Abstract
Interleukins, a group of cytokines participating in inflammation and immune response, are proved to be involved in the formation and development of pulmonary fibrosis. In this article, we reviewed the relationship between interleukins and pulmonary fibrosis from the clinical, animal, as well as cellular levels, and discussed the underlying mechanisms in vivo and in vitro. Despite the effects of interleukin-targeted treatment on experimental pulmonary fibrosis, clinical applications are lacking and unsatisfactory. We conclude that intervening in one type of interleukins with similar functions in IPF may not be enough to stop the development of fibrosis as it involves a complex network of regulation mechanisms. Intervening interleukins combined with other existing therapy or targeting interleukins affecting multiple cells/with different functions at the same time may be one of the future directions. Furthermore, the intervention time is critical as some interleukins play different roles at different stages. Further elucidation on these aspects would provide new perspectives on both the pathogenesis mechanism, as well as the therapeutic strategy and drug development.
Subject terms: Pathogenesis, Cell biology
Introduction
Interleukins (ILs) are a type of cytokines with immunoregulatory functions and derived from a variety of cells, including macrophages, T lymphocytes, mast cells, stromal cells, epithelial cells, and neutrophils, etc.1,2. In 1977, IL-1 was first discovered and at least 38 interleukins have been found thereafter. According to the sequence homology, as well as their main functions and receptors, interleukins are divided into IL-1 family, γc family, chemokine family, IL-10 family, IL-6/IL-12 family, and IL-17 family1. They are classified as type-1 (Th1-like) and type-2 (Th2-like) cytokines based on their immune responses as well3. Their functions are also complex and diverse. Generally speaking, interleukins regulate the immune system by participating in innate immune responses, promoting the proliferation and differentiation of immune cells, and specifically recruiting inflammatory cells4–8. In addition, some interleukins are also critical for inflammation responses and hematopoiesis9–13. A number of studies have evidenced that interleukins are associated with some autoimmune diseases (e.g., rheumatoid arthritis) and tumorigenesis14.
Pulmonary fibrosis (PF) is a chronic, progressive fibrotic lung pathological change that can be observed in idiopathic pulmonary fibrosis (IPF), systemic sclerosis, silicosis, and other lung diseases, characterized by damage to the alveolar structure and the replacement of normal lung tissue by deposited extracellular matrix, which resulted in respiratory failure and death15,16. The most common idiopathic interstitial pneumonia is IPF, which manifests as usual interstitial pneumonia (UIP) with temporal and spatial heterogeneity17,18. Incidence of IPF is estimated to range between 2 and 30 cases per 100,000 people per year, with a median survival of 2–3 years from diagnosis19,20. The treatment for IPF is very limited. Although Pirfenidone and Nintedanib have been recommended for clinical use, the efficacy is still insufficient to cure the disease21. Therefore, clarifying the pathogenesis of IPF is of great significance to therapeutic strategy.
Damage, aberrant senescence, and apoptosis of alveolar epithelial cells (AECs), as well as dysfunctional repair after injury leading to tissue fibrotic changes, is regarded as the core pathogenesis of IPF18,22–26. The immune response is also involved in the development of IPF27,28. Under the action of chemokines, circulating immune cells are recruited to lesions, where there are abnormal AECs and accumulated fibroblasts. Th1/Th2 imbalance promotes pulmonary fibrosis through pro-fibrotic factors and inflammatory cytokines29. Furthermore, immune regulatory mechanisms dominated by macrophages and/or dendritic cells (DCs) have also been documented in IPF30–34. These phenomena caused by immune cells seem to be closely associated with cytokines. A hypothesis called the “phagocytosis-secretion-immunity” network of macrophages explained the relationship between immune cells, cytokines, and pulmonary fibrosis35. Therefore, the unbalanced secretion of cytokines may be the key cause of aberrant cell function that results in immunologic derangement in pulmonary fibrosis.
Interleukins and pulmonary fibrosis in clinical research
Interleukin levels in lung tissue, bronchoalveolar lavage fluid (BALF), or blood are altered in patients with pulmonary fibrosis (Table 1). IL-1β and IL-17A in BALF, as well as IL-2, IL-10, IL-12 in serum, were higher in IPF patients as compared to healthy subjects36,37. Interleukin levels not only change between subjects with and without pulmonary fibrosis, but also between different stages of pulmonary fibrosis. For example, peripheral blood levels of IL-6 and IL-9 increased in patients with acute exacerbation IPF (AE-IPF), compared with those with stable IPF38,39.
Table 1.
Changes of interleukin levels in patients with pulmonary fibrosis.
| Interleukin | Group | Sample | Method | Fold change | Ref. |
|---|---|---|---|---|---|
| IL-1β | IPF vs. HC | BALF | ELISA | ↑, 2.23 | 36 |
| IL-2 | IPF vs. HC | Serum | ELISA | ↑, 10 | 37 |
| IL-6 | AE-IPF vs. HC | Serum | Protein microarray analysis | ↑, 1.36 | 39 |
| AE-IPF vs. stable IPF | Serum | ↑, 1.19 | 39 | ||
| IL-8 | IPF vs. HC | Serum | ELISA | ↑, 2.67 | 37 |
| IPF vs. HC | Serum and BALF | ELISA | ↑, >10 | 40 | |
| IL-9 | AE-IPF vs. stable IPF | Serum | Protein microarray analysis | ↑, 1.50 | 39 |
| IL-10 | IPF vs. HC | Serum | ELISA | ↑, 10.12 | 37 |
| IL-12 | IPF vs. HC | Serum | ELISA | ↑, 6.92 | 37 |
| IL-17A | IPF vs. HC | BALF | ELISA | ↑, 9.67 | 36 |
| IL-33 | IPF vs. HC | BALF | ELISA | ↑, 3.59 | 42 |
AE-IPF acute exacerbation-idiopathic pulmonary fibrosis, BALF bronchoalveolar lavage fluid, HC healthy control, IPF idiopathic pulmonary fibrosis.
Conversely, interleukin levels reflect the degree of inflammation and poor prognosis in pulmonary fibrosis and can be used to assess disease progression and severity. For instance, IL-8 (a member of the CXC chemokine family) level in serum reflects the degree of neutrophilic alveolitis in IPF, and its level in lung tissue is negatively correlated with lung function37,40. Moreover, high and low concentrations of IL-8 in plasma are correlated with a median survival of 1.9 and 5.1 years, respectively41. And mortality was increased by 6.7% for each 1 pg/mL in serum concentrations of IL-838,39.
Disorders of interleukins might affect the formation and development of pulmonary fibrosis. IL-33 (a member of the IL-1 family) and thymic stromal lymphopoietin co-stimulate the upstream and downstream signals of IL-13 42. An increased IL-13 level and its inducible proteins and factors (such as periostin and CCL2) in IPF may accelerate the process of pulmonary fibrosis by inhibiting epithelial wound healing43.
Interleukins and pulmonary fibrosis in animal models
The level and function of interleukins are also altered in animal models of pulmonary fibrosis (Fig. 1 and Table 2). Interleukins can promote inflammation by regulating immune cell aggregation, thereby affecting pulmonary fibrosis. In bleomycin-treated mice, IL-1β (an IL-1 subtype with pro-inflammatory activity), induced and activated by inflammasome in damaged lung tissue, promotes recruitment of neutrophils and lymphocytes, leading to inflammation at the injury site, as well as pulmonary fibrosis44. Similarly, IL-5 (a type-2 cytokine) is increased in lung tissue of bleomycin-induced mice and promotes eosinophil recruitment, as well as pulmonary fibrosis45.
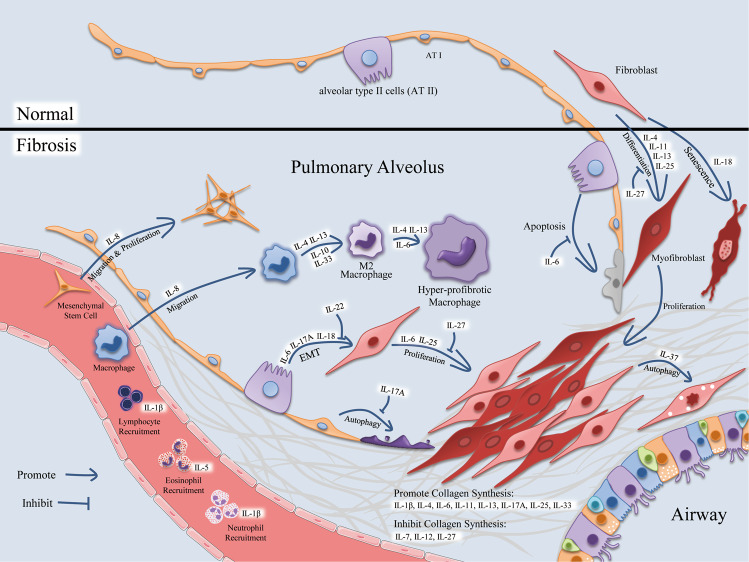
Fig. 1. Interleukins affect the morphology and functions of various cells in pulmonary fibrosis.
(1) Mesenchymal stem cells: IL-8 facilitates the migration and proliferation of mesenchymal stem cells. (2) Macrophages: IL-8 also induces migration of macrophages. IL-4, IL-13, IL-10, and IL-33 promote macrophages to transform into M2 phenotype, which is further promoted by IL-4, IL-6, and IL-13 to transform into a hyper-profibrotic phenotype. (3) Other immune cells: IL-1β induces recruitment of lymphocytes and neutrophils, while IL-5 induces recruitment of eosinophils. (4) Alveolar epithelial cells: IL-17A inhibits autophagy of alveolar epithelial cells, and IL-6 suppresses apoptosis of alveolar type II cells (AT II). IL-6, IL-17A, and IL-18 promote EMT of AT II, whereas IL-22 inhibits this process. (5) Fibroblasts: IL-6 and IL-25 promote the proliferation of fibroblasts. IL-4, IL-11, IL-13, and IL-25 induce differentiation of fibroblasts, whereas IL-27 suppresses both events. IL-18 contributes to the senescence of fibroblasts, and IL-37 facilitates autophagy of fibroblasts. IL-1β, IL-4, IL-6, IL-11, IL-13, IL-17A, IL-25, and IL-33 promote collagen synthesis, while IL-7, IL-12, and IL-27 inhibit collagen synthesis.
Table 2.
Mechanisms of interleukins in pulmonary fibrosis.
| Name | Subtype | Pro-/anti-Inflammatorya | Pro-/anti-Fibrosisb | Mechanisms | Ref. |
|---|---|---|---|---|---|
| IL-1α | IL-1 family | + | + | IL-1α promotes the conversion of fibroblasts to a pro-inflammatory phenotype, which secretes inflammatory cytokines. | 66 |
| IL-1β | IL-1 family | + | + | IL-1β promotes the recruitment of neutrophils and lymphocytes, leading to inflammation at the injury site and pulmonary fibrosis. | 44 |
| + | IL-1β stimulates fibroblasts to synthesize collagen and produce fibrin. | 67,68 | |||
| IL-4 | γc (IL-2) family and type-2 cytokine | / | + | IL-4 stimulates fibroblasts to induce collagen gene expression. | 55,56 |
| + | IL-4 promotes the conversion of fibroblasts to myofibroblasts by activating JNK/ERK signaling, reducing COX gene expression in fibroblasts, and inhibiting PGE2 production. | 57,71 | |||
| − | IL-4 inhibits T cell inflammation and limits lung injury. | 58 | |||
| IL-5 | Type-2 cytokine | / | + | IL-5 promotes lung eosinophil recruitment, which induces the expression of cytokines leading to fibrosis. | 45 |
| IL-6 | IL-6/IL-12 family | ± | + | In bleomycin-treated mice, M2-like macrophages induce the formation of the IL-6/sIL-6Ra complex, which stimulates IL-6 trans-signaling in lung fibroblasts and other cells to promote ECM production and cell proliferation. | 51,52 |
| + | In the bleomycin-induced fibrotic microenvironment, IL-6, secreted by polarized M2-like macrophages, cooperates with IL-4 and IL-13 to promote aggravation of fibrosis in mice. | 53 | |||
| + | IL-6 regulates proliferation and apoptosis of IPF fibroblasts by ERK signaling and STAT3 signaling. | 72–75 | |||
| + | IL-6, secreted by ATIIs in a paracrine way, activates fibroblasts and promotes pulmonary fibrosis through STAT3 signaling. | 88 | |||
| ± | IL-6 acts on ATIIs at the early stage of pulmonary fibrosis and macrophages at the late stage, playing an anti-fibrotic and pro-fibrotic role, respectively. | 54 | |||
| IL-7 | γc (IL-2) family | / | − | IL-7 mediates the increase of Smad7 through JAK/STAT signaling and exerts an anti-fibrotic effect. | 69 |
| − | IL-7 was found to inhibit TGF-β-mediated phosphorylation of PKC-δ (protein kinase C-δ) in fibroblasts from fibrotic lung, but not from the normal counterpart. | 70 | |||
| IL-8 | CXC chemokine family | / | + | IL-8 derived from MPCs promotes proliferation, differentiation, and migration of MPCs in an autocrine manner, and also induces macrophage migration to the fibroblastic foci through receptors CXCR1/2. | 95 |
| IL-9 | γc (IL-2) family and type-2 cytokine | ± | − | IL-9 exerts an anti-inflammatory activity and a protective role in bleomycin-induced pulmonary fibrosis. | 61 |
| − | Overexpression of IL-9 promotes PGE2 production of macrophages to inhibit silica-induced pulmonary fibrosis. | 62 | |||
| + | Neutralization of IL-9 by a specific Ab reduces silica-induced lung inflammation and fibrosis in mice. | 63 | |||
| IL-10 | IL-10 family | − | − | IL-10 inhibits downregulation of IFN-γ and upregulation of TGF-β1 by IFN-γ signaling in bleomycin-induced mice, thereby delaying the process of pulmonary fibrosis. | 46 |
| + | Long-term overexpression of IL-10 can promote fibrosis by activating M2 macrophages. | 86,87 | |||
| IL-11 | IL-6/IL-12 family | / | + | IL-11 promotes fibrin synthesis and fibrosis via the transduction of non-classical ERK signal in an autocrine way. | 78 |
| + | IL-11 stimulates fibroblast phenotype transformation and promotes collagen synthesis regulated by ERK kinase in vitro. | 79 | |||
| IL-12 | IL-6/IL-12 family and type-1 cytokine | / | − | IL-12 may induce transformation of Th2 cells to Th1 cells, thereby upregulating IFN-γ expression, inhibiting collagen production by fibroblasts to suppress fibrosis. | 96 |
| IL-13 | Type-2 cytokine | / | + | IL-13 significantly increases the expression of α-SMA and collagen I in IPF lung fibroblasts, while it has no remarkable effect on normal fibroblasts. | 76 |
| + | IL-13 induces differentiation of fibroblasts to myofibroblasts via multiple mechanisms: (1) Regulating the JNK signal. (2) Promoting fibroblast proliferation by inhibiting COX expression and PGE2 production. (3) Promoting differentiation of fibroblasts via upregulating YY1 (Yin Yang 1) expression in AKT signaling. | 57,71,77 | |||
| IL-17A | IL-17 family | + | + | IL-17A induces an immunosuppressive microenvironment and suppresses activation of autophagy in the fibrotic lung tissue. | 47 |
| + | IL-17A induces EMT in A549 cells by activating the classical Smad2/3 pathway and non-classical ERK1/2 pathway. | 91 | |||
| IL-18 | IL-1 family | + | + | IL-18 promotes lung fibrosis by downregulating the anti-senescence protein Klotho in lung fibroblasts. | 48 |
| + | IL-18 induces EMT by upregulating α-SMA, transcription factor Snail-1, and downregulating E-cadherin, thus participating in the development of bleomycin-induced pulmonary fibrosis. | 49 | |||
| IL-22 | IL-10 family | − | − | IL-22 exerts an anti-fibrotic effect via inhibiting EMT by targeting alveolar epithelia. | 92,93 |
| IL-23 | IL-6/IL-12 family | / | + | IL-23 mediates the production of IL-17 by γδ T cells or CD4+ T cells to promote pulmonary fibrosis. | 98,99 |
| IL-25 (IL-17E) | IL-17 family and type-2 cytokine | / | + | IL-25 is secreted by AECs and can promote proliferation, differentiation, and collagen synthesis of fibroblasts by binding to IL-17BR. | 80,81 |
| + | IL-25 induces ILC2 to release IL-13, which promotes fibrosis. | 100 | |||
| IL-27 | IL-6/IL-12 family | − | − | IL-27 inhibits fibroblast proliferation and differentiation, collagen synthesis, as well as TIMP1 expression, whereas promotes secretion of MMP2 and MMP9. | 83 |
| − | IL-27 regulates the differentiation of T cells, affecting the release of other cytokines against lung fibrosis. | 101 | |||
| IL-32γ | Not classified | + | − | Recombinant IL-32γ exerts an anti-fibrotic effect by inhibiting the integrin-mediated activation of FAK/paxillin, a critical pathway in fibroblast activation. | 84 |
| IL-33 | IL-1 family | / | + | Polarization of M1 macrophages to M2 macrophages, promoted by IL-33 via IL-33/ST2 signal, is one of the pathological features in IPF. | 85 |
| IL-37 | IL-1 family | − | − | IL-37 plays an anti-fibrotic effect via promoting the expression of autophagy activation marker LC3II and inducing autophagy in fibroblasts. | 50 |
ATIIs type II alveolar cells, COX cyclooxygenase, ECM extracellular matrix, EMT epithelial-mesenchymal transition, ERK extracellular signal-regulated kinase, ILC2 type 2 congenital lymphocytes; JNK c-Jun N-terminal kinase, MMP matrix metalloproteinase, MPCs mesenchymal progenitor cells, PGE2: prostaglandin E2, PKC-δ protein kinase C-δ, TGF-β1 transforming growth factor-β1, TIMP tissue inhibitor of metalloproteinase, YY1: Yin Yang 1, α-SMA α-smooth muscle actin.
aPro-inflammatory: +; anti-inflammatory: −; dual role: ±; neither or unknown: /.
bPro-fibrosis: +; anti-fibrosis: −; dual role: ±.
Altered interleukin levels in animals with pulmonary fibrosis could impact their weight, survival rate, etc. As an anti-inflammatory and anti-fibrotic cytokine, IL-10 inhibits the downregulation of IFN-γ and upregulation of TGF-β1 in bleomycin-induced pulmonary fibrosis mice, thereby reducing the number of infiltrated inflammatory cells and development of lung fibrosis. Overexpression of IL-10 reduces weight loss and survival rate drop in bleomycin-instillation mice46. IL-17A, a pro-inflammatory cytokine also known as IL-17, inhibits the activation of autophagy and autophagy-related cell death in bleomycin-induced lung injury to promote fibrosis. Intravenous anti-IL-17A neutralizing antibody increased survival of bleomycin-injured mice47.
Interleukins influence pulmonary fibrosis mainly through inflammation and immune response, but also via other ways. In bleomycin mice models, IL-18, a pro-inflammatory cytokine, induces the senescence of lung fibroblasts by downregulating the anti-senescence protein Klotho48. Besides, IL-18 induces EMT by upregulating α-SMA, transcription factor Snail-1, and downregulating E-cadherin, thus participating in the development of bleomycin-induced pulmonary fibrosis49. After bleomycin treatment, intranasal instillation of IL-37 (an anti-inflammatory interleukin) plays an anti-fibrotic effect via promoting the expression of autophagy activation marker LC3II and inducing autophagy in fibroblasts. But, regrettably, expression of IL-37 decreases in the lung tissue of IPF patients and mouse models50.
IL-6 (acts as both a pro-inflammatory and an anti-inflammatory cytokine) is a member of the IL-6/IL-12 cytokine family. In bleomycin-treated mice, M2-like macrophages induce the formation of the IL-6/sIL-6Ra complex, which stimulates IL-6 trans-signaling in lung fibroblasts and other cells to promote ECM production and cell proliferation51,52. In the bleomycin-induced fibrotic microenvironment, M2 macrophages polarize and secrete IL-6, which, together with IL-4 and IL-13 (both are type-2 cytokines produced by Th2 cells), activates M2-like macrophages possessing hyper-profibrotic phenotype, then the hyper-profibrotic macrophages accumulate and finally induce extracellular matrix deposition and aggravate pulmonary fibrosis53. Also, the elevated IL-6 level may be related to decreased lung function52.
The above studies suggested that interleukins exacerbate or alleviate pulmonary fibrosis via a variety of ways. And some interleukins may serve a dual role in pulmonary fibrosis. For example, IL-6 may act on ATIIs and is anti-fibrotic in the early stage, but act on fibroblasts, as well as macrophages and play a pro-fibrotic role in the late stage54. IL-4 was initially thought to be pro-fibrotic and then was believed to have no effect on pulmonary fibrosis, while Huaux et al. reported that it has different roles between early and late stages, similar to IL-6 56–60. The role of IL-4 in pulmonary fibrosis may be controversial and needs further investigation.
IL-9 is a secreted protein that belongs to the γc family and type-2 cytokines. The role of IL-9 in pulmonary fibrosis is also controversial. Arras et al. found that IL-9 exerts an anti-inflammatory activity and a protective role in bleomycin-induced pulmonary fibrosis61. Furthermore, overexpression of IL-9 promotes PGE2 production of macrophages to inhibit silica-induced pulmonary fibrosis62. However, Sugimoto et al. showed that neutralization of IL-9 by a specific Ab reduces silica-induced lung inflammation and fibrosis in mice63.
Main target cells of interleukins in pulmonary fibrosis
Fibroblasts
Fibroblasts play an essential role in the progression of pulmonary fibrosis. Cytokines induce excessive secretion of collagen from fibroblasts and promote the differentiation from fibroblasts to myofibroblasts64,65. Recently, evidence suggested that interleukins directly interact with fibroblasts to promote or inhibit pulmonary fibrosis.
In vitro study has demonstrated that IL-1α (a pro-inflammatory subtype of the IL-1 family) secreted by the alveolar epithelia under stress directly promotes the formation of pro-inflammatory phenotypes of fibroblasts, which further secrete other cytokines to promote pulmonary fibrosis66. IL-1β, another IL-1 subtype released by lung macrophages, stimulates fibroblasts to synthesize collagen and produce fibrin67,68.
A study demonstrates that IL-37, an anti-inflammatory interleukin, decreases collagen deposition by fibroblasts and alleviates pulmonary fibrosis via inhibiting TGF-β signal transduction. In addition, IL-37 also promotes the autophagy of fibroblasts and regulates cell proliferation, as well as metabolism by inhibiting PI3K/AKT, ERK, and MAPK signaling pathways to protect against fibrosis50.
IL-7, a member of the γc family, can induce the synthesis of inflammatory mediators by monocytes and also has anti-fibrotic effects. It appears to have different sensitivities to abnormal and normal fibroblasts. In pulmonary fibrosis, TGF-β signaling induces fibroblast activation and collagen synthesis, while Smad7 can block this process by inhibiting TGF-β signaling. IL-7 mediates the increase of Smad7 through JAK/STAT signaling and exerts an anti-fibrotic effect. Especially, IL-7 only works this way in fibroblasts from IPF patients, not from healthy subjects69. Besides, IL-7 was found to inhibit TGF-β-mediated phosphorylation of PKC-δ (protein kinase C-δ) in fibroblasts from fibrotic lung, but not from the normal counterpart70.
IL-4 may play a dual role in pulmonary fibrosis. On the one hand, it induces the gene expression of collagen in lung fibroblasts and promotes the differentiation of fibroblasts to myofibroblasts via activation of the JNK/ERK pathway in a time-dependent and dose-dependent manner. The differentiation is also related to the reduction of COX gene expression in fibroblasts and the inhibition of PGE2 production71. On the other hand, IL-4 inhibits T cell inflammation and limits lung injury58.
As mentioned above, IL-6 plays different roles via acting on different cells at different stages. Also, IL-6 plays opposing roles between fibroblasts from normal subjects and IPF patients. IL-6 is anti-proliferative in normal lung fibroblasts, whereas is strongly pro-proliferative in IPF fibroblasts72. In addition, IL-6 upregulates the expression of pro-apoptotic protein Bax and promotes Fas-induced apoptosis through the STAT-3 signaling pathway in normal fibroblasts, while it induces the expression of anti-apoptotic molecule Bcl-2 and proliferation of IPF fibroblasts by ERK signaling pathway73. Also, IL-6 promotes the proliferation of IPF lung fibroblasts via the IL-6/STAT3 axis and trans-signaling51,74,75.
Similar to IL-6, IL-13 has different sensitivities to fibroblasts from normal people and IPF patients. Murray et al. found that IL-13 significantly promotes the expression of α-SMA and collagen I in IPF lung fibroblasts, while the normal fibroblasts do not respond to IL-13 76. Moreover, IL-13 induces differentiation of fibroblasts to myofibroblasts via multiple mechanisms: (1) Regulating the JNK signal. (2) Promoting fibroblast proliferation by inhibiting COX expression and PGE2 production. (3) Promoting differentiation of fibroblasts via upregulating YY1 (Yin Yang 1) expression in AKT signaling57,71,77.
IL-11, a member of the IL-6/IL-12 family, can be secreted by multiple cells. Studies have shown that human primary fibroblasts specifically express IL-11 and its receptor IL-11RA. IL-11 promotes fibrin synthesis and fibrosis via transduction of non-classical ERK signal in an autocrine way78. In addition, IL-11 stimulates fibroblast phenotype transformation and promotes collagen synthesis regulated by ERK kinase in vitro, thereby promoting the development of pulmonary fibrosis79.
IL-25, also known as IL-17E because of the homology with IL-17 family members, is a type-2 cytokine and pro-fibrotic factor. It is secreted by AECs and can promote proliferation, differentiation, and collagen synthesis of fibroblasts by binding to IL-17BR80,81.
IL-27, a heterodimeric cytokine that belongs to the IL-6/IL-12 family, is generally believed to be anti-fibrotic82. It inhibits the proliferation and differentiation of fibroblasts via inactivating JAK/STAT and TGF-β1/Smad signaling. In addition, IL-27 promotes fibroblasts to secrete MMP2 (matrix metalloproteinase 2) and MMP9, as well as inhibits the expression of TIMP1 (tissue inhibitor of metalloproteinase 1) to resist pulmonary fibrosis83.
IL-32 can induce the production of several pro-inflammatory mediators. Hong et al. showed that recombinant IL-32γ exerts an anti-fibrotic effect by inhibiting integrin-mediated activation of FAK/paxillin, a critical pathway in fibroblast activation84.
Macrophages
Under the effect of cytokines, macrophages are involved in the inflammatory response and fibrotic diseases by changing cell phenotypes30. Studies have shown that IL-8, IL-33, and IL-10 contribute to pulmonary fibrosis by targeting macrophages.
Macrophages infiltration occurs in IPF lungs. Macrophages migrate to lesion regions under the action of chemokines. Polarization of M1 macrophages to M2 macrophages, promoted by IL-33 via IL-33/ST2 signal85, is one of the pathological features in IPF. Besides, long-term overexpression of IL-10 (considered to be anti-fibrotic) can promote fibrosis by activating M2 macrophages86,87.
Alveolar epithelia
Alveolar epithelial are critical cells in the pathogenesis of pulmonary fibrosis. Therefore, cytokines that affect the repair or apoptosis of alveolar epithelia have a definite effect on the development of pulmonary fibrosis. The activated Wnt/β-catenin signaling pathway induces type II alveolar cells (ATIIs) to secrete IL-1β, which enhances TGF-β signaling and promotes the release of IL-6 88. IL-1β and IL-6 induce EMT to promote fibrosis via TGF-β signaling and STAT3 signaling, respectively88,89. IL-1β also induces epithelial wound repair90.
In bleomycin mice models, endogenous IL-6 regulates ATIIs through STAT3/Akt signaling in an autocrine or paracrine manner. It originates from ATIIs at the inflammatory stage after bleomycin administration. Blocking IL-6 at this stage accelerates pulmonary fibrosis, possibly by enhancing apoptosis of ATIIs54.
In vitro evidence suggested that IL-18 and IL-17A both induce EMT49,91. Also, in pulmonary fibrosis, IL-22 (an anti-inflammatory cytokine of the IL-10 family) exerts an anti-fibrotic effect via inhibiting EMT by targeting alveolar epithelia, which is the only target cell type of IL-22 in the lung92,93.
Other types of cells
In addition to the target cells described above, other types of cells can also be targeted by interleukins and affect the progression of pulmonary fibrosis94. For example, IL-8, secreted by mesenchymal progenitor cells (MPCs), promotes MPCs of IPF lungs to proliferate, differentiate and migrate in an autocrine manner. Besides, IL-8 stimulates macrophages to migrate to the fibroblastic foci through receptors CXCR1/295.
IL-12 (a type-1 cytokine) may induce the transformation of Th2 cells to Th1 cells, thereby upregulating IFN-γ expression, inhibiting collagen production by fibroblasts to suppress fibrosis96. IL-33 from fibroblasts and innate immune cells was found to increase IL-13 production by Th2 cells, macrophages, and type II congenital lymphocytes (ILC2) to promote lung fibrosis97. Besides, in vivo or in vitro studies demonstrated that IL-25 targets ILC2, while IL-23 and IL-27 (members of the IL-6/IL-12 family) target T lymphocytes to regulate pulmonary fibrosis98–101. The specific mechanisms are listed in Table 2.
Conclusion and future perspectives
In vivo and in vitro studies have shown that altered interleukin levels participate in the formation and development of pulmonary fibrosis by regulating inflammation, immune response, autophagy, senescence, EMT, etc. (Fig. 1). And the target cells of interleukins are mainly fibroblasts, macrophages, and epithelial cells (Table 3).
Table 3.
Target cells of interleukins in pulmonary fibrosis.
| Target cell | Interleukins |
|---|---|
| Fibroblasts | IL-1α, IL-1β, IL-4, IL-6, IL-7, IL-11, IL-13, IL-18, IL-25, IL-27, IL-32γ, IL-37 |
| Mesenchymal stem cells | IL-8 |
| Alveolar epithelial cells | IL-1β, IL-6, IL-17A, IL-18, IL-22 |
| Macrophages | IL-4, IL-6, IL-8, IL-10, IL-13 Il-33 |
| T cells | IL-12, IL-23, IL-27, IL-33 |
| ILC2s | IL-25 |
Most interleukins exert either anti-fibrotic or pro-fibrotic effects, whereas few show a dual role or are still controversial as we mentioned above. The sources, signal pathways, and target cells of interleukins, as well as their stability and metabolism in pulmonary fibrosis, need to be clarified. Also, it is important to keep the experimental conditions consistent with previous studies, so that the results and conclusions are comparable. Moreover, some studies were using interleukin overexpressing transgenic mice to investigate the role of a particular interleukin in pulmonary fibrosis, however, the extreme abundance of an individual factor will affect the entire cytokine network and immune regulation, the results from such models may therefore not totally reflect the real state.
Despite the effects of interleukin-targeted treatment on experimental pulmonary fibrosis, clinical applications are lacking and unsatisfactory. Phase II clinical trials (NCT01266135; NCT01629667; NCT01872689; NCT02345070) showed that although IL-13 monoclonal antibodies QAX-576, Tralokinumab, and Lebrikizumab had acceptable safety and tolerability, and Romilkimab (SAR156597, a bispecific Ig-G4 antibody that binds and neutralizes both circulating IL-4 and IL-13) appeared to reduce the occurrence of acute exacerbations in IPF patients, none of these drugs reached the expected efficacy102–104. These clinical trials suggest that intervening in one type of interleukins with similar functions in IPF may not be enough to stop the development of fibrosis as it involves a complex network of regulation mechanisms. Intervening interleukins combined with other existing therapy (add-on trial) or targeting interleukins affecting multiple cells/with different functions at the same time may be one of the future directions. For example, therapies that inducing IL-22 production to promote lung epithelial regeneration and using IL-12 to inhibit collagen production by fibroblasts may be able to help improve pulmonary fibrosis. VEGF, FGF, and PDGF signaling pathways or Wnt/β-catenin signaling pathways may be targeted in synergy with ILs. Furthermore, the intervention time is critical as some interleukins play different roles at different stages. These should be tested in experimental animal models first before going to the clinical setting.
Acknowledgments
Funding
This work was supported by the National High-Level Talents Program (X.T.), the National Natural Science Foundation of China (81770015, X.T.), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155), Open Project of State Key Laboratory of Respiratory Disease (SKLRD-OP-201906), Special Fund for Science and Technology Innovation of Guangdong Province (2020B1111330001), and Guangzhou Institute of Respiratory Health Open Project (funds provided by China Evergrande Group)-Project No. 2020GIRHHMS16.
Author contributions
X.T. conceived and designed the manuscript, provided guidance, and edited the manuscript. All authors wrote the manuscript and critically revised it.
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Edited by A. Rufini
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi Xin She, Qing Yang Yu
References
- 1.Akdis, M., Aab, A., Altunbulakli, C., Azkur, K., Costa, R. A., Crameri, R. et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 138, 984–1010 (2016). [DOI] [PubMed]
- 2.Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genomics. 2010;5(1):30–55. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996;9(4):532–62. doi: 10.1128/CMR.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010). [DOI] [PubMed]
- 5.Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018). [DOI] [PMC free article] [PubMed]
- 6.Leonard WJ, Lin J-X, O’Shea JJ. The γ family of cytokines: basic biology to therapeutic ramifications. Immunity. 2019;50(4):832–50. doi: 10.1016/j.immuni.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Fickenscher H, Hör S, Küpers H, Knappe A, Wittmann S, Sticht H. The interleukin-10 family of cytokines. Trends Immunol. 2002;23(2):89–96. doi: 10.1016/S1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 8.Richmond J, Tuzova M, Cruikshank W, Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell Physiol. 2014;229(2):139–47. doi: 10.1002/jcp.24441. [DOI] [PubMed] [Google Scholar]
- 9.Moschen AR, Tilg H, Raine TIL-12. IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019;16(3):185–96. doi: 10.1038/s41575-018-0084-8. [DOI] [PubMed] [Google Scholar]
- 10.Monin, L., Gaffen, S. L. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 10, a028522 (2018). [DOI] [PMC free article] [PubMed]
- 11.Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13(9):1307–15. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- 12.Unver N, McAllister F. IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018;41:10–7. doi: 10.1016/j.cytogfr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tait Wojno ED, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–70. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. Biology of IL-38 and its role in disease. Immunol. Rev. 2018;281(1):191–6. doi: 10.1111/imr.12612. [DOI] [PubMed] [Google Scholar]
- 15.Bagnato, G., Roberts, W. N, Roman, J. & Gangemi, S. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur. Respirat. Rev. 26, 160125 (2017). 10.1183/16000617.0125-2016. [DOI] [PMC free article] [PubMed]
- 16.Pollard KM. Silica, silicosis, and autoimmunity. Front. Immunol. 2016;7:97. doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen L, Ruppert C, Ochs M. Tissue remodelling in pulmonary fibrosis. Cell Tissue Res. 2017;367(3):607–26. doi: 10.1007/s00441-016-2543-2. [DOI] [PubMed] [Google Scholar]
- 18.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–52. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Resp. Crit. Care. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 21.Raghu G, Rochwerg B, Zhang Y, Garcia CAC, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. an update of the 2011 Clinical Practice Guideline. Am. J. Resp. Crit. Care. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 22.Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir. Res. 2018;19(1):32. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc. Am. Thorac. Soc. 2006;3(4):293–8. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Wang Y, Pandupuspitasari NS, Wu G, Xiang X, Gong Q, et al. Endoplasmic reticulum stress, a new wrestler, in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Transl. Res. 2017;9(2):722–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Birch J, Barnes PJ, Passos JF. Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharm. Therapeut. 2018;183:34–49. doi: 10.1016/j.pharmthera.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Bilgili, H., Bialas, A. J., Gorski, P. & Piotrowski, W. J. Telomere abnormalities in the pathobiology of idiopathic pulmonary fibrosis. J. Clin. Med. 8, 1232 (2019). [DOI] [PMC free article] [PubMed]
- 27.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001;345(7):517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 28.Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018;5:43. doi: 10.3389/fmed.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune mechanisms in pulmonary fibrosis. Am. J. Resp. Cell Mol. 2016;55(3):309–22. doi: 10.1165/rcmb.2016-0121TR. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang C-Y. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Resp. Res. 2018;19(1):170. doi: 10.1186/s12931-018-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsoumakidou M, Karagiannis KP, Bouloukaki I, Zakynthinos S, Tzanakis N, Siafakas NM. Increased bronchoalveolar lavage fluid CD1c expressing dendritic cells in idiopathic pulmonary fibrosis. Respiration. 2009;78(4):446–52. doi: 10.1159/000226244. [DOI] [PubMed] [Google Scholar]
- 32.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, et al. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am. J. Resp. Crit. Care. 2010;182(3):385–95. doi: 10.1164/rccm.200907-1164OC. [DOI] [PubMed] [Google Scholar]
- 33.Tort Tarres M, Aschenbrenner F, Maus R, Stolper J, Schuette L, Knudsen L, et al. The FMS-like tyrosine kinase-3 ligand/lung dendritic cell axis contributes to regulation of pulmonary fibrosis. Thorax. 2019;74(10):947–57. doi: 10.1136/thoraxjnl-2018-212603. [DOI] [PubMed] [Google Scholar]
- 34.Shin JS. Unexpected role of dendritic cells in pulmonary fibrosis. Thorax. 2019;74(10):925–6. doi: 10.1136/thoraxjnl-2019-213510. [DOI] [PubMed] [Google Scholar]
- 35.Liu G, Zhai H, Zhang T, Li S, Li N, Chen J, et al. New therapeutic strategies for IPF: Based on the “phagocytosis-secretion-immunization” network regulation mechanism of pulmonary macrophages. Biomed. Pharmacother. 2019;118:109230. doi: 10.1016/j.biopha.2019.109230. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1 beta-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010;207(3):535–52. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, Mpaka M, et al. Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Resp. Med. 2006;100(5):938–45. doi: 10.1016/j.rmed.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Papiris SA, Tomos IP, Karakatsani A, Spathis A, Korbila I, Analitis A, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine. 2018;102:168–72. doi: 10.1016/j.cyto.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Weng D, Chen X-Q, Qiu H, Zhang Y, Li Q-H, Zhao M-M, et al. The role of infection in acute exacerbation of idiopathic pulmonary fibrosis. Mediat Inflamm. 2019;2019:5160694. doi: 10.1155/2019/5160694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziegenhagen MW, Zabel P, Zissel G, Schlaak M, Muller-Quernheim J. Serum level of interleukin 8 is elevated in idiopathic pulmonary fibrosis and indicates disease activity. Am. J. Resp. Crit. Care. 1998;157(3 Pt 1):762–8. doi: 10.1164/ajrccm.157.3.9705014. [DOI] [PubMed] [Google Scholar]
- 41.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am. J. Resp. Crit. Care. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JU, Chang HS, Lee HJ, Jung CA, Bae DJ, Song HJ, et al. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm. Med. 2017;17(1):39. doi: 10.1186/s12890-017-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am. J. Resp. Cell Mol. 2014;50(5):985–94. doi: 10.1165/rcmb.2013-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 2007;117(12):3786–99. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gharaee-Kermani M, McGarry B, Lukacs N, Huffnagle G, Egan RW, Phan SH. The role of IL-5 in bleomycin-induced pulmonary fibrosis. J. Leukoc. Biol. 1998;64(5):657–66. doi: 10.1002/jlb.64.5.657. [DOI] [PubMed] [Google Scholar]
- 46.Kurosaki F, Uchibori R, Sehara Y, Saga Y, Urabe M, Mizukami H, et al. AAV6-mediated IL-10 expression in the lung ameliorates bleomycin-induced pulmonary fibrosis in mice. Hum. Gene Ther. 2018;29(11):1242–51. doi: 10.1089/hum.2018.024. [DOI] [PubMed] [Google Scholar]
- 47.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 2011;187(6):3003–14. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 48.Zhang LM, Zhang J, Zhang Y, Fei C, Wang L, Yi ZW, et al. Interleukin-18 promotes fibroblast senescence in pulmonary fibrosis through down-regulating Klotho expression. Biomed. Pharmacother. 2019;113:108756. doi: 10.1016/j.biopha.2019.108756. [DOI] [PubMed] [Google Scholar]
- 49.Zhang LM, Zhang Y, Fei C, Zhang J, Wang L, Yi ZW, et al. Neutralization of IL-18 by IL-18 binding protein ameliorates bleomycin-induced pulmonary fibrosis via inhibition of epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019;508(2):660–6. doi: 10.1016/j.bbrc.2018.11.129. [DOI] [PubMed] [Google Scholar]
- 50.Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS, et al. IL-37 attenuates lung fibrosis by inducing autophagy and regulating TGF-beta1 production in mice. J. Immunol. 2019;203(8):2265–75. doi: 10.4049/jimmunol.1801515. [DOI] [PubMed] [Google Scholar]
- 51.Le TT, Karmouty-Quintana H, Melicoff E, Le TT, Weng T, Chen NY, et al. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J. Immunol. 2014;193(7):3755–68. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shieh, J. -M., Tseng, H. -Y., Jung, F., Yang, S. -H, Lin, J. -C. Elevation of IL-6 and IL-33 levels in serum associated with lung fibrosis and skeletal muscle wasting in a bleomycin-induced lung injury mouse model. Mediat. Inflamm. 2019, 7947596 (2019). 10.1155/2019/7947596. [DOI] [PMC free article] [PubMed]
- 53.Ayaub EA, Dubey A, Imani J, Botelho F, Kolb MRJ, Richards CD, et al. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci. Rep. 2017;7(1):13281. doi: 10.1038/s41598-017-13511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi T, Tanaka K, Fujita T, Umezawa H, Amano H, Yoshioka K, et al. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir. Res. 2015;16:99. doi: 10.1186/s12931-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillery P, Fertin C, Nicolas JF, Chastang F, Kalis B, Banchereau J, et al. Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures potential role in fibrosis. FEBS Lett. 1992;302(3):231–4. doi: 10.1016/0014-5793(92)80448-P. [DOI] [PubMed] [Google Scholar]
- 56.Sempowski GD, Derdak S, Phipps RP. Interleukin-4 and interferon-gamma discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J. Cell Physiol. 1996;167(2):290–6. doi: 10.1002/(SICI)1097-4652(199605)167:2<290::AID-JCP13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie TIL-4. and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J. Allergy Clin. Immunol. 2001;107(6):1001–8. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 58.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J. Immunol. 2003;170:2083–92. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- 59.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Resp. Cell Mol. 2002;27(4):419–27. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 60.Izbicki G, Or R, Christensen TG, Segel MJ, Fine A, Goldstein RH, et al. Bleomycin-induced lung fibrosis in IL-4-overexpressing and knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283(5):L1110–L6. doi: 10.1152/ajplung.00107.2002. [DOI] [PubMed] [Google Scholar]
- 61.Arras M, Louahed J, Heilier J-F, Delos M, Brombacher F, Renauld J-C, et al. IL-9 protects against bleomycin-induced lung injury: involvement of prostaglandins. Am. J. Pathol. 2005;166(1):107–15. doi: 10.1016/S0002-9440(10)62236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arras M, Louahed J, Simoen V, Barbarin V, Misson P, van den Brule S, et al. B lymphocytes are critical for lung fibrosis control and prostaglandin E2 regulation in IL-9 transgenic mice. Am. J. Respir. Cell Mol. Biol. 2006;34(5):573–80. doi: 10.1165/rcmb.2004-0383OC. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto N, Suzukawa M, Nagase H, Koizumi Y, Ro S, Kobayashi K, et al. IL-9 Blockade Suppresses Silica-induced Lung Inflammation and Fibrosis in Mice. Am. J. Resp. Cell Mol. 2019;60(2):232–43. doi: 10.1165/rcmb.2017-0287OC. [DOI] [PubMed] [Google Scholar]
- 64.Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix biology: journal of the International Society for. Matrix Biol. 2018;71-72:112–27. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolb M, Bonella F, Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Resp. Med. 2017;131:49–57. doi: 10.1016/j.rmed.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 66.Suwara MI, Green NJ, Borthwick LA, Mann J, Mayer-Barber KD, Barron L, et al. IL-1 alpha released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 2014;7(3):684–93. doi: 10.1038/mi.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993;150:4188–96. [PubMed] [Google Scholar]
- 68.Kline JN, Schwartz DA, Monick MM, Floerchinger CS, Hunninghake GW. Relative release of interleukin-1 beta and interleukin-1 receptor antagonist by alveolar macrophages. A study in asbestos-induced lung disease, sarcoidosis, and idiopathic pulmonary fibrosis. Chest. 1993;104(1):47–53. doi: 10.1378/chest.104.1.47. [DOI] [PubMed] [Google Scholar]
- 69.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Invest. 2002;109(7):931–7. doi: 10.1172/JCI0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Keane MP, Zhu LX, Sharma S, Rozengurt E, Strieter RM, et al. Interleukin-7 and transforming growth factor-beta play counter-regulatory roles in protein kinase C-delta-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J. Biol. Chem. 2004;279(27):28315–9. doi: 10.1074/jbc.C400115200. [DOI] [PubMed] [Google Scholar]
- 71.Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 2003;132(2):168–76. doi: 10.1159/000073718. [DOI] [PubMed] [Google Scholar]
- 72.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, et al. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am. J. Pathol. 2003;163(1):345–54. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moodley YP, Misso NLA, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am. J. Resp. Cell Mol. 2003;29(4):490–8. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- 74.O’Donoghue RJ, Knight DA, Richards CD, Prele CM, Lau HL, Jarnicki AG, et al. Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. Embo. Mol. Med. 2012;4(9):939–51. doi: 10.1002/emmm.201100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epstein Shochet G, Brook E, Bardenstein-Wald B, Shitrit D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Resp. Res. 2020;21(1):56. doi: 10.1186/s12931-020-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int. J. Biochem. Cell Biol. 2008;40(10):2174–82. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Yao H, Lin X, Xu H, Dean D, Zhu Z, et al. IL-13 induces YY1 through the AKT pathway in lung fibroblasts. PLoS ONE. 2015;10(3):e0119039. doi: 10.1371/journal.pone.0119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schafer S, Viswanathan S, Widjaja AA, Lim W-W, Moreno-Moral A, DeLaughter DM, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552(7683):110–5. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng B, Dong JR, D’Agostino G, Viswanathan S, Widjaja AA, Lim WW, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2019;11(511):14. doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 80.Xu, X., Geng, J., Li, S., Jiang, D., Wang, C. Dai, H. The expression and role of IL-25 and its receptor in idiopathic pulmonary fibrosis. Chest149, A213 (2016).
- 81.Xu X, Luo S, Li B, Dai H, Zhang J. Feature Article: IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp. Biol. Med. 2019;244(9):770–80. doi: 10.1177/1535370219843827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fabbi M, Carbotti G, Ferrini S. Dual roles of IL-27 in cancer biology and immunotherapy. Mediat. Inflamm. 2017;2017:3958069. doi: 10.1155/2017/3958069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong Z, Zhao X, Tai W, Lei W, Wang Y, Li Z, et al. IL-27 attenuates the TGF-beta1-induced proliferation, differentiation and collagen synthesis in lung fibroblasts. Life Sci. 2016;146:24–33. doi: 10.1016/j.lfs.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Hong GH, Park SY, Kwon HS, Bang BR, Lee J, Kim SY, et al. IL-32gamma attenuates airway fibrosis by modulating the integrin-FAK signaling pathway in fibroblasts. Respir. Res. 2018;19(1):188. doi: 10.1186/s12931-018-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS. Zhang L, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014;134(6):1422–32 e11. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, et al. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300(3):L341–L53. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E, et al. Hydrogel-based delivery of Il-10 improves treatment of bleomycin-induced lung fibrosis in mice. Biomaterials. 2019;203:52–62. doi: 10.1016/j.biomaterials.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aumiller V, Balsara N, Wilhelm J, Gunther A, Konigshoff M. WNT/beta-catenin signaling induces IL-1 beta expression by alveolar epithelial cells in pulmonary fibrosis. Am. J. Resp. Cell Mol. 2013;49(1):96–104. doi: 10.1165/rcmb.2012-0524OC. [DOI] [PubMed] [Google Scholar]
- 89.Doerner AM, Zuraw BL. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Resp. Res. 2009;10:100. doi: 10.1186/1465-9921-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1beta augments in vitro alveolar epithelial repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279(6):L1184–90. doi: 10.1152/ajplung.2000.279.6.L1184. [DOI] [PubMed] [Google Scholar]
- 91.Ahmad, A., Wang, T., Liu, Y., Zou, J. -F., Cheng, Z. -S. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-β1 mediated Smad2/3 and ERK1/2 activation. PLoS ONE12, e0183972 (2017). [DOI] [PMC free article] [PubMed]
- 92.Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, et al. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis. Mediat. Inflamm. 2013;2013:209179. doi: 10.1155/2013/209179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am. J. Resp. Cell Mol. 2004;31(2):220–6. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 94.Shimbori C, Upagupta C, Bellaye P-S, Ayaub EA, Sato S, Yanagihara T, et al. Mechanical stress-induced mast cell degranulation activates TGF-β1 signalling pathway in pulmonary fibrosis. Thorax. 2019;74(5):455–65. doi: 10.1136/thoraxjnl-2018-211516. [DOI] [PubMed] [Google Scholar]
- 95.Yang L, Herrera J, Gilbertsen A, Xia H, Smith K, Benyumov A, et al. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314(1):L127–L36. doi: 10.1152/ajplung.00200.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281(1):L92–L7. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 97.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front. Immunol. 2018;9:2432. doi: 10.3389/fimmu.2018.02432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS ONE. 2011;6(8):e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl Acad. Sci. USA. 2014;111(1):367–72. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong Z, Lu X, Yang Y, Zhang T, Li Y, Chai Y, et al. IL-27 alleviates the bleomycin-induced pulmonary fibrosis by regulating the Th17 cell differentiation. BMC Pulm. Med. 2015;15:13. doi: 10.1186/s12890-015-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raghu, G., Richeldi, L., Crestani, B., Wung. P., Bejuit, R. & Esperet, C. et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772). Eur. Respirat. J.52, (2018). [DOI] [PubMed]
- 103.Parker, J. M, Glaspole, I. N., Lancaster, L. H., Haddad, T J., She, D., Roseti, S.L. et al. A Phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am. J. Resp. Crit Care197, 94–103 (2018). [DOI] [PubMed]
- 104.Swigris JJ, Ogura T, Scholand M, Glaspole I, Maher TM, Kardatzke D, et al. The RIFF Study (Cohort A): a phase II, randomized, double-blind, placebo-controlled trial of lebrikizumab as monotherapy in patients with idiopathic pulmonary fibrosis. Am. J. Resp. Crit. Care. 2018;197:2. doi: 10.1164/rccm.201712-2403ED. [DOI] [Google Scholar]