Abstract
Decreased glomerular filtration rate (GFR) is linked to poor survival. The predictive value of creatinine estimated GFR (eGFR) and cystatin C eGFR in critically ill patients may differ substantially, but has been less studied. This study compares long-term mortality risk prediction by eGFR using a creatinine equation (CKD-EPI), a cystatin C equation (CAPA) and a combined creatinine/cystatin C equation (CKD-EPI), in 22,488 patients treated in intensive care at three University Hospitals in Sweden, between 2004 and 2015. Patients were analysed for both creatinine and cystatin C on the same blood sample tube at admission, using accredited laboratory methods. During follow-up (median 5.1 years) 8401 (37%) patients died. Reduced eGFR was significantly associated with death by all eGFR-equations in Cox regression models. However, patients reclassified to a lower GFR-category by using the cystatin C-based equation, as compared to the creatinine-based equation, had significantly higher mortality risk compared to the referent patients not reclassified. The cystatin C equation increased C-statistics for death prediction (p < 0.001 vs. creatinine, p = 0.013 vs. combined equation). In conclusion, this data favours the sole cystatin C equation rather than the creatinine or combined equations when estimating GFR for risk prediction purposes in critically ill patients.
Subject terms: Kidney diseases, Risk factors, Prognostic markers
Introduction
Glomerular filtration rate (GFR) is the best routinely available estimate for kidney function and essential for detection and management of both acute kidney injury (AKI) and chronic kidney disease (CKD). Loss of kidney function by decreased estimated glomerular filtration rate (eGFR) is associated with poor survival1. Creatinine is the most frequently used biomarker for eGFR. Yet, creatinine may vary with factors not related to kidney function per se such as muscle mass, gender, ethnicity, and dietary factors2,3. Cystatin C is an alternative biomarker for eGFR-estimation which does not depend on muscle mass and thus fairly constant with age and gender4,5. Still, creatinine is the most frequently used estimate of eGFR in critically ill patients6,7. Patients in intensive care are often bedfast and may have loss of muscle mass and altered distribution volumes due to severe illness. An ongoing loss of muscle mass and low protein intake may possibly lead to a decrease in creatinine in plasma, leading to potential risk of eGFR overestimation8. It may therefore be hypothesized that creatinine is a less informative biomarker in the estimation of eGFR than cystatin C. Cystatin C may on the other hand be influenced by cortisol, obesity and other traditional risk factors or possibly inflammation9–13, which varies in critically ill. The performance of creatinine and cystatin C in estimating long-term mortality is less studied in critically ill patients.
The aim of this study was to study the predictive value of creatinine and cystatin C in critically ill patients by investigating if cystatin C improves the association between eGFR and mortality, compared to creatinine, in this particular patient group using reclassification and model discrimination with C-statistics. The study includes a large number of intensive care patients from three Swedish University Hospitals and compares eGFR calculated by creatinine and cystatin C, respectively, and mortality risk at a median follow-up of 5 years.
Results
Baseline characteristics and mortality risks in eGFR subgroups
Out of the 22,488 included patients, 10,392 (46%) were admitted to a general intensive care unit, 2597 (11%) to a neurosurgical intensive care unit, 5132 (23%) to a cardiothoracic intensive care unit and 4367 (19%) were admitted to a coronary care unit. Table 1 shows the baseline characteristics of the study participants. Cardiovascular diseases, including hypertension and diabetes mellitus, infections, and trauma were the most common diagnoses among the included patients.
Table 1.
Basic characteristics of all study participants and in the subgroup general ICU in median (interquartile interval).
| All | General ICU | |
|---|---|---|
| Number | 22,488 | 10,392 |
| Age | 65 (53–74) | 62 (45–73) |
| Female gender (%) | 36 | 40 |
| Mortality within 7 days (%) | 7 | 11 |
| Mortality within 14 days (%) | 8 | 13 |
| Mortality within 30 days (%) | 11 | 16 |
| Mortality within 90 days (%) | 14 | 21 |
| Total mortality during follow-up (%) | 37 | 49 |
| Plasma Creatinine, µmol/L | 81 (64–112) | 85 (63–134) |
| Plasma Cystatin C, mg/L | 1.06 (0.81–1.52) | 1.07 (0.75–1.75) |
| eGFR crea, ml/min/1,73 m2 | 81 (53–98) | 79 (42–103) |
| eGFR comb, ml/min/1,73 m2 | 75 (47–99) | 73 (38–106) |
| eGFR cyst C, ml/min/1,73 m2 | 68 (44–95) | 68 (37–105) |
| Discharge diagnoses | ||
| Infectious disease (%) | 24.8 | 36.4 |
| Diabetes mellitus (%) | 15.7 | 13.4 |
| Hypertension (%) | 29.8 | 20.7 |
| Cardiovascular disease (%) | 38.3 | 19.4 |
| Cerebrovascular disease (%) | 10.5 | 6.1 |
| Liver and biliary tract diseases (%) | 3.7 | 6.8 |
| Kidney diseases (%) | 8.7 | 13.4 |
| Trauma (%) | 13.0 | 20.9 |
| Intoxications (%) | 0.7 | 1.3 |
| Charlson Comorbidity Index (95% CI) | 1.56 (1.54–1.59) | 1.85 (1.80–1.89) |
During follow-up (median [interquartile interval] 5.1 [2.3–7.1] years, corresponding to 106,036 person-years in total) 8401 (37%) participants died. Hazard ratios with 95% confidence intervals for mortality of all causes for each equation versus the reference point at 95 ml/min/1.73 m2 are shown in cubic spline curves (Fig. 1). At eGFR values below 30–40 ml/min/1.73 m2 the mortality risk was significantly higher with an equation containing cystatin C, alone or in combination with creatinine, compared to the equation with only creatinine. Harrell´s C statistics (95% confidence interval) for Cox regression models predicting mortality was 0.640 (0.631–0.649) for eGFRCr, 0.664 (0.655–0.673, P < 0.001 vs. eGFRCr) for eGFRComb and 0.667 (0.658–0.676, P < 0.001 vs. eGFRCr and P = 0.013 vs eGFRComb) for eGFRCyst. Thus, the equation with only Cystatin C significantly increased the C-statistics for the prediction of death as compared to the equations with Creatinine, both sole and combined.
Figure 1.
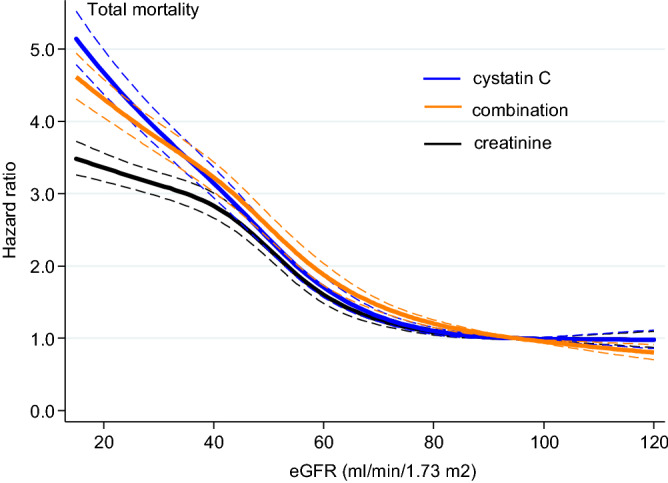
The hazard ratios and 95% confidence intervals (in thin dotted lines) for mortality by each equation from univariate Cox proportional-hazard models are shown in regression spline curves. The reference point is set to 95 ml/min/1.73 m2.
Incidence rates for mortality according to the eGFR categories > 60, 60–30, 30–20 and < 20 mL/min/1.73m2, defined by respective equation, are presented in Table 2. Overall, decreasing eGFR, irrespective of equation, significantly associated with mortality risk (Table 2, Fig. 1).
Table 2.
All-cause mortality estimates by different eGFR equations using Cox regression models.
| ml/min/1.73 m2 | N at risk/N of events | IR (95% CI) | HR (95% CI) | |
|---|---|---|---|---|
| eGFR Creatinine | ≥ 60 | 15,749/4404 | 5.4 (5.2–5.6) | Ref. |
| 60–30 | 4381/2384 | 14.2 (13.6–14.7) | 2.4 (2.3–2.5) | |
| 30–20 | 1112/744 | 20.6 (19.2–22.2) | 3.4 (3.2–3.7) | |
| < 20 | 1246/869 | 21.8 (20.4–23.3) | 3.6 (3.3–3.8) | |
| eGFR Cystatin C | ≥ 60 | 13,271/3200 | 4.5 (4.3–4.7) | Ref. |
| 60–30 | 6103/2974 | 11.7 (11.2–12.1) | 2.4 (2.3–2.6) | |
| 30–20 | 1526/1027 | 21.2 (20.0–22.6) | 4.1 (3.8–4.4) | |
| < 20 | 1588/1200 | 27.3 (25.8–28.9) | 5.0 (4.7–5.4) | |
| eGFR Combined | ≥ 60 | 14,556/3734 | 4.8 (4.7–5.0) | Ref. |
| 60–30 | 5069/2649 | 13.2 (12.7–13.7) | 2.5 (2.4–2.6) | |
| 30–20 | 1347/902 | 20.6 (19.3–21.9) | 3.7 (3.5–4.0) | |
| < 20 | 1516/1116 | 25.4 (23.9–26.9) | 4.4 (4.2–4.8) |
IR incidence rate, HR hazard ratio.
Comparing eGFRCyst with eGFRCr using reclassification analysis
Overall, patients reclassified to a lower GFR-category by using the cystatin C-based equation, as compared to the creatinine-based equation, had significantly higher mortality risk (Table 3) compared to the referent patients not reclassified. Conversely, patients reclassified to a higher GFR-category by the cystatin C-based formula, as compared to the creatinine-based equation, had significantly lower mortality risk. These associations were also seen in the subgroup of patients at general ICU. Adjusting for age, gender and cci did in some comparisons give weaker and no longer significant associations. However, in reclassification to lower category by cystatin C almost all associations were still present after adjustments. Altogether, the calculated NRI for the cystatin C equation compared to creatinine equation was 0.13, P < 0.001, indicating improved reclassification by the cystatin C equation.
Table 3.
Mortality risk when classified according to eGFR recalculated with cystatin C, as compared to creatinine, in the whole study population (all) and in general ICU.
| Evaluated (N) | Reclassified to higher eGFR | Not reclassified | Reclassified to lower eGFR | ||||
|---|---|---|---|---|---|---|---|
| N (%) | HR (95% CI) | N (%) | HR (95% CI) | N (%) | HR (95% CI) | ||
| All | |||||||
| 2960 events | 1444 events | ||||||
| eGFR ≥ 60 | 15,749 | NA | NA | 12,658 (80) | ref | 3091 (20) | 2.37 (2.23–2.53) |
| eGFR ≥ 60, adj | 15,749 | NA | NA | 12,658 (80) | ref | 3091 (20) | 1.51 (1.41–1.61) |
| 228 events | 1452 events | 704 events | |||||
| eGFR 60–30 | 4381 | 588 (13) | 0.69 (0.60–0.79) | 2822 (64) | ref | 971 (22) | 1.80 (1.64–1.97) |
| eGFR 60–30, adj | 4381 | 588 (13) | 0.84 (0.73–0.97) | 2822 (64) | ref | 971 (22) | 1.62 (1.48–1.77) |
| 132 events | 293 events | 319 events | |||||
| eGFR 30–20 | 1112 | 257 (23) | 0.71 (0.58–0.88) | 488 (40) | ref | 406 (37) | 1.40 (1.20–1.64) |
| eGFR 30–20, adj | 1112 | 257 (23) | 0.92 (0.74–1.13) | 488 (40) | ref | 406 (37) | 1.46 (1.24–1.71) |
| 161 events | 708 events | ||||||
| eGFR ≤ 20 | 1246 | 276 (22) | 0.70 (0.59–0.83) | 970 (78) | ref | NA | NA |
| eGFR ≤ 20, adj | 1246 | 276 (22) | 0.87 (0.73–1.03) | 970 (78) | ref | NA | NA |
| General ICU | |||||||
| 1720 events | 716 events | ||||||
| eGFR ≥ 60 | 6523 | NA | NA | 5398 (83) | ref | 1125 (17) | 2.74 (2.52–3.00) |
| eGFR ≥ 60, adj | 6523 | NA | NA | 5398 (83) | ref | 1125 (17) | 1.63 (1.49–1.79) |
| 183 events | 847 events | 405 events | |||||
| eGFR 60–30 | 2144 | 397 (19) | 0.56 (0.48–0.66) | 1243 (58) | ref | 504 (24) | 1.44 (1.28–1.62) |
| eGFR 60–30, adj | 2144 | 397 (19) | 0.68 (0.58–0.80) | 1243 (58) | ref | 504 (24) | 1.39 (1.23–1.56) |
| 111 events | 202 events | 222 events | |||||
| eGFR 30–20 | 740 | 203 (27) | 0.63 (0.50–0.79) | 272 (37) | ref | 265 (36) | 1.25 (1.03–1.51) |
| eGFR 30–20, adj | 740 | 203 (27) | 0.81 (0.64–1.02) | 272 (37) | ref | 265 (36) | 1.34 (1.10–1.62) |
| 147 events | 553 events | ||||||
| eGFR ≤ 20 | 985 | 245 (25) | 0.69 (0.58–0.83) | 740 (75) | ref | NA | NA |
| eGFR ≤ 20, adj | 985 | 245 (25) | 0.84 (0.70–1.01) | 740 (75) | ref | NA | NA |
Adj = adjusted for age, gender and Charlson Comorbidity Index. HR = hazard ratio.
Comparing eGFRCyst with eGFRComb using reclassification analysis
Generally, patients reclassified to a lower GFR-category by using the cystatin C-based equation, as compared to the combined equation, had significantly higher mortality risk (Table 4) compared to the referent patients not reclassified. Also, patients reclassified to a higher GFR-category by the cystatin C-based formula, as compared to the combined equation, had significantly lower mortality risk. Adjusting for age, gender and cci did in some comparisons give weaker and no longer significant associations, particularly in the subgroup general ICU. Altogether, the calculated NRI for the cystatin C equation compared to the combined equation was 0.04, P < 0.001, indicating an improved reclassification.
Table 4.
Mortality risk when classified according to eGFR recalculated with cystatin C, as compared to the combination formula, in the whole study population (all) and in general ICU.
| Evaluated (N) | Reclassified to higher eGFR | Not reclassified | Reclassified to lower eGFR | ||||
|---|---|---|---|---|---|---|---|
| N (%) | HR (95% CI) | N (%) | HR (95% CI) | N (%) | HR (95% CI) | ||
| All | |||||||
| 3072 events | 662 events | ||||||
| eGFR ≥ 60 | 14,556 | NA | NA | 12,977 (89) | ref | 1579 (11) | 1.99 (1.83–2.17) |
| eGFR ≥ 60, adj | 14,556 | NA | NA | 12,977 (89) | ref | 1579 (11) | 1.38 (1.27–1.51) |
| 126 events | 2169 events | 354 events | |||||
| eGFR 60–30 | 5069 | 292 (6) | 0.81 (0.68–0.97) | 4275 (84) | ref | 502 (10) | 1.82 (1.62–2.03) |
| eGFR 60–30, adj | 5069 | 292 (6) | 0.94 (0.78–1.12) | 4275 (84) | ref | 502 (10) | 1.67 (1.49–1.87) |
| 138 events | 585 events | 178 events | |||||
| eGFR 30–20 | 1344 | 236 (18) | 0.84 (0.70–1.00) | 875 (65) | ref | 233 (17) | 1.30 (1.10–1.53) |
| eGFR 30–20, adj | 1344 | 236 (18) | 0.96 (0.80–1.16) | 875 (65) | ref | 233 (17) | 1.27 (1.07–1.50) |
| 113 events | 1003 events | ||||||
| eGFR ≤ 20 | 1516 | 185 (12) | 0.70 (0.57–0.84) | 1331 (88) | ref | NA | NA |
| eGFR ≤ 20, adj | 1516 | 185 (12) | 0.81 (0.66–0.98) | 1331 (88) | ref | NA | NA |
| General ICU | |||||||
| 1812 events | 316 events | ||||||
| eGFR ≥ 60 | 6150 | NA | NA | 5614 (91) | ref | 536 (9) | 2.32 (2.06–2.62) |
| eGFR ≥ 60, adj | 6150 | NA | NA | 5614 (91) | ref | 536 (9) | 1.48 (1.31–1.67) |
| 100 events | 1211 events | 208 events | |||||
| eGFR 60–30 | 2290 | 202 (9) | 0.64 (0.52–0.78) | 1818 (79) | ref | 270 (12) | 1.45 (1.25–1.68) |
| eGFR 60–30, adj | 2290 | 202 (9) | 0.71 (0.58–0.87) | 1818 (79) | ref | 270 (12) | 1.42 (1.23–1.65) |
| 113 events | 375 events | 102 events | |||||
| eGFR 30–20 | 805 | 177 (22) | 0.79 (0.64–0.98) | 501 (62) | ref | 127 (16) | 1.15 (0.92–1.43) |
| eGFR 30–20, adj | 805 | 177 (22) | 0.90 (0.73–1.12) | 501 (62) | ref | 127 (16) | 1.31 (1.05–1.64) |
| 102 events | 766 events | ||||||
| eGFR ≤ 20 | 1145 | 158 (14) | 0.71 (0.58–0.88) | 987 (86) | ref | NA | NA |
| eGFR ≤ 20, adj | 1145 | 158 (14) | 0.82 (0.67–1.01) | 987 (86) | ref | NA | NA |
Adj = adjusted for age, gender and Charlson Comorbidity Index.
Comparing eGFRComb with eGFRCr using reclassification analysis
Patients reclassified to a lower GFR-category by using the combined equation, as compared to the sole creatinine equation, had significantly higher mortality risk (Table 5) compared to the referent patients not reclassified. Patients reclassified to a higher GFR-category by the combined formula, as compared to the sole creatinine equation, had significantly lower mortality risk. Adjusting for age, gender and CCI did not substantially alter the associations. Altogether, the calculated NRI for the combined equation compared to the sole creatinine equation was 0.11, P < 0.001, indicating improved reclassification.
Table 5.
Mortality risk when classified according to eGFR recalculated with a combination formula, as compared to creatinine, in the whole study population (all) and in general ICU.
| Evaluated (N) | Reclassified to higher eGFR | Not reclassified | Reclassified to lower eGFR | ||||
|---|---|---|---|---|---|---|---|
| N (%) | HR (95% CI) | N (%) | HR (95% CI) | N (%) | HR (95% CI) | ||
| All | |||||||
| 3616 events | 788 events | ||||||
| eGFR ≥ 60 | 15,749 | NA | NA | 14,225 (90) | ref | 1524 (10) | 2.51 (2.33–2.72) |
| eGFR ≥ 60, adj | 15,749 | NA | NA | 14,225 (90) | ref | 1524 (10) | 1.52 (1.40–1.65) |
| 118 events | 1803 events | 463 events | |||||
| eGFR 60–30 | 4381 | 331 (8) | 0.59 (0.49–0.71) | 3417 (78) | ref | 633 (14) | 1.75 (1.57–1.94) |
| eGFR 60–30, adj | 4381 | 331 (8) | 0.76 (0.63–0.92) | 3417 (78) | ref | 633 (14) | 1.56 (1.41–1.73) |
| 55 events | 411 events | 278 events | |||||
| eGFR 30–20 | 1112 | 120 (11) | 0.62 (0.47–0.82) | 640 (58) | ref | 351 (32) | 1.51 (1.30–1.76) |
| eGFR 30–20, adj | 1112 | 120 (11) | 0.86 (0.65–1.15) | 640 (58) | ref | 351 (32) | 1.49 (1.27–1.73) |
| 59 events | 810 events | ||||||
| eGFR ≤ 20 | 1246 | 112 (9) | 0.62 (0.48–0.81) | 1139 (91) | ref | NA | NA |
| eGFR ≤ 20, adj | 1246 | 112 (9) | 0.75 (0.58–0.98) | 1139 (91) | ref | NA | NA |
| General ICU | |||||||
| 2035 events | 401 events | ||||||
| eGFR ≥ 60 | 6523 | NA | NA | 5933 (91) | ref | 590 (9) | 2.88 (2.59–3.22) |
| eGFR ≥ 60, adj | 6523 | NA | NA | 5933 (91) | ref | 590 (9) | 1.66 (1.48–1.85) |
| 93 events | 1069 events | 273 events | |||||
| eGFR 60–30 | 2144 | 217 (10) | 0.52 (0.42–0.65) | 1593 (74) | ref | 334 (16) | 1.49 (1.30–1.70) |
| eGFR 60–30, adj | 2144 | 217 (10) | 0.68 (0.55–0.84) | 1593 (74) | ref | 334 (16) | 1.42 (1.25–1.63) |
| 46 events | 291 events | 198 events | |||||
| eGFR 30–20 | 739 | 99 (13) | 0.54 (0.39–0.73) | 405 (55) | ref | 235 (32) | 1.39 (1.16–1.67) |
| eGFR 30–20, adj | 739 | 99 (13) | 0.76 (0.56–1.04) | 405 (55) | ref | 235 (32) | 1.40 (1.16–1.68) |
| 53 events | 647 events | ||||||
| eGFR ≤ 20 | 985 | 99 (10) | 0.61 (0.46–0.81) | 886 (90) | ref | NA | NA |
| eGFR ≤ 20, adj | 985 | 99 (10) | 0.73 (0.55–0.97) | 886 (90) | ref | NA | NA |
Adj = adjusted for age, gender and Charlson Comorbidity Index.
Discussion
This study includes intensive care patient data from three Swedish University Hospitals and shows that eGFR estimated with cystatin C, alone or in combination with creatinine, was more closely associated with risk of death of all causes as compared to creatinine-based eGFR. More specifically, the sole cystatin C eGFR equation predicted mortality better than the combined equation. The associations were found in the whole sample as well as in the more critically ill subgroup at the general ICU. The underlying mechanisms for these associations are uncertain but may relate to either the a superiority of cystatin C as a GFR-biomarker in this setting or possible non-GFR effects of cystatin C associated with mortality or a combination of the two.
The theory of cystatin C being a more suitable GFR-biomarker than creatinine in critically ill patients arises from the well-known disadvantages of creatinine as a biomarker of GFR. The production rate of creatinine is mainly determined by the patients muscle mass. Generally, the reliability of creatinine as an accurate GFR-biomarker assumes a steady-state production, distribution and clearance of creatinine. Critically ill, bedfast, patients may for several reasons not be in steady-state due to ongoing loss of muscle mass14 or altered distribution volume due to fluid accumulation i.e. increased total water volume15. Other factors may include impared liver function, low meat intake, trauma or fever. All together these factors may potentially lead to a risk of falsely low creatinine values and hence eGFR overestimation in the critically ill patients6, 16, 17. Theoretically, Cystatin C may have advantages over creatinine in estimating eGFR. Cystatin C is produced by all nucleated cells and is not dependent of muscle mass18. Further Cystatin C is freely filtered in glomeruli and not affected by malnutrition19. One concern is that cystatin C generally may have a high turnover in critical illnesses such as sepsis and/or inflammation causing falsely too high concentrations possibly leading to underestimating eGFR4, 10, 11. However, a causal role between inflammation and cystatin C has been under debate and is difficult to establish20. Despite the theoretical advantages that cystatin C may have over creatinine as an eGFR-biomarker in critically ill patients, it has not been convincingly shown that cystatin C alone is more accurately related to golden standard measured GFR with iohexol clearance than creatinine alone. A study by Delanaye et al. showed that cystatin C was more closely related to measured GFR than creatinine alone in critically ill patients21. Another study showed that combined creatinine-cystatin C eGFR-equations or a mean of cystatin C eGFR and creatinine eGFR show the highest agreement with iohexol clearance in critically ill17. This high agreement between the combined equation and measured GFR has also been reported by the CKD-EPI group in patients with chronic kidney disease and in the general population22, 23. Thus, these findings do not point out cystatin C alone as the most accurate biomarker for eGFR so this is not the whole explanation for the superior performance of cystatin C in prognosticating mortality.
Temporary acute kidney injury is common in critically ill patients and clearly related to fatal outcome8, 24 but according to our data even one single measurement of Cystatin C at admission indicated a long-term mortality risk. Could non-GFR related factors underlie the strong association between cystatin C and long-term mortality? Cystatin C has been correlated with mortality independently of renal function in ICU patients8, a finding which is in line with this theory. Another study corroborates with this finding and found that cystatin C was correlated to all-cause mortality despite normal creatinine levels25. Traditional cardiovascular risk factors such as diabetes, obesity, smoking, hypertension, insulin resistance and inflammation and also non-traditional risk factors (symmetric dimetylarginine) have been linked to cystatin C10–13, and these diseases were highly prevalent as discharge diagnoses in this patient cohort. It cannot be ruled out that the reason why cystatin C predicts mortality better than creatinine is probably, at least in part, due to non-GFR related CVD factors affecting cystatin C. Similar results that the sole cystatin C equation outperformes combined equations or sole creatinine equations for mortality estimation have been shown for other patient groups than critically ill such as unselected patients seeking health care26. Further, a meta-analysis of community-based and CKD cohorts by the CKD-prognosis consortium, where cystatin C-based eGFR equations, alone or in combination with creatinine, strengthened the association between eGFR and death at all different levels of eGFR. However, despite the consistent superiority of cystatin C based eGFR over creatinine based GFR in previous studies, a recent large scale Mendelian randomization analyses, predominantly based on community based studies, did not support a causal role of circulating cystatin C in the development of cardiovascular disease27. Additional studies are warranted to provide further insights into the underlying mechanisms of these associations.
All analyses were performed at accredited University Hospital laboratories with methods traceble to the international standard calibration which is a strength in this study. One laboratory changed the creatinine method from Jaffe to enzymatic during the study period. Jaffe methods are known to potentially overestimate creatinine, however at the time of the study inclusion the Jaffe methods at Swedish hospital laboratories were recalibrated to harmonize with enzymatic methods28. Hence, the creatinine methods used in this study are sufficiently comparable for the included subjects and for the study aim. There were no loss to follow up due to the high quality of Swedish registry data29. We are not aware of any larger study comparing creatinine and cystatin C for risk prediction purposes in critically ill patients and we believe that since three large University Hospitals contributed with data the generalizability to critically ill patients in general increase. We did not include the factor for African-Americans in the formulas in this study based on the knowledge that study participants are predominantly Caucasian. This should not have biased our results but we acknowledge however that the study results may have limited generalizability to other ethnicities than Caucasian. Charlson comorbidity index only records prior hospital care, comorbidity treated in primary care may therefore have been missed which is a disadvantage. Given the possible non-GFR related connection between inflammation and cystatin C that has been proposed it is a disadvantage that the study lacks an inflammatory variable as a covariate in the models. Concerns have been raised that the net reclassification index may provide false positive findings during certain circumstances30, however, given the consistency of the superiority of cystatin C based GFR throughout the full range of eGFR provide support for the validity of our findings.
A sole cystatin C equation for eGFR consistently predicted mortality risk better as compared to the sole creatinine-based equation or the combined creatinine/cystatin C equation in patients at intensive care units. Thus, our data favours the use of the sole cystatin C equation rather than the combined cystatin C-creatinine equation when estimating GFR for risk prediction purposes in critically ill patients.
Methods
Study population
The retrospective observational study is based on simultaneous measurements of plasma creatinine and cystatin C on adult patients at admission to intensive care units at Uppsala, Karolinska and Lund University Hospitals, Sweden, from 2004 to 2015. These hospitals perform the vast majority of all cystatin C analyses in Sweden. The patients’ samples were analysed at Uppsala, Karolinska and Lund University Hospital Laboratories, respectively, on the same fresh plasma sample tube. Valid quantitative result of creatinine, cystatin C, age and gender were extracted from the laboratory information systems. All Swedish citizens and those with residence permit have personal identity numbers. Only patients with a complete personal identity number, 16 years of age and older, were included. If participants had more than one measurement only the first measurement was included in the study. In total, 22,488 unique patients with simultaneous measurements of plasma creatinine and cystatin C were included. Swedish Ethical Review Authority in Uppsala approved the research protocol, Dnr 2013/441. All methods were carried out in accordance with relevant guidelines and regulations and reporting followed the STROBE Statement. Informed consent from subjects was waived by the Swedish Ethical Review Authority in Uppsala since only anonymised registry data was analysed.
Measurement of creatinine, cystatin C and estimation of glomerular filtration rate (eGFR)
Plasma creatinine (µmol/L) was analysed with a modified kinetic Jaffe method 2004–2008 and an enzymatic method 2009–2015 on Architect ci8200 (Abbott Laboratories, Abbott Park, Ill., USA) in Uppsala, modified kinetic Jaffe method on UniCel DXC800 (Beckman Coulter, Brea, CA) at Karolinska and an enzymatic method on Roche Cobas c501 (Roche Diagnostics, Rotkreuz, Switzerland) in Lund. All methods were IDMS calibrated by the manufacturer and all three hospital laboratories were accredited. eGFRCr, in mL/min/1.73 m2, was estimated using The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation from 200931. Plasma cystatin C was analysed with reagents from Dade Behring on a BN ProSpec analyzer (Siemens Healthcare Diagnostics) at Uppsala and Karolinska University Hospitals until 2007 and thereafter with an assay from Gentian (Gentian, Moss, Norway), traceable to the international calibrator ERM-DA471/IFCC, on Architect ci8200 in Uppsala32, 33, and on UniCel DXC800 at Karolinska. Cystatin C was analysed with reagents from Roche, traceable to the international calibrator ERM-DA471/IFCC34, on Roche Cobas c501 in Lund. eGFRCyst in mL/min/1.73 m2 was calculated from plasma cystatin C using CAPA35. The international IFCC-equation Caucasian, Asian, Pediatric, and Adult cohorts (CAPA) is practically assay-independent since ERM-DA471/IFCC calibrated cystatin C-assays from 7 diagnostic companies are used. Further, the equation is based on Caucasian and Asian, both pediatric and adult cohorts. eGFRComb in mL/min/1.73m2 was calculated using the CKD-EPI combined creatinine/cystatin C equation (2012)22.
Comorbidity data and endpoint definition
Comorbidity data from 2004 and forward for calculating the Charlson Comorbidity Index (CCI) i.e. in hospital care prior to the study intensive unit care was collected from the National Patient Register that collects data from all in-patient hospital visits in Sweden. CCI is described in detail in Quan et al.36. In brief, the CCI categorizes comorbidities of patients based on the ICD-10 diagnosis codes and sorted into categories. Included categories were myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, rheumatic disease, lever disease, diabetes with complications, diabetes without complication, hemiplegia or paraplegia, renal disease and HIV/AIDS. Each comorbidity category weights from 1 to 6 (based on mortality risk) and the sum of all the weights is the comorbidity score for a patient. A score of zero indicates no comorbidities. A higher score is associated with a higher mortality risk. Three thousand five hundred and ten patients (16%) had no prior hospital care recorded in the National Patient Register. The endpoint mortality was defined using the Swedish Cause of Death Register for all participants and there was no loss to follow up. Both registers are administered by the Swedish National Board of Health and Welfare and records data for all Swedish residents.
Statistics
The associations of eGFRCr, eGFRCyst and eGFRComb and mortality, respectively, were analysed in Cox proportional hazard regression models. The univariate hazard ratio was computed for each 1 ml/min/1.73 m2 of eGFR from 15 to 120 using a reference point at 95 ml/min/1.73 m237 and shown as regression spline curves. The Harrell´s C statistics38, 95% confidence intervals and p-values were calculated using the “somersd” package with the “lincom” command by splitting the study population randomly into a training set and a test set. We also divided the participants into risk categories according to the European Society of Cardiology (ESC) clinical cardiovascular prevention guidelines39 and the clinical decision limit for dialysis in the intensive care unit 20 ml/min/1.73 m2. Thus, the variables were entered into the models in the eGFR categories > 60, 60–30, 30–20 and < 20 mL/min/1.73 m2. The study population were classified to an eGFR category by the creatinine equation and the combined equation and proportion of participants who were reclassified to a higher or lower eGFR category by the cystatin C CAPA equation was assessed for mortality risk compared to the participants not reclassified using Cox proportional hazards models. Models were adjusted for the potential confounding variables age, gender and CCI since the patients who reclassified to a higher risk category with cystatin C were generally older, of male gender and had higher CCI.
Overall improvement in reclassification based the eGFR categories > 60, 60–30, 30–20 and < 20 mL/min/1.73 m2 was evaluated using net reclassification improvement (NRI) according to Pencina et al.40 P values < 0.05 were regarded as statistically significant. Calculations were performed with Stata 13 (Stata Corp., College Station, TX, USA).
Abbreviations
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- HR
Hazard ratio
- IFCC
International federation of clinical chemistry
- NRI
Net reclassification improvement
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.H.K., M.B., B.R., A.D. and A.L. The first draft of the manuscript was written by J.H.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Uppsala University.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the reason that the datasets used contain information that potentially could identify individual patients. Authors are willing to share their data on reasonable request and after case-by-case assessment of such request by a local ethics committee.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peralta CA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin. Chem. 1992;38:1933–1953. doi: 10.1093/clinchem/38.10.1933. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1567–1576. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am. J. Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 6.Lipcsey M, Furebring M, Rubertsson S, Larsson A. Significant differences when using creatinine, modification of diet in renal disease, or cystatin C for estimating glomerular filtration rate in ICU patients. Upsala J. Med. Sci. 2011;116:39–46. doi: 10.3109/03009734.2010.526724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1) Critical care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell M, et al. Cystatin C is correlated with mortality in patients with and without acute kidney injury. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2009;24:3096–3102. doi: 10.1093/ndt/gfp196. [DOI] [PubMed] [Google Scholar]
- 9.Åkerfeldt T, Helmersson J, Larsson A. Postsurgical inflammatory response is not associated with increased serum cystatin C values. Clin. Biochem. 2010;43:1138–1140. doi: 10.1016/j.clinbiochem.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Schei J, et al. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin. J. Am. Soc. Nephrol. 2016;11:280–286. doi: 10.2215/CJN.07360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 2013;83:1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathisen UD, et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J. Am. Soc. Nephrol. 2011;22:927–937. doi: 10.1681/ASN.2010050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melsom T, et al. Estimated GFR is biased by non-traditional cardiovascular risk factors. Am. J. Nephrol. 2015;41:7–15. doi: 10.1159/000371557. [DOI] [PubMed] [Google Scholar]
- 14.Puthucheary ZA, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 15.Prowle JR, Chua HR, Bagshaw SM, Bellomo R. Clinical review: volume of fluid resuscitation and the incidence of acute kidney injury—a systematic review. Crit. Care. 2012;16:230. doi: 10.1186/cc11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravn B, Prowle JR, Martensson J, Martling CR, Bell M. Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit. Care Med. 2017;45:e932–e940. doi: 10.1097/CCM.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 17.Ravn B, et al. Creatinine versus cystatin C based glomerular filtration rate in critically ill patients. J. Crit. Care. 2019;52:136–140. doi: 10.1016/j.jcrc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Kashani KB, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit. Care Med. 2017;45:e23–e29. doi: 10.1097/CCM.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 19.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am. J. Kidney Dis. 2013;62:595–603. doi: 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubb A, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand. J. Clin. Lab. Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delanaye P, et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15:9. doi: 10.1186/1471-2369-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksen BO, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 24.Bell M, et al. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2005;20:354–360. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- 25.Lassus J, et al. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur. Heart J. 2007;28:1841–1847. doi: 10.1093/eurheartj/ehl507. [DOI] [PubMed] [Google Scholar]
- 26.Helmersson-Karlqvist J, Arnlov J, Larsson A. Cystatin C-based glomerular filtration rate associates more closely with mortality than creatinine-based or combined glomerular filtration rate equations in unselected patients. Eur. J. Prev. Cardiol. 2016;23:1649–1657. doi: 10.1177/2047487316642086. [DOI] [PubMed] [Google Scholar]
- 27.van der Laan SW, et al. Cystatin C and cardiovascular disease: a Mendelian randomization study. J. Am. Coll. Cardiol. 2016;68:934–945. doi: 10.1016/j.jacc.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helmersson-Karlqvist J, Ridefelt P, Boija EE, Nordin G. Lower creatinine concentration values and lower inter-laboratory variation among Swedish hospital laboratories in 2014 compared to 1996: results from the Equalis external quality assessment program. Clin. Chem. Lab. Med. 2019;57:838–844. doi: 10.1515/cclm-2018-0670. [DOI] [PubMed] [Google Scholar]
- 29.Almgren T, et al. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J. Hypertens. 2007;25:1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 30.Pepe MS, Fan J, Feng Z, Gerds T, Hilden J. The Net Reclassification Index (NRI): a misleading measure of prediction improvement even with independent test data sets. Stat. Biosci. 2015;7:282–295. doi: 10.1007/s12561-014-9118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Åkerblom A, et al. Cystatin C- and creatinine-based estimates of renal function and their value for risk prediction in patients with acute coronary syndrome: results from the PLATelet inhibition and patient outcomes (PLATO) study. Clin. Chem. 2013;59:1369–1375. doi: 10.1373/clinchem.2012.200709. [DOI] [PubMed] [Google Scholar]
- 33.Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. Calibration of the siemens cystatin C immunoassay has changed over time. Clin. Chem. 2011;57:777–778. doi: 10.1373/clinchem.2010.159848. [DOI] [PubMed] [Google Scholar]
- 34.Grubb A, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem. Lab. Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 35.Grubb A, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin. Chem. 2014;60:974–986. doi: 10.1373/clinchem.2013.220707. [DOI] [PubMed] [Google Scholar]
- 36.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 37.Shlipak MG, et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Perk J, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur. Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to the reason that the datasets used contain information that potentially could identify individual patients. Authors are willing to share their data on reasonable request and after case-by-case assessment of such request by a local ethics committee.


