Abstract
The development of bispecific antibodies that redirect the cytotoxic activity of CD3+ T cells to tumours is a promising immunotherapeutic strategy for the treatment of haematological malignancies and solid cancers. Since the landmark FDA approval at the end of 2014 of the anti-CD3 × anti-CD19 bispecific antibody blinatumomab (Blincyto®) for the treatment of relapsed/refractory B-cell acute lymphoblastic leukaemia, ~100 clinical trials investigating the safety and efficacy of CD3+ bispecific T-cell redirectors for cancer have been initiated. However, despite early success, numerous challenges pertaining to CD3+ T-cell redirection in the context of cancer exist, including the recruitment of counterproductive CD3+ T-cell subsets, the release of systemic cytokines, the expansion of immune checkpoint molecules, the presence of an immunosuppressive tumour microenvironment, tumour antigen loss/escape, on-target off-tumour toxicity and suboptimal potency. The aim of the present review is to discuss novel approaches to overcome the key challenges associated with CD3+ bispecific T-cell redirection in order to achieve an optimal balance of anti-tumour activity and safety.
Subject terms: Oncology, Translational research
Background
The development of antibody therapeutics has transformed the field of cancer immunotherapy. The first successful monoclonal antibody (mAb) therapy in oncology was rituximab (Rituxan®), a mouse–human chimeric immunoglobulin G1 (IgG1) mAb targeting the CD20 antigen on B cells for the treatment of non-Hodgkin’s lymphoma.1 Signalling-induced cell death and Fc-mediated effector functions, including antibody-dependent cellular cytotoxicity (ADCC) and complement fixation, have been identified as key processes that contribute to the multifactorial mechanism of the action of rituximab.2,3
The concept of engineering mAbs directed against cancer antigens expressed on haematological malignancies was subsequently extended to solid cancers and was followed by the design of novel bispecific antibody formats capable of engaging multiple targets. CD3+ bispecific T-cell redirection antibody therapeutics, for example, mechanistically function by bridging T cells with cancer cells. In so doing, the cytolytic activity of CD3+ T cells can be redirected towards tumour cells to facilitate their elimination, independent of the usual requirement for the T cell to be bound to a major histocompatibility complex (MHC) molecule (MHC restriction)4 (Fig. 1). A major advantage associated with bispecific antibodies is that functional activity can be achieved, which would otherwise not be achievable in the context of monovalent antibody combinations. Nearly 25 years after its initial conceptualisation, the first bispecific CD3+ T-cell redirector, catumaxomab (Removab®), was approved by the European Union (EU) for the treatment of malignant ascites in 2009. However, this anti-CD3 × anti-epithelial cell adhesion molecule (EpCAM) antibody was shown to induce off-target hepatotoxicity in patients due to its binding to hepatic macrophages and was later withdrawn from the market due to commercial reasons.5,6
Fig. 1. Mechanism of action of CD3+ bispecific T-cell redirection in cancer.
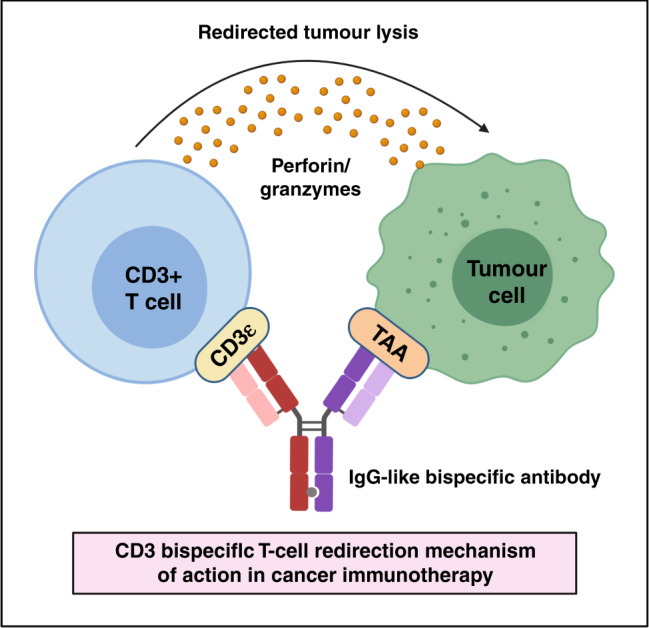
The schematic depicts an IgG-like bispecific antibody simultaneously binding a tumour-associated antigen (TAA) on a cancer cell and CD3 epsilon on a T cell to redirect the cytotoxic activity of T cells to these tumour cells.
The Food Drug Administration (FDA) subsequently approved the anti-CD3 × anti-CD19 bispecific T-cell engager blinatumomab (Blincyto®) for the treatment of patients with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia (ALL).7 FDA approval of this drug was based on the results of the multicentre, open-label, single-arm Phase 2 BLAST clinical trial (NCT01207388), in which 78% of patients with B-cell ALL achieved a complete minimal residual disease (MRD) response after just one cycle of treatment. Compared with MRD non-responders, patients who achieved complete MRD responses also demonstrated longer overall survival (12.5 versus 38.9 months; P = 0.002) and relapse-free survival (5.7 versus 23.6 months; P = 0.002).8 The unprecedented clinical success of blinatumomab has since laid the foundation for the development of CD3+ T-cell-engaging bispecific antibodies. Although blinatumomab is structurally formatted as a bispecific T-cell engager (BiTE)®, additional bispecific antibody designs have been described for CD3+ T-cell redirection (Fig. 2). Next-generation CD3+ bispecific T-cell redirectors are under preclinical and clinical investigation. To date, ~100 clinical trials have been initiated for CD3+ bispecific T-cell redirection in cancer.5,9–11
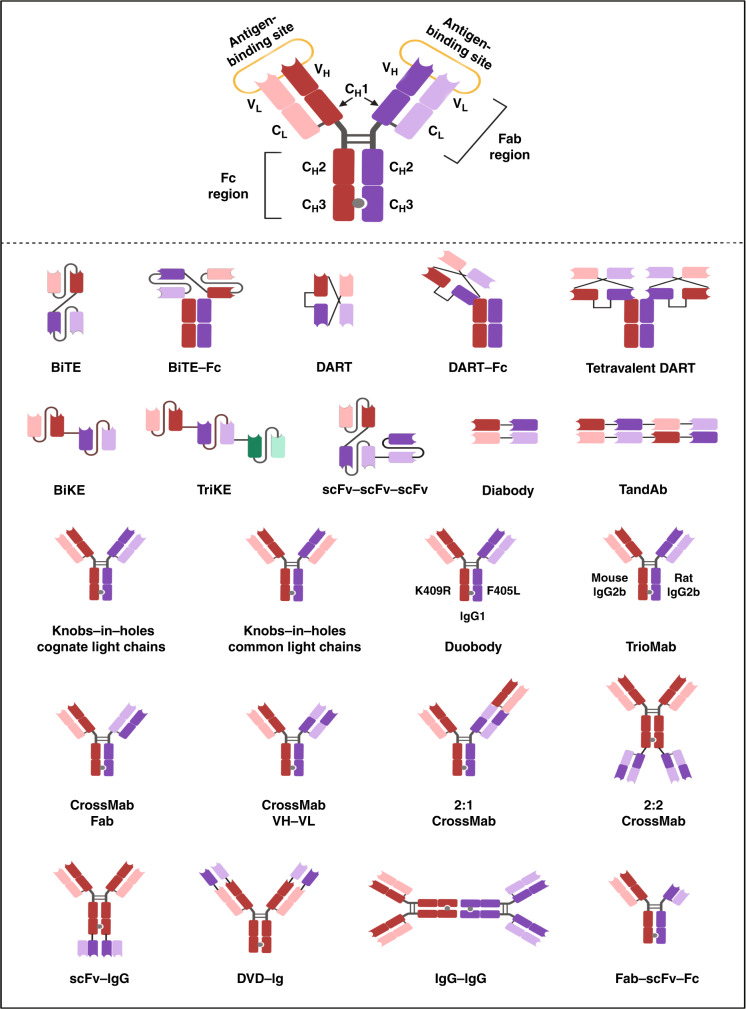
Fig. 2. Bispecific antibody constructs for CD3+ T-cell redirection.
A typical IgG-like bispecific antibody (shown) consists of two heavy chains and two light chains, subdivided into variable domains and constant domains. The Fab region (antigen-binding domain) and Fc region (effector-function domain) are indicated. VH variable heavy, VL variable light, CH constant heavy, CL constant light. Various bispecific antibody formats exist for CD3+ T-cell redirection: bispecific T-cell engager (BiTE), BiTE-Fc; dual-affinity re-targeting (DART), DART-Fc; tetravalent DART; bispecific killer cell engager (BiKE); tri-specific killer cell engager (TriKE); single-chain variable fragment (scFv)-scFv-scFv; diabody; tandem diabody (TandAb); knobs-in-holes cognate light chains; knobs-in-holes common light chains; DuoBody; TrioMab; CrossMab Fab; CrossMab VH-VL; 2:1 CrossMab; 2:2 CrossMab; scFv-IgG; DVD-Ig; IgG-IgG; and Fab-scFv-Fc. DuoBody bispecific antibodies are formatted on an IgG1 backbone and are generated by controlled Fab arm exchange of complementary CH3 domain mutations that promote heterodimerisation (K409R refers to a lysine (K) to arginine (R) amino acid substitution; F405L refers to phenylalanine (F) to leucine (L) amino acid substitution).
Blinatumomab is now approved for the treatment of patients with Philadelphia-negative and -positive relapsed/refractory ALL, as well as those patients with ALL who are in remission but show signs of MRD; however, its use is associated with elevated levels of cytokine release and neurotoxicity, although these toxicities can be clinically managed.10 Indeed, CD3+ T-cell redirection has been reported to be associated with a number of challenges that might limit its use or reduce its anti-tumour efficacy, including the recruitment of counterproductive CD3+ T-cell subsets, dose-limiting cytokine storm, the presence of an immunosuppressive tumour microenvironment (TME), T-cell dysfunction and exhaustion due to expression of immune checkpoint molecules, tumour antigen escape, on-target off-tumour toxicity and suboptimal potency, all of which will be outlined in this article (Table 1). Continued progress in the clinic and momentum in the field of cancer immunotherapy will therefore rely on addressing these major obstacles, and therefore novel strategies to overcome the challenges associated with the use of T-cell redirection are also described herein.
Table 1.
Mitigation strategies to overcome the challenges associated with CD3+ bispecific T-cell redirection in cancer.
| Challenges associated with CD3+ T-cell redirection in cancer | Resulting consequences | Mitigation strategies |
|---|---|---|
| Recruitment of pan T-cell populations | Activation/redirection of counterproductive CD3+ T-cell subsets (e.g., Tregs, naive/exhausted T cells, CD4+ T cells that secrete excessive amounts of cytokines) | Selective redirection of CD8+ cytotoxic T lymphocytes or CD8+ tissue-resident memory T cells |
| Cytokine release syndrome | Cytokine storm (life-threatening immune-related adverse event); dose-limiting; efficacy-limiting; narrow therapeutic window | Pharmacological intervention with IL-6R antagonists, TNF-α blockers, or corticosteroids; quantitative cytokine modelling to improve clinical dosing strategy; sequence-based antibody discovery to decouple cytokine release from anti-tumour activity |
| Expression of immune checkpoint molecules | T-cell dysfunction and exhaustion lead to suppressed anti-tumour immunity | Combination therapy with co-inhibitory receptor antagonists (e.g., anti-PD-1, anti-CTLA-4) or co-stimulatory receptor agonists (e.g., anti-4-1BB, anti-CD28) |
| Immunosuppressive tumour microenvironment | Hostile TME components (e.g., stromal cells, immune cells, cytokines, soluble factors) contribute to suppression of anti-tumour immune response | Combination therapy with IL-10 inhibitors, adenosine receptor antagonists, or VLA4 inhibitors; oncolytic adenoviruses armed with CD3 redirectors that target immunosuppressive stromal cells |
| Tumour antigen escape | Resistance to CD3+ T-cell redirection | Development of CD3+ T-cell redirectors with dual tumour antigen targeting |
| Lack of tumour antigen selectivity | On-target off-tumour toxicity | Protease-activated T-cell engagers; hemibodies; development of a multivalent avidity-based antigen-binding strategy to generate bispecific antibodies that selectively redirect T cells towards tumour cells while avoiding healthy tissue |
| Suboptimal potency | Diminished anti-tumour immune response | Consideration of target antigen copy number and size; modulation of binding domain positioning (e.g., epitope distance) and valency |
Recruitment of pan T-cell populations
CD3+ bispecific T-cell engagers function by redirecting all T-cell populations towards cancer cells to facilitate efficient tumour cell killing. However, several disadvantages are associated with polyclonal T-cell activation and redirection, including the recruitment of ‘counterproductive’ CD3+ T-cell subsets, such as naive/exhausted T cells, regulatory T (TREG) cells and other CD4+ T-cell populations.12–14 In fact, resistance to blinatumomab has been attributed to the activation and recruitment of CD3+ CD4+ CD25hi FoxP3+ TREG cells, which have been shown to suppress T-cell proliferation and hamper CD8+-mediated tumour cell lysis, ultimately compromising the efficacy of blinatumomab treatment in patients with precursor B-cell ALL.15 In addition to TREG cells, excessive recruitment of other CD4+ T-cell populations, including the helper T (TH) cell subsets TH1 and TH17, can be detrimental because these cells can rapidly secrete a milieu of cytokines leading to severe immune-related adverse events such as cytokine storm, which is outlined below.16,17
Improving the selectivity of T-cell-engaging bispecific antibodies
The selective recruitment of CD8+ cytotoxic T lymphocytes (CTLs)—so-called foot soldiers of the immune system—to tumour cells represents a more productive approach to drive potent anti-tumour immune responses compared with non-specific pan CD3+ T-cell redirection, as professional CD8+ CTLs are equipped with perforin/granzymes and can initiate killing immediately upon crosslinking with tumour cells.18,19 However, a bispecific antibody targeting the CD8 co-receptor and prostate stem cell antigen (PSCA) constructed to test this approach was able to efficiently engage only pre-activated CD8+ T cells and redirect them for tumour cell lysis—freshly isolated CD8+ T cells could not be activated/redirected—thus signifying the importance of both pre-activation and co-ligation in inducing selective tumour killing.17 The tissue-resident memory (TRM) CD8+ CD69+ CD103+ T-cell phenotype has also emerged as an important T-cell population for selective retargeting to tumour cells, as this memory CD8+ T-cell subset expresses high levels of cytotoxic molecules and is associated with a good clinical outcome in cancer.20 Menares et al.21 also demonstrated, in 2019, a crosstalk between TRM T cells and dendritic cells that leads to an amplified CD8+ CTL-mediated anti-tumour immune response. In view of these results, research efforts are underway to improve the selectivity of T-cell-engaging bispecific antibodies in favour of more potent tumour cell killing accompanied by reduced toxicity.
Cytokine release syndrome
Cytokine release syndrome is a severe immune response that is characterised by the rapid, systemic release of pro-inflammatory cytokines, including interleukin (IL)-6, IL-10, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). Elevated levels of these core cytokines induce immune cells to produce excessive amounts of additional cytokines, creating an amplified phenomenon referred to as a ‘cytokine storm’.22,23 This immune-related adverse event is frequently observed in response to chimeric antigen receptor (CAR)-T cell therapy or T-cell redirection therapy and presents a major challenge in the context of CD3+ T-cell redirection because the infused bispecific antibodies indiscriminately bind to all available T cells, leading to a massive release of cytokines. Indeed, blinatumomab and two FDA-approved CAR-T cell therapies, tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta), have been shown to induce cytokine release syndrome in patients with leukaemia and lymphoma.24 Clinical manifestations of cytokine release syndrome include fever, fatigue, myalgia, respiratory distress, capillary leakage, vasodilatory shock and eventual organ system failure.22 CD19-CAR-T cells armed with anti-CD3 × anti-HER2 or anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibodies have been developed to address the cytokine release syndrome.25 This approach is self-limiting and holds potential for reducing the risk of the occurrence of a cytokine storm as the amount of bispecific antibody on the bispecific antibody-armed CAR-T cell decreases after multiple cell divisions.
Managing the cytokine release syndrome using pharmacological approaches
A number of pharmacological approaches have been investigated to overcome the cytokine release syndrome associated with T-cell redirection therapies. Monocyte activation mediated by TNF-α generated by T-cell triggering has been demonstrated to be a primary source of systemic cytokine release after treatment with anti-CD3 × anti-HER2 bispecific antibodies and, interestingly, TNF-α blockade alone was sufficient to impair cytokine release without affecting the cytotoxic activity of the T-cell-redirecting antibodies.26 Furthermore, administration of the immunosuppressant dexamethasone prior to treatment with an anti-CD3 × anti-glypican 3 (GPC3) bispecific antibody led to significant inhibition of cytokine release without compromising the anti-tumour activity in a humanised mouse model.27 In another study, pre-treatment with the anti-IL-6 receptor antibody tocilizumab prior to administration of an anti-CD3 × anti-prostate-specific membrane antigen (PSMA) bispecific antibody attenuated the cytokine release syndrome while maintaining therapeutic activity in patients with castration-resistant metastatic prostate cancer.28 However, in this same study, dexamethasone treatment prior to bispecific antibody therapy was shown to negatively impact anti-tumour efficacy, as evidenced by suppression of T-cell proliferation and inhibition of tumour lysis.28 Similarly, the data from a Phase 1 clinical trial showed that dexamethasone treatment did not increase the maximum tolerated dose of an anti-CD3 × anti-carcinoma embryonic antigen (CEA) bispecific antibody (NCT01284231). Taken together, the results of these studies suggest that co-administration of CD3+ T-cell-redirecting therapies with steroids, anti-TNF-α blockers or IL-6 receptor targeting antibodies might, in certain circumstances, help to mitigate the cytokine storm associated with T-cell redirection.29 The outcomes of such combinatorial approaches are context-dependent and could be influenced by the specific mechanism of a given T-cell redirector. Thus, a deeper mechanistic understanding of the relationship between T-cell activation and the cytokine storm is warranted. The identification of clinical biomarkers that are predictive of a cytokine release syndrome will aid in the future development of novel therapeutic agents and approaches that are capable of mitigating the cytokine storm in the context of T-cell redirection. Of note, regression modelling has been used to identify a cytokine signature in patients that is predictive of the development of the cytokine release syndrome after CAR-T cell treatment.30
Novel strategies to mitigate the cytokine release syndrome
Although the cytokine release syndrome has the potential to be managed in part as described above, research efforts are focused on developing novel strategies to mitigate the syndrome to increase the therapeutic window for T-cell-engaging therapies. The unprecedented cytokine storm observed in six healthy volunteers within 90 min of receiving TGN1412, a super-agonist antibody targeting the T-cell costimulatory receptor CD28, sent shockwaves throughout the scientific community as this life-threatening inflammatory response was not predicted by preclinical toxicology models.24,31 Preventing future occurrences of this magnitude in other T-cell-based immunotherapies quickly became a priority. Since this devastating Phase 1 clinical trial, different clinical dosing regimens for T-cell-engaging therapeutic antibodies have been considered. A priming dose strategy has been tested in the clinic to mitigate the toxicities induced by the cytokine release syndrome—this approach involves administration of a lower initial dose followed by a higher maintenance dose (NCT02152956).32,33 As the methodology of selecting an optimal priming dosing regimen is empirical by nature, quantitative cytokine modelling was later introduced to improve the design of clinical dosing strategies to achieve efficacy while minimising cytokine release syndrome-related toxicities. Indeed, a novel pharmacokinetic–pharmacodynamic (PK–PD) model developed in 2019 simulates the cytokine release profiles in response to treatment with T-cell-redirecting therapies.33 A quantitative systems pharmacology model was subsequently established to enable the accurate prediction of safety- and efficacy-related biomarkers to reduce the risk of cytokine storm with the anti-CD3 × anti-CD20 bispecific antibody mosunetuzumab in non-Hodgkin lymphoma (NCT02500407).34
Although quantitative modelling has been used to determine the optimal dosing to mitigate the cytokine release syndrome associated with current T-cell-engaging antibodies, next-generation CD3+ T-cell-recruiting bispecific antibodies have been engineered to directly address the challenge of limiting the dose to prevent cytokine release syndrome. Initial anti-CD3+ antibody generation efforts were often biased towards high-affinity CD3 binders, which elicited not only potent tumour cell killing, but also high levels of cytokine release. The identification of naturally occurring anti-CD3 antibodies with varying binding affinities and T-cell activation profiles soon became a priority. A next-generation sequence (NGS)-based antibody discovery platform established in 2019 to identify anti-CD3 antibodies from humanised rats that bind to multiple CD3 epitopes with a diverse range of binding strengths was used to identify lead anti-CD3-binding domains, which were then formatted as bispecific antibodies.35 In particular, the anti-CD3 × anti-BCMA bispecific T-cell redirector that emerged from this antibody screen was shown to elicit robust tumour antigen-specific killing in a mouse xenograft model with almost undetectable cytokine release. Importantly, this work provides proof of concept for uncoupling of tumour cell cytotoxicity from cytokine release, thus relieving the dose constraints associated with T-cell engagers and broadening the therapeutic index. Collectively, these results show that PK–PD modelling and sequence-based antibody discovery strategies can be used to mitigate cytokine release syndrome-related toxicities from T-cell-redirecting antibody therapeutics.
Additional next-generation T-cell-recruiting bispecific antibodies have been described, including an elegant body of work that demonstrated the use of bispecific antibodies to retarget ex vivo expanded T cells to solid and haematological malignancies such as non-Hodgkin’s lymphoma, multiple myeloma, pancreatic cancer, breast cancer and prostate cancer. These armed T-cell infusions not only show preclinical and clinical anti-tumour efficacy, with or without stem cell transplantation, but, importantly, avoid cytokine release syndrome. The lack of apparent dose-limiting toxicity provides a rationale for the use of bispecific antibody-armed activated T-cell infusions as a strategy to overcome the challenge of cytokine release syndrome.36–41
Expression of immune checkpoint molecules
Inhibitory checkpoint molecules and stimulatory checkpoint molecules function alongside each other to ensure that the immune system can mount a response against ‘foreign’ bodies but that this response is controlled, in terms of type, magnitude and duration, in order to maintain self-tolerance and prevent autoimmunity. It is well established that inhibitory molecules expressed on the surface of T cells or tumour cells can promote T-cell dysfunction and exhaustion, leading to a suppressed anti-tumour immune response. Examples of such co-inhibitory immune checkpoint molecules include programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), lymphocyte-activation gene 3 (LAG3) and T-cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT).42 Enrichment of immune checkpoint receptor expression was observed in CD8+ CD69+ CD103+ human T cells with a TRM phenotype, and anti-PD-1 treatment led to a significant expansion of this T-cell population in melanoma patients.43 Moreover, anti-PD-1 therapy was shown to enhance the infiltration of tissue-resident CD8+ memory T cells in a mouse model of melanoma.44
CD3+ bispecific T-cell redirection combined with immune checkpoint co-inhibitors
Combinatorial immunotherapy approaches have been designed to counteract the mechanisms of T-cell dysfunction/exhaustion using inhibitory checkpoint-blocking antibodies to improve the clinical outcomes of individuals receiving CD3+ bispecific T-cell redirection therapies. Interestingly, resistance to blinatumomab treatment has been attributed to an increase in PD-L1 expression on CD19+ leukaemic cells.45 Since this discovery, a number of clinical trials evaluating the safety and efficacy of blinatumomab in combination with pembrolizumab (anti-PD-1 therapy) for the treatment of lymphoma or leukaemia have been initiated (NCT03160079, NCT03605589, NCT03340766, NCT03512405). Furthermore, the addition of the dual immune-checkpoint blockade to blinatumomab therapy is under investigation in the clinic (e.g., a Phase 1 trial of blinatumomab and nivolumab (an anti-PD-1 antibody) with or without ipilimumab (an anti-CTLA-4 antibody) for the treatment of leukaemia is underway (NCT02879695)). A bifunctional checkpoint inhibitory T-cell engager (CiTE) that redirects T cells to CD33 (expressed on acute myeloid leukaemia (AML) cells), combined with the extracellular domain of PD-1 to bind weakly to, but inhibit engagement of PD-L1, demonstrated early preclinical success in mitigating adaptive immune escape in patients with AML.46 Notably, anti-CD3 × anti-PD-1 × anti-HIV and anti-CD3 × anti-TIGIT × anti-HIV trifunctional T-cell engagers have demonstrated efficacy in enhancing CD8+ T-cell effector functions in SHIV-infected rhesus macaques;47 this model can probably be applied in the context of cancer. Immune checkpoint inhibition coupled with CD3+ bispecific T-cell redirection has also been examined in solid tumours. The safety and efficacy of MGD007 (anti-CD3 × anti-gpA33 bispecific antibody) in combination with MGA012 (an anti-PD-1 antibody) is currently under clinical investigation in a Phase 1/2 trial for metastatic colorectal cancer (NCT03531632). A quantitative systems pharmacology model and biomarker strategy revealed that combination therapy comprising an anti-CD3 × anti-CEA bispecific antibody with anti-PD-L1 treatment led to an enhancement of anti-tumour activity in patients with colorectal cancer.48
CD3+ bispecific T-cell redirection combined with immune checkpoint co-stimulators
Although antagonising co-inhibitory receptors on T cells has proven to be a promising approach for enhancing the anti-tumour activity of CD3+ T-cell redirectors, there is also evidence that agonistic antibodies that are capable of activating co-stimulatory receptors on T cells, including members of the tumour necrosis factor receptor (TNFR) superfamily (e.g., 4-1BB, OX40, GITR, CD27) or immunoglobulin superfamily (e.g., CD28, ICOS), can be used to boost immune responses to CD3+ T-cell-engaging therapies. Notably, an agonistic anti-4-1BB antibody was shown to restore the functionality of exhausted CD8+ tumour-infiltrating lymphocytes in a mouse model of melanoma.49 Likewise, stimulation of 4-1BB signalling with an agonistic anti-4-1BB antibody led to the prolonged persistence of cytotoxic T lymphocytes and their enhanced function in a B16F10-OVA melanoma mouse model.50 Anti-4-1BB co-stimulation was also demonstrated to enhance the anti-tumour activity of an anti-CD3 × anti-PSMA bispecific antibody both in vitro and in a syngeneic mouse model of prostate cancer.51 Furthermore, combination therapy of fibroblast activation protein (FAP)-4-1BBL with a CEA-targeted T-cell bispecific antibody plus CD19-4-1BBL with a CD20-targeted T-cell bispecific antibody led to significant tumour regression in mouse models and intratumoural accumulation of activated CD8+ cytotoxic T cells. Mechanistically, in the presence of T-cell receptor signalling, tumour antigen-targeted 4-1BB agonists induce 4-1BB stimulation in response to crosslinking with tumour antigen-expressing cells without relying on FcγR-mediated crosslinking.52 As well as 4-1BB, other co-stimulatory molecules such as CD28 have been shown to improve anti-tumour immune responses to CD3+ T-cell-redirecting antibodies. Treatment of cancer cells with anti-CD3 × anti-tumour antigen and anti-CD28 × anti-tumour antigen cross-interacting bispecific antibodies led to enhanced tumour cell-dependent T-cell activation in vitro.53 Moreover, anti-CD28 × anti-PSMA and anti-CD28 × anti-mucin 16 (MUC16) bispecific antibodies were shown to potentiate in vivo anti-tumour activity of CD3+ bispecific T-cell redirectors in humanised immunocompetent mouse models of prostate and ovarian cancer, respectively.54 Importantly, combination therapy did not elicit toxicity in cynomolgus monkeys in vivo. These results indicate that the expression profile of co-stimulatory (and co-inhibitory) molecules on patient T cells or tumour cells can inform of potential combination therapies with CD3+ T-cell redirectors.
Immunosuppressive TME
Fundamental differences exist in the disease phenotype of haematological malignancies and solid cancers, an obvious one being the highly immunosuppressive TME that is a characteristic feature of solid tumours. Major components of the TME of solid tumours include anti-tumour immune cells together with stromal cells, such as cancer-associated fibroblasts (CAFs), TREG cells, myeloid-derived suppressor cells (MDSCs) and tumour-associated macrophages (TAMs), that function in suppressing anti-tumour immunity.55–57 Immunosuppressive cytokines and soluble factors including adenosine, IL-10, transforming growth factor β (TGF-β), and indoleamine 2,3-dioxygenase (IDO) also reside in the hostile TME.
Targeting the hostile TME in conjunction with CD3+ bispecific T-cell redirection
Disrupting the reactive stroma that surrounds solid tumours represents a viable approach to increase the anti-tumour activity of T-cell-engaging bispecific antibody therapeutics. In fact, adenosine and IL-10 have been demonstrated to directly inhibit CD8+ T-cell function, thus providing a rationale for combination therapy of CD3+ T-cell redirectors with adenosine receptor antagonists or IL-10 inhibitors.58,59 Furthermore, an oncolytic adenovirus armed with an anti-CD3 × anti-FAP-α bispecific antibody redirected the cytotoxic activity of T cells towards CAFs residing in the stroma, thereby eliminating these stromal cells and facilitating the penetration and spread of the adenovirus to ultimately enhance the viral oncolysis of cancer cells.60 In addition, anti-CD3 × anti-CD206 and anti-CD3 × anti-folate receptor β (FRβ) T-cell engagers demonstrated preferential T-cell-mediated killing of M2 polarised TAMs in vitro,61 and localisation of the expression of these T-cell engagers to tumours using the oncolytic virus enadenotucirev caused a reversal of the immunosuppressive TME of solid tumours via targeted TAM depletion and potent cancer cell cytotoxicity.
As with solid cancers, the TME of haematological malignancies also presents a challenge to T-cell-redirecting therapies. The bone marrow niche in haematological cancers is a specialised microenvironment that contains soluble growth factors necessary for the survival of resident cancer stem cells (CSCs). The persistence of malignant progenitor cells such as CSCs in the bone marrow is linked to MRD and is a predominant source of resistance to antibody therapeutics. Nair-Gupta et al.62 demonstrated in 2020 that co-culture of bone marrow stromal cells with AML or multiple myeloma cells prevented the lysis of tumour cells by anti-CD3 × anti-CD123 or anti-CD3 × anti-BCMA bispecific T-cell redirectors in vitro and in vivo. Strikingly, however, inhibition of the integrin VLA4, which mediates interactions between tumour and stromal cells, not only reversed this stromal-mediated immunosuppression, but restored the sensitivity of leukaemic and myeloma tumour cells to CD3+ bispecific T-cell redirectors, as evidenced by robust in vitro and in vivo anti-tumour activity. Thus, targeting the hostile TME in combination with CD3+ bispecific T-cell redirection therapies constitutes a promising approach to overcome stroma-mediated immunosuppression and elicit greater anti-tumour immune responses.
Physiologically relevant models to assess combinatorial approaches
Given the complexity of the TME, designing optimal preclinical models that recapitulate human physiology and the disease phenotype remains a priority—the development of preclinical models that translate to human immunity was described by Hegde et al.63 as being a top key challenge in cancer immunotherapy. Three-dimensional multicellular tumour spheroids and ‘tumour on a chip’ microfluidics devices are examples of next-generation platforms that have been developed to increase preclinical-to-clinical translation of drug candidates.64,65 Such complex in vitro models are engineered to incorporate extracellular matrix components, soluble factors, tissue barriers, hypoxia gradients, interstitial pressure and vascular perfusion to recreate organ-specific architecture. Stromal cells, immune cells and endothelial cells can also be integrated into these micro-physiological tumour models to enable a deeper understanding of the interactions between cancer cells and their surrounding TME. Indeed, lysis mediated by T-cell-receptor (TCR)-engineered T cells of hepatocellular carcinoma cells embedded in a 3D collagen microenvironment was demonstrated using a microfluidics platform.66 Moreover, proof of concept was established for the assessment of tumour cell cytotoxicity by TCR-engineered T cells under hypoxic and inflammatory conditions in the microfluidics device. Physiologically relevant complex in vitro models can therefore represent powerful preclinical tools that can be used to evaluate such combinatorial immunotherapies to better predict translational success.
Tumour antigen escape and lack of tumour antigen specificity
The loss of expression of tumour antigens is another factor that can compromise the efficacy of CD3+ bispecific T-cell redirection therapies. For example, 10–20% of patients with B-cell ALL have been shown to experience resistance to blinatumomab treatment due to CD19 antigen loss.67 The emergence of CD19− leukaemic blasts during blinatumomab therapy has been mechanistically linked to disruptions in the trafficking of CD19 to the membrane.
Overcoming tumour antigen escape
The development of novel CD3+ T-cell redirectors with the ability to target two tumour antigens is a potential strategy that can be used to overcome tumour antigen escape and restore anti-tumour immunity.67 In fact, the strategy of simultaneously targeting two tumour antigens using bispecific tandem CARs has already been demonstrated to mitigate tumour antigen escape.68,69 A single-nucleotide polymorphism (SNP) present in the V domain of CD33 is known to limit the anti-tumour activity of anti-CD33 antibodies due to antigen loss, but the development of a novel bispecific antibody that targets CD3 and the C2 domain of CD33 resulted in T-cell-mediated tumour lysis independent of the SNP genotype.70 These findings indicate a therapeutic benefit in designing CD3+ T-cell redirectors that target the C2 domain and not the V domain of the CD33 epitope for the treatment of AML.
Improving tumour antigen specificity
Identifying antigenic targets that are specific to tumour cells and not expressed on healthy tissue is another challenge associated with developing CD3+ bispecific T-cell redirectors, especially for the treatment of solid malignancies. Even low-level antigen expression on normal cells can lead to detrimental on-target off-tumour toxicities in which the cytolytic activity of CD3+ T cells is directed towards healthy tissue. Preclinical studies suggest that tuning the affinity of CD3+ bispecific T-cell engagers towards target antigens is a rational approach to achieve an optimal balance of anti-tumour activity and safety.71 To reduce the occurrence of T-cell autoreactivity towards antigen-expressing vital organs, a multivalent avidity-based antigen-binding design strategy has been developed to generate an anti-CD3 × anti-HER2 bispecific antibody capable of selectively killing HER2-expressing tumour cells.72 Selective redirection of CD3+ T cells towards HER2+ cancer cells was achieved by engineering the anti-CD3 × anti-HER2 bispecific antibody with two low-affinity anti-HER2 Fab arms that engage HER2-overexpressing tumour cells with a high antigen density relative to healthy tissue. This seminal work provides proof of concept for redirecting the cytotoxic activity of T cells to tumour cells while sparing normal tissue and could be applicable to other solid tumour targets that are challenged by on-target off-tumour toxicity.
Another means to address the lack of tumour antigen specificity associated with CD3+ T-cell redirection involves the use of a conditionally active COBRA (COnditional Bispecific Redirected Activation) T-cell engager platform. COBRAs are prodrugs that are selectively activated upon cleavage by matrix metalloproteases (MMPs) in the protease-rich TME.73 This selective activation enables them to remain as inactive prodrugs in normal tissue where the proteolytic activity is tightly regulated but become active T-cell engagers in the context of tumours, where proteases are ubiquitously expressed. Indeed, COBRA constructs with an MMP-9 cleavable linker targeting anti-CD3, anti-EGFR and anti-human serum albumin were shown to induce tumour regression in a xenograft mouse model of colorectal cancer.73 Additional protease-activated T-cell engagers have also been described. Tumour specificity was demonstrated using an anti-folate receptor 1 (FOLR1) T-cell bispecific antibody engineered with an anti-idiotypic anti-CD3 ‘mask’ joined to an anti-CD3 Fab region via a protease-cleavable linker. ‘Unmasking’ of the anti-CD3 targeting moiety by cleavage by active proteases in the TME led to selective killing of FOLR1-expressing tumour cells in vitro and in vivo, while sparing normal cells with low FOLR1 expression.74 Next-generation designs of masked antibodies that are capable of being selectively unmasked at tumour sites have been optimised. Incorporation of a heterodimeric coiled-coil domain into an antibody construct was shown to be an effective and generalisable approach for masking the complementary-determining regions (CDRs) of an antibody, with selective cleavage of coiled-coil peptides by tumour-associated proteases restoring antigen binding while overcoming on-target off-tumour toxicity.75 Moreover, preclinical proof-of-concept studies demonstrated that such probody therapeutics improved the safety profile of EGFR-targeting antibodies and broadened the therapeutic index.76 A first-in-human Phase 1/2 clinical trial evaluating the safety and tolerability of a PD-L1 probody therapeutic (CX-072) as monotherapy or in combination with ipilimumab or vemurafenib for the treatment of solid tumours or lymphomas (NCT03013491) is currently underway.
In addition to protease-activated T-cell engagers, a novel split T-cell engaging antibody format—referred to as a hemibody (e.g., an antigen-specific scFv fused to the VH or VL region of an anti-CD3 antibody)—has been described. Simultaneous engagement of two complementary hemibodies with their respective antigens on a single target cell activated the CD3-binding site and redirected CD3+ T cells for tumour cell lysis. Using this approach, hemibodies can selectively kill dual-antigen-positive tumour cells while sparing cells that express a single antigen, thereby overcoming the limitation of off-target toxicity commonly associated with CD3+ T-cell redirection.77
Suboptimal potency
Improving the potency of CD3+ bispecific T-cell redirectors is an ongoing challenge. Both the size of the target antigen and the distance of the target epitope to the T-cell membrane have been identified as important factors contributing to the anti-tumour activity of CD3+ T-cell engagers.78,79 Interestingly, anti-CD3 × anti-chondroitin sulphate proteoglycan 4 (CSPG4) bispecific antibodies demonstrated greater potency, in terms of T-cell-mediated tumour lysis, when the size of the target antigen was smaller. Moreover, binding of the anti-CD3 × anti-CSPG4 bispecific antibody to the membrane-proximal domain D3 of CSPG4 rather than the distal domain elicited a more potent anti-tumour immune response by the redirected T cells.78 Additional factors, such as the copy number and valency of tumour antigens, have also been shown to affect the potency of bispecific T-cell redirectors.79 Collectively, these preclinical data suggest that awareness of target antigen size/copy number and modulation of binding domain positioning and valency should be considered in the design of more potent, best-in-class CD3+ T-cell redirectors.
Concluding remarks and future directions
Antibody-based therapy—particularly CD3+ bispecific T-cell redirection—has revolutionised the field of cancer immunotherapy.80 From a preclinical aspect, anti-tumour activity in both haematological malignancies and solid cancers has been achieved with CD3+ T-cell engaging bispecific antibodies by addressing some of the major obstacles inherent to this therapeutic class, such as the expression of inhibitory immune checkpoint molecules, the presence of an immunosuppressive TME, suboptimal potency, escape of tumour antigens, cytokine storm and on-target off-tumour toxicity. Encouragingly, many of these novel CD3+ bispecific T-cell antibodies have progressed to clinical trials and next-generation designs of CD3+ bispecific T-cell redirectors are being characterised.
Intriguingly, preclinical evidence has demonstrated that in vitro BiTE-mediated tumour cell cytotoxicity is enhanced by the expression of CD80/CD86 co-stimulatory molecules on tumour cells.81,82 The engagement of CD80/CD86-activating ligands on cancer cells with their cognate receptor (CD28) on naive T cells provides co-stimulation to support T-cell activity.83 These data are further corroborated by robust evidence suggesting that CAR-T cell potency is enhanced upon the incorporation of co-stimulatory domains (e.g., CD28, 4-1BB) into the CAR backbones.84 However, although co-stimulation via CD28 has provided benefit to BiTEs and CAR-T cells alike by enhancing anti-tumour activity without inducing toxicity, cases have arisen where too much co-stimulation has induced life-threatening immune-related adverse events such as cytokine storm, as observed using the anti-CD28 super-agonist TGN1412.24 As such, optimising co-stimulatory signalling is important for achieving anti-tumour activity while minimising toxicity. Accordingly, simultaneous multiple interaction T-cell-engaging (SMITE) bispecific antibodies, comprising a combination of two BiTE antibodies, have been developed.85 In fact, T-cell co-stimulation with an anti-CD28 × anti-ROR1 BiTE was shown to enhance the anti-tumour activity of a CD3 × ROR1 BiTE in K562 cells transduced with ROR1. In addition, co-activation of T cells with a CD28 × PD-L1 BiTE and a paired CD3 × CD19 BiTE was demonstrated to overcome BiTE resistance by reversing immune checkpoint inhibition in PD-L1-expressing cancer cells.85 Furthermore, a bispecific antibody retargeted CAR, comprising an extracellular folate receptor fused to intracellular TCR and CD28 costimulatory domains in tandem, has been developed.86 This work demonstrated proof of concept for combining bispecific antibodies with genetically engineered T cells, which led to simultaneous TCR activation/co-stimulation and induced a tumour-antigen-specific T-cell response. CD3+-redirecting tri-specific antibodies have also been designed to bridge T cells to tumour cells in the presence of co-stimulation. For example, the anti-CD3 × anti-CD28 × anti-CEA tri-specific antibody was shown to mediate tumour-specific cytolysis of CEA-expressing cancer cells in vitro.87 Along the same lines, an anti-CD3 × anti-CD28 × anti-CD38 tri-specific T-cell redirector antibody has been developed (Fig. 3), which, when administered in vivo, led to the proliferation of effector/memory T-cell populations and the suppression of tumour growth in CD38-expressing melanoma.88 Next-generation CD3+ T-cell engagers that exploit signalling through co-stimulatory molecules appear to represent promising antibody therapeutics for cancer immunotherapy.
Fig. 3. Next-generation anti-CD3 × anti-CD28 × anti-CD38 tri-specific T-cell-engaging antibody.
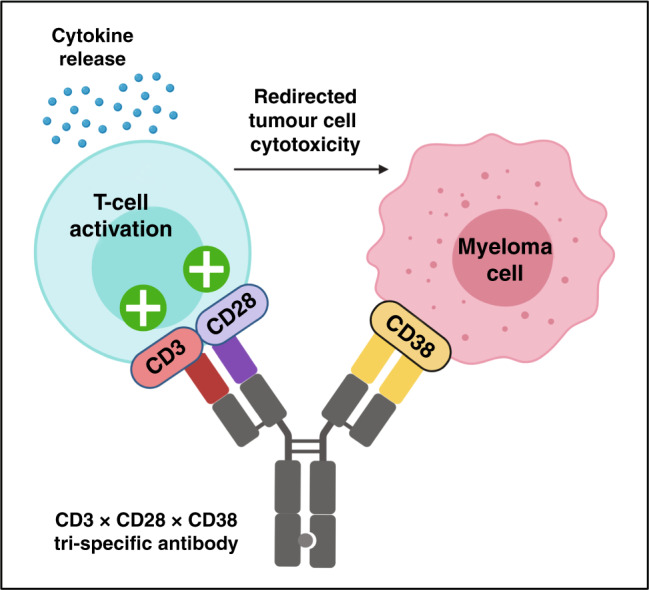
The diagram depicts a tri-specific antibody targeting anti-CD3ε and anti-CD28 on T cells as well as anti-CD38 on multiple myeloma cells. Co-engagement of CD3, CD28 and CD38 results in efficient T-cell stimulation and activation, cytokine release, and redirection of T-cell-mediated cytotoxicity to myeloma cells.
A novel T-cell redirection strategy called STAb immunotherapy, which involves the endogenous secretion of T cell-redirecting bsAbs (bispecific antibodies), has also emerged as a promising platform in oncology.89 In this approach, T cells are genetically engineered to secrete bispecific T-cell-redirecting antibodies intratumourally (known as ‘on-tumour’ STAbs) or at a distant site (‘off-tumour’ STAbs). This platform addresses some of the challenges associated with systemic antibody administration, such as inadequate tumour penetration, on-target off-tumour toxicity, and short serum half-life. Proof of concept for STAb cancer immunotherapy has been demonstrated in preclinical in vitro and in vivo studies.90–92
Overall, substantial progress has been made towards the development of CD3+ bispecific T-cell redirectors for cancer immunotherapy. A diverse panel of haematological and solid tumour targets have been characterised for CD3+ T-cell redirection, and the safety and efficacy of ~100 antibodies in this therapeutic class are currently under investigation in the clinic for the treatment of haematological cancers and solid tumours (Tables 2 and 3). Nonetheless, a number of challenges associated with CD3+ T-cell redirection exist that can limit anti-tumour efficacy. As many of these challenges are common to other cancer immunotherapy approaches such as CAR-T cell therapy,93 lessons learned from these studies can potentially be applied to CD3+ T-cell redirection. Future directions in the field of cancer immunotherapy are likely to involve identifying optimal combination therapies with CD3+ bispecific T-cell redirectors that enhance anti-tumour immunity while minimising toxicity.
Table 2.
Clinical trials of CD3+ bispecific T-cell redirectors for haematological cancers.
| Targets | Clinical trial Phase | Clinical trial identifier | Disease indication(s) | Organisation(s) | Antibody |
|---|---|---|---|---|---|
| CD3 × CD19 | Launched | NCT01207388 | B-cell precursor ALL | Amgen | Blinatumomab; Blincyto; AMG 103 |
| CD3 × CD19 | Phase 1 | NCT03571828 | B-cell NHL; DLBCL; MCL; FL | Amgen | AMG 562 |
| CD3 × CD19 | Phase 1 | NCT04056975 | B-cell lymphoma | Generon | A-319 |
| CD3 × CD19 | Phase 1 | CTR20191955 | B-cell lymphoma | Lvzhu Biopharmaceutical | K193 |
| CD3 × CD20 | Phase 1/2 | B-cell NHL; CLL | Regeneron | REGN1979 | |
| CD3 × CD20 | Phase 1 | NCT04082936 | B-cell NHL; DLBCL; MCL; FL; MZL | IGM Biosciences | IGM-2323 |
| CD3 × CD20 | Phase 1 | NCT02924402 | B-cell NHL; CLL; small lymphocytic lymphoma | Xencor | Plamotamab; XmAb13676 |
| CD3 × CD20 | Phase 1/2 | B-cell NHL; CLL; DLBCL | Genentech; Hoffmann-La Roche | Mosunetuzumab; BTCT4465A; RO7030816 | |
| CD3 × CD20 | Phase 1/2 | NCT03625037 | DLBCL; MCL; FL | Genmab | GEN3013 |
| CD3 × CD20 | Phase 1 | B-cell NHL; DLBCL; FL | Hoffmann-La Roche | Glofitamab; RO7082859 | |
| CD3 × CD20 | Phase 1 | B-cell NHL; multiple myeloma; plasma cell neoplasm | Barbara Ann Karmanos Cancer Institute | CD20Bi-ATC | |
| CD3 × CD33 | Phase 1 | NCT03915379 | Myelodysplastic syndrome; AML | Janssen | JNJ-67571244 |
| CD3 × CD33 | Phase 1 | NCT02520427 | Myelodysplastic syndrome; AML | Amgen | AMG 330 |
| CD3 × CD33 | Phase 1 | NCT03224819 | AML | Amgen | AMG 673 |
| CD3 × CD33 | Phase 1 | NCT03516760 | AML | Celgene | GEM333 |
| CD3 × CD33 | Phase 1 | Myelodysplastic syndrome; AML | Amphivena Therapeutics | AMV564 | |
| CD3 × CD38 | Phase 1/2 | NCT03309111 | Multiple myeloma | Glenmark Pharmaceuticals | GBR 1342 |
| CD3 × CD38 | Phase 1 | NCT03445663 | Multiple myeloma | Amgen | AMG 424 |
| CD3 × CD123 | Phase 1 | NCT03647800 | Myelodysplastic syndrome; AML | Aptevo Therapeutics | APVO436 |
| CD3 × CD123 | Phase 1/2 | AML; ALL; other haematologic malignancies | MacroGenics | Flotetuzumab; MGD006 | |
| CD3 × CD123 | Phase 1 | NCT02730312 | B-cell ALL; AML; CLL; blastic plasmacytoid dendritic cell neoplasm | Xencor | Vibecotamab; XmAb14045 |
| CD3 × CD123 | Phase 1/2 | NCT03594955 | B-cell ALL; AML; myelodysplastic syndrome | Sanofi | SAR440234 |
| CD3 × CD123 | Phase 1 | NCT02715011 | AML | Janssen | JNJ-63709178 |
| CD3 × BCMA | Phase 1 | Multiple myeloma | Boehringer Ingelheim; Amgen | BI 836909; AMG 420 | |
| CD3 × BCMA | Phase 1 | NCT03287908 | Multiple myeloma | Amgen | AMG 701 |
| CD3 × BCMA | Phase 1 | NCT03933735 | Multiple myeloma | AbbVie; TeneoBio | TNB-383B |
| CD3 × BCMA | Phase 1 | NCT03269136 | Multiple myeloma | Pfizer | PF-06863135 |
| CD3 × BCMA | Phase 1 | Multiple myeloma | Janssen | JNJ-64007957 | |
| CD3 × BCMA | Phase 1 | NCT03486067 | Multiple myeloma | Celgene | CC-93269 |
| CD3 × BCMA | Phase 1/2 | NCT03761108 | Multiple myeloma | Regeneron; Sanofi | REGN5458 |
| CD3 × BCMA | Phase 1 | NCT04083534 | Multiple myeloma | Regeneron; Sanofi | REGN5459 |
| CD3 × BCMA × ALB | Phase 1/2 | NCT04184050 | Multiple myeloma | Harpoon Therapeutics; AbbVie | HPN217 |
| CD3 × CLEC12A | Phase 1 | NCT03038230 | AML | Merus | Tepoditamab; MCLA-117 |
| CD3 × FcRH5 | Phase 1 | NCT03275103 | Multiple myeloma | Genentech | RO7187797; BFCR4350A |
| CD3 × FLT3 | Phase 1 | NCT03541369 | AML | Amgen | AMG 427 |
| CD3 × GPRC5D | Phase 1 | Multiple myeloma | Janssen | JNJ-64407564 |
BCMA B-cell maturation antigen, ALB albumin, CLEC12A C-type lectin domain family 12 member A, FcRH5 Fc receptor-homology 5, FLT3 Fms-like tyrosine kinase 3, GPRC5D G protein-coupled receptor class C group 5 member D, AML acute lymphoblastic leukaemia, NHL non-Hodgkin lymphoma, DLBCL diffuse large B-cell lymphoma, MCL mantle cell lymphoma, FL follicular lymphoma, CLL chronic lymphocytic leukaemia, MZL marginal zone lymphoma, AML acute myeloid leukaemia.
Table 3.
Clinical trials of CD3+ bispecific T-cell redirectors for solid cancers.
| Targets | Clinical trial Phase | Clinical trial identifier | Disease indication(s) | Organisation(s) | Antibody |
|---|---|---|---|---|---|
| CD3 × B7-H3 | Phase 1 | NCT03406949 | Advanced solid tumours | MacroGenics | Orlotamab; MGD009 |
| CD3 × CEACAM | Phase 1 | Gastrointestinal adenocarcinoma | Amgen; MedImmune | AMG 211; MEDI-565 | |
| CD3 × CEACAM | Phase 1/2 | Colorectal cancer; NSCLC | Hoffmann-La Roche | Cibisatamab; RO6958688 | |
| CD3 × CLDN18.2 | Phase 1 | NCT04260191 | Gastric and gastro-oesophageal junction adenocarcinoma | Amgen | AMG 910 |
| CD3 × DLL3 | Phase 1 | NCT03319940 | SCLC | Amgen | AMG 757 |
| CD3 × EGFRvIII | Phase 1 | NCT03296696 | Glioblastoma | Amgen | AMG 596 |
| CD3 × EGFR | Phase 1/2 | Pancreatic cancer; colorectal cancer; glioblastoma | Barbara Ann Karmanos Cancer Institute; University of Virginia | EGFR2Bi-ATC; EGFR-BATs | |
| CD3 × EpCAM | Phase 1 | CTR20181212 | Malignant ascites | Wuhan YZY Biopharma | M701 |
| CD3 × EpCAM | Withdrawn from market | Withdrawn from market | Malignant ascites | Trion Pharma | Catumaxomab; Removab |
| CD3 × GD2 | Phase 1/2 | NCT02173093 | Neuroblastoma | University of Virginia | GD2Bi-aATC |
| CD3 × gpA33 | Phase 1/2 | Colorectal cancer | MacroGenics | MGD007 | |
| CD3 × gp100 | Phase 1/2 | Uveal melanoma; malignant melanoma | Immunocore; University of Pennsylvania | IMCgp100 | |
| CD3 × GPC3 | Phase 1 | NCT02748837 | Solid tumours | Chugai Pharmaceutical | ERY974 |
| CD3 × GUCY2c | Phase 1 | NCT04171141 | Gastrointestinal tumours; colorectal cancer; oesophageal adenocarcinomas | Pfizer | PF-07062119 |
| CD3 × HER2 | Phase 1/2 | Prostate cancer; breast cancer; leptomeningeal metastases | Barbara Ann Karmanos Cancer Institute; University of Virginia | HER2Bi-ATC; HER2-BATs | |
| CD3 × HER2 | Phase 1 | NCT03448042 | Solid tumours | Genentech | BTRC4017A |
| CD3 × HER2 | Phase 1/2 | NCT03983395 | Breast cancer | Glenmark Pharmaceuticals | GBR 1302; ISB 1302 |
| CD3 × HER2 | Phase 1 | CTR20171194 | Advanced solid tumours | Wuhan YZY Biopharma | M802 |
| CD3 × MAGE-A4 | Phase 1/2 | NCT03973333 | Advanced solid tumours | Immunocore Ltd | IMC-C103C |
| CD3 × MSLN × ALB | Phase 1/2 | NCT03872206 | Ovarian cancer; fallopian tube cancer; peritoneal cancer; pancreatic cancer; mesothelioma | Harpoon Therapeutics | HPN536 |
| CD3 × MUC1 | Phase 2 | Kidney cancer; breast cancer; lung cancer; gastric cancer; pancreatic cancer; colorectal cancer; liver cancer | Fuda Cancer Hospital, Guangzhou; Benhealth Biopharmaceutical | Activated CIK and CD3-MUC1 | |
| CD3 × MUC16 | Phase 1/2 | NCT03564340 | Ovarian cancer; fallopian tube cancer; primary peritoneal cancer | Regeneron; Sanofi | REGN4018 |
| CD3 × MUC17 | Phase 1 | NCT04117958 | Gastric and gastro-oesophageal junction cancer | Amgen | AMG 199 |
| CD3 × P-cadherin | Phase 1 | NCT02659631 | Triple-negative breast cancer; colorectal cancer; NSCLC | Pfizer | PF-06671008 |
| CD3 × PRAME | Phase 1/2 | NCT04262466 | Advanced solid tumours | Immunocore Ltd | IMC-F106C |
| CD3 × PSCA | Phase 1 | NCT03927573 | Pancreatic cancer; breast cancer; urogenital cancer; NSCLC | Celgene | GEM3PSCA |
| CD3 × PSMA | Phase 1 | NCT03926013 | Prostate cancer; renal cell carcinoma | Janssen | JNJ-63898081 |
| CD3 × PSMA | Phase 1 | NCT03792841 | Prostate cancer | Amgen | AMG 160 |
| CD3 × PSMA | Phase 1 | NCT01723475 | Prostate cancer | Amgen; Bayer | Pasotuxizumab; AMG 212 |
| CD3 × PSMA | Phase 1 | NCT02262910 | Prostate cancer | Aptevo Therapeutics | ES414 |
| CD3 × PSMA × ALB | Phase 1 | NCT03577028 | Prostate cancer | Harpoon Therapeutics | HPN424 |
| CD3 × SSTR2 | Phase 1 | NCT03411915 | Neuroendocrine tumours; gastrointestinal stromal tumours | Xencor | Tidutamab; XmAb18087 |
| CD3 × STEAP1 | Phase 1 | NCT04221542 | Prostate cancer | Amgen | AMG 509 |
| CD3 × 5T4 | Phase 1/2 | NCT04424641 | Solid tumours | Genmab; AbbVie | GEN1044 |
CEACAM carcinoembryonic antigen-related adhesion molecule, CLDN18.2 claudin-18 isoform 2, DLL3 delta-like canonical notch ligand 3, EGFRvIII epidermal growth factor receptor vIII, EGFR epidermal growth factor receptor, EpCAM epithelial cell adhesion molecule, gpA33 glycoprotein A33, gp100 glycoprotein 100, GUCY2c guanylate cyclase 2c, HER2 human epidermal growth factor receptor 2, MAGE-A4 melanoma-associated antigen A4, MSLN mesothelin, ALB albumin, MUC1 mucin 1, MUC16 mucin 16, MUC17 mucin 17, PRAME preferentially expressed antigen in melanoma, PSCA prostate stem cell antigen, PSMA prostate-specific membrane antigen, SSTR2 somatostatin receptor type 2, STEAP1 six transmembrane epithelial antigen of the prostate 1, NSCLC non-small cell lung cancer, SCLC small-cell lung cancer.
Acknowledgements
The authors thank Bidisha Dasgupta and Anna Sberna for providing information regarding CD3+ T-cell redirection molecules in development.
Author contributions
All authors (A.S., S.D. and I.S.G.) made a substantial contribution to all aspects of the preparation of this paper, including conceiving the work that led to the submission, drafting and revising the paper and approving the final version. First authorship is shared by A.S. and S.D.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
S.D. and I.S.G. are paid employees of the Janssen Pharmaceutical Companies of Johnson & Johnson and receive salary and other compensation.
Funding information
This work was funded by the Janssen Pharmaceutical Companies of Johnson & Johnson.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ajit Singh, Sundee Dees
References
- 1.Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-Cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goebeler ME, Bargou RC. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020;17:418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 4.Deshaies RJ. Multispecific drugs herald a new era of biopharmaceutical innovation. Nature. 2020;580:329–338. doi: 10.1038/s41586-020-2168-1. [DOI] [PubMed] [Google Scholar]
- 5.Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 6.Borlak J, Langer F, Spanel R, Schondorfer G, Dittrich C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget. 2016;7:28059–28074. doi: 10.18632/oncotarget.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharm. Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Gokbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–1531. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharm. Ther. 2019;201:103–119. doi: 10.1016/j.pharmthera.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Nie S, Wang Z, Moscoso-Castro M, D’Souza P, Lei C, Xu J, et al. Biology drives the discovery of bispecific antibodies as innovative therapeutics. Antib. Therapeutics. 2020;3:18–62. doi: 10.1093/abt/tbaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Wang J. T cell-redirecting bispecific antibodies in cancer immunotherapy: recent advances. J. Cancer Res. Clin. Oncol. 2019;145:941–956. doi: 10.1007/s00432-019-02867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedi RM. T cell responses: naïve to memory and everything in between. Adv. Physiol. Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golubovskaya V, Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers. 2016;8:36. doi: 10.3390/cancers8030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chraa D, Naim A, Olive D, Badou A. T lymphocyte subsets in cancer immunity: friends or foes. J. Leukoc. Biol. 2019;105:243–255. doi: 10.1002/JLB.MR0318-097R. [DOI] [PubMed] [Google Scholar]
- 15.Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31:2181–2190. doi: 10.1038/leu.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalk I, Feldmann A, Koristka S, Arndt C, Cartellieri M, Ehninger A, et al. Characterization of a novel single-chain bispecific antibody for retargeting of T cells to tumor cells via the TCR co-receptor CD8. PLoS ONE. 2014;9:95517. doi: 10.1371/journal.pone.0095517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman A, Arndt C, Topfer K, Stamova S, Krone F, Cartellieri M, et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- 20.Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, et al. Targeting resident memory T cells for cancer immunotherapy. Front. Immunol. 2018;9:1722. doi: 10.3389/fimmu.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menares E, Galvez-Cancino F, Caceres-Morgado P, Ghorani E, Lopez E, Diaz X, et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat. Commun. 2019;10:4401. doi: 10.1038/s41467-019-12319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey N. Cytokine release syndrome: who is at risk and how to treat. Best. Pr. Res Clin. Haematol. 2017;30:336–340. doi: 10.1016/j.beha.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, SchloBer A, Schlaak M, et al. Cytokine release syndrome. JITC. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 25.Thakur A, Scholler J, Schalk DL, June CH, Lum LG. Enhanced cytotoxicity against solid tumors by bispecific antibody-armed CD19 CAR-T cells: a proof-of-concept study. J. Cancer Res. Clin. Oncol. 2020;146:2007–2016. doi: 10.1007/s00432-020-03260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Piskol R, Ybarra R, Chen YJJ, Li J, Slaga D, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 2019;11:8861. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 27.Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci. Transl. Med. 2017;9:4291. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 28.Kauer J, Horner S, Osburg L, Muller S, Marklin M, Heitmann JS, et al. Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies. J. Immunother. Cancer. 2020;8:000621. doi: 10.1136/jitc-2020-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019;11:851–857. doi: 10.2217/imt-2019-0074. [DOI] [PubMed] [Google Scholar]
- 30.Teachy DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat. Rev. Immunol. 2012;12:317–318. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs K, Godwin J, Foster M, Vey N, Uy GL, Rizzieri DA, et al. Lead-in dose optimization to mitigate cytokine release syndrome in AML and MDS patients treated with flotetuzumab, a CD123 x CD3 DART molecule for T-cell redirected therapy. Blood. 2017;130:3856. [Google Scholar]
- 33.Chen X, Kamperschroer C, Wong G, Xuan D. A modeling framework to characterize cytokine release upon T-cell-engaging bispecific antibody treatment: methodology and opportunities. Clin. Transl. Sci. 2019;12:600–608. doi: 10.1111/cts.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosseini I, Gadkar K, Stefanich E, Li CC, Sun LL, Chu YW, et al. Mitigating the risk of cytokine release syndrome in a Phase I trial of CD20/CD3 bispecific antibody mosunetuzumab in NHL: impact of translational system modeling. npj Syst. Biol. Appl. 2020;6:28. doi: 10.1038/s41540-020-00145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinklein ND, Pham D, Schellenberger U, Buelow B, Boudreau A, Choudhry P, et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. mAbs. 2019;11:639–652. doi: 10.1080/19420862.2019.1574521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lum LG, Thakur A, Liu Q, Deol A, Al-Kadhimi Z, Ayash L, et al. CD20-targeted T cells after stem cell transplantation for high risk and refractory non-Hodgkin’s lymphoma. Biol. Blood Marrow Transplant. 2013;19:925–933. doi: 10.1016/j.bbmt.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lum LG, Thakur A, Kondadasula SV, Al-Kadhimi Z, Deol A, Tomaszewski EN, et al. Targeting CD138-/CD20+ clonogenic myeloma precursor cells decreases these cells and induces transferable antimyeloma immunity. Biol. Blood Marrow Transplant. 2016;22:869–878. doi: 10.1016/j.bbmt.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lum LG, Thakur A, Choi M, Deol A, Kondadasula V, Schalk D, et al. Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. Oncoimmunology. 2020;9:1773201. doi: 10.1080/2162402X.2020.1773201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lum LG, Thakur A, Al-Kadhimi Z, Colvin GA, Cummings FJ, Legare RD, et al. Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin. Cancer Res. 2015;21:2305–2314. doi: 10.1158/1078-0432.CCR-14-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaishampayan U, Thakur A, Rathore R, Kouttab N, Lum LG. Phase I study of anti-CD3 x anti-Her2 bispecific antibody in metastatic castrate resistant prostate cancer patients. Prostate Cancer. 2015;2015:285193. doi: 10.1155/2015/285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakur A, Rathore R, Kondadasula SV, Uberti JP, Ratanatharathorn V, Lum LG. Immune T cells can transfer and boost anti-breast cancer immunity. Oncoimmunology. 2018;7:1500672. doi: 10.1080/2162402X.2018.1500672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin. Cancer Res. 2016;22:1856–1864. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 44.Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martinez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohnke T, Krupka C, Tischer J, Knosel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J. Hematol. Oncol. 2015;8:111. doi: 10.1186/s13045-015-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann M, Krupka C, Deiser K, Lindl B, Mocikat R, Metzeler KH, et al. Development of a bifunctional checkpoint inhibitory T cell engager (CiTE) to reverse adaptive immune escape in AML. Blood. 2018;132:4069. doi: 10.1182/blood-2018-05-849802. [DOI] [PubMed] [Google Scholar]
- 47.Promsote W, Ambrozak D, Almasri C, Liu C, DeMouth M, Hataye J, et al. Tri-functional T-cell engagers target immune checkpoint inhibitors PD-1 and TIGIT to enhance CD8 effector T-cell functions in chronically SHIV-infected rhesus macaques. J. Immunol. 2020;204:169.31. [Google Scholar]
- 48.Ma H, Wang H, Sove RJ, Wang J, Giragossian C, Popel AS. Combination therapy with T cell engager and PD-L1 blockade enhances the antitumor potency of T cells as predicted by a QSP model. J. Immunother. Cancer. 2020;8:001141. doi: 10.1136/jitc-2020-001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 2017;214:381–400. doi: 10.1084/jem.20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weigelin B, Bolanos E, Teijeira A, Martinez-Forero I, Labiano S, Azpilikueta A, et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc. Natl Acad. Sci. USA. 2015;112:7551–7556. doi: 10.1073/pnas.1506357112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu D, Tavare R, Haber L, Aina OH, Vazzana K, Ram P, et al. A PSMA-targeting CD3 bispecific antibody induces antitumor responses that are enhanced by 4-1BB costimulation. Cancer Immunol. Res. 2020;8:596–608. doi: 10.1158/2326-6066.CIR-19-0518. [DOI] [PubMed] [Google Scholar]
- 52.Claus C, Ferrara C, Xu W, Sam J, Lang S, Uhlenbrock F, et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci. Transl. Med. 2019;11:5989. doi: 10.1126/scitranslmed.aav5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willems A, Schoonooghe S, Eeckhout D, De Jaeger G, Grooten J, Mertens N. CD3 x CD28 cross-interacting bispecific antibodies improve tumor cell dependent T-cell activation. Cancer Immunol. Immunother. 2005;54:1059–1071. doi: 10.1007/s00262-005-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skokos D, Waite JC, Haber L, Crawford A, Hermann A, Ullman E, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 2020;12:7888. doi: 10.1126/scitranslmed.aaw7888. [DOI] [PubMed] [Google Scholar]
- 55.Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vafa O, Trinklein ND. Perspective: designing T-cell engagers with better therapeutic windows. Front. Oncol. 2020;10:446. doi: 10.3389/fonc.2020.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mastelic-Gavillet B, Rodrigo BN, Decombaz L, Wang H, Ercolano G, Ahmed R, et al. Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8+ T cells. J. Immunother. Cancer. 2019;7:257. doi: 10.1186/s40425-019-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, et al. Interleukin-10 directly inhibits CD8+ T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity. 2018;48:299–312. doi: 10.1016/j.immuni.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Sostoa J, Fajardo CA, Moreno R, Ramos MD, Farrera-Sal M, Alemany R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott EM, Jacobus EJ, Lyons B, Frost S, Freedman JD, Dyer A, et al. Bi-and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer. 2019;7:320. doi: 10.1186/s40425-019-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nair-Gupta P, Rudnick SI, Luistro L, Smith M, McDaid R, Li Y, et al. Blockade of VLA4 sensitizes leukemic and myeloma tumor cells to CD3 redirection in the bone marrow microenvironment. Blood Cancer J. 2020;10:65. doi: 10.1038/s41408-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar V, Varghese S. Ex vivo tumor-on-a-chip platforms to study intercellular interactions within the tumor microenvironment. Adv. Health. Mater. 2019;8:1801198. doi: 10.1002/adhm.201801198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavesi A, Tan AT, Koh S, Chia A, Colombo M, Antonecchia E, et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cell against solid tumors. JCI Insight. 2017;2:89762. doi: 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–104. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 68.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Investig. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair-Gupta P, Diem M, Reeves D, Wang W, Schulingkamp R, Sproesser K, et al. A Novel C2 domain binding CD33xCD3 bispecific antibody with potent T-cell redirection activity against acute myeloid leukemia. Blood Adv. 2020;4:906–919. doi: 10.1182/bloodadvances.2019001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staflin K, Zuch de Zafra CL, Schutt LK, Clark V, Zhong F, Hristopoulos M, et al. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight. 2020;5:133757. doi: 10.1172/jci.insight.133757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slaga D, Ellerman D, Lombana TN, Vij R, Li J, Hristopoulos M, et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci. Transl. Med. 2018;10:5775. doi: 10.1126/scitranslmed.aat5775. [DOI] [PubMed] [Google Scholar]
- 73.Panchal A, Seto P, Wall R, Hillier BJ, Zhu Y, Krakow J, et al. COBRATM: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors. mAbs. 2020;12:1792130. doi: 10.1080/19420862.2020.1792130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geiger M, Stubenrauch KG, Sam J, Richter WF, Jordan G, Eckmann J, et al. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat. Commun. 2020;11:3196. doi: 10.1038/s41467-020-16838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trang VH, Zhang X, Yumul RC, Zeng W, Stone IJ, Wo SW, et al. A coiled-coil masking domain for selective activation of therapeutic antibodies. Nat. Biotechnol. 2019;37:761–765. doi: 10.1038/s41587-019-0135-x. [DOI] [PubMed] [Google Scholar]
- 76.Desnoyers LR, Vasiljeva O, Richardson JH, Yang A, Menendez EEM, Liang TW, et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 2013;5:144. doi: 10.1126/scitranslmed.3006682. [DOI] [PubMed] [Google Scholar]
- 77.Banaszek A, Bumm TGP, Nowotny B, Geis M, Jacob K, Wolfl M, et al. On target restoration of a split T cell-engaging antibody for precision immunotherapy. Nat. Commun. 2019;10:5387. doi: 10.1038/s41467-019-13196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol. Immunother. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ellerman D. Bispecific T-cell engagers: towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods. 2019;154:102–117. doi: 10.1016/j.ymeth.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 80.Runcie K, Budman DR, John V, Seetharamu N. Bi-specific and tri-specific antibodies—the next big thing in solid tumor therapeutics. Mol. Med. 2018;24:50. doi: 10.1186/s10020-018-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG330. Blood. Cancer J. 2015;5:340. doi: 10.1038/bcj.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feucht J, Kayser S, Gorodezki D, Hamieh M, Doring M, Blaeschke F, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7:76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azuma M. Co-signal molecules in T-cell activation: historical overview and perspective. Adv. Exp. Med. Biol. 2019;1189:3–23. doi: 10.1007/978-981-32-9717-3_1. [DOI] [PubMed] [Google Scholar]
- 84.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunol. 2019;8:1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Correnti CE, Laszlo GS, de van der Schueren WJ, Godwin CD, Bandaranayake A, Busch MA, et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32:1239–1243. doi: 10.1038/s41375-018-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urbanska K, Lynn RC, Stashwick C, Thakur A, Lum LG, Powell DJ., Jr Targeted cancer immunotherapy via combination of designer bispecific antibody and novel gene-engineered T cells. J. Transl. Med. 2014;12:347. doi: 10.1186/s12967-014-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang XB, Zhao BF, Zhao Q, Piao JH, Liu J, Lin Q, et al. A new recombinant single chain trispecific antibody recruits T lymphocytes to kill CEA (carcinoma embryonic antigen) positive tumor cells in vitro efficiently. J. Biochem. 2004;135:555–565. doi: 10.1093/jb/mvh065. [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer. 2020;1:86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 89.Blanco B, Compte M, Lykkemark S, Sanz L, Alvarez-Vallina L. T cell-redirecting strategies to ‘STAb’ tumors: beyond CARs and bispecific antibodies. Trends Immunol. 2019;40:243–257. doi: 10.1016/j.it.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 90.Compte M, Blanco B, Serrano F, Cuesta AM, Sanz L, Bernad A, et al. Inhibition of tumor growth in vivo by in situ secretion of bispecific anti-CEA x anti-CD3 diabodies from lentivirally transduced human lymphocytes. Cancer Gene Ther. 2007;14:380–388. doi: 10.1038/sj.cgt.7701021. [DOI] [PubMed] [Google Scholar]
- 91.Iwahori K, Kakarla S, Velasquez MP, Yu F, Yi Z, Gerken C, et al. Engager T cells: a new class of antigen-specific T cells that redirect bystander T cells. Mol. Ther. 2015;23:171–178. doi: 10.1038/mt.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Compte M, Cuesta AM, Sanchez-Martin D, Alonso-Camino V, Vicario JL, Sanz L, Alvarez-Vallina L. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cells. 2009;27:753–760. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies. 2019;8:41. doi: 10.3390/antib8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.