Abstract
The widespread and poorly regulated use of antibiotics in animal production in low- and middle-income countries (LMICs) is increasingly associated with the emergence and dissemination of antibiotic resistance genes (ARGs) in retail animal products. Here, we compared Escherichia coli from chickens and humans with varying levels of exposure to chicken meat in a low-income community in the southern outskirts of Lima, Peru. We hypothesize that current practices in local poultry production result in highly resistant commensal bacteria in chickens that can potentially colonize the human gut. E. coli was isolated from cloacal swabs of non-organic (n = 41) and organic chickens (n = 20), as well as from stools of market chicken vendors (n = 23), non-vendors (n = 48), and babies (n = 60). 315 E. coli isolates from humans (n = 150) and chickens (n = 165) were identified, with chickens showing higher rates of multidrug-resistant and extended-spectrum beta-lactamase phenotypes. Non-organic chicken isolates were more resistant to most antibiotics tested than human isolates, while organic chicken isolates were susceptible to most antibiotics. Whole-genome sequencing of 118 isolates identified shared phylogroups between human and animal populations and 604 ARG hits across genomes. Resistance to florfenicol (an antibiotic commonly used as a growth promoter in poultry but not approved for human use) was higher in chicken vendors compared to other human groups. Isolates from non-organic chickens contained genes conferring resistance to clinically relevant antibiotics, including mcr-1 for colistin resistance, blaCTX-M ESBLs, and blaKPC-3 carbapenemase. Our findings suggest that E. coli strains from market chickens are a potential source of ARGs that can be transmitted to human commensals.
Keywords: AMR, genomics, LMIC, poultry, Escherichia coli, one health, WGS, Peru
Introduction
Antimicrobial resistance (AMR) in human pathogens has become a major global health threat (O’Neill, 2014; World Health Organization [WHO], 2017b), with bacterial infections increasingly failing to first-line and “last-resort” antibiotic therapies. Decades of widespread antibiotic use in medicine and agriculture (Silbergeld et al., 2008) have resulted in the emergence and spread of various resistance determinants in microbial populations. In particular, the increasing demand for animal protein has led to a dramatic modernization of agriculture, including the regular use of antibiotics in feed to promote animal growth in addition to their therapeutic use. At low (sub-inhibitory) but constant dosages, antibiotics serve as growth promoters by reducing the levels of pathogenic strains and altering the microbiota to allow the host for more nutrient uptake (Evans and Wegener, 2003). This selective pressure has dramatically increased the rate of resistance to various drugs in the microbiota of farm animals, including commensals and pathogens alike (Woolhouse et al., 2015; Robinson et al., 2016; Liu et al., 2018; Nadimpalli et al., 2018; Van Boeckel et al., 2019). Resistant strains can be transmitted from animals to humans through meat consumption, direct animal contact, and exposure to environmental runoff (Hoelzer et al., 2017). Furthermore, horizontal gene transfer can enable the rapid exchange of resistance determinants between different bacterial lineages across hosts and environments (Marshall and Levy, 2011; Woolhouse et al., 2015). Because most antibiotic resistance genes (ARGs) are found in bacteria isolated from both humans and animals, the direction of transfer of most such genes and resistant organisms can be difficult to demonstrate.
We previously surveyed the antibiotic resistomes in the guts of healthy adults in a peri-urban community south of Lima and found high diversity and abundance of genes encoding resistance to amphenicol antibiotics (Pehrsson et al., 2016). A recent study in Cambodia compared E. coli isolates from humans, meat, and fish and found moderate levels of amphenicol resistance in human isolates (Nadimpalli et al., 2019). Although used widely until the 1980s, chloramphenicol is now rarely prescribed in human medicine in Peru and is banned from food animal production since 2013 (Diario Oficial El Peruano, 2013). However, florfenicol (a fluorinated thiamphenicol analog) is widely employed in broiler farming therapeutically and as a growth promoter and available in various commercial feed premixes (FAO, 2014). This has led to the hypothesis that amphenicol resistance in human commensals did not emerge from clinical use, but in food animal populations due to extensive veterinary use of chloramphenicol, florfenicol, and other related compounds. Chickens, most of which now are grown under local intensive farming systems, provide the primary source of animal protein for the Peruvian population (World Bank Group, 2017). Average per capita consumption was estimated at 49.5 kg in 2018, and up to 80.5 kg per person per year in the capital of Lima (Ministerio de Agricultura y Riego [MINAGRI], 2018).
We hypothesize that current practices in poultry production and handling in LMICs result in highly resistant chicken commensals that can potentially colonize the human gut. To test this, we assessed the distribution of resistant E. coli and associated ARGs in market chickens, chickens grown without antibiotics (organic chickens), and residents from a low-income, peri-urban community in Lima, with varying levels of exposure to poultry.
Materials and Methods
Study Site
Local market stalls in Villa El Salvador (VES) and its neighboring district, San Juan de Miraflores (SJM) in southern Lima, were visited to purchase whole chickens. Human fecal samples were collected from the community surrounding the VES market (see Supplementary Table 1). These neighboring districts share similar demographic characteristics and contain various urban informal settlements (Instituto Nacional de Estadística e Informática [INEI], 2017). Informal housing arrangements, lack of running water, and inadequate sanitation in most households make these sites representative of peri-urban settlements in other LMICs, which are considered hotspots for AMR (Nadimpalli et al., 2020). We also collected laying hens’ samples from an organic free-range farm in Vegueta (VEG), located approximately 150 km north of Lima.
Samples
Humans
Fecal samples were collected in March 2018 from three resident groups in the VES community: chicken vendors (n = 23) working in the markets where chickens were purchased, babies (n = 60) between 1 and 24 months old from an ongoing cohort study in the community, and non-vendor adults (n = 48). Fresh feces were collected by individuals and legal guardians as instructed. Fecal samples were swabbed and placed vials with Cary-Blair transport medium, stored at 4°C, and transferred to the laboratory for further processing. Ethical approval was obtained from Institutional Review Boards at Universidad Peruana Cayetano Heredia and Asociación Benéfica Prisma.
Market (Non-organic) Chickens
Forty-one recently slaughtered whole chickens were purchased in 14 market stalls of VES and SJM from March to April of 2018. Whole chickens and market stands were selected by convenience. We have no information on the exact rearing conditions or origin of these chickens. However, almost all of the chicken meat sold in Lima originates from conventional local production systems that heavily rely on routine antibiotic use as a standard industry practice. Chickens were taken to field laboratories for the collection of intestinal contents. Cloacal and intestinal swabs were put in sterile tubes with saline solution and transferred within 2 h to the laboratory for bacterial culture.
Organic Laying Hens
Cloacal swabs from 20 laying hens from the sole Certified Humane® (Humane Farm Animal Care [HFAC], 2018) organic free-range farm in Lima were obtained in May of 2019 to have a set of isolates originating from poultry raised without antibiotics as a comparison group to the market chickens. Cloacal swabs were put in sterile tubes with Cary-Blair transport medium, stored at 4°C, and transferred to the laboratory for processing.
Culture and Isolation
Samples were streaked in CHROMagar Orientation Media (CHROMagar Microbiology, Paris, France) for rapid differentiation and presumptive identification of E. coli. Up to 3–5 dark pink to red colonies indicative of E. coli were re-streaked to MacConkey agar (Becton Dickinson, Heidelberg, Germany) for lactose fermentation confirmation and then selected for species confirmation with a conventional biochemical profiling panel (Garrity et al., 2005). Those confirmed as E. coli (n = 315) were included in the study and stored in Tryptic soy broth (TSB, Becton Dickinson) with glycerol at −20°C until DNA extraction.
Antibiotic Susceptibility Testing
Disk diffusion tests were performed with CLSI 2018 standards, using susceptible, intermediate, and resistant definitions for Enterobacteriaceae (Clinical and Laboratory Standards Institute (CLSI), 2018). A total of 18 antibiotics were used (see Supplementary Table 2). Extended-spectrum β-lactamase (ESBL) activity was detected using the cefotaxime-ceftazidime-cefepime-aztreonam with amoxicillin with clavulanic acid test, according to EUCAST standards (The European Committee on Antimicrobial Susceptibility Testing [EUCAST], 2017). We interpreted florfenicol susceptibility using chloramphenicol’s CLSI breakpoints as there are no approved cut-off values for E. coli (White et al., 2000; Clinical and Laboratory Standards Institute (CLSI), 2018). We did not report on colistin phenotypic resistance due to the lack of recommended cut-off values for colistin disk diffusion testing (Ezadi et al., 2018). A multidrug-resistant drug isolate was defined as expressing phenotypic resistance to three or more antibiotic classes (Magiorakos et al., 2012).
DNA Extraction and WGS
DNA was extracted from 1 ml TSB culture using the GeneJet Genomic DNA purification kit (Thermo Fisher Scientific, Waltham, MA, United States) following the manufacturer’s instructions. DNA was eluted in 200 μl Tris-EDTA buffer and quantified using the Qubit dsDNA BR Kit (Thermo Fisher Scientific). We selected a subset of 118 isolates for WGS on the Illumina MiSeq platform. We randomized isolate selection within each study group to include representative drug susceptibility patterns. Libraries were prepared from 1 ng gDNA with the Nextera XT kit (Illumina, San Diego, CA, United States). Batches of 24 libraries were indexed and sequenced with MiSeq v3 sequencing kits to generate 300 bp paired-end reads and yield a mean of 84x genome coverage (minimum 17x, maximum 163x). Raw Illumina reads were uploaded to GenBank under BioProject PRJNA633873.
Genomic and Phylogenetic Analyses
Raw reads were assessed with FastQC v0.11.9, trimmed with Trimmomatic v0.36.6 (Bolger et al., 2014), assembled with SPAdes v.3.10.0 (Bankevich et al., 2012), and annotated with Prokka v1.5 (Seemann, 2014). MLST was determined from de novo assemblies using the CGE pipeline (Thomsen et al., 2016) based on the Enterobase scheme1 accessed through PubMLST2. ARGs were annotated by querying assemblies against the CARD database (Alcock et al., 2020) at >90% identity. We clustered ARG-containing contigs with CD-HIT (Li and Godzik, 2006) at an 80% similarity threshold over the contig’s length. Plasmid typing was done using the PlasmidFinder database (Carattoli et al., 2014) and BLAST (Ye et al., 2006) to identify assemblies containing an Inc reference gene, with a threshold of 90% identity and E-value <1e-35. Prokka-annotated assemblies were used as input for Roary v3.13.0 (Page et al., 2015) to determine the pangenome and perform a core gene alignment of all sequenced isolates using blastp identity threshold of 95%. Variable positions were extracted from an alignment of 2,233 core genes (2,252,390 bp) and used to build a maximum-likelihood phylogenetic tree with RAxML v8.2.4 (Stamatakis, 2014) with the general time-reversible (GTR) substitution model and gamma correction for rate heterogeneity. SNP-dists v0.7.0 was used to build a pairwise SNP distance matrix from the pangenome alignment. A published genome of Escherichia fergusonii (Manninger et al., 2016) was used to root the phylogenetic tree. CLC Genomics Workbench v20.0 (QIAGEN Bioinformatics) was used to visualize and annotate the tree.
Statistical Analysis
The proportion of resistant isolates was tabulated for each sample type. Comparisons of proportions were evaluated using the Chi-square test or Fisher’s exact test as appropriate. Data management and statistical analysis were performed with a confidence level of 95% using STATA 16 (StataCorp, College Station, TX, United States) and R (v3.5.2).
Results
Antimicrobial Susceptibility
Chickens
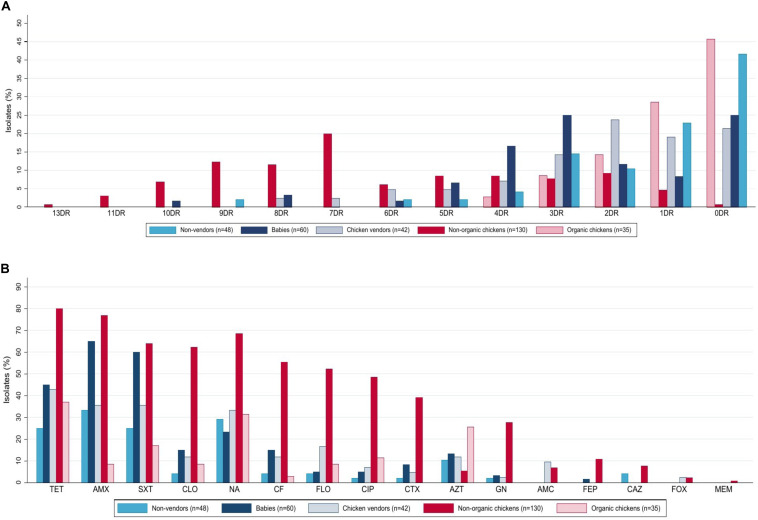
Escherichia coli isolates were obtained from market (non-organic) (n = 130) and organic (n = 35) chickens. Multidrug-resistant (MDR) rates were higher in non-organic animals (76.9 vs. 11.4%, p < 0.001, Chi-square test). Only the non-organic chicken isolates were ESBL producers (39.2%, n = 51), and presented resistance to at least five antibiotic families (46.2%, n = 60), including chloramphenicol (62.3%, n = 81), florfenicol (52.3%, n = 68), and meropenem (0.8%, n = 1). These isolates presented the highest resistance levels to almost every antimicrobial tested. In contrast, the organic chicken isolates were susceptible gentamicin, amoxicillin with clavulanic acid, cefotaxime, cefepime, ceftazidime, and cefoxitin (Figure 1). A comparison of resistance rates is detailed in Table 1.
FIGURE 1.
Phenotypic antibiotic resistance of 315 E. coli isolates from humans and chickens in Lima, Peru. (A) Isolates were grouped by number of antibiotics to which they were resistant based on disk-diffusion assays. (B) Based on resistance to 16 antibiotics. (A) Percentage of resistances to different drugs, DR, drug resistance. (B) Resistance patterns to different antibiotics, TET, tetracycline; AMX, amoxicillin; SXT, trimethoprim/sulfamethoxazole; NA, nalidixic acid; CLO, chloramphenicol; CF, cefalotin; FLO, florfenicol; CIP, ciprofloxacin; CTX, cefotaxime; AZT, azithromycin; GN, gentamicin; AMC, Amoxicillin with Clavulanic Acid; FEP, cefepime; CAZ, ceftazidime; FOX, cefoxitin; MEM, meropenem.
TABLE 1.
Resistance profiles and bivariate analysis of E. coli isolates from chickens and humans.
| Results | Humans | Chickens | Total (n=315) | pe | |||||||||
| Total (n=150) | N (%) |
pa | pb | pc | Total (n=165) | N (%) |
pd | ||||||
| Non-vendor adults (n=48) | Babies (n=60) | Vendors (n=42) | Non-organic (n=130) | Organic (n=35) | |||||||||
| Multidrug-resistance | |||||||||||||
| Yes | 56 (37.3) | 11 (22.9) | 29 (48.3) | 16 (38.1) | 0.007 | 0.305 | 0.117 | 104 (63) | 100 (76.9) | 4 (11.4) | <0.001 | 160 (50.8) | <0.001 |
| ESBL | |||||||||||||
| Yes | 6 (4) | 1 (2.1) | 4 (6.7) | 1 (2.4) | 0.379* | 0.646* | 1.000* | 51 (30.9) | 51 (39.2) | 0 (0) | <0.001 | 57 (18.1) | <0.001 |
| Amphenicols | |||||||||||||
| Cloramphenicol | 16 (10.7) | 2 (4.2) | 9 (15) | 5 (11.9) | 0.107* | 0.655 | 0.245* | 84 (50.9) | 81 (62.3) | 3 (8.6) | <0.001 | 100 (31.8) | <0.001 |
| Florfenicol | 12 (8) | 2 (4.2) | 3 (5) | 7 (16.7) | 1.000* | 0.087* | 0.077* | 71 (43.1) | 68 (52.3) | 3 (8.6) | <0.001 | 83 (26.4) | <0.001 |
| Tetracyclines | |||||||||||||
| Tetracycline | 57 (38) | 12 (25) | 27 (45) | 18 (42.9) | 0.032 | 0.830 | 0.073 | 117 (70.9) | 104 (80) | 13 (37.1) | <0.001 | 174 (55.2) | <0.001 |
| Sulfonamides | |||||||||||||
| Trimethoprim/sulfamethoxazole | 63 (42) | 12 (25) | 36 (60) | 15 (35.7) | <0.001 | 0.016 | 0.268 | 89 (53.9) | 83 (63.9) | 6 (17.1) | <0.001 | 152 (48.3) | 0.034 |
| Aminoglycosides | |||||||||||||
| Gentamicin | 4 (2.7) | 1 (2.1) | 2 (3.3) | 1 (2.4) | 1.000* | 1.000* | 1.000* | 36 (21.8) | 36 (27.7) | 0 (0) | <0.001 | 40 (12.7) | <0.001 |
| Macrolides | |||||||||||||
| Azithromycin | 18 (12) | 5 (10.4) | 8 (13.3) | 5 (11.9) | 0.643 | 0.831 | 1.000* | 16 (9.7) | 7 (5.4) | 9 (25.7) | <0.001 | 34 (10.8) | 0.511 |
| Penicillins | |||||||||||||
| Amoxicillin | 70 (46.7) | 16 (33.3) | 39 (65) | 15 (35.7) | 0.001 | 0.004 | 0.813 | 103 (62.4) | 100 (76.9) | 3 (8.6) | <0.001 | 173 (54.9) | 0.005 |
| Amoxicillin with Clavulanic Acid | 4 (2.7) | 0 (0) | 0 (0) | 4 (9.5) | N.A. | 0.026* | 0.044* | 9 (5.5) | 9 (6.9) | 0 (0) | 0.207* | 13 (4.1) | 0.214 |
| Cephalosporins | |||||||||||||
| Cefalotin | 16 (10.7) | 2 (4.2) | 9 (15) | 5 (11.9) | 0.107* | 0.655 | 0.245* | 73 (44.2) | 72 (55.4) | 1 (2.9) | <0.001 | 89 (28.3) | <0.001 |
| Cefotaxime | 8 (5.3) | 1 (2.1) | 5 (8.3) | 2 (4.8) | 0.223* | 0.697* | 0.597* | 51 (30.9) | 51 (39.2) | 0 (0) | <0.001 | 59 (18.7) | <0.001 |
| Cefepime | 1 (0.7) | 0(0) | 1 (1.7) | 0 (0) | 1.000* | 1.000* | N.A. | 14 (8.5) | 14 (10.8) | 0 (0) | 0.042* | 15 (4.7) | 0.001 |
| Ceftazidime | 2 (1.3) | 2 (4.2) | 0(0) | 0(0) | 0.195* | N.A. | 0.497* | 10 (6.1) | 10 (7.7) | 0 (0) | 0.122* | 12 (3.8) | 0.029 |
| Cefoxitin | 1 (0.7) | 0 (0) | 0 (0) | 1 (2.4) | N.A. | 0.412* | 0.467* | 3 (1.8) | 3 (2.3) | 0 (0) | 1.000* | 4 (1.3) | 0.624* |
| Carbapenems | |||||||||||||
| Meropenem | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N.A. | N.A. | N.A. | 1 (0.6) | 1 (0.8) | 0 (0) | 1.000* | 1 (0.3) | 1.000* |
| Quinolones | |||||||||||||
| Nalidixic Acid | 42 (28) | 14 (29.2) | 14 (23.3) | 14 (33.3) | 0.492 | 0.265 | 0.670 | 100 (60.6) | 89 (68.5) | 11 (31.4) | <0.001 | 142 (45.1) | <0.001 |
| Ciprofloxacin | 7 (4.7) | 1 (2.1) | 3 (5) | 3 (7.1) | 0.627* | 0.688* | 0.336 | 67 (40.6) | 63 (48.5) | 4 (11.4) | <0.001 | 74 (23.5) | <0.001 |
P-values: Chi-square test and confidence level of 95%, * Fisher exact test and confidence level of 95%.pa: babies and non-vendor adults, pb: babies and chicken vendors, pc: chicken vendors and non-vendor adults, pd: non-organic and organic chickens, pe: humans and chickens. ESBL, Detection of Extended Spectrum Beta-lactamases; N.A., not applicable.
Humans
Human isolates (n = 150) were obtained from babies aged 0–2 years (40%, n = 60), adult non-vendors (32%, n = 48), and chicken vendors in local markets (28%, n = 42). MDR isolates were more frequent in chicken vendors (38.1%, n = 16) compared to non-vendors (22.9%, n = 11). Isolates from chicken vendors presented higher resistance rates to florfenicol (16.7%, n = 7) compared to non-vendor adults (4.2%, p = 0.077, Fisher’s exact test) and babies (5%, p = 0.087, Fisher’s exact test). However, they were not more resistant to chloramphenicol (11.9 vs. 4.2%, p = 0.245, Fisher’s exact test). E. coli isolates from babies presented high resistance levels to tetracycline (45%, n = 27), trimethoprim/sulfamethoxazole (60%, n = 36), amoxicillin (65%, n = 39), azithromycin (13.3%, n = 8), chloramphenicol (15%, n = 9), cefalotin (15%, n = 9), cefotaxime (8.3%, n = 5), and gentamicin (3.3%, n = 2).
Chickens Versus Humans
Overall, resistance rates were higher among chicken E. coli compared to human isolates (Table 1), including MDR (63 vs. 37.3%, p < 0.001, Chi-square test) and ESBL-producing E. coli (30.9 vs. 4.0%, p < 0.001, Chi-square test). Additionally, we found higher florfenicol resistance in 43.1% (n = 71) of chicken isolates and 16.7% (n = 7) of chicken vendors compared to other groups. Further resistance results are shown in Table 1.
Genomic Analysis
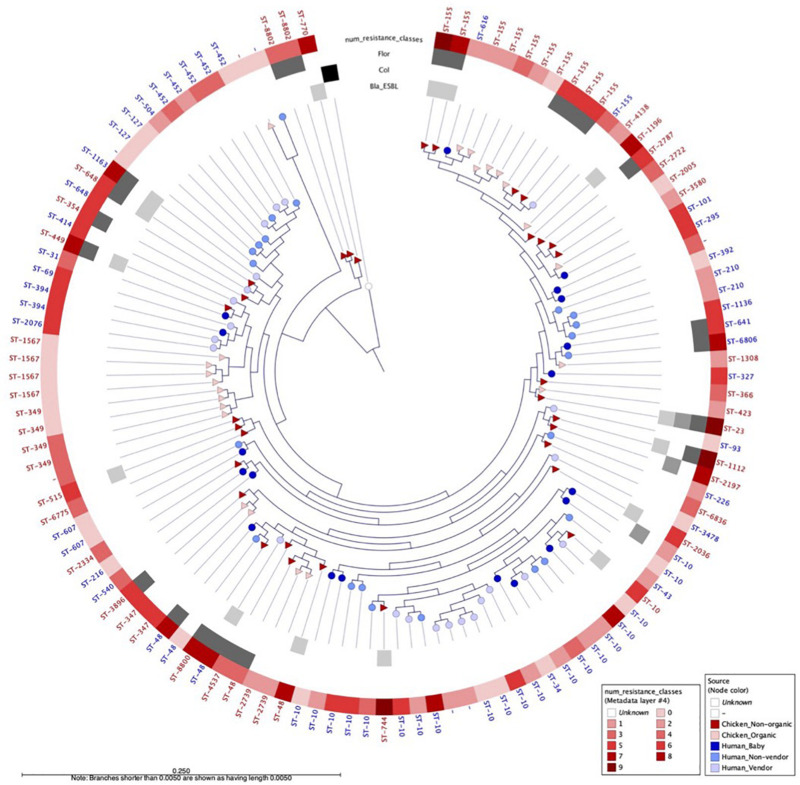
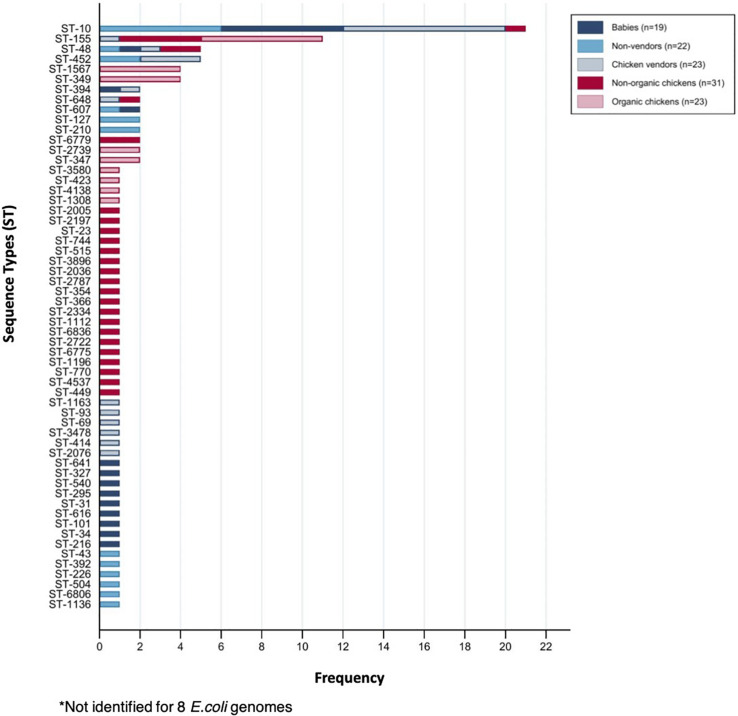
We selected a random subset of 118 isolates from babies (n = 19), adults (n = 22), chicken vendors (n = 23), non-organic chickens (n = 31), and organic chickens (n = 23) to further understand the flow of E. coli phylogroups and ARGs between animals and humans. The genomic dataset had a mean N50 of 102,136 bp (SD = 49,584 bp) and a mean total length of 4,490,970 bp (SD = 1,222,343 bp). Pangenome analysis using Roary identified a core genome (i.e., genes found in ≥99% of isolates) of 2,304 genes and an accessory genome (found in ≤15% of isolates) of 26,135 genes. To assess the genomic similarity between isolates, we built a maximum-likelihood phylogenetic tree from the pangenome alignment (Figure 2) and calculated all pairwise SNP distances (Supplementary Figure 1). We identified 58 sequence types (ST) and 14 clonal complexes in the dataset (Figure 3). ST-10 (n = 21), ST-155 (n = 11), ST-48 (n = 5), and ST-648 (n = 2) were assigned to isolates of both animal and human origin. Highly similar isolates (differing in less than 100 SNPs across their pangenomes) were only found within host groups. STs shared by humans and chickens were more distantly related: ST-155 isolates (differing in 951 SNPs) were found in organic chickens and babies; ST-10 (1,046 SNPs), ST-155 (1,141 SNPs), ST-48 (1,542 SNPs), and ST-648 (13,470 SNPs) were shared by chicken vendors, non-vendors and market chickens.
FIGURE 2.
Maximum likelihood phylogenetic tree built from the alignment of 2,233 E. coli core genes from 118 human and animal isolates, using E. fergusonii as outgroup. Nodes are shaped by host type (circle = human, triangle = chicken) and colored by sampling group. Outer gray circles indicate presence of ESBL, mcr-1, and floR genes. Outer red circles indicate the number of antibiotic classes to which the isolate is resistant. WGS-based sequence type (ST) is indicated for each isolate.
FIGURE 3.
Sequence types (ST) identified among sequenced E. coli isolates.
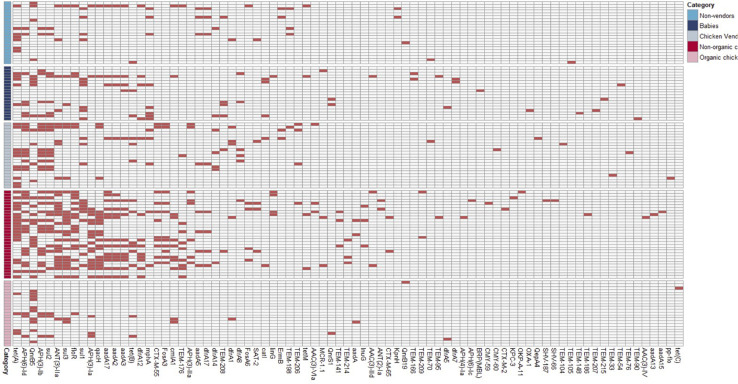
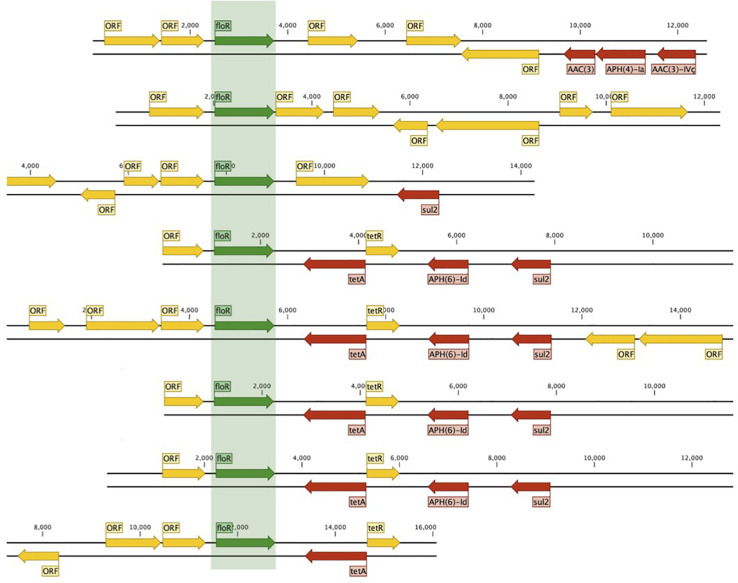
We identified 604 ARG hits and 81 unique ARGs in the dataset (Figure 4) with a mean of 5.1 genes (95%CI: 4.2–6.0) per isolate. Detected ARGs are associated with resistance to beta-lactams (n = 30), aminoglycosides (n = 18), trimethoprim (n = 7), amphenicols (n = 4), tetracyclines (n = 4), quinolones (n = 4), sulfonamides (n = 3), fosfomycins (n = 2), lincosamides (n = 2), macrolides (n = 1), glycopeptides (n = 1), polymyxins (n = 1), streptogramins (n = 1), and streptothricins (n = 1). Fifteen isolates (13 from market chickens and two from vendors) were positive for ESBLs; we found blaCTX-M-55 in 73% (11/15) of them, in plasmid contigs that shared >96% sequence similarity between chickens and vendors. We found the blaKPC-3 gene encoding carbapenem resistance in one market chicken isolate. Additionally, three isolates (two from market chickens and one from a baby) had the mcr-1 colistin resistance gene (Figure 2). Forty-five plasmid replicon markers were identified in both humans and chickens (Supplementary Data Set 1 and Supplementary Figure 2). The most frequent markers were IncFIB (AP001918) (42.4%), Col (pHAD28) (35.6%), and IncFII (28%). Some markers were found in only one host type, such as IncB/O/K/Z_4 (p < 0.001) and Col156 (p = 0.003) in humans, and IncHI1B (p < 0.001) in chickens (Supplementary Figure 2). We did not find significant differences in plasmid markers among CTX-M, mcr-1, and blaKPC-3 producers from chickens and humans (Supplementary Table 3). We identified the floR gene in 18.6% (22/118) of genomes, and their contigs clustered into eight unique (>80% identity) sequences that matched to plasmid replicons of the IncF family (Figure 5). They shared a common theme in which floR was often found along with other antibiotic resistance genes (tetA, APH(6)-Id, sul2) and proteins predicted to be involved in horizontal gene transfer and DNA recombination (transposases, resolvases, recombinases, relaxases). This suggests that floR has been transferred on multiple occasions to MDR plasmids commonly shared by animal and human hosts. The cmlA1 and catA1 genes (which encode resistance to chloramphenicol but not florfenicol) were found in 11 and four genomes, respectively. A summary of the genomic analysis is described in Supplementary Data Set 2. All 604 ARG hits are listed in Supplementary Table 4.
FIGURE 4.
Antimicrobial resistance genes detected among sequenced E. coli.
FIGURE 5.
Mobilization of floR in conjugative plasmids from animals and humans. Eight unique (80% ID clustering) floR-containing plasmids were found in 22/118 humans and chicken isolates.
Discussion
We compared the resistance rates, genotype distributions, and ARGs present in commensal E. coli isolates from human and chicken populations. 315 E. coli isolates from humans (n = 150) and chickens (n = 165) were identified, with chickens showing higher rates of MDR (63 vs. 37.3%) and ESBL (30.9 vs. 4%) phenotypes. Poultry production is one of the largest and most widespread industries in Peru, making use of large quantities of various antimicrobials critical for human medicine (Page and Gautier, 2012; World Health Organization [WHO], 2017a). Despite their importance for treatment and disease prevention, their extended and unregulated use as growth promoters increases selective pressure for MDR bacteria (Diarra and Malouin, 2014). Our results highlight the potential consequences of this practice in poultry production.
Given that many LMICs are now transitioning to industrial models of animal production, there is a concern that extensive animal exposure to antibiotics may result in the “spillover” of resistant bacteria and ARGs into humans. Although ARG transfer has been extensively studied in pathogenic organisms, the vast majority of transfer events occur silently among non-pathogenic bacteria in host-associated and environmental microbial communities (Smillie et al., 2011; Pehrsson et al., 2016; Wang et al., 2017). E. coli and members of the Enterobacteriaceae are well adapted to the gut environment, acquiring diverse functions and ARGs to colonize their hosts (Szmolka and Nagy, 2013). It is thus likely that ARGs can accumulate in commensal strains to enrich the human gut resistome, and later be mobilized into pathogenic strains to become multidrug-resistant (Penders et al., 2013).
Increased global consumer awareness of how animal meat is produced has increasingly lead to the establishment of organic and free-range farms (Holtcamp, 2011). This production model aims to stop the widespread use of antibiotics as prophylactics and growth promoters in chickens under the premise that it will reduce AMR rates in exposed bacteria due to an absence of this selective pressure (Tang et al., 2017). The lower rates of AMR found in organic chickens compared to conventionally raised ones support this assertion. Furthermore, organic chicken isolates were entirely susceptible to gentamicin, amoxicillin with clavulanic acid, cefotaxime, cefepime, ceftazidime, and cefoxitin; the first three, together with florfenicol, are frequently found as active ingredients in local commercially available premixes aimed toward infection prevention and enhancement of growth performance.
The number of peri-urban communities has increased dramatically in recent decades in Peru and other LMICs, on par with poorly regulated neighborhood markets. Despite regulatory authorities’ supervision, many small markets function clandestinely for slaughtering to meet the consumers’ demand for “fresh” goods. Such consumer preferences, combined with other external factors, result in the poultry industry trading around 80% of its chicken production live (De León, 2009). Consequently, poultry butchering and handling practices in market stalls and related environments (including households) pose a risk of exposure to fecal cross-contamination from the viscera, a possible transfer route of animal-derived E. coli into the human gut. Despite their close contact with chickens and regular manipulation of viscera, E. coli isolates from chicken vendors did not fully match the resistance patterns observed in chicken isolates; this may be in part because the use of antibiotics to treat human infections also determine the resistance patterns of E. coli in the human gut. Shared STs (e.g., ST-10, ST-155, ST-48) were found in both chicken vendors and market chickens, coinciding with previous reports of globally successful STs linked to zoonotic transmission (Cohen Stuart et al., 2012; Yamaji et al., 2018; Falgenhauer et al., 2019; Hussain et al., 2019). However, shared STs differed in 900+ SNPs across their core genomes, which rules out a direct transmission between hosts and may reveal host-specific adaptations in E. coli.
Florfenicol, which is not approved for use in humans, was the only antibiotic tested for which resistance levels were significantly higher in chicken vendors than other human groups. We found florfenicol resistance in 43% of non-organic chicken isolates and 17% of chicken vendors. The floR gene was found in 17 E. coli genomes from chickens and five from humans and was associated with conjugative plasmids that were highly similar between humans and animal isolates (Figure 5). The high diversity of floR-carrying plasmids and the fact that they were identified in 15 different STs may reflect a strong selective pressure to maintain resistance to florfenicol in chicken E. coli populations. The floR gene confers resistance to florfenicol and chloramphenicol via an efflux pump mechanism (Bischoff et al., 2005; Braibant et al., 2005; Van Hoek et al., 2011) and is readily transferred among Gram-negative bacterial lineages via conjugative plasmids (Kruse and Sorum, 1994; Singer et al., 2004). We hypothesize that resistance to florfenicol in humans may occur via the colonization by floR-positive strains of animal origin or plasmid conjugation from animal strains into human commensals, both facilitated by improper handling of chicken meat by both vendors and consumers. This identifies floR as a potential marker of antibiotic resistance in humans that can be traced directly to antibiotic use in animals.
Resistance to last-resort drugs such as colistin and carbapenems is increasing worldwide (Peyclit et al., 2019). The blaKPC-3 gene and phenotypic resistance to meropenem were observed in one market chicken isolate. The blaKPC-2 gene had been described in Klebsiella pneumoniae from Peruvian hospital settings (Horna et al., 2017; Roach et al., 2020) but this is, to our knowledge, the first report of KPC-3 in Peru; its origins and spread into animal populations warrant further study. Three isolates harboring the colistin resistance mcr-1 gene were found in humans and chickens. Colistin is used to treat human infections caused by carbapenem-resistant bacteria (Nation et al., 2017) and mcr-1 has already been reported in local E. coli and K. pneumoniae clinical isolates (Ugarte-Silva et al., 2018; Deshpande et al., 2019). The import and trade of colistin in veterinary products was banned in Peru in 2019 (Diario Oficial El Peruano, 2019) but they were still in use in poultry farms at the time of sampling.
Despite our initial assumption that babies would present lower rates of resistance compared to adults, they had similar resistance profiles to chicken vendors and had higher rates of phenotypic resistance to amoxicillin and trimethoprim/sulfamethoxazole than adults. This supports the findings of a previous study in Peru that found older age protective against resistance (Kalter et al., 2010). Children are prone to play in soils and have a higher risk of colonization with enteropathogens via the fecal-oral route (Marquis et al., 1990; Lietzau et al., 2007; Fuhrimann et al., 2016). The effect may be exacerbated in this community, where water and adequate sanitation are not available in all households. Surveys collected during an ongoing cohort study in VES (unpublished data) indicate that the most commonly used antibiotics in this group were amoxicillin and amoxicillin with clavulanic acid, followed by trimethoprim/sulfamethoxazole and erythromycin, consumed between the first 2 months up to 2 years at a rate of 3.8 courses per child-year (Nadimpalli et al., 2020). Predictably, 63.9% of the baby isolates in our study exhibited resistance to amoxicillin and 52.5% to trimethoprim/sulfamethoxazole. Other antibiotics administered to this group but with no evidence of resistance were cephalexin, clarithromycin, azithromycin, ciprofloxacin, and furazolidone. Community-level education campaigns on antibiotic awareness, combined with behavior change interventions, could help limit the transmission of ARGs and resistant bacteria to babies.
Many reports have identified high levels of AMR in food animals and retail meats in the United States (Davis et al., 2018; Liu et al., 2018), China (Liu et al., 2017; Wu et al., 2018; He et al., 2019), and Europe (Gelbíčová et al., 2019; Mellor et al., 2019). Other studies have assessed ARG dissemination between isolates of human, animal, and environmental origin in LMICs (Nadimpalli et al., 2019; Subbiah et al., 2020). Our study is innovative because we compared animals raised with and without antibiotics, along with humans with varying levels of exposure to chicken meat, and used WGS to identify resistant isolates and ARGs among human and animal populations within the same community. However, it presents limitations: (i) We focused exclusively on E. coli, and our results do not account for the effects in other commensal species nor the transfer of mobile genetic elements (MGEs) between them; (ii) We included only one isolate for each subject, so we were unable to assess within-host E. coli diversity; (iii) The timeline for our collection of human stool samples and chicken intestinal and cloacal isolates do not overlap for much of the study; (iv) Illumina-based sequencing generated short reads that made it challenging to reconstruct full plasmid sequences. The use of long-read sequencing should vastly improve assemblies and provide new insights into the exchange and recombination of mobile genetic elements between hosts.
There are very few studies that can clearly link antibiotic use on farms with antibiotic resistance in humans, in part because of the lack of national antibiotic consumption surveys on farms and the high degree of HGT that occurs in enterobacterial genomes (Smillie et al., 2011; Partridge et al., 2018). WHO’s 2017 Global Action Plan on AMR calls for strengthening national surveillance capacities (World Health Organization [WHO], 2017b). Surveillance data on antibiotic use and resistance rates in poultry may serve stakeholders to make evidence-based decisions and policies, as is the case with high-income countries. AMR surveillance studies conducted in South America are scarce compared to other LMICs (Bantar et al., 2000; García et al., 2012; Baker et al., 2017; Bazzo et al., 2018). As the antibiotic resistome expands through the accumulation of gene cassettes or novel plasmids, and with further ARG transfer from animals into commensal human strains, last-resort drugs such as colistin and carbapenems will become increasingly ineffective to combat pathogenic microorganisms.
This study highlights the potential dissemination of resistance genes in Escherichia coli from market chickens into human populations. Policy change is needed to curb the misuse of antibiotics in agriculture, which in the past has been successful at reducing the environmental burden of resistance without hurting the productivity of farmers (Aarestrup et al., 2010; Marshall and Levy, 2011). It is estimated that Peru will increase antimicrobial use in livestock by 160% from 2010 to 2030 (Van Boeckel et al., 2015). To offset this scenario, the National Multi-sectoral Action Plan to Combat Antimicrobial Resistance is set to provide a set of milestones involving regulations of antimicrobial use in food animals by 2021 (Ministerio de Salud [MINSA], 2019). We support the view that restricting non-therapeutic supplementation of antibiotics in animal feed and regulating the drug classes used to treat disease will help prevent the dissemination of AMR from animals into humans. Our research may serve as a baseline for future interventions aimed at limiting the spread of AMR in the environment.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA633873/, PRJNA633873.
Ethics Statement
The studies involving human participants were reviewed and approved by the Comité Institucional de Ética en Investigacion, Universidad Peruana Cayetano Heredia. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
PT, RG, DB, MM, and GS designed the study. MM, GS, AD-B, BA, LC, and MC collected the samples and conducted the experiments. CC-V, PT, GS, MH, MP, and AL analyzed the datasets. PT, GS, AD-B, and MM wrote the manuscript. RG, DB, AL, and MP reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Maya Nadimpalli for comments on the manuscript and Javier Valdivia from our industry partner, for providing access to the organic chicken flocks for sampling.
Funding. This study was supported by the Innóvate Perú grant #289-2017, CONCYTEC grant #088-2018, NIH D43 TW010074, and R01 AI108695-01A1 grants. PT and GS were supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009343 and the University of California Global Health Institute. GS was supported by the CONCYTEC-FONDECYT-World Bank Group contract number E033-01-08-2018-FONDECYT/Banco Mundial-Programas de Doctorado en Áreas Estratégicas y Generales. AL was supported by the training grant D43TW007393 awarded by the Fogarty International Center of the U.S. National Institutes of Health. The funders had no involvement in the conduct or publication of this research. The opinions expressed are those of the authors and do not necessarily reflect the views of the sponsors.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.635871/full#supplementary-material
Pairwise SNP distances between all pairs of E. coli isolates.
Plasmid replicon marker sequences among sequenced E. coli.
Location, type, and sample size of humans and chickens included in the study.
Antibiotics used for resistance profiling.
Plasmid markers and bivariate analysis of sequenced E. coli from chickens and humans. ∗Fisher exact test and confidence level of 95%.
Antimicrobial resistance genes hits among 118 sequenced E. coli.
Plasmid markers detection among 118 E. coli isolates.
Genomic analysis of 118 E. coli isolates.
References
- Aarestrup F. M., Jensen V. F., Emborg H. D., Jacobsen E., Wegener H. C. (2010). Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am. J. Vet. Res. 71 726–733. 10.2460/ajvr.71.7.726 [DOI] [PubMed] [Google Scholar]
- Alcock B. P., Raphenya A. R., Lau T. T. Y., Tsang K. K., Bouchard M., Edalatmand A., et al. (2020). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48 D517–D525. 10.1093/nar/gkz935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. S., Campos J., Pichel M., Della Gaspera A., Duarte-Martínez F., Campos-Chacón E., et al. (2017). Whole genome sequencing of Shigella sonnei through PulseNet Latin America and Caribbean: advancing global surveillance of foodborne illnesses. Clin. Microbiol. Infect. 23 845–853. 10.1016/j.cmi.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantar C., Famiglietti A., Goldberg M., Altschuler M., Bardi L., Casellas J. M., et al. (2000). Three-year surveillance study of nosocomial bacterial resistance in Argentina. Int. J. Infect. Dis. 4 85–90. 10.1016/S1201-9712(00)90099-7 [DOI] [PubMed] [Google Scholar]
- Bazzo M. L., Golfetto L., Gaspar P. C., Pires A. F., Ramos M. C., Franchini M., et al. (2018). First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015-16. J. Antimicrob. Chemother. 73 1854–1861. 10.1093/jac/dky090 [DOI] [PubMed] [Google Scholar]
- Bischoff K. M., White D. G., Hume M. E., Poole T. L., Nisbet D. J. (2005). The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple-drug resistance in swine Escherichia coli. FEMS Microbiol. Lett. 243 285–291. 10.1016/j.femsle.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braibant M., Chevalier J., Chaslus-Dancla E., Pagès J. M., Cloeckaert A. (2005). Structural and functional study of the phenicol-specific efflux pump floR belonging to the major facilitator superfamily. Antimicrob. Agents Chemother. 49 2965–2971. 10.1128/AAC.49.7.2965-2971.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A., Zankari E., Garciá-Fernández A., Larsen M. V., Lund O., Villa L., et al. (2014). In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58 3895–3903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) (2018). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals: CLSI standard VET01, 5th Edn. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Cohen Stuart J., van den Munckhof T., Voets G., Scharringa J., Fluit A., Hall M. L., et al. (2012). Comparison of ESBL contamination in organic and conventional retail chicken meat. Int. J. Food Microbiol. 154 212–214. 10.1016/j.ijfoodmicro.2011.12.034 [DOI] [PubMed] [Google Scholar]
- Davis G. S., Waits K., Nordstrom L., Grande H., Weaver B., Papp K., et al. (2018). Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 18:174. 10.1186/s12866-018-1322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De León I. (2009). An Institutional Assessment of Antitrust Policy: The Latin American Experience. Alphen aan den Rijn: Kluwer Law International B.V. [Google Scholar]
- Deshpande L. M., Hubler C., Davis A. P., Castanheira M. (2019). Updated prevalence of mcr-like genes among Escherichia coli and klebsiella pneumoniae in the SENTRY program and characterization of mcr-1.11 variant. Antimicrob. Agents Chemother. 63 e2450-18. 10.1128/AAC.02450-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diario Oficial El Peruano (2013). Resolución Directoral N° 0072-2013-MINAGRI-SENASA-DIAIA – Norma Legal Diario Oficial El Peruano. Available online at: https://busquedas.elperuano.pe/normaslegales/prohiben-importacion-y-comercializacion-de-diversos-principi-resolucion-directoral-n-0072-2013-minagri-senasa-diaia-991128-1/ (accessed October 16, 2020). [Google Scholar]
- Diarra M. S., Malouin F. (2014). Antibiotics in canadian poultry productions and anticipated alternatives. Front. Microbiol. 5:282. 10.3389/fmicb.2014.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Wegener H. C. (2003). Antimicrobial growth promoters and Salmonella spp., campylobacter spp. in poultry and swine, denmark. Emerg. Infect. Dis. 9 489–492. 10.3201/eid0904.020325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezadi F., Ardebili A., Mirnejad R. (2018). Antimicrobial susceptibility testing for polymyxins: challenges, issues, and recommendations. J. Clin. Microbiol. 57:e1390-18. 10.1128/JCM.01390-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgenhauer L., Imirzalioglu C., Oppong K., Akenten C. W., Hogan B., Krumkamp R., et al. (2019). Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in ghana. Front. Microbiol. 9:3358. 10.3389/fmicb.2018.03358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrimann S., Winkler M. S., Stalder M., Niwagaba C. B., Babu M., Kabatereine N. B., et al. (2016). Disease burden due to gastrointestinal pathogens in a wastewater system in Kampala, Uganda. Microb. Risk Anal. 4 16–28. 10.1016/j.mran.2016.11.003 [DOI] [Google Scholar]
- García C., Rijnders M. I. A., Bruggeman C., Samalvides F., Stobberingh E. E., Jacobs J. (2012). Antimicrobial resistance and molecular typing of Staphylococcus aureus bloodstream isolates from hospitals in Peru. J. Infect. 65 406–411. 10.1016/j.jinf.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Garrity G., Brenner D. J., Krieg N. R., Staley J. R. (2005). “Volume 2: the proteobacteria, Part B: the Gamma proteobacteria,” in Bergey’s Manual® of Systematic Bacteriology, (New York, NY: Springer; ), 1106. 10.1007/0-387-28022-7 [DOI] [Google Scholar]
- Gelbíčová T., Baráková A., Florianová M., Jamborová I., Zelendová M., Pospíšilová L., et al. (2019). Dissemination and comparison of genetic determinants of mcr-mediated colistin resistance in Enterobacteriaceae via retailed raw meat products. Front. Microbiol. 10:2824. 10.3389/fmicb.2019.02824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T., Wang R., Liu D., Walsh T. R., Zhang R., Lv Y., et al. (2019). Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 4 1450–1456. 10.1038/s41564-019-0445-2 [DOI] [PubMed] [Google Scholar]
- Hoelzer K., Wong N., Thomas J., Talkington K., Jungman E., Coukell A. (2017). Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet. Res. 13:211. 10.1186/s12917-017-1131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtcamp W. (2011). Poultry relief? Organic farming may reduce drug resistance. Environ. Health Perspect. 119:a489. 10.1289/ehp.119-a489b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horna G., Velasquez J., Fernández N., Tamariz J., Ruiz J. (2017). Characterisation of the first KPC-2-producing Klebsiella pneumoniae ST340 from Peru. J. Glob. Antimicrob. Resist. 9 36–40. 10.1016/j.jgar.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Humane Farm Animal Care [HFAC] (2018). HFAC Standards for Production of Egg Laying Hens. Available online at: http://certifiedhumane.org/wp-content/uploads/Std18.Layers.3A-5.pdf (accessed October 16, 2020). [Google Scholar]
- Hussain A., Shaik S., Ranjan A., Suresh A., Sarker N., Semmler T., et al. (2019). Genomic and functional characterization of poultry Escherichia coli from india revealed diverse extended-spectrum β-lactamase-producing lineages with shared virulence profiles. Front. Microbiol. 10:2766. 10.3389/fmicb.2019.02766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Estadística e Informática [INEI] (2017). Compendio Estadístico Provincia de Lima 2017. Available online at: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1477/libro.pdf (accessed October 16, 2020). [Google Scholar]
- Kalter H. D., Gilman R. H., Moulton L. H., Cullotta A. R., Cabrera L., Velapatiño B. (2010). Risk factors for antibiotic-resistant Escherichia coli carriage in young children in Peru: community-based cross-sectional prevalence study. Am. J. Trop. Med. Hyg. 82 879–888. 10.4269/ajtmh.2010.09-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse H., Sorum H. (1994). Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Appl. Environ. Microbiol. 60 4015–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Godzik A. (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Lietzau S., Raum E., von Baum H., Marre R., Brenner H. (2007). Household contacts were key factor for children’s colonization with resistant Escherichia coli in community setting. J. Clin. Epidemiol. 60 1149–1155. 10.1016/j.jclinepi.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Liu B. T., Song F. J., Zou M., Zhang Q. Di, Shan H. (2017). High incidence of Escherichia coli strains coharboring mcr-1 and blaNDM from chickens. Antimicrob. Agents Chemother. 61:e2347-16. 10.1128/AAC.02347-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. M., Stegger M., Aziz M., Johnson T. J., Waits K., Nordstrom L., et al. (2018). Escherichia coli ST131-H22 as a foodborne uropathogen. MBio 9:e00470-18. 10.1128/mBio.00470-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A. P., Srinivasan A., Carey R. B., Carmeli Y., Falagas M. E., Giske C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- Manninger P., Koziol A., Carrillo C. D. (2016). Draft whole-genome sequences of Escherichia fergusonii strains isolated from beef trim (GTA-EF02), ground beef (GTA-EF03), and chopped kale (GTA-EF04). Genome Announc. 4:e00185-16. 10.1128/genomeA.00185-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis G. S., Ventura G., Gilman R. H., Porras E., Miranda E., Carbajal L., et al. (1990). Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am. J. Public Health 80 146–149. 10.2105/AJPH.80.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. M., Levy S. B. (2011). Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24 718–733. 10.1128/CMR.00002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor K. C., Petrovska L., Thomson N. R., Harris K., Reid S. W. J., Mather A. E. (2019). Antimicrobial resistance diversity suggestive of distinct Salmonella typhimurium sources or selective pressures in food-production animals. Front. Microbiol. 10:708. 10.3389/fmicb.2019.00708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Agricultura y Riego [MINAGRI] (2018). Sistema Integrado de Estadísticas Agraria: MINAGRI – DGESEP – DEA. Boletín Estadístico Mensual de la Producción y Comercialización de Productos Avícolas. Available online at: http://siea.minagri.gob.pe/siea/?q=publicaciones/boletin-estadistico-mensual-de-la-produccion-y-comercializacion-avicola (accessed October 16, 2020). [Google Scholar]
- Ministerio de Salud [MINSA] (2019). Plan Multisectorial Para Enfrentar la Resistencia a los Antimicrobianos 2019-2021. Available online at: http://extwprlegs1.fao.org/docs/pdf/per188340anx.pdf (accessed October 16, 2020). [Google Scholar]
- Nadimpalli M. L., Marks S. J., Montealegre M. C., Gilman R. H., Pajuelo M. J., Saito M., et al. (2020). Urban informal settlements as hotspots of antimicrobial resistance and the need to curb environmental transmission. Nat. Microbiol. 2020 56 787–795. 10.1038/s41564-020-0722-0 [DOI] [PubMed] [Google Scholar]
- Nadimpalli M., Delarocque-Astagneau E., Love D. C., Price L. B., Huynh B. T., Collard J. M., et al. (2018). Combating global antibiotic resistance: emerging one health concerns in lower-and middle-income countries. Clin. Infect. Dis. 66 963–969. 10.1093/cid/cix879 [DOI] [PubMed] [Google Scholar]
- Nadimpalli M., Vuthy Y., de Lauzanne A., Fabre L., Criscuolo A., Gouali M., et al. (2019). Meat and fish as sources of extended-spectrum β-lactamase– producing Escherichia coli Cambodia. Emerg. Infect. Dis. 25 126–131. 10.3201/eid2501.180534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation R. L., Garonzik S. M., Thamlikitkul V., Giamarellos-Bourboulis E. J., Forrest A., Paterson D. L., et al. (2017). Dosing guidance for intravenous colistin in critically ill patients. Clin. Infect. Dis. 64 565–571. 10.1093/cid/ciw839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J. (2014). Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London: The Review on Antimicrobial Resistance. [Google Scholar]
- Page A. J., Cummins C. A., Hunt M., Wong V. K., Reuter S., Holden M. T. G., et al. (2015). Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31 3691–3693. 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. W., Gautier P. (2012). Use of antimicrobial agents in livestock. Rev. Sci. Tech. 31 145–188. 10.20506/rst.31.1.2106 [DOI] [PubMed] [Google Scholar]
- Partridge S. R., Kwong S. M., Firth N., Jensen S. O. (2018). Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31:e00088-17. 10.1128/CMR.00088-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrsson E. C., Tsukayama P., Patel S., Mejía-Bautista M., Sosa-Soto G., Navarrete K. M., et al. (2016). Interconnected microbiomes and resistomes in low-income human habitats. Nature 533 212–216. 10.1038/nature17672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J., Stobberingh E. E., Savelkoul P. H. M., Wolffs P. F. G. (2013). The human microbiome as a reservoir of antimicrobial resistance. Front. Microbiol. 4:87. 10.3389/fmicb.2013.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyclit L., Baron S. A., Rolain J. M. (2019). Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol 9:193. 10.3389/fcimb.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2014). Request for Revision/Information to the Database on Countries’ Needs for MRLs: Codex Committee on Residues of Veterinary Drugs in Foods (CCRVDF). Available online at: http://www.fao.org/fao-who-codexalimentarius/committees/committee/related-circular-letters/en/?committee=CCRVDF (accessed October 16, 2020). [Google Scholar]
- Diario Oficial El Peruano (2019). Resolución Directoral No 0091-2019-MINAGRI-SENASA-DIAIA. Lima: Diario El Peruano. [Google Scholar]
- Roach D., Waalkes A., Abanto J., Zunt J., Cucho C., Soria J., et al. (2020). Whole genome sequencing of peruvian Klebsiella pneumoniae identifies novel plasmid vectors bearing carbapenem resistance gene NDM-1. Open Forum Infect. Dis. 7:ofaa266. 10.1093/ofid/ofaa266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. P., Wertheim H. F. L., Kakkar M., Kariuki S., Bu D., Price L. B. (2016). Animal production and antimicrobial resistance in the clinic. Lancet 387 e1–e3. 10.1016/S0140-6736(15)00730-8 [DOI] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Genome Anal. 30 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Silbergeld E. K., Graham J., Price L. B. (2008). Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 29 151–169. 10.1146/annurev.publhealth.29.020907.090904 [DOI] [PubMed] [Google Scholar]
- Singer R. S., Patterson S. K., Meier A. E., Gibson J. K., Lee H. L., Maddox C. W. (2004). Relationship between phenotypic and genotypic florfenicol resistance in Escherichia coli. Antimicrob. Agents Chemother. 48 4047–4049. 10.1128/AAC.48.10.4047-4049.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie C. S., Smith M. B., Friedman J., Cordero O. X., David L. A., Alm E. J. (2011). Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480 241–244. 10.1038/nature10571 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah M., Caudell M. A., Mair C., Davis M. A., Matthews L., Quinlan R. J., et al. (2020). Antimicrobial resistant enteric bacteria are widely distributed amongst people, animals and the environment in Tanzania. Nat. Commun. 11:228. 10.1038/s41467-019-13995-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmolka A., Nagy B. (2013). Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 4:258. 10.3389/fmicb.2013.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K. L., Caffrey N. P., Nóbrega D. B., Cork S. C., Ronksley P. E., Barkema H. W., et al. (2017). Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet. Heal. 1 e316–e327. 10.1016/S2542-5196(17)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing [EUCAST] (2017). Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 7.1. Available online at: https://www.eucast.org/ (accessed October 16, 2020). [Google Scholar]
- Thomsen M. C. F., Ahrenfeldt J., Cisneros J. L. B., Jurtz V., Larsen M. V., Hasman H., et al. (2016). A bacterial analysis platform: an integrated system for analysing bacterial whole genome sequencing data for clinical diagnostics and surveillance. PLoS One 11:e0157718. 10.1371/journal.pone.0157718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte-Silva R. G., Olivo López J. M., Corso A., Pasteran F., Albornoz E., Sahuanay B., et al. (2018). Resistencia a colistín mediado por el gen mcr-1 identificado en cepas de Escherichia coli y Klebsiella pneumoniae. primeros reportes en el Perú. Anal. Fac. Med. 79 213–220. 10.15381/anales.v79i3.15313 [DOI] [Google Scholar]
- Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., Robinson T. P., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U.S.A. 112 5649–5654. 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T. P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N. G., et al. (2019). Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 365:eaaw1944. 10.1126/science.aaw1944 [DOI] [PubMed] [Google Scholar]
- Van Hoek A. H. A. M., Mevius D., Guerra B., Mullany P., Roberts A. P., Aarts H. J. M. (2011). Acquired antibiotic resistance genes: an overview. Front. Microbiol. 2:203. 10.3389/fmicb.2011.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang R., Li J., Wu Z., Yin W., Schwarz S., et al. (2017). Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2:16260. 10.1038/nmicrobiol.2016.260 [DOI] [PubMed] [Google Scholar]
- White D. G., Hudson C., Maurer J. J., Ayers S., Zhao S., Lee M. D., et al. (2000). Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 38 4593–4598. 10.1128/jcm.38.12.4593-4598.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M., Ward M., Van Bunnik B., Farrar J. (2015). Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 370:20140083. 10.1098/rstb.2014.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Group (2017). Gaining Momentum in Peruvian Agriculture: Opportunities to Increase Productivity and Enhance Competitiveness. Report Production Under Peruvian Agriculture Opportunities World Bank Group. Washington, DC: World Bank Group [Google Scholar]
- World Health Organization [WHO] (2017a). Critically Important Antimicrobials for Human Medicine, 5th Revision 2016. Geneva: WHO. [Google Scholar]
- World Health Organization [WHO] (2017b). Global Action Plan on Antimicrobial Resistance. Geneva: WHO. [Google Scholar]
- Wu C., Wang Y., Shi X., Wang S., Ren H., Shen Z., et al. (2018). Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008-2014. Emerg. Microbes Infect. 7:30. 10.1038/s41426-018-0033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji R., Friedman C. R., Rubin J., Suh J., Thys E., McDermott P., et al. (2018). A population-based surveillance study of shared genotypes of Escherichia coli isolates from retail meat and suspected cases of urinary tract infections. mSphere 3:e00179-18. 10.1128/msphere.00179-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., McGinnis S., Madden T. L. (2006). BLAST: improvements for better sequence analysis. Nucleic Acids Res. 34 W6-9. 10.1093/nar/gkl164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pairwise SNP distances between all pairs of E. coli isolates.
Plasmid replicon marker sequences among sequenced E. coli.
Location, type, and sample size of humans and chickens included in the study.
Antibiotics used for resistance profiling.
Plasmid markers and bivariate analysis of sequenced E. coli from chickens and humans. ∗Fisher exact test and confidence level of 95%.
Antimicrobial resistance genes hits among 118 sequenced E. coli.
Plasmid markers detection among 118 E. coli isolates.
Genomic analysis of 118 E. coli isolates.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA633873/, PRJNA633873.