental deprivation, or exposure to violence), they converge in their effects and thus can be grouped together as “childhood adversity” (Sapolsky, 2017; Smith and Pollak, 2020). This cumulative approach emphasizes the high prevalence of co-occurring adversity types and focuses more on the number of adverse life events influencing development than the nature of these events (Hughes et al., 2017; Smith and Pollak, 2020). In contrast, another perspective rooted in developmental psychopathology proposes a dimensional approach to measuring childhood adversity, arguing that threat and deprivation are two central dimensions of childhood adversity whose influences on neurobiological development are substantially distinct (McLaughlin and Sheridan, 2016; Sheridan and McLaughlin, 2014). Considering childhood maltreatment through the lens of violations in the expectable environment (Nelson and Gabard-Durnam, 2020), child maltreatment reflects experiences that are expected but do not occur (i.e., neglect), or that do occur but are atypical in some way (i.e., abuse). Consistent with the conceptualization of threat and deprivation according to the dimensional approach (McLaughlin and Sheridan, 2016; Sheridan and McLaughlin, 2014), abuse refers to acts of commission involving harm, or threat of harm, whereas neglect refers to acts of omission involving deprivation (Rogosch & Cicchetti, 1994). The main goal of the current study was to address these two competing perspectives by examining cumulative maltreatment experiences versus differentiated measurement of abuse and neglect experiences related to brain development.
Extant neuroscience work presents structural and functional brain sequelae of caregiving adversity, such as abuse and neglect. The neural processes affected in individuals with a history of childhood maltreatment are predominantly in fronto-limbic networks (including the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, hippocampus, and amygdala). For the effects of child maltreatment on the control system, a few functional neuroimaging studies using inhibitory control tasks indicate that youths who experienced neglectful and/or abusive care demonstrate heightened activation in the dorsomedial frontal regions which have been linked to inhibitory control and conflict/error processing (Bruce et al., 2013; Lim et al., 2015; Mueller et al., 2010). First, maltreated foster pre-adolescents (90 % experienced physical neglect and 55 % physical abuse) showed higher activation in the anterior cingulate cortex and middle frontal gyrus compared to their nonmaltreated counterparts (Bruce et al., 2013). Second, physically abused adolescents showed higher activation in the anterior cingulate cortex as well as bilateral pre-supplementary and supplementary motor area, compared to nonmaltreated adolescents (Lim et al., 2015). Third, neglected adolescents exhibited higher activation in the inferior frontal cortex and striatum compared to nonmaltreated adolescents (Mueller et al., 2010). Finally, Blair et al. (2019) examined how abuse and neglect were associated with brain activation during an emotion-based inhibitory control task and found that abuse (but not neglect) was differentially related to brain regions that were involved response control versus emotional processing. Specifically, greater abuse was related to lower activation in the brain regions involved in response control and motor responding (such as the inferior parietal lobule and postcentral gyrus) but higher activation in the brain regions involved in responding to or representing affective information (such as the rostromedial frontal cortex, middle temporal gyrus, and superior temporal gyrus) among adolescents.
Turning to functional neuroimaging studies examining maltreatment and the valuation system, findings indicate that maltreatment is associated with a blunted neural response to reward cues in the orbito-striatal network. Specifically, adolescents with emotional neglect showed blunted development of ventral striatum activity related to reward expectancy over two years (Hanson et al., 2015), and this finding is consistent with another study demonstrating that young adults with childhood maltreatment exhibited blunted anticipatory reward activity in the left basal ganglia relative to young adults without maltreatment experience (Dillon et al., 2009). Similarly, adolescents primarily with neglect and emotional abuse showed blunted striatum activation related to expected value representation for both approach and avoidance trials, compared to nonmaltreated adolescents (Gerin et al., 2017). Unexpectedly, Gerin and colleagues (2017) found that maltreated adolescents showed greater activation in the putamen during expected value representation while avoiding possible punishment (avoidance trials). This result suggests altered brain activation during reinforcement learning among maltreated youths; that is, for those whose environments are laden with threat-related cues, avoiding probable punishment may be particularly rewarding.
Although prior research has focused on maltreatment effects on reward processing, choosing high-risk yet high-reward options may be explained not only by the neural processes reacting to the value of the reward but also by the neural processes evaluating the risk associated with the rewarding options. Indeed, value-based decision-making research has shown that risky choices are driven by neural computations associated with the likelihood of receiving rewards as well as the value of rewards (d’Acremont and Bossaerts, 2008; Mohr et al., 2010). However, it is not known how maltreatment experiences may be related to neural processing of risk valuation. The current investigation integrates the two lines of developmental neuroscience research—adversity effects of the valuation system and adversity effects of the control system—while focusing on risk processing in the valuation system in order to better understand how developmental trajectories of the two primary neural systems related to risk taking may be differentially affected by experiences of maltreatment.
Although different subtypes of child maltreatment tend to co-occur, experiences of different subtypes appear to be distinct enough to differentially influence neurodevelopment. To date, no prospective longitudinal study has examined how abuse and neglect may be differentially related to developmental trajectories of brain functioning throughout adolescence. Following the dimensional model of childhood adversity (McLaughlin and Sheridan, 2016; Sheridan and McLaughlin, 2014), the current longitudinal study elucidates the effect of child maltreatment on neurodevelopment by evaluating differential contributions of two core dimensions underlying maltreatment: threat (i.e., abuse) and deprivation (i.e., neglect). We further tested whether there are cumulative effects of maltreatment, regardless of dimensions, based on the view that varied types of early adversity converge in producing similar problems later on (e.g., Sapolsky, 2017; Smith and Pollak, 2020). We used conditional latent growth curve modeling to examine dimensional versus cumulative effects of child maltreatment on developmental trajectories of the valuation and the control systems in adolescence.
1. Method
1.1. Participants
The sample included 167 adolescents (53 % males) from a southeastern state in the United States, who participated in annual assessments across four years, with a subset participating in a fifth follow-up year. Adolescents were 13–14 years of age at Time 1 (M = 14.07, SD = 0.54 for Time 1, M = 15.05, SD = 0.54 for Time 2, M = 16.07, SD = 0.56 for Time 3, and M = 17.01, SD = 0.55 for Time 4). About 78 % of adolescents identified as White, 14 % Black or African-American, 6% as more than one race, 1% as American Indian or Alaska Native, and 1% Asian. Median annual family income was in the $35,000-$50,000 range, with varying levels of family economic status (50 % “poor/near poor” and 50 % “non-poor” according to income-to-needs ratio). Among the primary caregivers (137 mothers, 21 fathers, and 9 others), 34 % had a high school degree or less, 24 % some college education, 24 % bachelor’s degree, and 18 % graduate degree. Inclusion criteria included being age 13–14 at Time 1 with vision corrected to be able to see the computer display clearly. Exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for >10 min, orthodontia impairing image acquisition, severe psychopathology (e.g., psychosis), and other contraindications to magnetic resonance imaging (MRI).
At Time 1, 157 adolescents participated. At Time 2, 10 adolescents were added (to offset annual attrition) for a final sample of 167 (150 at Time 2, 147 at Time 3, and 150 at Time 4). Across all four years, 24 adolescents did not participate at all four time points for reasons including: ineligibility for tasks (n = 2), declined participation (n = 17), and lost contact (n = 5) during the follow-up assessments. At Time 5, 126 adolescents (52 % males; 80 % White, Mage =18.39, SD = 0.67) participated in a follow-up study where they retrospectively reported on childhood maltreatment. Rate of participation was not significantly predicted by income, sex, race or study variables (ps> .18).
1.2. Procedures
Data included in the present study were collected as part of a larger project. Adolescent participants and their primary caregivers were recruited via email announcements, flyers, and snowball sampling (word-of-mouth). Data collection was administered at university offices where participants completed self-report questionnaires, behavioral and neuroimaging tasks, and were interviewed by trained research assistants. The study duration was on average five hours long and participants were compensated monetarily for their time. All procedures were approved by the institutional review board of the university and written informed consent or assent was received from all participants.
1.3. Measures
1.3.1. Maltreatment
Maltreatment was measured using the Maltreatment and Abuse Chronology of Exposure scale (MACE; Teicher and Parigger, 2015), which evaluates the severity of exposure to different types of maltreatment during each year of childhood (ages 1–18). Adolescents were asked to retrospectively indicate at which ages they experienced the events, described across 52 items. Abuse was represented by the subscales of physical abuse (6 items), sexual abuse (7 items), verbal abuse (4 items), and non-verbal abuse (6 items). Neglect was represented by the subscales of physical neglect (5 items) and emotional neglect (5 items). Sample items include, “Intentionally pushed, grabbed, shoved, slapped, pinched, punched or kicked you” (physical abuse) and “were there to take care of you and protect you (physical neglect, reverse coded)”. The current analyses used retrospective reports of maltreatment from ages 0–17, committed by caregiver figures (parents, stepparents, or other adults living in the house) with the exception of sexual abuse for which perpetrators included caregiver figures, adults not living in the house, and peers (see Supplementary Table S1 for frequency of maltreatment by subtype and age of exposure). Subscale scores were scaled using an algorithm provided by Teicher and Parigger (2015). Higher scores indicate higher maltreatment. Previous research has demonstrated good to excellent test-retest reliability for all the maltreatment subtypes used in the current study (Teicher and Parigger, 2015). We created a composite of abuse by summing the subscale scores of physical abuse, sexual abuse, verbal abuse, and non-verbal abuse, and a composite of neglect by summing the subscale scores of physical neglect and emotional neglect.
1.3.2. Risk processing
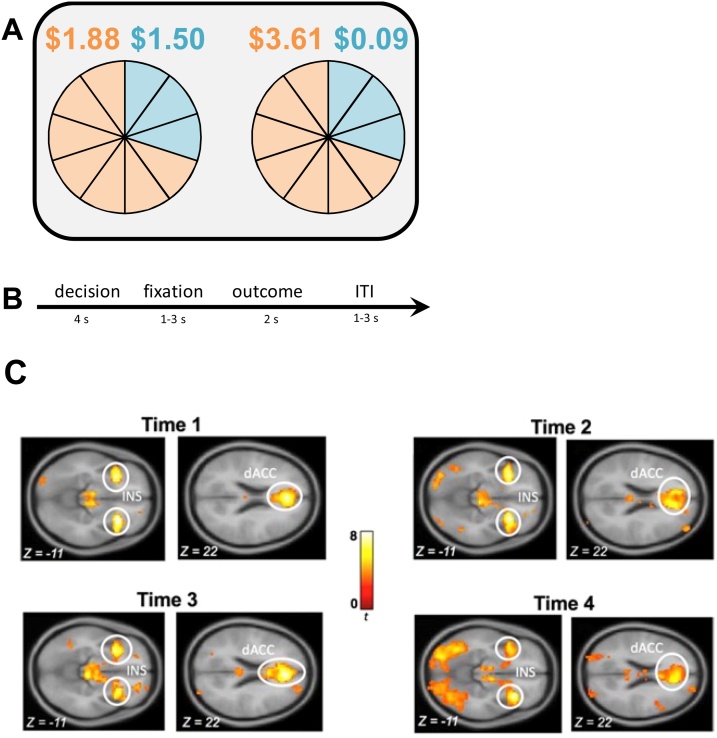
At each time point, adolescents engaged in a modified economic lottery choice task (Holt and Laury, 2002) while their blood-oxygen-level-dependent (BOLD) responses were recorded. On each trial, adolescents were asked to choose between two gambles, where one gamble was always riskier (higher coefficient of variation; CV) than the other (see Fig. 1A-B). The CV was computed by dividing the standard deviation of a gamble by the expected value (i.e., probability-weighted outcome) of that gamble. For each gamble, there was a high and low monetary outcome, each associated with a specific probability that varied across a total of 72 trials (approximately 25 min to complete). To incentivize performance, participants were compensated based on their winnings from four randomly selected trials.
Fig. 1.
Schematic Display of the Lottery Choice Task and Blood-Oxygen-Level-Dependent Responses to Risk (Coefficient of Variation).
Note. (A) In the lottery choice task, adolescents were asked to choose between pairs of uncertain gambles. For each gamble, there was a high and low monetary outcome, each associated with a specific probability. The associations between outcomes and probabilities are represented with corresponding colors (orange or blue). (B) Each trial consisted of a decision phase, a fixation phase, an outcome phase, and an inter-trial-interval (ITI). (C) During the decision phase increased activation was found in the insula (INS) and dorsal anterior cingulate cortex (dACC) during riskier gambles as was indicated by the coefficient of variation (CV). Figure adapted from Asscheman et al. (2020).
1.3.3. Cognitive control
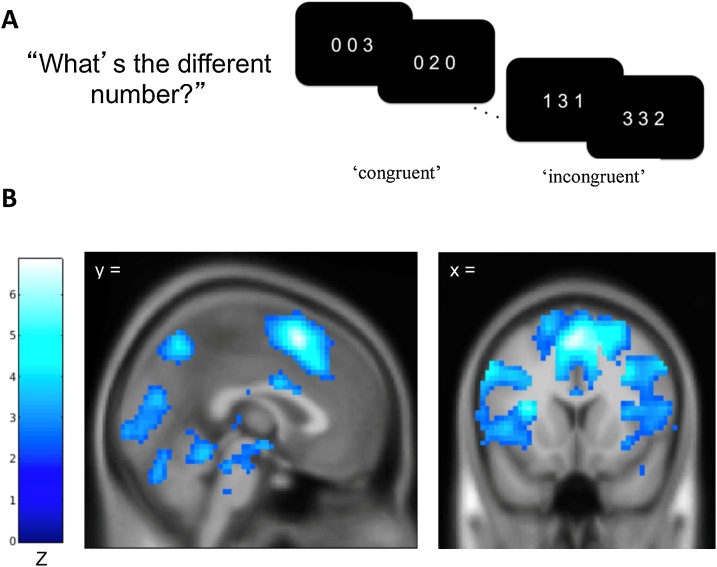
At each time point adolescents completed a multi-source interference task (MSIT; Bush, et al., 2003) while their BOLD responses were recorded. In each trial adolescents were presented with three digits and were tasked with reporting the identity of the different digit (not like the other two) by pressing a button. In neutral trials, the target’s identity matched the digit’s presented location, but in interference trials, the target’s identity was not congruent with the digit’s presented location (see Fig. 2A). Consistent with previous research (Bush et al., 2003), we calculated mean response times for each condition. We found a significant positive effect of MSIT interference on response time for correct responses, such that response time was higher for interference trials compared to neutral trials [t(153) = 69.58 at Time 1, t(148) = 69.41 at Time 2, t(142) = 63.30 at Time 3, and t(142) = 59.87 at Time 4, all ps < .001].
Fig. 2.
Schematic Display of the Multi-Source Interference Task (MSIT) and Activation Maps Showing Significant Activation for the Interference-Neutral Contrast.
Note. A) In the multi-source interference task (MSIT), adolescents were asked to identify the digit that differed from two other concurrently presented digits, ignoring its position in the sequence. B) Map showing a significant negative linear relationship between the time points and the interference effect on BOLD using the Sandwich Estimator Toolbox. Displayed using voxel-wise false discovery rate corrected threshold of p < .05 and gray matter mask.
1.4. fMRI data acquisition and analysis
MRI data were acquired on a 3 T Siemens Tim Trio scanner with a standard 12-channel head matrix coil. Structural images were acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence with the following parameters: repetition time (TR) =1200 ms, echo time (TE) =2.66 ms, field of view (FoV) = 245 × 245 mm, and 192 slices with the spatial resolution of 1 × 1 × 1 mm. Echo-planar images were collected using the following parameters: slice thickness = 4 mm, 34 axial slices, FoV = 220 × 220 mm, TR = 2 s, TE =30 ms, flip angel = 90 degrees, voxel size = 3.4 × 3.4 × 4 mm, 64 × 64 grid, and slices were hyperangulated at 30 degrees from anterior-posterior commissure. Imaging data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). First, functional scans were corrected for rigid head motion using a six-parameter rigid body transformation. After realignment, the mean functional image was co-registered to the anatomical image, normalized to MNI template, and smoothed using a 6 mm full-width-half-maximum Gaussian filter. Six rigid body realignment parameters were included to account for the effect of in-scanner motion, and low-frequency signal was removed using a high-pass filter with cutoff of 0.006 Hz (168 s per SPM). Six rigid body realignment parameters were included to account for the effect of in-scanner motion, and low-frequency signal was removed using a high-pass filter with cutoff of 0.00781 Hz (128 s which was the default for SPM) for risk processing data, and 0.006 Hz (168 s) for cognitive control data to better capture the expected signal (see Henson, 2007, pp 200–203).
1.4.1. Neural risk processing
For each individual, a general linear model (GLM) was constructed including decision and outcome events of the task modeled with a duration of four and two seconds, respectively. To assess neural risk processing, a parametric regressor of decision phase activation representing the risk level (i.e., CV) for chosen gambles was entered into the model. The CV is a scale-free metric and has been shown to be superior in explaining choice behavior compared to other economic measures of risk (i.e., standard deviation or variance) because outcomes are coded by the relative risk as opposed to the absolute outcome (Weber et al., 2004). Additionally, the individual level GLM included a parametric regressor indicating whether participants received high or low monetary outcomes during the outcome phase, one regressor for the button press, and six motion regressors. These regressors were included to characterize the error term in the neural model. At the group level of the GLM, whole brain analysis was conducted to determine how CV for chosen gambles modulated BOLD responses during the decision phase. All statistical inferences were made at a cluster-corrected threshold of p < .05 with a Family-Wise Error (FWE) correction, with an initial cluster-forming uncorrected threshold of p < .001.
1.4.2. Neural cognitive control
Preprocessed MRI data were analyzed by first entering them into a first-level analysis General Linear Model (GLM) in SPM8, in which interference and neutral blocks were modeled using boxcars convolved with the canonical haemodynamic response function (HRF) with six motion regressors. Additionally, framewise displacement (FD) was calculated from the realignment parameters, with rotational displacement converted to millimeters using the surface of a sphere of radius 50 mm (Power et al., 2012; Siegel et al., 2014). Volumes with FD > 0.9 mm were censored by adding a volume-specific regressor for each scrubbed volume in the GLM. This frame censoring approach was used because it appeared to be particularly beneficial to analyzing the repeated measures data simultaneously. For each GLM, an interference greater than neutral contrast map was generated by subtracting the neutral beta map from the positive beta map. These contrast maps were entered into four second-level GLMs in SPM8, one for each time point, using root mean frame displacement as a regressor non-interest. To better understand how activation changed across time points we further entered the first-level contrast maps into a longitudinal group-level model using the Sandwich Estimator Toolbox version 2.1.0 (Guillaume et al., 2014) using root mean frame displacement as a regressor non-interest to address age-related changes to within-scanner motion (Satterthwaite et al., 2012). A significant effect of MSIT interference on BOLD signal was observed at each time point, in line with previously reported effects of the MSIT (see Fig. 2B, Kim-Spoon et al., 2019). Furthermore, we observed significant changes in the interference effect on BOLD responses across four time points (see Fig. 2B), as reported by Kim-Spoon et al. (2019). Regions of interest were defined around peaks of time point related change in BOLD responses using cluster-derived masks with a cluster defining voxel-wise false discovery rate corrected (FDR) threshold of p < 1e-5 and a gray matter mask.
1.5. Data analytic plan
Models were tested using Structural Equation Modeling (SEM) in Mplus statistical software version 8 (Muthén & Muthén, 1998-2018). Model fit was assessed by χ2 value, degrees of freedom, corresponding p-value, Root Mean Square Error of Approximation (RMSEA), and Confirmatory Fit Index (CFI). RMSEA values less than .08 and CFI values greater than .90 were considered an acceptable fit (Bentler, 1990; Browne and Cudeck, 1993). Little’s MCAR test indicated that the missing data pattern for all study variables resembled a Completely at Random pattern (χ2 = 329.91, df = 356, p = .836). Therefore, full information maximum likelihood (FIML) estimation procedure was used to address missing data given its superiority to those obtained with listwise deletion or other ad hoc methods (Schafer & Graham, 2002).
For testing patterns of neurodevelopment, we first tested univariate growth curve models of risk processing and cognitive control. Linear and nonlinear models were tested to fit the baseline model for the observed data patterns across the four time points. The first latent factor was the intercept, with all factor loadings fixed to one. To keep temporal precedence in the prediction of neurodevelopment from maltreatment experiences, we set the intercept of the neural outcomes to Time 4. The second latent factor was the slope, indicating growth of the function and change over time. The two growth factors were allowed to covary. Nested model comparisons were used to determine the shape of the trajectories. The χ2 difference test was used to compare these nested models and the most parsimonious model with acceptable fits was chosen as the best-fitting model. In the no growth model, non-significant change in slope was assumed. In the linear growth model, a linear pattern of change was assumed with factor loadings fixed to -3, -2, -1, and 0 from Time 1 through Time 4. The latent basis growth model allowed the data to estimate the shape of growth by fixing the first and last time points (to -1 and 0, respectively) and freely estimating the second and third time points. Finally, conditional growth curve modeling was used to test the effects of maltreatment on neurodevelopment. We compared a dimensional model including abuse and neglect with a cumulative model including only a single composite representing cumulative maltreatment. Regression paths were estimated from maltreatment predictors to the growth factors of risk processing and cognitive control.
2. Results
Prior to analysis, statistical outliers (n = 26 across all neural variables and all time points) were Winsorized to the next value that was not an outlier (i.e., within 3.29 SD), resulting in all variables with acceptable skewness and kurtosis (< 3 and < 10, respectively). Table 1 depicts descriptive statistics and correlations for all study variables. Multivariate GLM analyses testing demographic covariates indicated that sex (p = .299), race (p = .553), and family income (p = .167) were not significant predictors of the study variables (six maltreatment subtypes, four risk processing variables, and four cognitive control variables). Because these variables were not significant predictors, they were not included in the main analyses.
Table 1.
Descriptive Statistics and Bivariate Correlations of Maltreatment, Insula-dACC Activation during Risk Processing and Fronto-parietal Activation during Cognitive Control.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | M | SD | Min | Max | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Maltreatment Composite | – | 10.21 | 9.66 | 0.00 | 46.00 | |||||||||
| 2. Abuse Composite | .92* | – | 7.17 | 7.75 | 0.00 | 32.00 | ||||||||
| 3. Neglect Composite | .63* | .26* | – | 3.03 | 4.09 | 0.00 | 14.00 | |||||||
| 4. Insula-dACC T1 | .00 | .00 | .00 | – | 0.04 | 0.05 | −0.08 | 0.21 | ||||||
| 5. Insula-dACC T2 | .02 | .07 | −.09 | .35* | – | 0.61 | 0.77 | −1.25 | 3.44 | |||||
| 6. Insula-dACC T3 | .06 | .14 | −.12 | .28* | .35* | – | 0.57 | 0.76 | −1.33 | 3.36 | ||||
| 7. Insula-dACC T4 | −.01 | .04 | −.10 | .29* | .40* | .45* | – | 0.83 | 1.15 | −3.07 | 4.87 | |||
| 8. Fronto-parietal T1 | .06 | .04 | .07 | −.04 | .11 | .09 | .08 | – | 0.47 | 0.25 | −0.19 | 1.09 | ||
| 9. Fronto-parietal T2 | −.06 | −.08 | .00 | −.05 | .07 | .02 | −.03 | .31* | – | 0.33 | 0.20 | −0.18 | 0.83 | |
| 10. Fronto-parietal T3 | −.29* | −.29* | −.15 | −.19 | −.06 | −.02 | −.06 | .33* | .27* | – | 0.27 | 0.23 | −0.30 | 0.92 |
| 11. Fronto-parietal T4 | .01 | .01 | .01 | −.12 | .20* | .02 | .02 | .39* | .29* | .17 | 0.26 | 0.20 | −0.19 | 0.89 |
Notes. dACC = dorsolateral anterior cingulate cortex; T1 = Time 1; T2 = Time 2; T3 = Time 3; T4 = Time 4. * p < .05.
2.1. Neural activation during risk processing
Results indicated that the bilateral insula and dorsal anterior cingulate cortex (dACC) belonged to the largest regions, with the greatest t-values, among the regions activated in response to the differing levels of risk. Additionally, they were the most consistently activated brain regions across all four waves of our longitudinal assessments. In contrast, activation in the ventral striatum, orbitofrontal cortex, and lateral prefrontal cortex was not consistent across assessments. Accordingly, the main analyses focused on the bilateral insula and dACC. On each assessment, the CV of the chosen gamble was significantly associated with BOLD responses in the insula and dACC, such that choosing riskier gambles was related to higher BOLD responses in the insula and dACC (Fig. 1C). This finding is consistent with the robust literature implicating the insular cortex and dACC as key regions involved in risk processing (see meta-analysis by Mohr et al., 2010; Platt and Huettel, 2008; Schonberg et al., 2011). Eigenvariate values were extracted for the left and the right insula and dACC using a 6 mm sphere around the peak voxel coordinates of each region (for coordinates see Supplementary Tables S2−5). We created a neural risk processing composite by averaging the scores of the eigenvariate values for bilateral insula and dACC, with higher scores indicating higher BOLD responses. Because confirmatory factor analysis models with two indicators have to impose equality constraints on the two factor loadings, thus equal weighting (see Little, Lindengerger, and Nesselroade, 1999), confirmatory factor analyses testing measurement models were not needed. Correlations between bilateral insula and dACC were .90 at Time 1, .82 at Time 2, .71 at Time 3, and .76 at Time 4 (all ps < .001).
2.2. Neural activation during cognitive control
At each time point, the GLM indicated a significant interference effect on BOLD responses (see Fig. 2B), as reported in Kim-Spoon et al. (2019). Our longitudinal model showed significant changes in the interference effect on BOLD responses in cognitive control regions identified by the MSIT across four time points (Kim-Spoon et al., 2019). The SwE derived map of time-related changes in BOLD was used to identify nine clusters of interest for an ROI analysis, including bilateral insula, bilateral middle frontal gyrus (MFG), left pre-supplementary motor area (pSMA), left rostral anterior cingulate cortex (rACC), left inferior parietal lobule (IPL), right precuneus, and left middle occipital gyrus (see Fig. 2C; for coordinates for peak regions within each time point, see Supplementary Tables S2−5). From each time-point, the first eigenvariate values in the interference minus neutral contrast was obtained, after adjusting for an F-contrast of the effect of interest.
Based on a series of confirmatory factor analyses of neural activation during cognitive control (see Kim-Spoon et al., 2020 for details), we calculated a neural cognitive control composite by averaging across seven indicators (left and right insula, left and right MFG, left pSMA, left IPL, and right precuneus) that significantly loaded on the same latent factor. We labeled the composite ‘fronto-parietal’ activation because those seven ROIs are located in the fronto-parietal network that is involved in cognitive control (Dosenbach et al., 2008; Sebastian et al., 2013).
2.3. Univariate growth curve modeling of Insula-dACC activation during risk processing and fronto-parietal activation during cognitive control
Three separate models were fit to determine the shape of the trajectories of insula-dACC activation during risk processing and fronto-parietal activation during cognitive control, respectively (see Table 2). In order to determine the most parsimonious model, we tested the univariate growth curve models two ways: with residuals free to vary and with residuals fixed to be equal across time.
Table 2.
Model Fit for Univariate Growth Models of Insula-dACC Activation during Risk Processing and Fronto-parietal Activation during Cognitive Control.
| Model Label | χ2 | df | p | RMSEA | CFI | Comparison | Δχ2 | Δdf | p(d) |
|---|---|---|---|---|---|---|---|---|---|
| Insula-dACC Activation during Risk Processing | |||||||||
|
224.71 | 9 | .000 | 0.38 | .00 | ||||
|
30.41 | 6 | .000 | 0.16 | .63 | 1 vs 2 | 194.30 | 3 | .000 |
|
2.73 | 4 | .604 | 0.00 | 1.00 | 2 vs 3 | 27.68 | 2 | .000 |
| Fronto-parietal Activation during Cognitive Control | |||||||||
| 1. No growth model | 97.93 | 8 | .000 | 0.27 | .00 | ||||
| 2. Linear growth model | 20.36 | 5 | .001 | 0.14 | .61 | 1 vs 2 | 77.57 | 3 | .000 |
| 3. Latent basis growth model | 4.15 | 3 | .245 | 0.05 | .97 | 2 vs 3 | 16.21 | 2 | .000 |
Notes. dACC = dorsolateral anterior cingulate cortex; RMSEA = root mean square error of approximation; CFI = comparative-fit index; Δχ2 = difference in likelihood ratio tests; Δdf = difference in df; p(d) = probability of the difference tests. The best-fitting models are in boldface.
In the risk processing models, we used the composite of bilateral insula and dACC activation. The univariate growth curve models with residuals constrained to be equal across time produced poor model fit. Freeing residuals to vary across time produced acceptable model fit. The latent basis growth model provided the best fit to the data compared to the no growth and linear growth models (see Table 2). In these models, a small, non-significant negative residual variance (σ2 < -0.001) was fixed to zero. The means of the intercept (M = 0.84, SE = .09, p < .001) and slope (M = 0.80, SE = .09, p < .001) were positive and significant, indicating significant increases over time. The variance of the intercept (σ2 = 0.49, SE = .12, p < .001) and slope (σ2 = 0.46, SE = .11, p < .001) were also significant, indicating significant individual differences in levels and change in risk processing. The intercept and slope factors covaried with each other (cov = .47, SE = .12, p < .001).
In the cognitive control models, we used the fronto-parietal activation composite. The univariate growth curve models with residuals constrained to be equal across time produced acceptable model fit and also were more parsimonious than the models with freed residuals. These models included correlations between residuals at adjacent time points. As shown in Table 2, the latent basis growth model provided the best fit. The mean of the slope was significant (M = -0.21, SE = .02, p < .001), whereas the variance was not significant (σ2 = .01, SE = .01, p = .442). Thus, the result suggested significant decreases over time with non-significant individual differences in change rates. The mean of the intercept was significantly different from zero (M = 0.26, SE = .02, p < .001) and there were significant individual differences in levels (σ2 = 0.01, SE = .00, p = .041). The intercept and slope factors did not covary with each other (cov = -.00, SE = .01, p = .595).
2.4. Maltreatment predicting neurodevelopment during adolescence
To examine how maltreatment experiences are related to adolescent brain development involved in risk processing and cognitive control, we tested two models: The cumulative maltreatment model using a single composite of maltreatment, and the dimensional model using two composites of abuse and neglect. In these models, we ran the analyses both with N = 167 (entire sample) and N = 126 (participants who had data on maltreatment), and the results were highly consistent. Therefore, we reported the findings using N = 167.
2.4.1. Cumulative vs. Dimensional models of maltreatment predicting neurodevelopment of risk processing
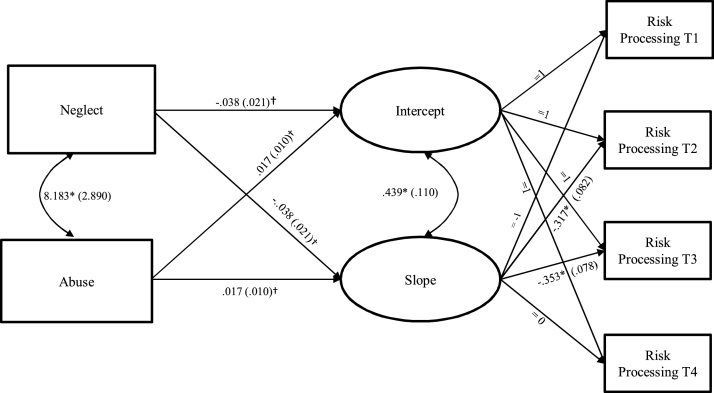
For the cumulative model in which the maltreatment composite predicted growth factors (intercept and slope) of insula-dACC activation during risk processing, the model fit was acceptable (χ2 = 3.19, df = 6, p = .784, RMSEA = 0.00, CFI = 1.00), but maltreatment did not significantly predict the intercept (b = 0.00, SE = .01, p = .756; β = .03) or the slope (b = 0.00, SE = .01, p = .754; β = .04). Next, the dimensional model in which neglect and abuse simultaneously predicted growth factors of insula-dACC activation during risk processing yielded acceptable model fit (χ2 = 3.50, df = 8, p = .899, RMSEA = 0.00, CFI = 1.00). As seen in Fig. 3, the effects of neglect and abuse on the intercept and slope were approaching significance, and replication is warranted. Nevertheless, it is noteworthy that higher levels of neglect were related to lower levels of insula-dACC activation at Time 4 (b = -0.04, SE = .02, p = .061; β = -.22) and smaller increases in insula-dACC activation over time (b = -0.04, SE = .02, p = .061; β = -.23). The effects of abuse were in the opposite direction (b = 0.02, SE = .01, p = .104; β = .19 for the intercept and b = 0.02, SE = .01, p = .100, β = .19 for the slope) and indicated that higher levels of abuse were related to higher levels of insula-dACC activation at Time 4 and larger increases in insula-dACC activation over time.
Fig. 3.
Growth Curve Model of Insula-dACC Activation during Risk Processing Predicted by Neglect and Abuse.
Notes. dACC = dorsolateral anterior cingulate cortex; EN = emotional neglect; PN = physical neglect; PA = physical abuse; SA = sexual abuse; VA = verbal abuse; and NVA = non-verbal abuse; T1 = Time 1; T2 = Time 2; T3 = Time 3; T4 = Time 4. For clarity of presentation, residuals are not shown. Unstandardized estimates (standard errors) are presented. “=” indicates fixed parameters.
* p ≤ .05; † p ≤ .10.
2.4.2. Cumulative vs. Dimensional models of maltreatment predicting neurodevelopment of cognitive control
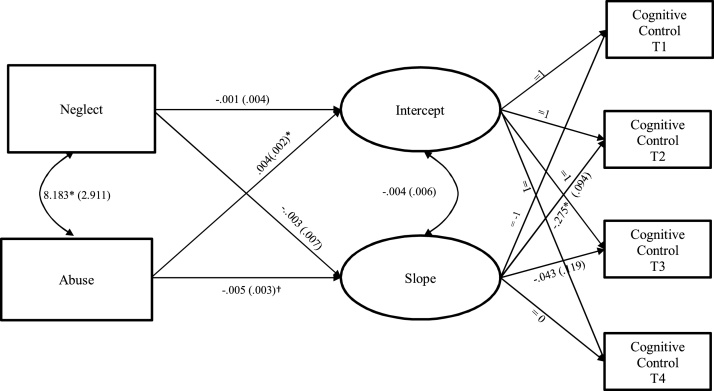
The cumulative model in which the single composite of maltreatment predicted growth factors of fronto-parietal activation during cognitive control yielded acceptable model fit (χ2 = 8.84, df = 5, p = .116, RMSEA = 0.07, CFI = .92). Maltreatment significantly predicted the intercept (b = -0.00, SE = .00, p = .045; β = -.26) and slope (b = -0.01, SE = .00, p = .047; β = -.41). The results indicated that higher levels of maltreatment were associated with lower levels of fronto-parietal activation at Time 4 and larger decreases in fronto-parietal activation over time. The dimensional model in which fronto-parietal activation growth factors were predicted by neglect and abuse separately yielded acceptable model fit (χ2 = 9.18, df = 7, p = .240, RMSEA = 0.04, CFI = .95). As seen in Fig. 4, examination of parameter estimates revealed that abuse was responsible for the significant effects of cumulative maltreatment both for the intercept (b = -0.00, SE = .00, p = .053, β = -.26) and slope (b = -0.01, SE = .00, p = .099, β = -.38) rather than neglect. Thus, the result indicated that higher levels of abuse were associated with steeper decreases in fronto-parietal activation over time. The effects of neglect were not significant for the intercept (b =- 0.00, SE = 0.00, p = .905, β = -.02) or for the slope (b = -0.00, SE = .01, p = .686, β = -.10).
Fig. 4.
Growth Curve Model of Fronto-parietal Activation during Cognitive Control Predicted by Neglect and Abuse.
Notes. EN = emotional neglect; PN = physical neglect; PA = physical abuse; SA = sexual abuse; VA = verbal abuse; and NVA = non-verbal abuse; T1 = Time 1; T2 = Time 2; T3 = Time 3; T4 = Time 4. For clarity of presentation, residuals and correlations between residuals are not shown. Unstandardized estimates (standard errors) are presented. “=” indicates fixed parameters.
* p ≤ .05; † p ≤ .10.
3. Discussion
We examined whether two core dimensions of child maltreatment—abuse versus neglect—are differentially associated with neurodevelopmental trajectories of risk-related decision making throughout adolescence. Certain theoretical views posit that different forms of childhood adversity (such as poverty, insensitive caregiving, and violence exposure) are similar enough in their effects to combine them into one category (Sapolsky, 2017; Smith and Pollak, 2020) that nevertheless can bring forth many different types of psychiatric disorders (Caspi et al., 2014). A competing view is that qualitatively distinct adverse experiences, such as threat and deprivation, are likely to have distinct sequelae in brain development (McLaughlin and Sheridan, 2016). In the current study, we compared a cumulative model in which a single composite of maltreatment (i.e., combination of abuse and neglect) predicted growth trajectories of neural processes, and a dimensional model in which two separate composites of abuse and neglect simultaneously predicted growth trajectories of neural processes. Our data provided evidence that abuse and neglect may be differentially related to developmental changes in neural processes of risky decision making. Specifically, neglect was a more prominent predictor for developmental changes in insula-dACC activation during risk processing, whereas abuse was a more prominent predictor for developmental changes in fronto-parietal activation during cognitive control. Thus, the results supported the view that there are differential effects of deprivation (i.e., neglect) and threat (i.e., abuse) on neurobiological development (McLaughlin and Sheridan, 2016; Sheridan and McLaughlin, 2014).
Our univariate growth curve modeling results showed that neural activation during risk processing in the insula and dACC increased from ages 13–17 years. Behaviorally, risk-preference on the lottery choice task decreased from ages 13–17 years in the current sample (Asscheman et al., 2020). Although longitudinal studies on the development of risk processing in the insula and dACC are lacking, prior cross-sectional research discovered that adults showed higher insula and dACC activation compared to adolescents and children during risky decisions (Paulsen et al., 2012). Our data further indicated that adolescents who experienced higher levels of neglect may exhibit slower increases in neural activation in the bilateral insula and dACC during risk processing (although this was marginally significant, so replication is warranted). The finding is consistent with prior research indicating that adolescents with higher childhood stress showed lower activation in the insula during the anticipation of potential losses, whereas adolescents with lower childhood stress showed greater insular activation when presented with cues signaling potential losses (Birn et al., 2017). These findings imply that early life stress, such as child neglect, negatively affects the development of risk-related neural processes that allow for avoidance of risk by attending to the magnitude of potential negative consequences.
The association between neglect and insula-dACC activation during risk processing is in line with the theoretical perspective positing that disruptions in early learning account for pervasive consequences of neglect on neurodevelopment (McLaughlin et al., 2016). Our data suggest that, for adolescents with more experiences of neglect, a lack of learning opportunities may contribute to neural insensitivity during the valuation of risk information. Neglect may constrain basic forms of learning which lay a foundation for risk valuation, because those basic learning processes depend on cognitive stimulation and social inputs provided through caregiver interactions (e.g., Sheridan et al., 2017). In addition, neglect may limit opportunities to learn cognitive skills related to risk evaluation via parental modeling. For example, prior research demonstrated a link between higher parental monitoring and greater risk sensitivity in the insular cortex (Lauharatanahirun et al., 2018). It is expected that adolescents with neglectful parents are less likely to engage in parent-child interactions where parents draw their adolescent’s attention to potentially risky outcomes and model these learning processes as encoded by the insular cortex.
In contrast to neglect, higher levels of abuse were associated with faster growth in neural activation during risk processing shown in the insula and dACC. Although this association between abuse and neural activation during risk processing was approaching statistical significance and replication of the finding is warranted, we note that our finding of the positive association between abuse and faster growth in insula-dACC activation during risk processing supports the stress acceleration hypothesis (Callaghan and Tottenham, 2016) which proposes that threat experience is related to accelerated brain maturation. Our finding is also in line with prior research demonstrating greater activation in the dACC during regulation of negative emotional stimuli among adolescents who experienced abuse (McLaughlin et al., 2015) and greater insular activation in response to emotional stimuli among pre-adolescents and early adolescents who experienced threat (i.e., physical and sexual abuse), compared to their counterparts without such adverse experiences (McCrory et al., 2011; McLaughlin et al., 2015).
Turning from risk processing to cognitive control, we found that the cumulative maltreatment composite (combining abuse and neglect subscales) was associated with steeper developmental decreases in fronto-parietal activation during cognitive control as well as lower levels of activation at Time 4. This finding clarifies prior cross-sectional findings that document differences in brain activation during inhibitory control tasks between adolescents with versus without maltreatment experiences (Bruce et al., 2013) by demonstrating that such differences reflect stress acceleration in maltreated adolescents. Further, testing the relative effects of abuse and neglect revealed that the effects were driven by abuse. Fronto-parietal activation decreased as adolescents’ behavioral cognitive control improved from ages 13–17 years in the current sample (Kim-Spoon et al., 2020). The observed decreases in fronto-parietal activation are consistent with prior research demonstrating age-related decreases in brain activation during cognitive control, reflecting more efficient neural functioning with development (Crone and Steinbeis, 2017; Luna et al., 2010). The association between abuse and steeper decreases in fronto-parietal activation supports the stress acceleration hypothesis (Callaghan and Tottenham, 2016). Our finding extends previous findings from a cross-sectional neuroimaging study reporting that abuse, but not neglect, was related to lower activation in brain regions involved in response control among adolescents (Blair et al., 2019). Taken together, the findings of abuse on neural cognitive control are consistent with the prior finding demonstrating that early experiences of threat, but not deprivation, are associated with accelerated biological aging (Sumner et al., 2019).
Comparing the dimensional model (examining abuse and neglect separately) to the cumulative model (abuse and neglect combined), our findings suggest that cumulative approaches may be accompanied by missed opportunities. First, although the effects of cumulative maltreatment on insula-dACC activation during risk processing were not significant, when considering abuse and neglect separately, detrimental effects of neglect on neurodevelopment of risk processing emerged. Second, we found opposing effects of abuse and neglect (albeit these effects were not statistically significant): neglect was negatively related, but abuse positively related, to greater insula-dACC activation during risk processing. These opposing effects may explain the non-significant effects of cumulative maltreatment on neural risk processing. In addition, by examining the relative effects of abuse and neglect, we obtained a more nuanced understanding of maltreatment experiences such that the observed effects of cumulative maltreatment on fronto-parietal activation during cognitive control were driven by abuse rather than neglect. Finally, it was abuse, but not neglect, that manifested stress acceleration effects on brain development related to both risk processing and cognitive control.
As such, our findings support the dimensional approach, in which distinct underlying dimensions and sequelae of maltreatment are represented by threat versus deprivation (McLaughlin and Sheridan, 2016; Sheridan and McLaughlin, 2014). Consistent with our findings, Lambert et al. (2016) reported that cumulative risk scores obscure specificity in the associations of violence exposure (threat) and poverty (deprivation) when predicting cognitive control and emotional regulation. Given evidence that maltreated youths experience multiple subtypes of abuse and neglect (Manly et al., 2001), and that abuse and neglect sometimes show opposite effects on brain structure (e.g., amygdala volume; Teicher and Samson, 2016), the current results underscore the importance of examining co-occurring forms of maltreatment simultaneously, but as separate variables, to evaluate whether they show distinct associations with neural and behavioral outcomes.
The contributions of the current study should be considered in light of several limitations. First, although this study used prospective longitudinal data, because of its correlational nature the detected significant effects should not be interpreted as causal in nature. Second, child maltreatment was assessed using retrospective self-reports on the MACE. Although we used the MACE with 18- to 19-year-olds whose ages were close enough to childhood to provide as reliable recall as possible, retrospective self-reports could have been affected by recall bias. However, scientific studies and reviews indicate that the concern about unreliability of retrospective reports is rather exaggerated: there is no clear link between current psychiatric or mood status and less reliable or less valid recall of early experiences (Brewin et al., 1993). Furthermore, retrospective self-reports of maltreatment are verifiable (Chu et al., 1999) and are related to poor health and behavior outcomes regardless of concordance with official records (Negriff et al., 2017). Finally, the current study focused on examining how maltreatment experiences were related to neural activation of the valuation and the control systems. Given the evidence that the interplay between the valuation and the control systems contributes to risky behaviors (e.g., Kim-Spoon et al., 2017), investigating how between-system connectivity may be influenced by maltreatment experiences is a fruitful direction for future research.
These limitations notwithstanding, the current study had notable strengths. Our large community sample offered a more nuanced understanding of the maltreatment effects on brain development, compared to the majority of prior human neuroimaging research that compared relatively small numbers of healthy controls to maltreated individuals in clinical samples (which often have confounding psychiatric disorders such as post-traumatic stress disorder). Thus, our findings elucidate the effects of normative variability in adverse experiences on brain functioning underlying risky decision making. Additionally, our sample included adolescents from an Appalachian region of southwestern Virginia, which includes understudied rural communities that face unique challenges such as relative low income, geographical isolation, and limited prosocial recreational opportunities. Youths from these rural communities show relatively higher incidences of health risk behaviors (e.g., Moreland et al., 2013), providing implications for preventive intervention efforts. Methodologically, we used a novel approach to modeling individual differences in within-person changes in brain activation variables based on multivariate repeated measures of fMRI data.
In closing, the current investigation advances the literature on the link between distinct forms of maltreatment and neurodevelopment underlying risk-related decision making (i.e., the valuation and the control systems). We evaluated how child maltreatment collectively—or abuse and neglect differentially—contribute to brain development during adolescence, using longitudinal data of child maltreatment spanning from age 1 through age 17 and brain functioning spanning from age 13 through age 17. Most importantly, this investigation represents the first evidence suggesting that, at the neurobiological level, distinct types of child adversity (abuse and neglect) are differentially related to adolescents’ developmental trajectories of insula-dACC activation during risk processing and fronto-parietal activation during cognitive control. Our findings highlight the effects that adversity can have on the brain and that can be distinguished when measuring qualitatively different experiences engendered by threat (e.g., abuse) versus deprivation (e.g., neglect) as well as different brain regions and functions that are targeted by such experiences.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any agency of the U.S. government.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse (R01 DA036017 to Jungmeen Kim-Spoon and Brooks King-Casas and F31 DA042594 to Nina Lauharatanahirun). We thank the former and current JK Lifespan Development Lab members for their help with data collection. We are grateful to the adolescents and parents who participated in our study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100939.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Asscheman J.S., Deater-Deckard K., Lauharatanahirun N., van Lier P.A.C., Koot S., King-Casas B., Kim-Spoon J. Associations between peer attachment and neural correlates of risk processing across adolescence. Dev. Cogn. Neurosci. 2020;42 doi: 10.1016/j.dcn.2020.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Roeber B.J., Pollak S.D. Early childhood stress exposure, reward pathways, and adult decision making. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2017;114:13549–13554. doi: 10.1073/pnas.1708791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Aloi J., Crum K., Meffert H., White S.F., Taylor B.K., Blair R.J. Association of different types of childhood maltreatment with emotional responding and response control among youths. JAMA Network Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4604. e194604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C.R., Andrews B., Gotlib I.H. Psychopathology and early experience: A reappraisal of retrospective reports. Psychol. Bull. 1993;113:82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Browne M.W., Cudeck R. Alternative ways of assessing model fit. In: Bollen K., Long S., editors. Testing Structural Equation Models. Sage; Beverly Hills, CA: 1993. pp. 136–162. [Google Scholar]
- Bruce J., Fisher P.A., Graham A.M., Moore Iii W.E., Peake S.J., Mannering A.M. Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Developmental Psychopathology. 2013;4:931–941. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Shin L.M., Holmes J., Rosen B.R., Vogt B.A. The Multi-Source Interference Task: Validation study with fMRI in individual subjects. Mol. Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts R.M., Belsky D.W., Goldman-Mellor S.J., Harrington H., Israel S., Moffitt T.E. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.A., Frey L.M., Ganzel B.L., Matthews J.A. Memories of childhood abuse: Dissociation, amnesia, and corroboration. Am. J. Psychiatry. 1999;156:749–755. doi: 10.1176/ajp.156.5.749. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Steinbeis N. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn. Sci. (Regul. Ed.) 2017;21:205–215. doi: 10.1016/j.tics.2017.01.003. [DOI] [PubMed] [Google Scholar]
- d’Acremont M., Bossaerts P. Neurobiological studies of risk assessment: A comparison of expected utility and mean-variance approaches. Cogn. Affect. Behav. Neurosci. 2008;8:363–374. doi: 10.3758/CABN.8.4.363. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Holmes A.J., Birk J.L., Brooks N., Lyons-Ruth K., Pizzagalli D.A. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. (Regul. Ed.) 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin M., Puetz V., Blair R., White S., Sethi A., Hoffmann F., McCrory E. A neurocomputational investigation of reinforcement-based decision making as a candidate latent vulnerability mechanism in maltreated children. Dev. Psychopathol. 2017;29:1689–1705. doi: 10.1017/S095457941700133X. [DOI] [PubMed] [Google Scholar]
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. NeuroImage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. Efficient experimental design for fMRI. In: Penny W., Friston K., Ashburner J., Keibel S., Nichols T., editors. Statistical Parametric Mapping. Elsevier; 2007. (1st ed., pp. 193–210). [DOI] [Google Scholar]
- Holt C.A., Laury S.K. Risk aversion and incentive effects. Am. Econ. Rev. 2002;92:1644–1655. doi: 10.1257/000282802762024700. [DOI] [Google Scholar]
- Hughes K., Bellis M.A., Hardcastle K.A., Sethi D., Butchart A., Mikton C., Dunne M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health. 2017;2 doi: 10.1016/S2468-2667(17)30118-4. e356-e366. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J., Kahn R.E., Lauharatanahirun N., Deater-Deckard K., Bickel W.K., Chiu P.H., King-Casas B. Executive functioning and substance use in adolescence: Neurobiological and behavioral perspectives. Neuropsychologia. 2017;100:79–92. doi: 10.1016/j.neuropsychologia.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Deater-Deckard K., Brieant A., Lauharatanahirun N., Lee J., King-Casas B. Brains of a feather flocking together? Peer and individual neurobehavioral risks for substance use across adolescence. Dev. Psychopathol. 2019;31:1661–1674. doi: 10.1017/S0954579419001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Herd T., Brieant A., Elder J., Lee J., Deater-Deckard K., King-Casas B. 2020. Longitudinal Neuroimaing Study of Cognitive Control Using Latent Growth Modeling: Developmental Changes and Brain-behavior Association . Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H.K., King K.M., Monahan K.C., McLaughlin K.A. Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev. Psychopathol. 2016;29:929–940. doi: 10.1017/s0954579416000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauharatanahirun N., Maciejewski D., Holmes C., Deater-Deckard K., Kim-Spoon J., King-Casas B. Neural correlates of risk processing among adolescents: Influences of parental monitoring and household chaos. Child Dev. 2018;89:784–796. doi: 10.1111/cdev.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hart H., Mehta M.A., Simmons A., Mirza K., Rubia K. Neural correlates of error processing in young people with a history of severe childhood abuse: An fMRI study. Am. J. Psychiatry. 2015;172:892–900. doi: 10.1176/appi.ajp.2015.14081042. [DOI] [PubMed] [Google Scholar]
- Little T.D., Lindenberger U., Nesselroade J.R. On selecting indicators for multivariate measurement and modeling with latent variables: When“ good” indicators are bad and“ bad” indicators are good. Psychol. Methods. 1999;4:192–211. [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly J.T., Kim J.E., Rogosch F.A., Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Dev. Psychopathol. 2001;13:759–782. [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., Mechelli A., Bird G., Kelly P.A., Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: A dimensional approach to childhood adversity. Curr. Dir. Psychol. Sci. 2016;25:239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Nelson C.A. Neglect as a violation of species-expectant experience: Neurodevelopmental consequences. Biol. Psychiatry. 2016;82:462–471. doi: 10.1016/j.biopsych.2017.02.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr P.N.C., Biele G., Heekeren H.R. Neural processing of risk. J. Neurosci. 2010;30:6613–6619. doi: 10.1523/jneurosci.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland J.J., Raup-Krieger J.L., Hecht M.L., Miller-Day M.M. The conceptualization and communication of risk among rural appalachian adolescents. J. Health Commun. 2013;18:668–685. doi: 10.1080/10810730.2012.743620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., Peloso E., Mandell D., Leibenluft E., Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L.K. and Muthén, B.O. (1998-2017). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén.
- Negriff S., Schneiderman J.U., Trickett P.K. Concordance between self-reported childhood maltreatment versus case record reviews for child welfare-affiliated adolescents: Prevalence rates and associations with outcomes. Child Maltreat. 2017;22:34–44. doi: 10.1177/1077559516674596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Gabard-Durnam L.J. Early adversity and critical periods: Neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020;43:133–143. doi: 10.1016/j.tins.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D., Carter R.M., Platt M., Huettel S., Brannon E. Neurocognitive development of risk aversion from early childhood to adulthood. Front. Hum. Neurosci. 2012;5 doi: 10.3389/fnhum.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt M.L., Huettel S.A. Risky business: The neuroeconomics of decision making under uncertainty. Nat. Neurosci. 2008;11:398. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Penguin Press.; New York: 2017. Behave: The Biology of Humans at Our Best and Worst. [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T., Fox C.R., Poldrack R.A. Mind the gap: Bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci. (Regul. Ed.) 2011;15:11–19. doi: 10.1016/j.tics.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A., Pohl M.F., Klöppel S., Feige B., Lange T., Stahl C., Tüscher O. Disentangling common and specific neural subprocesses of response inhibition. NeuroImage. 2013;64:601–615. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Sheridan M., McLaughlin K. Dimensions of early experience and neural development: Deprivation and threat. Trends Cogn. Sci. (Regul. Ed.) 2014;18:580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., Peverill M., Finn A.S., McLaughlin K.A. Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev. Psychopathol. 2017;29:1777–1794. doi: 10.1017/S0954579417001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 2020:1–27. doi: 10.1177/1745691620920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J.A., Colich N.L., Uddin M., Armstrong D., McLaughlin K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry. 2019;85:268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Parigger A. The’ Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10:414–423. doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual research review: Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E.U., Shafir S., Blais A.R. Predicting risk sensitivity in humans and lower animals: Risk as variance or coefficient of variation. Psychol. Rev. 2004;111:430. doi: 10.1037/0033-295X.111.2.430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.