Abstract
Background
The Bacillus Calmette-Guérin (BCG), the only vaccine against tuberculosis (TB) currently in use, has shown beneficial effects against unrelated infections and to enhance immune responses to vaccines. However, there is little evidence regarding the influence of BCG vaccination on pertussis.
Methods
Here, we studied the ability of BCG to improve the immune responses to diphtheria, tetanus, and acellular (DTaP) or whole-cell pertussis (DTwP) vaccination in a mouse model. We included MTBVAC, an experimental live-attenuated vaccine derived from Mycobacterium tuberculosis, in our studies to explore if it presents similar heterologous immunity as BCG. Furthermore, we explored the potential effect of routine BCG vaccination on pertussis incidence worldwide.
Findings
We found that both BCG and MTBVAC when administered before DTaP, triggered Th1 immune responses against diphtheria, tetanus, and pertussis in mice. Immunization with DTaP alone failed to trigger a Th1 response, as measured by the production of IFN-γ. Humoral responses against DTaP antigens were also enhanced by previous immunization with BCG or MTBVAC. Furthermore, exploration of human epidemiological data showed that pertussis incidence was 10-fold lower in countries that use DTaP and BCG compared to countries that use only DTaP.
Interpretation
BCG vaccination may have a beneficial impact on the protection against pertussis conferred by DTaP. Further randomized controlled trials are needed to properly define the impact of BCG on pertussis incidence in a controlled setting. This could be a major finding that would support changes in immunization policies.
Funding
This work was supported by the Ministry of “Economía y Competitividad”; European Commission H2020 program, “Gobierno de Aragón”; CIBERES; “Fundação Butantan”; Instituto de Salud Carlos III and “Fondo FEDER”.
Keywords: Live-tuberculosis vaccines, Heterologous immunity, Non-specific effects, DTP, BCG, MTBVAC
Research in context.
Evidence before this study
We searched PubMed using the following terms: “vaccine interference”, “BCG non-specific”, “BCG heterologous”, “BCG potentiates”, “BCG DTP”, “pertussis tuberculosis”, “diphtheria tuberculosis”, “tetanus tuberculosis” and “MTBVAC”. We included “Similar Articles” suggested by PubMed when we found articles that matched the scope of our study. The search started in August 2014, before we designed the mouse experiments, continued during the development of the experimental and then epidemiological studies and, finished in July 2020. Additional data were identified by searching published articles and reviews, and relevant books in the field (Plotkin's Vaccines, 7th Edition, 2018). Particularly relevant was the review published by Zimmermann and Curtis in Expert Rev Vaccines, 2018. They reported 8 studies on BCG effects on infant vaccination without finding statistical significance. Nevertheless, the majority of the studies found enhanced antibody levels in the BCG vaccinated group.
In the epidemiological study, we searched WHO web pages as mentioned in Methods. Of special relevance was the article published by Castro et al. in Clin Infect Dis. 2015, where they reported that BCG vaccination at birth could decrease hospitalization due to non-tuberculosis respiratory infections.
Added-value of this study
Some randomized controlled trials have demonstrated that BCG can potentiate immunity to other vaccines. However, this study provides for the first time epidemiological evidence that BCG may aid DTaP vaccine in the control of pertussis worldwide.
Implications of all the available evidence
The association of BCG and DTaP vaccination with lower pertussis incidence is an outstanding finding that, if confirmed by randomized controlled trials, can be valuable for the policy of immunization programs and an important point in the development of new tuberculosis vaccines.
Alt-text: Unlabelled box
1. Introduction
Vaccines work through the induction of specific immunological memory that promotes a lymphocyte response upon subsequent infection by a related pathogen. Some vaccines have also been shown to protect against unrelated pathogens and to reduce all-cause childhood mortality [1]. These non-specific effects of vaccines were first described in 1931 for the live-attenuated vaccine against tuberculosis (TB), the Bacillus Calmette-Guérin (BCG). A reduction in childhood mortality after BCG vaccination was observed, which could not be explained by the prevention of TB alone [2]. Despite all these pieces of evidence, without an immunological mechanism, the concept of non-specific effects of vaccines has often been received with scepticism. The World Health Organization (WHO) reported that the available evidence is based on very heterogeneous experimental designs [3]. Therefore, the Strategic Advisory Group of Experts on Immunization (SAGE) recommended further research of non-specific effects on BCG, and has proposed that the Immunization and Vaccines related Implementation Research Advisory Committee generate appropriate research questions and adequate studies [4,5]. Recently, in vitro animal and human studies have revealed “trained immunity/innate immune memory” as a conceivable biological mechanism, responsible for the non-specific effects of BCG. This special trait refers to the ability of innate immune cells (e.g. macrophages, monocytes, and NK cells) to be trained by vaccination with BCG through epigenetic and metabolic reprogramming. As a consequence, these cells become more responsive when exposed to unrelated pathogens [6]. In addition to trained innate immunity, BCG also induces long-lasting enhancement of adaptive immune responses [7,8]. This latter mechanism has been widely associated with the ability of BCG to enhance the immune response to other vaccines in general [9]. Previous vaccination with BCG has been demonstrated to enhance the pro-inflammatory and antibody response against the influenza virus vaccine [10]. A recent review evaluating the influence of BCG on the response to other vaccines found that BCG vaccination is associated with improved vaccine responses in five of the eight studies [11]. The reason for this variability remains unknown, although the authors mention some plausible explanations such as the BCG strain used [12] or the optimal timing of administration [13].
Moreover, BCG provides only limited protection against pulmonary TB in adolescents and adults, the most common form of the disease, and responsible for TB transmission [14]. Therefore, new improved anti-TB vaccines are needed. MTBVAC is a promising TB-vaccine candidate based on a live-attenuated Mycobacterium tuberculosis strain that contains major antigens absent in BCG, such as ESAT6 and CFP10 [15,16]. MTBVAC was attenuated by two independent unmarked deletions in the phoP and fadD26 virulence genes, and protection against TB challenge was demonstrated in mice, guinea pigs, and rhesus macaques [15,17]. It has completed Phase I clinical trials for safety and immunogenicity in healthy adults in Switzerland [18] and new-borns in South Africa [19]. MTBVAC is now in Phase IIa dose-finding trials in both adolescents and new-borns in South Africa (NCT02933281 and NCT03536117). As MTBVAC targets TB prevention in new-borns [20], it is important to demonstrate that the non-specific beneficial effects of BCG vaccination are maintained by MTBVAC [21].
The current vaccines against Bordetella pertussis infection have shown limitations in the protection of infants [22]. Whole-cell pertussis vaccines (wP) are reactogenic, limiting their acceptability and use beyond childhood [23]. Acellular vaccines (aP) have an improved safety profile but provide only relatively short-term protection, likely because of skewing of the immune response towards a Th2 profile [24], whereas wP vaccines favor Th1 immune responses, similar to natural infection [25]. A pertussis vaccination approach that combines the safety profile of aP with immunity induced by natural infection would thus be desirable. One approach would be the use of live-attenuated pertussis vaccines, such as BPZE1, currently in clinical development (NCT03541499) [26]. An alternative or complementary approach could make use of the non-specific effects of BCG [7,8] or in the future MTBVAC, to enhance the desired immune responses to pertussis vaccines.
Therefore, we examined here whether prior immunization with BCG or MTBVAC is associated with improved immune responses to diphtheria-tetanus-pertussis (DTP) vaccination, focusing particularly on pertussis Th1 and Th2 immune responses. Mice were vaccinated with BCG or MTBVAC, followed by immunizations with either DTaP or DTwP, and humoral and cellular immune responses were evaluated. In parallel, we assessed whether the global pertussis incidence varies according to the systematic vaccination with BCG and DTaP.
2. Methods
2.1. Ecological epidemiologic studies
To examine whether prior immunization with BCG is associated with improved pertussis control, an ecological epidemiologic study comparing pertussis incidence between countries that include BCG vaccination at birth in their national immunization programs (NIP) with those that do not, was performed.
The ecological epidemiologic study from 192 countries worldwide used epidemiological data from 2012 to 2017 obtained from the WHO (https://www.who.int/es), with population and socioeconomic data from the World Development Indicators webpage (https://datacatalog.worldbank.org/dataset/world-development-indicators) and BCG vaccination policies from the BCG Atlas website (http://www.bcgatlas.org). DTwP/DTaP information were extracted from the WHO (https://www.who.int/es) [27]. From these countries, 131 were analyzed after exclusion of those without BCG vaccine status [18], countries with missing data [10], with no pertussis incidence data [33], and those without information about DTwP/DTaP vaccination [4]. Yearly incidence rates of pertussis were calculated for each country considering the average rates for the studied period to obtain robust estimations. Income levels were taken into account and used to categorize countries in “low or lower-middle-income” (LIC) and “upper-middle or high income” (UIC) as a possible confounding variable. We also took into account the timing of the first BCG dose and the BCG vaccination coverage for countries with available data (http://www.bcgatlas.org).
Mumps and measles incidence rates were considered as control pathologies. Of 174 countries with BCG vaccination status reported, 59 were considered for mumps (115 countries with zero rates); and 116 were considered for measles (58 countries with zero rates).
Vaccination policy switches in European countries (Fig. S4) were obtained from the European centre for Disease Prevention and Control (ECDC) [28]. When the information was not available in the ECDC repository it was obtained from the corresponding national repository, for which a URL link is available on the ECDC website. Countries were selected for the analyses based on the availability of BCG, DTwP and DTaP vaccination policy data.
An additional analysis was performed considering only European countries with a double objective: 1) to replicate the potential effect of BCG vaccination on pertussis incidence, and 2) to assess whether the use of DTwP or DTaP could have any significant differential impact on disease incidence.
2.2. Bacterial culture conditions and vaccine preparation
MTBVAC [15] and BCG Pasteur 1173P [29] were cultured in Middlebrook 7H9 broth (BD) enriched with 10% (v/v) albumin, dextrose, catalase (ADC; Difco) and 0.05% (v/v) Tween-80 (Sigma) at 37 °C. Bacteria were harvested by centrifugation, suspended in Phosphate buffered saline (PBS) containing 5%glycerol, and frozen at −80 °C until use. Bacterial suspensions for vaccination were prepared in PBS from glycerol stocks and the corresponding viability was evaluated by plating serial dilutions onto Middlebrook 7H10 agar plates supplemented with 10% ADC and 0.5% glycerol.
The whole-cell DTwP vaccine was kindly provided by the Immunobiological section of the Butantan Institute. Each dose of DTwP is a mixture of up to 30 Lf of diphtheria toxoid, 25 Lf of tetanus toxoid, and 16 O.U. of whole-cell pertussis (wP) per dose adsorbed on aluminum hydroxide.
The acellular DTaP vaccine (Boostrix) was kindly provided by Manuel Mendez and Inmaculada Cuesta from Dirección General de Salud Pública, Gobierno de Aragón (Zaragoza, Spain). DTaP is a mixture of up to 2.5 Lf of diphtheria toxoid, 5 Lf of Tetanus toxoid, 8 μg of pertussis toxoid (PT), 8 μg of filamentous hemagglutinin, and 2.5 μg of pertactin per dose adsorbed in aluminum hydroxide and aluminum phosphate.
2.3. Animal strains, ethics, and facilities
The ARRIVE guidelines were followed for animal studies. To test DTwP, 5–7 week-old female BALB/c mice supplied by the Breeding Facility of the Butantan Institute were separated into groups of five animals to evaluate immune responses. They were kept under appropriate conditions during the study according to the Animal Care and Ethics Committee of the Butantan Institute under protocol 1253/14.
To test the DTaP, 5–7 week-old female BALB/c mice purchased from Javier Labs were separated into groups of five animals. They were maintained in the regulated “Centro de Investigaciones Biomédicas de Aragón” (CIBA, Zaragoza, Spain) facilities with reference number ES 50 297 0012 011. The procedures were carried out under Project Licences 50/14 approved by the Ethics Committee for Animal Experiments from the University of Zaragoza. The care and use of animals were performed according to the Spanish Policy for Animal Protection RD53/2013, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
2.4. Mouse immunization and procedures
Groups of 5 female BALB/c mice were immunized subcutaneously with a single dose of 100 μL containing 106 CFU of BCG or MTBVAC, or saline control (phosphate-buffered saline). DTwP was diluted to 0.8 and DTaP to 1/5 of the human dose, as previously described [30]. Vaccine formulation is described in Supplementary Material. Three doses of DTwP or DTaP, in a final volume of 100 μL, were administered subcutaneously in two-week intervals (Fig. 1a). We followed this vaccination schedule with the intent to adapt to mice the human WHO recommendations for infants [31]. A similar design was used in a subsequent experiment using a single dose of DTaP instead of three (Fig. 1b). The first dose of DTP (or saline control) was administered two weeks after saline, BCG, or MTBVAC immunization.
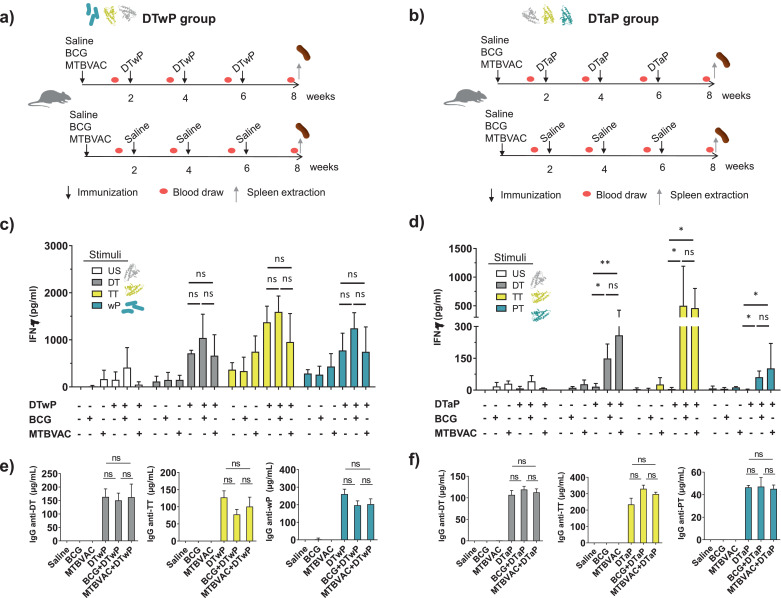
Fig. 1.
Effect of BCG or MTBVAC vaccination on the Th1 responses and IgG production induced by DTwP and DTaP vaccines. Experimental design. (a) Groups of ten BALB/c mice were immunized subcutaneously with 106 CFU of BCG, MTBVAC, or saline. Two weeks later Diphtheria-Tetanus-Pertussis vaccine (whole-cell DTwP (a) or acellular DTaP (b)) was administered subcutaneously to five mice in each group. Two additional DTwP or DTaP doses were given at two weeks intervals. Finally, eight weeks after BCG or MTBVAC immunization, total IgG against DTP antigens were measured and splenocytes were stimulated with DTP antigens to evaluate specific cytokine production. Two independent experiments were carried out, one in which DTwP was used and the other using DTaP. (c, d) IFN-γ production was measured upon stimulation of splenocytes with DT (Diphtheria Toxoid), TT (Tetanus toxoid), wP (Whole-Pertussis inactivated (c)), PT (Pertussis Toxoid (d)) or left unstimulated (US). (e, f) Eight weeks after BCG or MTBVAC immunization, and two weeks after the third DTP immunization, total IgG against DTP antigens were measured. Two independent experiments were carried out, N = 5 mice per group per condition. The groups that received BCG, MTBVAC, DTwP, and/or DTaP immunization are indicated with (+), and the absence of immunization is indicated with (-). Mann-Whitney U test (c, d) or unpaired t-test (e, f) P-values are indicated: * P-value < 0·05, ** P-value < 0·01. ns means statistically not significant.
2.5. Cellular immune response evaluation
Two weeks after the third dose of the DTP vaccine, mice were euthanized by carbon dioxide inhalation and the spleens were collected (Fig. 1a). Spleens were homogenized in a Wheaton tissue grinder and erythrocytes were lysed with sterile distilled water (DTwP experiments) or with a gentle MACS Dissociator in Red blood cell lysing buffer (SIGMA) (DTaP experiments). In both cases, cell suspensions (~ 5 × 106 cells/mL) were cultured in RPMI-1640 (Gibco) supplemented with 10% fetal serum bovine (Invitrogen), polymyxin B (250 ng/mL) and 100 units/mL penicillin/100 mg/mL streptomycin (Sigma). Splenocytes were kept at 37 °C in 5% CO2 for 48 h under stimulation with heat-inactivated diphtheria toxin (DT, 1 µg/mL; Calbiochem), formaldehyde inactivated Tetanus toxoid (TT, 1 µg/mL; Calbiochem), heat-inactivated Pertussis Toxin (PT, 1 μg/mL; Calbiochem) or heat-killed whole-cell pertussis (wP, 106 CFU). Culture supernatants were collected and cytokine concentrations were measured by Enzyme-Linked ImmunoSorbent Assay (ELISA) according to the manufacturer's instructions (Peprotech for DTwP experiments or MabTECH for DTaP experiments).
2.6. Humoral immune response for DT, TT, PT, and wP
Serum was collected from immunized mice ten days after each immunization (Fig. 1a). In the experiment using a single dose of DTaP, mice were bled two and four weeks after DTaP immunization (Supplementary Fig. S1b). The specific IgG titers were determined by ELISA. Immunoplates were coated with inactivated DT (1 µg/mL), TT (1 µg/mL), PT (1 μg/mL) or wP (105 CFU/mL). Goat anti-mouse IgG (1:10,000; Cat. No.1036–01, Southern Biotech, AL, USA) was used as a secondary antibody and rabbit anti-goat IgG conjugated with horseradish peroxidase (1:20,000; Cat. No. 6160–05, Southern Biotech, AL, USA) as the tertiary antibody. Colorimetric development was performed using 3,3′,5,5′-tetramethylbenzidine (TMB, DTaP experiments; Abcam), or in citrate buffer with H2O2 and o-phenylenediamine dihydrochloride (OPD, DTwP experiments). Standard curves for the determination of IgG concentration were established using either the mouse reference serum from Bethyl Technologies (DTaP experiments) or recombinant IgG (DTwP experiments).
2.7. Statistical analysis
In all experimental studies, GraphPad Prism 5.0 software was used for representation and statistical analyses. Statistical significances are indicated in the Figures (Fig. 1, Fig. S1 and Fig. 2) by asterisks as follows *P-value < 0·05, **P-value < 0·01 or ***P-value < 0·001. Outliers in experimental data were detected by the application of Grubbs' test with a significance level of 0·05 and then excluded from the analysis. The normality of experimental data was evaluated by the Shapiro-Wilk test. The unpaired t-test was used to compare levels of total IgG in mouse serum between groups vaccinated with DTaP/DTwP alone and groups vaccinated also with MTBVAC/BCG (Fig.1e, Fig. 1f, and Fig. 2b). Cytokine levels did not pass the normality test. Therefore, the Mann-Whitney U test was used to analyze them. We compared IFN-γ and IL-17A production after PT, TT, DT or wP stimulation between groups vaccinated with DTaP/DTwP alone and groups vaccinated also with MTBVAC/BCG (Fig.1c, Fig. 1d and Fig. S1).
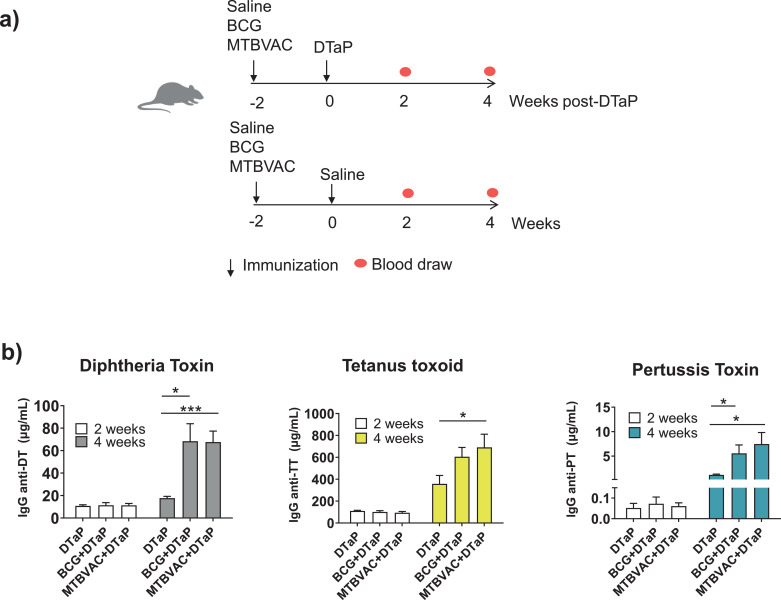
Fig. 2.
Effect of BCG or MTBVAC vaccination on IgG production after a single dose of DTaP. Experimental design. (a) Groups of ten BALB/c mice were immunized subcutaneously with 106 CFU of BCG, MTBVAC, or saline. Two weeks later Diphtheria-Tetanus-acellular Pertussis vaccine (DTaP) was administered subcutaneously to five mice in each group. (b) Two and four weeks after immunization with DTaP, specific IgG against DT, TT or PT were measured. Antibody titers in serum of immunized mice (c) were also analysed at four weeks post-DTaP immunization. OD450, Optical Density at 450 nm. N = 5 mice per group per condition. Unpaired t-test P-values are indicated: * P-value < 0·05, ** P-value < 0·01 and *** P-value < 0·001. DT (Diphtheria Toxoid), TT (Tetanus toxoid), PT (Pertussis Toxoid) US (unstimulated).
For the epidemiological studies, the Mann-Whitney U test was considered to assess differences in pertussis incidence rates (per 100,000 inhabitants) between countries according to vaccination status (BCG and DTaP/DTwP). This test was chosen to avoid the leverage effect of those countries with an extremely high incidence rate, which can affect drastically the normality of analyzed groups. We have also used Mann-Whitney U to analyze pertussis incidence rates between countries stratified by income levels to check if the different economic status of countries can affect the results (Fig. 3). Mumps and measles incidence rates were analyzed taking into account the same methodology (Fig. 4).
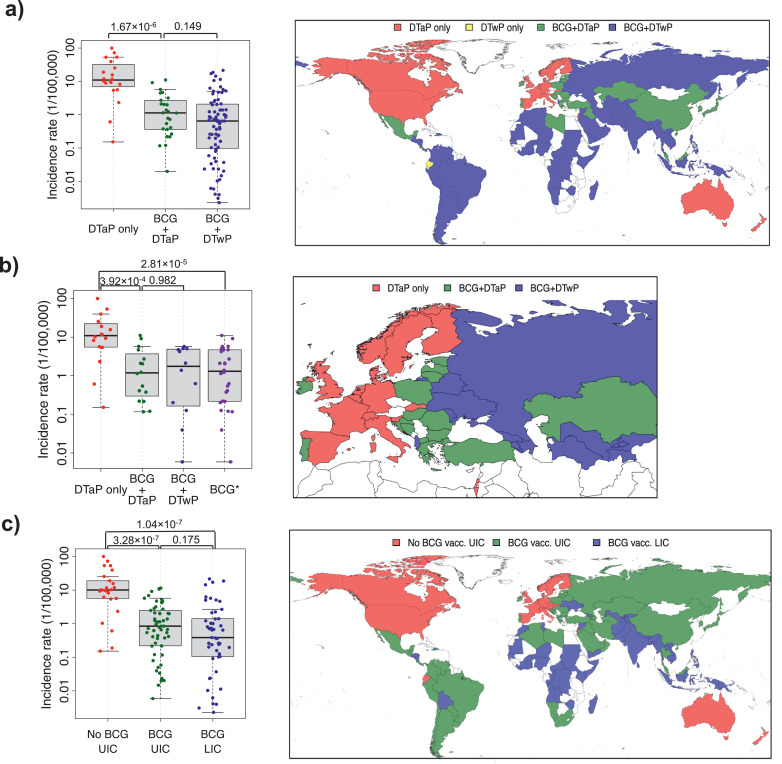
Fig. 3.
Pertussis incidence according to the use of BCG and the type of pertussis vaccine included in each country's national immunization program. (a) Worldwide pertussis incidence and (b) European incidence; or (c) pertussis incidence according to the income level. Adjacent maps show the vaccination status of each country analyzed in the corresponding graph. “DTaP only” includes countries where BCG is not part of their immunization program and DTaP is the pertussis vaccine used. “BCG+DTaP” includes all countries that use BCG and DTaP; and “BCG+DTwP” encompasses countries using BCG and DTwP. “BCG*” includes all the countries that use BCG irrespective of the type of pertussis vaccine used. LIC = Low or middle-lower income country. UIC = upper-middle or high-income country. Mann-Whitney U test P-values are displayed for the corresponding comparisons. DTwP (Diphtheria-Tetanus-whole-cell Pertussis vaccine), DTaP (Diphtheria-Tetanus-acellular Pertussis vaccine).
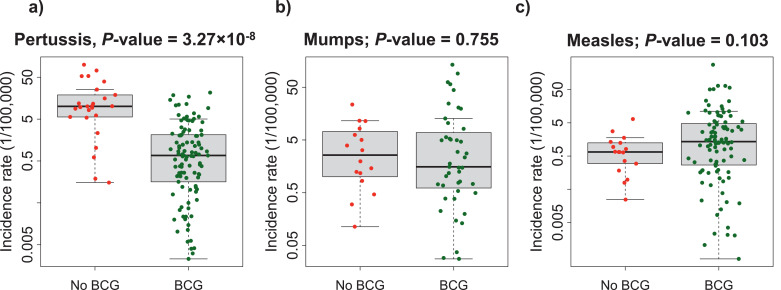
Fig. 4.
Worldwide pertussis, mumps and measles incidence, and BCG vaccination. Average pertussis (a), mumps (b), and measles (c) worldwide rates, classified by the use or not of BCG vaccine. Mann-Whitney U test P-values are displayed for the corresponding comparisons.
Association between BCG vaccination and pertussis incidence according to the timing of dose administration (at birth or later after birth) was evaluated by using the Mann-Whitney U test. In addition, we determined whether the possible effect of the BCG vaccine on pertussis incidence could be explained by BCG vaccination coverage. For this purpose, we considered ρ Spearman's coefficient to study the correlation between BCG vaccination coverage and the pertussis incidence for each country. We also have stratified this analysis by the DTaP/DTwP vaccination status (Fig. S5, Table S1).
The unpaired t-test was used to compare the three-dose DTaP vaccination coverage in a particular year between European countries that use or not BCG (Fig. S3)
To avoid underestimation of incidence rates for the different analyses, countries with zero incidence data for the different pathologies were excluded from the analyses, since this can potentially be associated with small population sizes (for instance, Barbados, Seychelles, or the Solomon Islands), or lack of data (for instance, Egypt, Mali, Mongolia), or even ineffective health reporting systems.
We considered 0·05 as the significance level. The statistical results and Figures related to the epidemiologic study have been obtained using R 3.4.2 for MAC OS X, [32], and specifically, maps were created using the R package maptools.
The sample size in the animal experiments was not calculated by statistical methods but was determined based on previous experience and publications [15,16,30]. The sample size in epidemiological studies was due to the data available in the World Health Organization website database [27].
No randomization specific methodology was applied to this study. Results from this study were not blinded for analysis.
2.8. Role of funding source
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
3. Results
3.1. BCG and MTBVAC enhance Th1 responses to DTAP antigens
To determine the influence of BCG or MTBVAC vaccination on DTP-specific cytokine responses in mice, groups were immunized with BCG, MTBVAC, or received saline and were then immunized with three consecutive doses of DTwP, DTaP, or saline (Fig. 1a and Fig. 1b). Two weeks after the last DTP dose, splenocytes were recovered and stimulated with DT, TT, PT, or with wP when DTwP was administered. Mice receiving DTwP showed comparable production of IFN-γ in stimulated splenocytes, independently of prior administration of BCG, MTBVAC, or saline (Fig. 1c). In contrast, for DTaP, previous vaccination with BCG or MTBVAC significantly enhanced IFN-γ production in splenocytes upon stimulation with DT, TT, or PT (Fig. 1d). Mice immunized with DTaP alone failed to mount significant IFN-γ responses against DTaP antigens (Fig. 1d), as expected because of intrinsic properties of DTaP, which has been shown to mount a weak Th1 response compared to DTwP [33].
We also analyzed the IL-17A production as this has been shown to have implications in protection and immunity against pertussis [[33], [34], [35]]. However, after DTaP vaccination, the IL-17A levels remained at background levels, irrespective of prior BCG, or MTBVAC vaccination (Fig. S1a). On the other hand, for DTwP; BCG, or MTBVAC appeared to slightly increase the IL-17A response, although this reached statistical significance only for the BCG vaccination (Fig. S1b).
3.2. BCG and MTBVAC vaccination stimulates IgG production against DTP antigens
Protection against diphtheria and tetanus is mediated by specific IgG. For protection against pertussis, aP-induced IgG is also important [36,37]. Therefore, we examined the influence of BCG or MTBVAC vaccination on the IgG responses induced by DTwP or DTaP. After three doses of DTwP or DTaP, there was no significant effect of BCG or MTBVAC vaccination on the IgG responses to DTP antigens (Fig. 1e and Fig. 1f). The kinetics of serum IgG production after each dose of DTP was also the same irrespective of previous immunization with BCG or MTBVAC (Fig. S2). However, when mice were immunized with a single dose of DTaP (Fig. 2a), prior BCG or MTBVAC vaccination resulted in a significant enhancement of the IgG responses to all three antigens, which lasted up to 4 weeks after DTaP immunization compared to mice that did not receive BCG or MTBVAC (Fig. 2b). Two weeks after DTaP vaccination, no significant IgG-DTP differences were observed between groups that received or not MTBVAC or BCG (Fig. 2b). This effect on DTaP-IgG responses after a single DTaP dose could have a clinical impact further explained in the Discussion section.
3.3. BCG vaccination impact on pertussis prevention worldwide
We next wondered whether the influence elicited by attenuated TB vaccines on DTaP immunogenicity observed in mice may be translated to protection against pertussis in humans. We, therefore, performed an extensive ecological analysis on the coverage of BCG and DTP vaccination in humans [38] and on the incidence of pertussis [39]. The worldwide incidence of pertussis was assessed taking into account the type of pertussis vaccine used (DTaP or DTwP); 26 countries use both BCG and DTaP vaccination (BCG+DTaP), 79 use BCG vaccination and DTwP (BCG+DTwP), 20 use only DTaP and 2 use only DTwP. The latter two countries were excluded from the analysis due to the small size of the group. In the BCG+DTaP countries, the pertussis incidence was 10-fold lower than in the DTaP countries (Fig. 3a; Table 1). No significant differences were observed in pertussis incidence between BCG+DTaP and BCG+DTwP countries (Fig. 3a; Table1).
Table 1.
Descriptive statistics for pertussis, mumps or measles incidence rates (per 100,000 inhabitants) according to the different stratifications for the different analyses performed for ecological study (Figs. 3 and 4). For each category number of countries, median, 1st-quantile (Q1), 3rd-quantile (Q3), range of values, and P-value is indicated. DTwP = Diphtheria-Tetanus-whole-cell Pertussis vaccine. DTaP = Diphtheria-Tetanus-acellular Pertussis vaccine. BCG = Bacillus Calmette-Guérin. LIC = Low or middle-lower income country. UIC = upper-middle or high-income country.* Number of countries with BCG differs in one country for which there is no information regarding DTaP/DTwP vaccines.** Number of countries without BCG differs in three countries for which there is no information regarding DTaP/DTwP vaccines.
| N° of countries | Median incidence rate | Q1-Q3 incidence rate | Range incidence rate | P-value | |
| Worldwide pertussis analysis | |||||
| BCG+DTaP* | 26 | 1·13 | 0·37–2·56 | 0·02–11·04 | vs. only DTaP 1·67 × 10−6 |
| BCG+DTwP* | 79 | 0·65 | 0·10–2·08 | 0·00–21·51 | vs. BCG+DTaP 0·149 |
| Only DTaP** | 20 | 10·99 | 7·57–28·95 | 0·15–99·66 | |
| Only DTwP** | 2 | 0·61 | 0·40–0·82 | 0·19–1·03 | |
| European pertussis analysis | |||||
| BCG+DTaP | 16 | 1·18 | 0·34–3·20 | 0·12–11·04 | vs. BCG+DTwP 0·982 |
| BCG+DTwP | 12 | 1·75 | 0·18–4·49 | 0·01–5·72 | |
| BCG | 28 | 1·31 | 0·22–4·67 | 0·01–11·04 | vs. only DTaP 2·81 × 10−5 |
| Only DTaP | 16 | 10·93 | 5·57–20·55 | 0·15–99·66 | vs BCG+DTaP 3·92 × 10−4 |
| Worldwide pertussis analysis considering income levels | |||||
| BCG UIC | 57 | 0.85 | 0·22–2·48 | 0·01–11·50 | vs. no BCG UIC 3·28 × 10−7 |
| BCG LIC | 49 | 0.39 | 0·11–1·41 | 0·00–21·51 | vs. BCG UIC 0·175 |
| No BCG UIC | 25 | 10·08 | 5·62–18·93 | 0·15–99·66 | vs. BCG LIC 1·04 × 10−7 |
| Worldwide pertussis analysis | |||||
| BCG* | 106 | 0·68 | 0·17–2·12 | 0·00–21·51 | 3·27 × 10–8 |
| No BCG** | 25 | 10·08 | 5·62–18·39 | 0·15–99·66 | |
| Worldwide mumps analysis | |||||
| BCG | 43 | 1·55 | 0·61–6·90 | 0·03–134·03 | 0·755 |
| No BCG | 16 | 2·59 | 1·09–6·74 | 0·11–23.53 | |
| Worldwide measles analysis | |||||
| BCG | 99 | 1·36 | 0·27–4·75 | 0·00–280·69 | 0·103 |
| No BCG | 17 | 0·66 | 0·30–1·25 | 0·02–6·50 | |
An additional analysis restricted to 44 countries of the European region included 16 only DTaP, 12 BCG+DTwP, and 16 BCG+DTaP countries. Similarly to the worldwide analysis, European countries using BCG showed lower pertussis incidences (Fig. 3b; Table1). This difference was also significant considering only countries that use DTaP (Fig. 3b; Table1). No difference in pertussis incidence was found between BCG+DTaP and BCG+DTwP countries (Fig. 3b; Table1).
We did not find evidence of an influence of pertussis vaccine coverage in our analyses since European BCG+DTaP countries and DTaP countries showed a similar degree of coverage, more than 90% (Fig. S3). Moreover, we did a descriptive analysis of the evolution in the number of pertussis cases in individual European countries. Interestingly, those countries that still maintain good BCG coverage in their immunization schedule show a trend to have fewer pertussis cases than countries where BCG vaccination ceased. In line with this observation, we also noticed an increase in pertussis cases after a period of BCG shortage in specific countries (Fig. S4).
We have considered other variables related to BCG vaccination to account for potential confounder effects. The time when BCG is administered could influence the overall mortality and affect immunogenicity to unrelated pathogens [40,41]. To address this factor, we compared countries in which the first BCG dose is administered at birth (89 countries) with those in which BCG is given later (15 countries). We did not observe any influence for countries with the DTaP vaccine, with the DTwP vaccine, or with DTaP/DTwP vaccine (Table S1; Fig. S5a).
We also considered the BCG vaccination coverage as a potential confounding variable (data available for 45 countries), but this did not find a significant correlation with pertussis incidence (r2 = −0·07, P-value = 0·816), neither for DTaP countries, nor for DTwP countries (r2 = −0·01, P-value = 0·954); nor (r2 = −0·01, P-value = 0·960) for all considered countries. This lack of significance is likely due to the small variability of BCG vaccination coverage, which is generally very high (median [IQR]: 95·0 [86·9–98·0]) (Fig. S5b).
3.4. Income level does not appear to modify the BCG effect on pertussis incidence
In order to assess the potential role of income level in the pertussis incidence, we compared 45 low or middle-lower income countries (LIC) with 82 upper-middle or high-income countries (UIC). All the 49 LIC countries used BCG vaccination, while only 57 out of 82 UIC had BCG vaccine included in their NIP before DTP. The pertussis incidence was higher in UIC countries not using BCG compared to either UIC countries using BCG, or LIC countries. No difference was found in pertussis incidence between LIC and UIC countries using BCG (Fig. 3; Table1).
3.5. Prior BCG vaccination does not influence measles and mumps incidence
To ensure we are comparing disease patterns rather than surveillance capacity, we considered mumps and measles as control outcomes. We could not use tetanus and diphtheria as controls because these diseases are near elimination in most countries [39], and their incidence is too low to provide meaningful data. Worldwide mumps incidence rates for 59 countries (of which 43 used neonatal BCG vaccination during the study period) and worldwide measles incidence rates for 116 countries (of which 99 used neonatal BCG vaccination during the study period) were compared. The analyses showed that while pertussis incidence rates were significantly higher in countries without a BCG vaccination program, BCG vaccination did not affect mumps nor measles incidence (Fig. 4; Table1).
4. Discussion
We provide mouse experimental and human ecological epidemiologic evidence suggesting that BCG vaccination may have a positive impact on vaccine-induced immunity against pertussis. To our knowledge, no previous epidemiological study has assessed the correlation between BCG vaccination and pertussis incidence. Our experimental animal model showed that both live-attenuated TB vaccines, BCG and MTBVAC, stimulate cellular Th1 immune responses and early induction of humoral immune responses to DTaP. This heterologous effect of BCG expands the beneficial properties of the vaccine beyond its application against TB and may have implications on the policy of its current use if our ecological findings can be confirmed by randomized controlled studies.
The main hypotheses to explain BCG non-specific effects are 1) BCG-induced trained innate immunity/innate immune memory and/or 2) enhancement of heterologous cellular and humoral responses [42]. The precise molecular mechanisms for the observations reported here are still under investigation, but the general hypothesis is that BCG can induce a long-lasting and enhanced Th1-like response with increased pro-inflammatory cytokine production after immunization with unrelated antigens [42]. We focused our studies on deciphering the influence of BCG on DTP-induced humoral and cellular immune responses.
In our experimental study, DTwP alone was sufficient to induce IFN-γ responses and no additional effect was observed in mice by prior immunization with BCG or MTBVAC. In contrast, when DTaP was used, prior immunization with BCG or MTBVAC allowed the induction of IFN-γ responses, which was not seen when DTaP was used alone. Whole-cell vaccines against pertussis, which have been shown to protect better than acellular pertussis vaccines, induce mixed Th1 and Th17 type immune responses, similar to what is seen after natural infection, whereas the acellular vaccines induce Th2 and Th17 but weak Th1 responses in mice [33]. Findings in humans are consistent with the data from animal models in that DTaP induces Th2 responses, while natural infection and DTwP induce Th1/Th17 responses [43]. Similar improved immune effects increasing Th1 response were shown when DTaP was used with an adjuvant derived from Seabuckthorn [44] or with outer membrane vesicles [45] instead of the current alum-based adjuvant. DTaP immunity potentiation by BCG or MTBVAC brings the immune response closer to that elicited by wP and may induce better protective immunity against B. pertussis infection [46,47].
Regarding the production of IgG to DTaP antigens, results in mice immunized with one dose of DTaP confirmed that BCG or MTBVAC increases specific IgG responses to unrelated vaccines, as the IgG response was enhanced four weeks after DTaP administration by prior BCG or MTBVAC vaccination. Limited studies have analyzed changes elicited by BCG on serological and cellular immune responses to DTP vaccines in humans after one or three doses [11,48]. Of these, the vast majority analyzed the BCG influence on DTP-IgG without finding statistical differences. Our results show that after three doses of DTwP or DTaP, IgG levels in the serum of immunized mice were similar, independent of previous use of BCG or MTBVAC. However, an extensive and very recent study found that neonatal BCG vaccination consistently increases IgG against DT and TT, being statistically significant for DT after 1 month of the third dose of DTaP [49]. Additionally, using BCG before one dose of influenza A (H1N1) vaccine significantly increased the IgG production against H1N1 [10].
The vaccination schedule of DTaP recommended by WHO [31] includes at least three doses to obtain optimal protection. The first dose is recommended at 6 weeks and the second and third doses, one and two months later, respectively. This implies a minimum of 14 weeks, during which newborns may be more susceptible to infection. In fact, infants younger than 1 year are estimated to represent more than half of all deaths caused by pertussis [50]. The fact that BCG and MTBVAC vaccination increases the levels of anti-DT, anti-TT, and anti-PT antibodies after a single dose of DTaP may therefore be more meaningful than increasing antibody responses after the third dose. Even if the effect were transient and similar to the protection of the newborn induced through maternal immunization with pertussis vaccines [51], it might be clinically relevant considering that this age represents the most vulnerable time-frame for the infant and when most of the cases occur.
Our ecological epidemiologic data suggest that countries maintaining BCG vaccination have lower pertussis incidence than those that interrupted its use. One limitation of our study is the lack of a DTwP only (in the absence of BCG) control group. Since there are not enough countries using DTwP without BCG in their immunization schedules (only 2), we have not been able to determine whether BCG enhances DTwP-induced protection. Our study has the limitations inherent to ecological epidemiologic studies: it is not possible to fully control the exposure and the pertussis outcome. This implies the possible existence of confounder effects that may affect the incidence of pertussis. We have considered analysis including the most plausible variables that could affect pertussis incidences, such as country income level or timing of BCG vaccine. The great value of this study is that worldwide data were analyzed; however, this may become a limitation due to the difficulty of obtaining homogenous and standardized data from so many different sources. Nevertheless, the specific effect of BCG on DTaP-induced protection against pertussis was controlled by the lack of such an effect on other childhood diseases such as mumps and measles. Interestingly, vaccines against these two diseases are also live-attenuated vaccines, and it is, therefore, possible that BCG may have a lower effect on disease incidences for which live-attenuated vaccines are widely used.
Factors such as the socio-economical level, gender, age, pertussis detection, and reporting rates, vaccine coverage, and circulating strain differences can influence the incidence of pertussis. We have found no influence on pertussis incidence by the most obvious factors: the income level (Fig. 3c) which is an important socioeconomic marker for the health system of each country and the DTaP coverage that is very high in all cases (Fig. S3). Regarding the pertussis detection, pertussis reporting rates, and the circulating strains we have minimized their influence by restricting the period of the analysis to five years. By doing so we minimize important changes in health policies or circulating strains. We have also performed an additional more restricted sub-analysis, limited to European countries (Fig. 3b), where the health policies, gender, age, and income levels are more homogeneous than globally. Furthermore, we have developed an additional analysis taking into account BCG vaccination coverage and the timing of administration of the first BCG dose for countries with available data. We did not observe significant differences according to BCG vaccination coverage, because the range of vaccination coverage is extremely narrow (median [IQR]: 95·0% [86·9%−98·0%]), nor did we see an influence of the time of BCG vaccination. Therefore, the extremely high statistical significance (P-value = 1·67 × 10−6, Fig. 3a) obtained between countries that use or do not use BCG, in combination with the consideration of possible confounding variables strongly suggests an association between BCG and DTaP vaccination and pertussis incidence reduction.
BCG has been associated with a reduction in hospitalization due to pulmonary infections [6]. A plausible contributing effect could be a reduction in hospitalizations caused by the respiratory pathogen B. pertussis. Our studies suggest that the BCG-related reduction of pertussis incidence may be due to the induction of cellular and early IgG responses against PT. To confirm this hypothesis, randomized controlled trials in children vaccinated with DTaP with or without prior BCG should be carried out, perhaps with special attention focused on the immune responses after the first vaccine dose.
A concern regarding the replacement of BCG by new generation live tuberculosis vaccines is whether they will maintain the non-specific beneficial effects seen with the BCG vaccination. Notably, of the 12 candidates in clinical trials in the TB vaccine pipeline, MTBVAC together with the BCG-derived VPM1002 are the only mycobacterial live-attenuated vaccines that aim to replace BCG, whereas the other candidates are based on subunit protein-adjuvant, viral vector vaccines, or inactivated whole-cell mycobacteria designed as BCG boosting strategies [52]. Of note, we have recently demonstrated that MTBVAC can induce heterologous protection against a lethal challenge with Streptococcus pneumoniae via the generation of trained immunity through the induction of glycolysis and glutaminolysis and the accumulation of histone methylation marks at the promoters of proinflammatory genes in human monocytes [53], which would suggest that MTBVAC is at least as efficient as BCG to trigger non-specific immunity. Additionally, our results here confirm that MTBVAC, like BCG, is associated with improved cellular and humoral immune responses to DTaP antigens in mice. This study might support further progress of MTBVAC clinical development into non-specific trials with EPI vaccines.
Altogether, our results suggest that BCG potentiates the DTaP vaccine and may help DTaP to improve the control of pertussis. Clinical trials will help to clarify the mechanism of these observations. BCG vaccination could be a rapid, accessible, and affordable way to enhance immunity, especially for infants that have not completed the immunization schedule with DTaP. If our findings can be confirmed by randomized controlled trials, they should be taken into account by vaccine policymakers that contemplate dropping neonatal BCG vaccination in countries in which DTaP is in use. Furthermore, if BCG were to be replaced in the future by other anti-TB vaccines, special attention should be paid to the impact of BCG cessation on incidences of diseases such as pertussis and potentially other infectious diseases, especially respiratory tract infections [[54], [55], [56]].
Glossary
Adjuvant - is a pharmacological or immunological agent that improves the immune response of a vaccine.
BCG – Bacille Calmette Guérin – current live attenuated vaccine against Tuberculosis based on attenuated Mycobacterium bovis
Confounding factor - is a variable that influences both the dependent variable and the independent variable, causing a spurious association.
DTP – Vaccine against Diphtheria, Tetanus, and Pertussis (whooping cough).
DTaP - Vaccine against Diphtheria, Tetanus, and Pertussis, including the acellular Pertussis vaccine.
DTwP - Vaccine against Diphtheria, Tetanus, and Pertussis, including the whole-cell Pertussis vaccine.
EPI - The Expanded Programme on Immunization of the WHO, which includes vaccines against diphtheria, whooping cough, tetanus, measles, poliomyelitis, and tuberculosis
Incidence - in epidemiology is a measure of the probability of occurrence of a given medical condition in a population within a specified period.
Live attenuated – live organisms with lower pathogenicity than the native strain
Low or lower-middle-income (LIC) – low/middle gross national income (GNI) per capita; the low dollar value of a country's final income in a year, divided by its population
MTBVAC – a new live attenuated vaccine obtained by phoP and fadD26 gene deletions in a strain of M. tuberculosis. This vaccine was constructed in a clinical isolate from lineage 4, the most distributed in Europe, America and Africa.
The national immunization program (NIP) - is the organizational component of Ministries of Health charged with preventing disease, disability, and death from vaccine-preventable diseases in children and adults.
Non-specific effects/ Heterologous effect – protective effects other than those on the targeted disease.
Ecological epidemiologic study - analyzes an specific outcome, namely at the population or group level, rather than individual level.
Trained immunity/innate immune memory - describes the long-term functional reprogramming of innate immune cells, which is evoked by exogenous or endogenous insults and which leads to an altered response towards a second challenge after the return to a non-activated state. Immune memory of the innate immune system and involves the epigenetic programming of myeloid lineage cells.
Upper-middle or high income (UIC) – middle/high gross national income (GNI) per capita; the high dollar value of a country's final income in a year, divided by its population
Vaccine coverage - refers to the proportion of a population that is appropriately immunized against a specific vaccine-preventable disease.
WHO recommendations – World Health Organization guidelines that can impact health policies or clinical interventions.
Whole-cell vaccines – contain the killed pathogen.
Contributors
Dr. Esther Broset and Dr. Jacobo Pardo-Seco had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conceptualization, E.B., J.G-A, C.M., L.C.C.L., and F.M.T; Methodology, N.A., J.G-A., A.I.K., and J.P-S.; Investigation, E.B., J.P-S., A.I.K., and A.I.D.; Writing – Original Draft, E.B., F.M.T., J.P-S, and A.I.K.; Writing–Review & Editing, J.G-A, C.M., L.C.C.L, C.L, A.I.D, I.R.C, and F.M.T.; Funding Acquisition, C.M., L.C.C.L and F.M.T.; Supervision, E.B., A.I.K., N.A., J.G-A., L.C.C.L., C.M., and F.M.T. All authors reviewed the final report.
Declaration of Competing Interests
Drr. Gonzalo-Asensio reports grants from Spanish Ministry of Science, Innovation and Universities, Grant numbers: PID2019–104690RB-I00 and BFU2015–72,190-EXP, during the conduct of the study; other from Biofabri, SL (Porriño, Spain), outside the submitted work. In addition, Dr. Gonzalo-Asensio has a patent "Tuberculosis Vaccine” PCT/ES2007/070,051 issued, and a patent "Compositions for use as a prophylactic agent to those at risk of infection of tuberculosis, or as secondary agents for treating infected tuberculosis patients" PCT/EP2019/054,106 issued.
Dr. Aguilo reports grants from Spanish Ministry of Science, Innovation and Universities, Grant number: RTC2018–097,625-B-100, during the conduct of the study; other from Biofabri, SL (Porriño, Spain), outside the submitted work. In addition, Dr. Aguilo has a patent "Compositions for use as a prophylactic agent to those at risk of infection of tuberculosis, or as secondary agents for treating infected tuberculosis patients" PCT/EP2019/054,106 issued.
Dr. Rivero Calle reports personal fees from GSK, Pfizer, Sanofi Pasteur and MSD for taking part in advisory boards and expert meetings outside the scope of the submitted work; and honoraria paid to her institution as sub-investigator in RCTs for GSK, Pfizer, Sanofi Pasteur, MSD, Sequirus, Janssen, Ablynx, Regeneron, Roche, Abbott, Novavax and MedImmune outside the submitted work.
Dr. MARTINON-TORRES reports other from Ablynx, grants and other from Jansen, personal fees, non-financial support and other from GSK, other from Regeneron, other from Medimmune, personal fees, non-financial support and other from Pfizer, grants, personal fees, non-financial support and other from MSD, personal fees and other from Sanofi Pasteur, personal fees and other from Novavax, grants from Astra Zeneca, other from Novartis, personal fees, non-financial support and other from Seqirus, other from Roche, other from Abbott, other from Instituto de Salud Carlos III, personal fees from Biofabri, outside the submitted work. In the case of Instituto de Salud Carlos III, “other” means “receives support for research activities”. In the case of, Ablynx, Jansen, GSK, Regeneron, Medimmune, Pfizer, MSD, Sanofi Pasteur, Novavax, Novartis, Seqirus, Roche, and Abott, “other” means “trials fees paid to the institution”.
Dr. Dacosta Urbieta has nothing to disclose. Dr. Kanno has nothing to disclose. Dr. Leite has nothing to disclose. Dr. Locht has nothing to disclose. Dr. Broset has nothing to disclose. Dr. Pardo-Seco has nothing to disclose.
Acknowledgments
Acknowledgments
The authors acknowledge the Controle Imunobiológico section of Instituto Butantan and Dirección General de Salud Pública, Gobierno de Aragón (Zaragoza, Spain) for kindly providing DTwP and DTaP vaccines, respectively, and Scientific and Technical Services from Instituto Aragonés de Ciencias de la Salud and Universidad de Zaragoza for their assistance. FM-T research activities received support from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud, GePEM ISCIII/PI16/01478/Cofinanciado FEDER and project ReSVinext ISCIII/PI16/01569/Cofinanciado FEDER; Consellería de Sanidade, Xunta de Galicia (RHI07/2-intensificación actividad investigadora, PS09749 and 10PXIB918184PR), Instituto de Salud Carlos III (Intensificación de la actividad investigadora 2007–2012, PI16/01569), Fondo de Investigación Sanitaria (FIS; PI070069, PI1000540, PI1901090) del plan nacional de I + D + I and ‘fondos FEDER’, and 2016-PG071 Consolidación e Estructuración REDES 2016GI-1344 G3VIP (Grupo Gallego de Genética Vacunas Infecciones y Pediatría, ED341D R2016/021). E.B, N.A, J.G.A and C.M activities were supported by the Spanish Ministry of “Economía y Competitividad” [Grant Nos BFU2015-72190-EXP and BES-2012-052937 to E.B.]; the Spanish Ministry of “Ciencia, Innovación y Universidades” [Grant No. RTI2018-097625-B-I00]; European Commission H2020 program European Union European & Developing Countries Clinical Trials Partnership (EDCTP) RIA2016V-1637 and “Gobierno de Aragón-Fondo Europeo de Desarrollo Regional (FEDER) 2014–2020: Construyendo Europa Desde Aragón”. L.C.C.L and A.I.K research received support from “Fundação Butantan”.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103254.
Appendix. Supplementary materials
References
- 1.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. 2013/05/18. [DOI] [PubMed] [Google Scholar]
- 2.Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. 1931;24(11):1481–1490. doi: 10.1177/003591573102401109. 1931/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evidence based recommendations on non-specific effects of BCG, DTP-containing and measles-containing vaccines on mortality in children under 5 years of age Background paper for SAGE discussions [Internet]. [cited 2020 Jul 25]. Available from: https://www.who.int/immunization/sage/meetings/2014/april/1_NSE_Backgroundpaper_final.pdf?ua=1.
- 4.WHO | Weekly Epidemiological Record, 2 June 2017, vol. 92, 22 (pp. 301–320). WHO [Internet]. 2017 [cited 2020 Oct 26]; Available from: http://www.who.int/wer/2017/wer9222/en/.
- 5.WHO | Weekly Epidemiological Record, 23 February 2018, vol. 93, 08 (pp. 73–96). WHO [Internet]. 2018 [cited 2020 Oct 26]; Available from: http://www.who.int/wer/2018/wer9308/en/
- 6.Martinon-Torres F. Vesikari T, Van Damme P., editors. Pediatric vaccines and vaccinations: a European textbook [Internet] Springer International Publishing; Cham: 2017. Expected and unexpected effects of vaccination; pp. 3–12. Available from. [DOI] [Google Scholar]
- 7.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A.B., Jacobs C. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6(2):152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen K.J., Larsen N., Biering-Sorensen S., Andersen A., Eriksen H.B., Monteiro I. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis. 2015;211(6):956–967. doi: 10.1093/infdis/jiu508. 2014/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina N.L., Zimmermann P., Curtis N. The impact of vaccines on heterologous adaptive immunity. Clin Microbiol Infect [Internet] 2019;25(12):1484–1493. doi: 10.1016/j.cmi.2019.02.016. http://www.sciencedirect.com/science/article/pii/S1198743X19300825 Available from. [DOI] [PubMed] [Google Scholar]
- 10.Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. Dec. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann P., Curtis N. The influence of BCG on vaccine responses - a systematic review. Expert Rev Vaccines. 2018 doi: 10.1080/14760584.2018.1483727. 2018/06/09. [DOI] [PubMed] [Google Scholar]
- 12.Anderson E.J., Webb E.L., Mawa P.A., Kizza M., Lyadda N., Nampijja M. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine. 2012;30(12):2083–2089. doi: 10.1016/j.vaccine.2012.01.053. Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen T.N., Birk N.M., Smits G., Jeppesen D.L., Stensballe L.G., Netea M.G. Vol. 35. Vaccine; 2017. pp. 2084–2091. (Bacille calmette-guerin (BCG) vaccination at birth and antibody responses to childhood vaccines. a randomised clinical trial). Apr. [DOI] [PubMed] [Google Scholar]
- 14.Fine P.E. Variation in protection by BCG: implications of and for heterologous immunity. Lancet [Internet] 1995;346(8986):1339–1345. doi: 10.1016/s0140-6736(95)92348-9. http://www.ncbi.nlm.nih.gov/pubmed/7475776 Available from. [DOI] [PubMed] [Google Scholar]
- 15.Arbues A., Aguilo J.I., Gonzalo-Asensio J., Marinova D., Uranga S., Puentes E. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine [Internet] 2013;31(42):4867–4873. doi: 10.1016/j.vaccine.2013.07.051. http://www.ncbi.nlm.nih.gov/pubmed/23965219 Available from. [DOI] [PubMed] [Google Scholar]
- 16.Aguilo N., Gonzalo-Asensio J., Alvarez-Arguedas S., Marinova D., Gomez A.B., Uranga S. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat Commun [Internet] 2017;8:16085. doi: 10.1038/ncomms16085. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White A.D., Sibley L., Sarfas C., Morrison A., Gullick J., Clark S., et al. MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies. npj Vaccines [Internet]. 2021 Dec 1 [cited 2021 Feb 4];6(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33397991/. [DOI] [PMC free article] [PubMed]
- 18.Spertini F., Audran R., Chakour R., Karoui O., Steiner-Monard V., Thierry A.C. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. 2015;3(12):953–962. doi: 10.1016/S2213-2600(15)00435-X. 2015/11/26. [DOI] [PubMed] [Google Scholar]
- 19.Tameris M., Mearns H., Penn-Nicholson A., Gregg Y., Bilek N., Mabwe S. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double-blind dose-escalation trial. Lancet Respir Med. 2019;7(9):757–770. doi: 10.1016/S2213-2600(19)30251-6. Sep. [DOI] [PubMed] [Google Scholar]
- 20.Marinova D., Gonzalo-Asensio J., Aguilo N., Martin C . MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev Vaccines. 2017;16(6):565–576. doi: 10.1080/14760584.2017.1324303. 2017/04/28. [DOI] [PubMed] [Google Scholar]
- 21.Kleinnijenhuis J., van Crevel R., Netea M.G. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):29–35. doi: 10.1093/trstmh/tru168. Jan. [DOI] [PubMed] [Google Scholar]
- 22.Martinon-Torres F., Heininger U., Thomson A., Wirsing von Konig C.H. Controlling pertussis: how can we do it? A focus on immunization. Expert Rev Vaccines. 2018;17(4):289–297. doi: 10.1080/14760584.2018.1445530. Apr. [DOI] [PubMed] [Google Scholar]
- 23.Miller D., Madge N., Diamond J., Wadsworth J., Ross E. Pertussis immunisation and serious acute neurological illnesses in children. BMJ. 1993;307(6913):1171–1176. doi: 10.1136/bmj.307.6913.1171. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancroft T., Dillon M.B.C., da Silva Antunes R., Paul S., Peters B., Crotty S. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cell Immunol. 2016:35–43. doi: 10.1016/j.cellimm.2016.05.002. 304–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varney M.E., Boehm D.T., DeRoos K., Nowak E.S., Wong T.Y., Sen-Kilic E. Bordetella pertussis whole cell immunization, unlike acellular immunization, mimics naive infection by driving hematopoietic stem and progenitor cell expansion in mice. Front Immunol. 2018;9:2376. doi: 10.3389/fimmu.2018.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorstensson R., Trollfors B., Al-Tawil N., Jahnmatz M., Bergstrom J., Ljungman M. A phase I clinical study of a live attenuated Bordetella pertussis vaccine–BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE. 2014;9(1):e83449. doi: 10.1371/journal.pone.0083449. 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Immunization schedules by diseases [Internet]. [cited 2020 Jul 25]. Available from: https://apps.who.int/immunization_monitoring/globalsummary/diseases?dc%5Bt%5D=d&dc%5Bgivenfrom%5D=0&dc%5Bgivento%5D=23741&dc%5Br%5D% 5B%5D=EURO&dc%5Bd%5D%5B%5D=Pertussis&commit=Ok+with+the+selection.
- 28.Vaccine scheduler | ECDC [Internet]. [cited 2020 Jul 25]. Available from: https://vaccine-schedule.ecdc.europa.eu/.
- 29.Brosch R., Gordon S.V., Garnier T., Eiglmeier K., Frigui W., Valenti P. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A. 2007/03/21. 2007;104(13):5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nascimento I.P., Dias W.O., Mazzantini R.P., Miyaji E.N., Gamberini M., Quintilio W. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect Immun. 2000/08/19. 2000;68(9):4877–4883. doi: 10.1128/iai.68.9.4877-4883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World health organization (WHO) immunization routine table [Internet]. Available from: http://www.who.int/immunization/policy/Immunization_routine_table2.pdf?ua=1.
- 32.Team R.C. R: a language and environment for statistical computing. [Internet]. Vienna, Austria. R Foundation Stat Comput. 2014 http://www.r-project.org/ Available from. [Google Scholar]
- 33.Ross P.J., Sutton C.E., Higgins S., Allen A.C., Walsh K., Misiak A. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013;9(4) doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solans L., Debrie A.S., Borkner L., Aguiló N., Thiriard A., Coutte L. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal Immunol [Internet] 2018;11(6):1753–1762. doi: 10.1038/s41385-018-0073-9. Nov 1 [cited 2020 Jul 25]Available from. [DOI] [PubMed] [Google Scholar]
- 35.Solans L., Gonzalo-Asensio J., Sala C., Benjak A., Uplekar S., Rougemont J. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004183. 2014/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065. doi: 10.1128/CVI.00131-10. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Europe C of. 2.7.16. Assay of pertussis vaccine (acellular). 9th Editio. Pharmacopoeia E, editor. Vol. 9th editor Strasbourg: European Pharmacopoeia; 2016.
- 38.World health organization (WHO) reported coverage [Internet]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragebcg.html.
- 39.World health organization (WHO) disease incidence: [Internet]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html.
- 40.Blok B.A., Charlotte L., De Bree J., Diavatopoulos D.A., Langereis J.D., Joosten L.A.B. Clinical infectious diseases interacting, nonspecific, immunological effects of bacille Calmette-Guérin and tetanus-diphtheria-pertussis inactivated polio vaccinations: an explorative, randomized trial. Clin Infect Dis ® [Internet] 2020;70(3):455–463. doi: 10.1093/cid/ciz246. https://academic.oup.com/cid/article-abstract/70/3/455/5421296 [cited 2020 Jul 25]Available from. [DOI] [PubMed] [Google Scholar]
- 41.Aaby P., Andersen A., Ravn H., Zaman K. Vol. 22. EBioMedicine; 2017. pp. 173–180. (Co-administration of bcg and diphtheria-tetanus-pertussis (DTP) vaccinations may reduce infant mortality more than the WHO-schedule of bcg first and then DTP. a re-analysis of demographic surveillance data from rural bangladesh). 2017/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butkeviciute E., Jones C.E., Smith S.G. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13:1193–1208. doi: 10.2217/fmb-2018-0026. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Lee S., Hendrikx L.H., Sanders E.A.M., Berbers G.A.M., Buisman A.-.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front Immunol. 2018;9:51. doi: 10.3389/fimmu.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayashankar B., Singh D., Tanwar H., Mishra K.P., Murthy S., Chanda S. Augmentation of humoral and cellular immunity in response to Tetanus and Diphtheria toxoids by supercritical carbon dioxide extracts of Hippophae rhamnoides L. leaves. Int Immunopharmacol. 2017;44:123–136. doi: 10.1016/j.intimp.2017.01.012. Mar. [DOI] [PubMed] [Google Scholar]
- 45.Gaillard M.E., Bottero D., Errea A., Ormazabal M., Zurita M.E., Moreno G. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine. 2014 Feb;32(8):931–937. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 46.Kapil P., Merkel T.J. Pertussis vaccines and protective immunity [Internet]. Vol. 59, Current Opinion in Immunology. Elsevier Ltd; 2019 [cited 2020 Jul 26]. p. 72–8. Available from: https://pubmed.ncbi.nlm.nih.gov/31078081/ [DOI] [PMC free article] [PubMed]
- 47.Van Twillert I., Han W.G.H., Van Els A.C.M. Vol. 73. 2015. p. 71. (Waning and aging of cellular immunity to Bordetella pertussis. Fems pathog dis [Internet]). [cited 2020 Jul 26]Available from/pmc/articles/PMC4626597/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blakney A.K., Tchakoute C.T., Hesseling A.C., Kidzeru E.B., Jones C.E., Passmore J.A.S. Delayed BCG vaccination results in minimal alterations in T cell immunogenicity of acellular pertussis and tetanus immunizations in HIV-exposed infants. Vaccine. 2015;33(38):4782–4789. doi: 10.1016/j.vaccine.2015.07.096. Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann P., Donath S., Perrett K.P., Messina N.L., Ritz N., Netea M.G. The influence of neonatal Bacille Calmette-Guerin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine. 2019;37(28):3735–3744. doi: 10.1016/j.vaccine.2019.03.016. Jun. [DOI] [PubMed] [Google Scholar]
- 50.Yeung K.H.T., Duclos P., Nelson E.A.S., Hutubessy R.C.W. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980. doi: 10.1016/S1473-3099(17)30390-0. Sep. [DOI] [PubMed] [Google Scholar]
- 51.Perrett K.P., Halperin S.A., Nolan T., Martinez Pancorbo C., Tapiero B., Martinon-Torres F. Immunogenicity, transplacental transfer of pertussis antibodies and safety following pertussis immunization during pregnancy: evidence from a randomized, placebo-controlled trial. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.10.105. Nov. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann S.H., Weiner J., von Reyn C.F. Novel approaches to tuberculosis vaccine development. Int J Infect Dis. 2017;56:263–267. doi: 10.1016/j.ijid.2016.10.018. 2016/11/07. [DOI] [PubMed] [Google Scholar]
- 53.Tarancón R., Domínguez-Andrés J., Uranga S., Ferreira A.V., Groh L.A., Domenech M. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog [Internet] 2020;16(4) doi: 10.1371/journal.ppat.1008404. https://pubmed.ncbi.nlm.nih.gov/32240273/ Apr 1 [cited 2020 Nov 3]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell [Internet] 2020;183(2):315–323. doi: 10.1016/j.cell.2020.08.051. [cited 2020 Nov 10]e9. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med [Internet] 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021. https://www.nejm.org/doi/full/10.1056/NEJMoa1714021 Jul 12 [cited 2020 Nov 10]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Castro M.J., Pardo-Seco J., Martinón-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis [Internet] 2015;60(11):1611–1619. doi: 10.1093/cid/civ144. https://academic.oup.com/cid/article/60/11/1611/356084 Jun 1 [cited 2020 Nov 10]Available from. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.