Abstract
Cardiac muscle thin filaments are composed of actin, tropomyosin, and troponin that change conformation in response to Ca2+ binding, triggering muscle contraction. Human cardiac troponin C (cTnC) is the Ca2+-sensing component of the thin filament. It contains structural sites (III/IV) that bind both Ca2+ and Mg2+ and a regulatory site (II) that has been thought to bind only Ca2+. Binding of Ca2+ at this site initiates a series of conformational changes that culminate in force production. However, the mechanisms that underpin the regulation of binding at site II remain unclear. Here, we have quantified the interaction between site II and Ca2+/Mg2+ through isothermal titration calorimetry and thermodynamic integration simulations. Direct and competitive binding titrations with WT N-terminal cTnC and full-length cTnC indicate that physiologically relevant concentrations of both Ca2+/Mg2+ interacted with the same locus. Moreover, the D67A/D73A N-terminal cTnC construct in which two coordinating residues within site II were removed was found to have significantly reduced affinity for both cations. In addition, 1 mM Mg2+ caused a 1.4-fold lower affinity for Ca2+. These experiments strongly suggest that cytosolic-free Mg2+ occupies a significant population of the available site II. Interaction of Mg2+ with site II of cTnC likely has important functional consequences for the heart both at baseline as well as in diseased states that decrease or increase the availability of Mg2+, such as secondary hyperparathyroidism or ischemia, respectively.
Keywords: contractility, myofilament, ITC, calorimetry, MD simulation, thermodynamic integration, molecular dynamics
Abbreviations: CaM, calmodulin; cTn, cardiac troponin; cTnC, cardiac troponin C; cTnI, cardiac troponin I; cTnT, cardiac troponin T; FF, fast-flow; ITC, isothermal titration calorimetry; PDB, Protein Data Bank; sTnC, skeletal muscle troponin TnC; TF, thin filament; TI, thermodynamic integration
Cardiac troponin (cTn) is a heterotrimeric complex that includes components for Ca2+ binding (cardiac troponin C [cTnC]), inhibition of contraction (cardiac troponin I [cTnI]), and tropomyosin binding (cardiac troponin T [cTnT]) (1). Ca2+ binding to site II of cTnC is the precursor to a series of structural perturbations in the thin filament (TF) that culminate in a force-generating reaction between the actin filament and myosin heads (1, 2, 3, 4, 5).
Human cTnC is a 161-amino acid protein composed of nine helices (N and A–H), which form four EF-hand or helix–loop–helix binding motifs (sites I–IV). Within these domains, residues in positions 1 (+x), 3 (+y), 5 (+z), 7 (−y), 9 (−x), and 12 (−z) contain oxygen atoms arranged in a pentagonal bipyramid allowing for coordination of metal cations (Figs. S1 and S2) (6, 7, 8). Skeletal muscle troponin TnC (sTnC) has four functional Ca2+-binding motifs (9, 10). cTnC has a similar overall structure to sTnC but a slightly different primary sequence; the insertion of a valine at residue 28, along with the substitutions D29L and D31A, has rendered site I of cTnC nonreceptive to Ca2+ binding (11, 12).
Ca2+ binding to sites III and IV in the C domain of cTnC occurs with high affinity (∼107 M−1) (∼10× higher than the N domain) and a slow exchange rate (∼100× slower than binding to the N domain) (13, 14, 15). Given the abundance of contractile filaments throughout cardiomyocytes, sites III/IV of cTnC buffer ∼80% of the 100 to 200 μM [Ca2+]in at resting concentrations of free Ca2+ (∼100 nM) (16). At resting free cytosolic Ca2+ concentrations, sites III and IV are usually saturated with Ca2+ (16). Mg2+ also binds at sites III and IV but with lower affinity (KA ∼ 104 M−1) (4). However, the cytosolic concentration of Mg2+ allows this cation to compete with and reduce the binding of Ca2+ to the “structural” sites (17, 18). The binding of Ca2+/Mg2+ to sites III and IV alters the structure of TnC and is a prerequisite for tethering to the rest of the TF (19, 20).
The C domain of cTnC is linked to the N domain by a linker region composed of a nine-turn α-helix (21, 22). Within the N domain (N-cTnC), Ca2+ binds the low-affinity (∼10−5 M) site II such that this site is only partially occupied at diastolic-free Ca2+ concentrations (∼0.1 μM) with very few sites being bound (23). The degree of occupancy is significantly higher at systolic-free Ca2+ concentrations (∼0.5–1.2 μM), which follow Ca2+-induced Ca2+ release (24). Ca2+ binding to site II provides the free energy to allow for exposure of a hydrophobic pocket, which is otherwise less favorable (25, 26). Helices B and C move away from helices N, A, and D to expose the hydrophobic cleft, with the short antiparallel β-sheet between EF-hands I and II acting as a hinge (27, 28, 29). Binding of the “switch peptide” of cTnI147–163 to this pocket facilitates exposure of this hydrophobic region (22, 30, 31).
A persistent and underinvestigated question is the role of cellular Mg2+ in the signaling of activation by cTnC. Of the total intracellular [Mg2+]i (∼10 mM), the majority is bound to cellular components such as ATP with only ∼0.5 to 1.0 mM being freely available in the cytosol (32, 33). In conditions with diminished Mg2+ buffering capacity, such as ATP-depleted states, the free [Mg2+] can increase significantly (34, 35) prior to being extruded from the cell (36), but it could also compete with Ca2+ for binding to cTnC.
Increase in Mg2+–ATP in both skeletal and cardiac tissues decreases the Ca2+ sensitivity of skinned fibers (37, 38, 39). These systems contain the entirety of the TF. The binding of Ca2+ normally induces a conformation change, which allows for exposure of the hydrophobic core of cTnC (40). It is unlikely that Mg2+ would cause this structural change. Indeed, previous findings suggest that Mg2+ competes for binding with Ca2+ but does not itself cause the same conformational changes (20).
Further evidence has been obtained through fluorescence-based studies of isolated cTnC (41, 42, 43, 44, 45, 46), the Tn complex (41, 43), and reconstituted fibers (43, 47), where Mg2+ appears to decrease Ca2+ sensitivity. In isolated TnC, the KA of the low-affinity sites (III/IV) for Ca2+ and Mg2+ was measured to be on the order of 106 and 102 M−1, respectively (42).
Interaction of Ca2+/Mg2+ with sites III/IV results in a large change in enthalpy (ΔH), in contrast to changes resulting from site I/II binding, which are small by comparison. Detection of heat changes associated with the interactions of metal ions and proteins is both challenging and a highly technique-dependent method, such that small changes may be deemed negligible (48, 49, 50). Experiments used to study this system decades ago were limited by the technology of the time. In contrast, modern isothermal titration calorimetry (ITC) is a sensitive method that can be used to define the thermodynamic parameters of binding without the use of labeling methods that could interfere. While there is a place for use of fluorophores to investigate cTnC function (51, 52, 53), even conservative substitutions (e.g., sTnC F29W) have been shown to modify Ca2+-binding properties (54). Modern ITC allows for the study of single-binding sites within isolated protein domains and can be used to detect heat changes as small as 0.1 μcal (55, 56, 57, 58).
We have used ITC to explore the binding of Mg2+ and Ca2+ to site II at the level of N-cTnC and full-length cTnC. Competitive binding to the N domain and mutations in the site II caused a reduction in apparent affinity, indicating interaction of both cations with the same locus in the protein. In addition, a double mutant removing two of the coordinating residues within the EF-hand of site II was used to investigate the impact on Ca2+ and Mg2+ binding to this site. In full-length cTnC, Mg2+ competed with and reduced Ca2+ binding to all three sites. These findings further corroborate and expand upon what has been shown by a few laboratories, but the findings are largely ignored by most; the role of Mg2+ in modulating the Ca2+ sensitivity of force production in cardiomyocytes is one that merits further discussion (40, 51, 52, 53).
Results
The ratio of the ligand to titrant in the single-binding site condition (as given by the stoichiometry—N) is a measure of the functional moles of protein and was approximately 1.00 in all the N-cTnC titrations (Table S2). Given the method of concentration determination, the number of binding sites, cooperativity, and the variable binding strength of each titrants, the N cannot be used in the same way for the full-length cTnC experiments (Table S3). Therefore, the values presented can be compared between conditions, but care should be taken when comparing these to other systems, such as the cTn complex or the reconstituted thin filaments (59, 60). Ease of manipulation of the N-cTnC/cTnC system contrasts with those that include the cTn/TF. Thus, the binding parameters measured here may not translate in absolute term when cTnC is incorporated into a more complex system; these limitations are further explored in the Discussion section.
Ca2+ and Mg2+ binding to apo-state N-cTnC
The interaction of N-cTnC with either Ca2+ or Mg2+ was found to be associated with a positive ΔH, so the interaction is driven by entropy (Fig. 1) consistent with previously published data (61, 62, 63, 64). Given the characteristic of the heat signals observed in the site II–containing system of N-cTnC, the endothermic component of the multiple binding site system (full-length cTnC) can be attributed to binding at this site within the N domain.
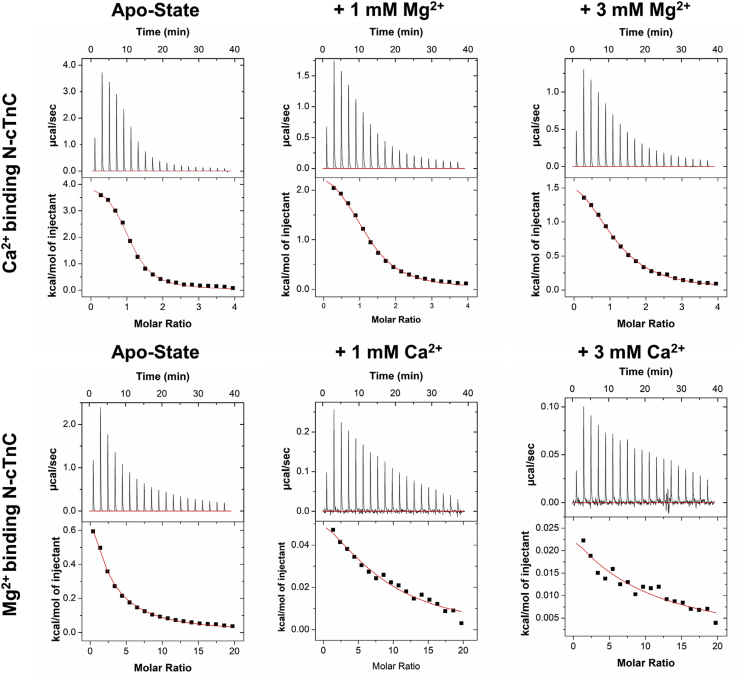
Figure 1.
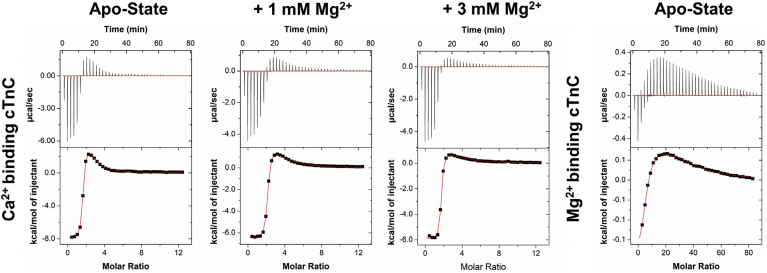
Representative isotherms of Ca2+and Mg2+binding to apo-state and preincubated N-cTnC. All titrations were carried out into 200 μM WT human N-cTnC in 50 mM Hepes at pH 7.2, 150 mM KCl, and 2 mM EDTA. The top row shows the titration of 4 mM Ca2+ into apo-state N-cTnC, followed by the same titration into 1 and 3 mM Mg2+ preincubated N-cTnC. The bottom row shows the titration of 20 Mg2+ into apo-state N-cTnC, followed by 1 and 3 mM Ca2+-preincubated N-cTnC. In the last injections, Mg2+ in the Ca2+-preincubation conditions, the heat changes approached the detection limits of the instrument. Thermograms were fit to a “single-binding site” model using Origin 7 MicroCal2000 ITC software package. N-cTnC, cardiac troponin C with N domain.
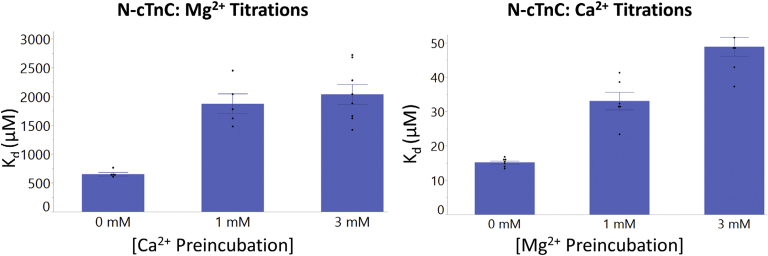
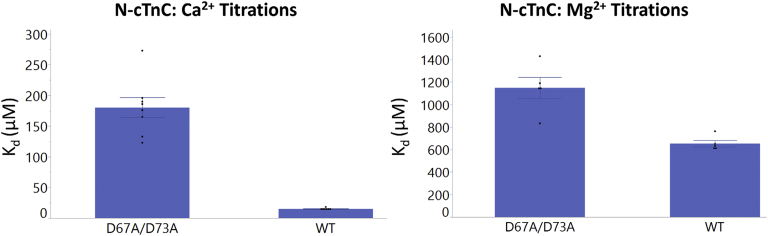
The affinity of N-cTnC for Ca2+ (Kd = 15.2 ± 0.5 μM) was found to be significantly greater (p = 0.001) and 42.9-fold different than for Mg2+ (Kd = 652.8 ± 28.4 μM) (Figs. 1 and 2; Table S2).
Figure 2.
Binding of Ca2+and Mg2+to apo-state and preincubated N-cTnC.Left panel, the affinity of site II for Mg2+ is compared in the apo-state and with Ca2+ preincubation in human N-cTnC. Right panel, the affinity of site II for Ca2+ is compared in the apo-state and with Mg2+ preincubation in N-cTnC. Statistical differences were assessed through ANOVA followed by Tukey's post hoc test. Ca2+ titrations were not significantly different. Titration of Mg2+ into N-cTnC preincubated with 1 and 3 mM Ca2+ was statistically indifferent, but both significantly differed from the apo-state titration (p < 0.0001). N-cTnC, cardiac troponin C with N domain.
The ΔH of the Ca2+–N-cTnC interaction (3.82 ± 0.04 kcal ∗ mol−1) was significantly greater (p < 0.0001) than that with Mg2+ (2.64 ± 0.10 kcal ∗ mol−1), indicating a greater enthalpic cost of binding for the Ca2+ titration. Moreover, the entropic contribution for the Ca2+ titrations (T ∗ ΔS = 10.39 ± 0.03 kcal ∗ mol−1) was more favorable (p < 0.0001) than the Mg2+ titrations (T ∗ ΔS = 6.99 ± 0.07 kcal ∗ mol−1) (Table S2).
As expected, the affinity of Ca2+ binding to apo-state N-cTnC (Kd = 15.2 ± 0.5 μM) was found to be greater than Mg2+ binding at this site, or when compared with the preincubation experiments (Fig. 2 and Table S2).
N-terminal cTnC
Mg2+ binding to Ca2+-preincubated N-cTnC
To investigate Mg2+ binding in the presence of Ca2+, apo-state N-cTnC was preincubated with three concentrations of Ca2+ (0, 1, and 3 mM), then titrated with 20 mM Mg2+ (Figs. 1 and 2 and Table S2). Moreover, the change in enthalpy in these conditions was lower with greater amounts of Ca2+ preincubated. Titration of Mg2+ into apo-state protein yielded a ΔH = 2.64 ± 0.10 kcal ∗ mol−1, lower than Ca2+ into apoprotein, which liberated 3.82 ± 0.04 kcal ∗ mol−1. Moreover, the Kd values were 1870.0 ± 171.5 and 2037.5 ± 172.2 μM for the 1 and 3 mM Ca2+ conditions, respectively, indicating a trend of decreasing affinity with increasing concentrations of Ca2+ preincubated with the protein sample and a more than two orders of magnitude lower affinity compared with the Ca2+ into WT condition. The reduction in affinity, ΔH, and lower ΔS associated with higher Ca2+ preincubation suggests that both metal cations may be binding to the same EF-hand–binding motif in site II of N-cTnC.
Ca2+ binding to Mg2+-preincubated N-cTnC
Apoprotein preincubated with Mg2+ was titrated with Ca2+ to assess the “apparent” affinity of the protein for Ca2+ when the site might be occupied with the other divalent cation. As expected, increasing the Mg2+ concentration significantly (p < 0.0001) reduced the ΔH associated with binding from 3.82 ± 0.04 kcal ∗ mol−1 in the apotitration to 1.73 ± 0.05 kcal ∗ mol−1 in the 3 Mm Mg2+ preincubated construct. The binding affinity changed from 15.2 ± 0.5 μM in the apo-state to 48.9 ± 2.8 μM in the 3 mM Mg2+-preincubated condition. The Ca2+ affinity was lower when comparing the apo-N-cTnC binding condition with higher concentrations of preincubated Mg2+ (Fig. 2 and Table S2).
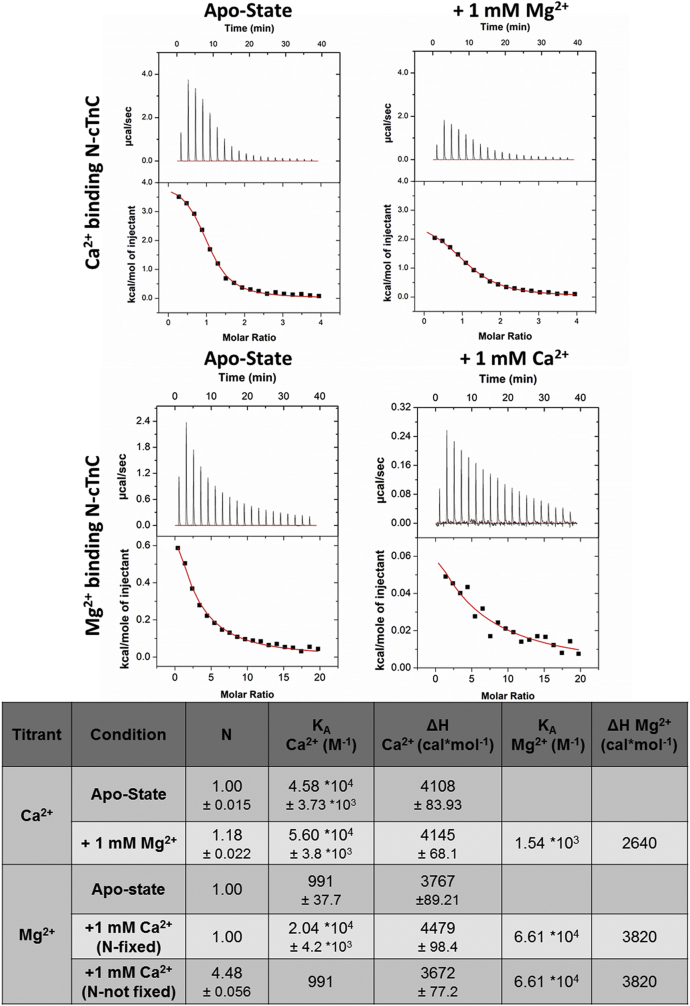
It is possible to directly obtain a measure of the binding of a secondary ion when a competition experiment is carried out. Figure 3 shows the binding of Ca2+ to N-cTnC in the apo-state and following preincubation with 1 mM Mg2+. This figure also shows the binding of Mg2+ to N-cTnC in the apo-state and following preincubation with 1 mM Ca2+. The values of the thermodynamic parameters obtained from this model for the apo-state titration were as follows: N = 1.00 ± 0.015 sites, KA = 4.58 ± 0.37 × 104 M−1, and ΔH = 4.11 ± 0.08 kcal/mol; these values are comparable to those obtained from the single-binding site model (Table S2). The parameters for the preincubation condition were as follows: N = 1.18 ± 0.02 sites, KA = 5.60 ± 0.38 × 104 M−1, and ΔH = 4.15 ± 0.07 kcal/mol for Ca2+; Mg2+ was estimated to bind with a KA of 1.54 × 103 M−1 and a ΔH of 2.64 kcal/mol; these values fall within the range obtained using the single-binding site model (Table S2). Moreover, the values obtained for the binding of Mg2+ to the apo-state N-cTnC and with preincubated Ca2+ are comparable to a single-binding site model (Fig. 3 and Table S2). The N-value obtained when applying the competitive binding model to the titration of Mg2+ into 1 mM Ca2+ preincubated N-cTnC (4.48 ± 0.06) is thought to result from a weak binding interaction and indicates a less reliable fit under the experimental conditions (Fig. 3).
Figure 3.
Competition model of Ca2+and Mg2+binding to apo-state and N-cTnC preincubated with 1 mM Mg2+or 1 mM Ca2+. All titrations were carried out into 200 μM WT human N-cTnC in 50 mM Hepes at pH 7.2, 150 mM KCl, and 2 mM EDTA. The top two panels show Ca2+ titration, and the bottom two panels show Mg2+ titrations. The top left panel shows the titration of 4 mM Ca2+ into apo-state N-cTnC, and the top right panel illustrates the same titration with 1 mM Mg2+-preincubated N-cTnC. The bottom left panel shows the titration of 20 mM Mg2+ into apo-state N-cTnC, and the bottom right panel illustrates the same titration with 1 mM Ca2+-preincubated N-cTnC. The thermograms were fit using a “competition” model using Origin 7 MicroCal2000 ITC software package, in which the concentration of the ion in the cell and an estimated KA value is input. The values for thermodynamic parameters obtained are listed in the table described. Thermodynamic parameters without a reported error value were fixed in the model. N-cTnC, cardiac troponin C with N domain.
Ca2+ and Mg2+ binding to apo-D67A/D73A N-cTnC
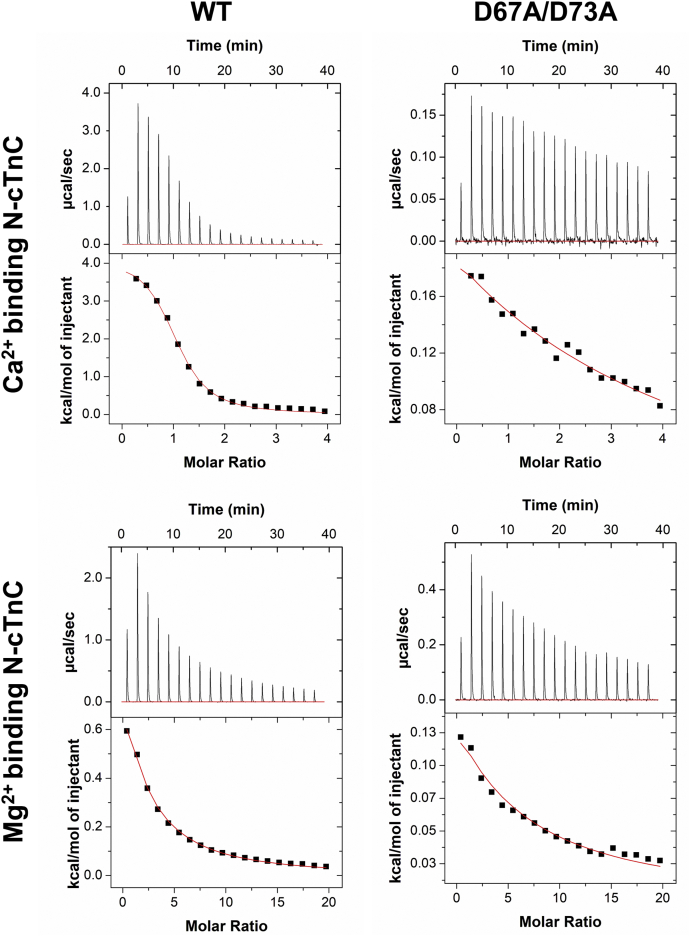
Point mutations (D67A and D73A) were made (Fig. S2), affecting two known Ca2+ coordinating residues in site II. Binding of both divalent cations was reduced by these mutations, but the Kd was lower for Ca2+ binding (180.3 ± 16.2 μM) compared with Mg2+ binding (1148.6 ± 95.0 μM) (Figs. 4 and 5 and Table S2). The double mutant caused a 11.9-fold alteration in Ca2+ binding, yet this difference was not found to be statistically significant (p = 0.88); it also altered Mg2+ binding 1.8-fold (p = 0.04); this change in Kd supports the binding of Mg2+ to the EF-hand of site II.
Figure 4.
Representative binding isotherms for binding of Ca2+and Mg2+WT and D67A/D73A N-cTnC. All titrations were carried out into 200 μM human N-cTnC in 50 mM Hepes at pH 7.2, 150 mM KCl, and 2 mM EDTA. Top row, titration of 4 mM Ca2+ into apo-state N-cTnC, followed by the same titration into the D67A/D73A mutant. Bottom row, titrations of 20 mM Mg2+ into apo-state N-cTnC, followed by the same titration into the D67A/D73A mutant. Thermograms were fit to a “single-binding site” model using Origin 7 MicroCal2000 ITC software package. N-cTnC, cardiac troponin C with N domain.
Figure 5.
Binding of Ca2+and Mg2+to WT and D67A/D73A N-cTnC. The effect of the D67A/D73A on Ca2+ and Mg2+ binding is assessed. ANOVA indicated that the binding affinity for both cations to human N-cTnC was lower when comparing the mutant and the WT (p < 0.0001). Tukey's post hoc test indicated that all four test conditions were significantly different. The effect on Ca2+ binding was more pronounced (11.9-fold reduction) compared with Mg2+ (1.8-fold reduction), but this is reconcilable with the number of coordinating residues needed to bind Ca2+ (6) versus Mg2+ (5); having four available coordinating residues was expected to affect Ca2+ binding to a greater extent. N-cTnC, cardiac troponin C with N domain.
Ca2+- and Mg2+-binding affinities from thermodynamic integration
Thermodynamic integration (TI) was performed to calculate absolute binding affinities (with change in Gibbs free energy reported) for the ions in the following systems: Ca2+ to WT N-cTnC, Ca2+ to D67A/D73A N-cTnC, Mg2+ to WT N-cTnC, and Mg2+ to D67A/D73A N-cTnC (Table 1). The average calculated binding affinities over five independent runs were −6.9 ± 1.3, −4.5 ± 2.4, −0.6 ± 2.8, and +0.4 ± 2.3 kcal ∗ mol−1, respectively. The TI-determined Ca2+-binding affinities were in good agreement with the ITC data. While the calculated absolute Mg2+-binding affinities were not in perfect agreement with the ITC data, they did show that Mg2+ had a weaker binding affinity than Ca2+ for all systems (−6.57 to −4.38 kcal ∗ mol−1 and −6.9 to −0.6 kcal ∗ mol−1 for ITC and TI, respectively, for WT system and −5.12 to −4.02 kcal ∗ mol−1 and −4.5 to +0.4 kcal ∗ mol−1 for ITC and TI, respectively, for D67A/D73A system). In addition, between the Mg2+-binding affinities, the binding affinity was consistently weaker for the D67A/D73A mutation. The ΔΔG values comparing ΔG between WT and D67A/D73A systems were similar for ITC and TI (0.36 kcal ∗ mol−1 and 1.0 kcal ∗ mol−1, respectively).
Table 1.
Average calculated binding affinities for Ca2+/Mg2+ interaction with site II of N-cTnC thermodynamic integration system
| System | ΔGTI (kcal ∗ mol−1) |
|---|---|
| Ca2+ to WT | −6.9 ± 1.3 |
| Ca2+ to D67A/D73A | −4.5 ± 2.4 |
| Mg2+ to WT | −0.6 ± 2.8 |
| Mg2+ to D67A/D73A | +0.4 ± 2.0 |
Averages were calculated over five independent runs.
Full-length cTnC
Ca2+ binding to apo-state full-length cTnC
Binding of Ca2+/Mg2+ to site II is characterized by an endothermic interaction as indicated by our titrations on the N-terminal domain in this and previous publications (63, 64). From this and the ITC work on full-length cTnC by others (65), we can deduce that the exothermic heat changes seen in Figure 6 result from interactions with site III/IV. The data (Fig. 6 and Table S3) show that Mg2+ binds to apo-state full-length cTnC at two distinct sets of sites.
Figure 6.
Representative isotherms for binding of Ca2+and Mg2+to full-length cTnC. All titrations were carried out into 100 μM full-length human WT cTnC. From left to right, the panels show the titration of 6 mM Ca2+ into apo-state full-length cTnC, 1 mM Mg2+ preincubated cTnC, and 3 mM Mg2+ preincubated cTnC. In the right most panel of this figure, 40 mM Mg2+ was titrated into full-length cTnC. The binding of Mg2+ occurs at two sets of different sites as seen in the isotherm, which contains both exothermic and endothermic components. All thermograms were fit to a “two sets-of-binding sites” model using Origin 7 MicroCal2000 ITC software package. cTnC, cardiac troponin C.
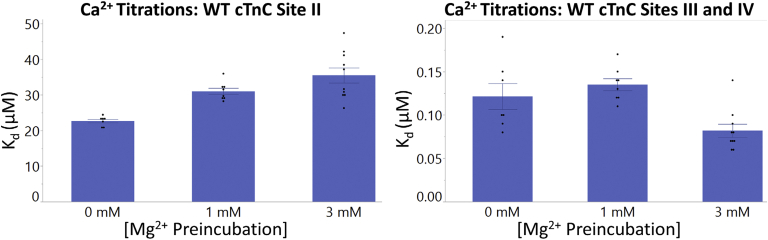
The binding of Ca2+ to sites III/IV occurred with an apparent Kd of 0.12 ± 0.02 μM, characterized by an exothermic component (ΔH = −8.12 ± 0.07 kcal ∗ mol−1) with a positive change in entropy (T ∗ ΔS = 1.24 ± 0.07 kcal ∗ mol−1). In the same full-length construct, the Kd associated with binding of Ca2+ to site II was 22.7 ± 0.5 μM. This interaction had a positive ΔH (3.71 ± 0.06 kcal ∗ mol−1) and was entropically driven (T ∗ ΔS = 10.0 ± 0.01 kcal ∗ mol−1) (Figs. 6 and 7 and Table S3).
Figure 7.
Binding of Ca2+and Mg2+to apo-state and preincubated full-length cTnC.Left panel, the affinity of site II for Ca2+ is compared in the apo-state and with Mg2+ preincubation in full-length human cTnC. Right panel, the affinity of sites III/IV for Ca2+ is compared in the apo-state and with Mg2+ preincubation in full-length cTnC. At site II, preincubation with 1 and 3 mM Mg2+ caused a statistically insignificant reduction in the affinities for Ca2+ binding (p = 0.52 and p = 0.14). At sites III/IV, preincubation with 1 mM or 3 mM Mg2+ did not significantly change affinity for Ca2+ binding. Statistical differences were assessed through two-way ANOVA followed by Tukey's post hoc test. cTnC, cardiac troponin C.
Mg2+ binding to apo-state full-length cTnC
Mg2+ binding to site II (Kd = 406.1 ± 7.9 μM) and sites III/IV (Kd = 16.7 ± 0.7 μM) was characterized by a positive ΔH (0.091 ± 0.001 kcal ∗ mol−1) and negative ΔH (−0.23 ± 0.01 kcal ∗ mol−1), respectively (Fig. 6 and Table S3). The difference in Kd values indicates that greater amounts of Mg2+ binding occur at the C-terminal sites, in comparison to the N-terminal site of cTnC. The interaction of Mg2+ with sites III/IV is two orders of magnitude weaker (p < 0.0001) than that seen for Ca2+. The interaction of Mg2+ with site II and sites III/IV was both entropically favorable (T ∗ ΔS = 4.71 ± 0.01 kcal ∗ mol−1 and T ∗ ΔS = 6.28 ± 0.03 kcal ∗ mol−1, respectively) and resulted in spontaneous interactions (ΔG = −4.62 ± 0.11 kcal ∗ mol−1 and ΔG = −6.51 ± 0.31 kcal ∗ mol−1, respectively).
Ca2+ binding to Mg2+-preincubated full-length cTnC
At greater concentrations, Mg2+ occupied a greater proportion of binding sites and limited binding Ca2+ to cTnC at all sites (Figs. 6 and 7 and Table S3). Binding of Ca2+ to site II was reduced by preincubation with 1 and 3 mM Mg2+ as indicated by an increase in Kd; however, these changes were not found to be statistically significant (p = 0.52 and p = 0.14), moreover ΔH was lowered (p < 0.0001 for both). Binding of Ca2+ to sites III/IV in the presence of 1 mM Mg2+ resulted in a Kd (0.14 ± 0.01 μM) that was not significantly different (p < 0.05) than seen for the 3 mM Mg2+ preincubation (Kd = 0.08 ± 0.01 μM) (Fig. 6 and Table S3).
At sites III/IV, for the 1 mM Mg2+ preincubation, the interaction proceeded with favorable enthalpy (ΔH = −6.87 ± 0.09 kcal ∗ mol−1) and entropy (T ∗ ΔS = 2.50 ± 0.10 kcal ∗ mol−1). For the 3 mM Mg2+ condition, the reaction was again exothermic (ΔH = −6.19 ± 0.06 kcal ∗ mol−1) with a positive change in T ∗ ΔS (3.50 ± 0.06 kcal ∗ mol−1).
Discussion
This study provides novel information regarding the thermodynamics that underlie the interaction between cTnC and two physiologically prevalent divalent cations. Our results significantly advance the understanding of the mechanisms and role of modifications in cellular Mg2+ in control of the cTnC–Ca2+ switch. Cellular-free Mg2+ is known to change in pathological conditions in the heart (66), but the mechanisms of its effects on activation of the myofilaments remain incompletely understood.
As seen in previous reports, we found the binding of Ca2+ to N-cTnC to be driven by entropy and unfavorable enthalpy (Table S2) (64, 65). The favorable ΔS may be due in part to the hydration enthalpy of Ca2+, which is thought to be on the order of ∼375 kcal ∗ mol−1 and slightly lower than that of Mg2+ (∼460 kcal ∗ mol−1) (67). It is also possible that the endothermic nature of these interactions results from other factors such as the exchange of protons that are transferred from the ligand to the buffer upon Ca2+ binding (61).
Measurement of Ca2+ binding to cTnC is often achieved indirectly by monitoring the fluorescence change and correlating this to the conformational change that results from the interaction. Fluorescent molecules such as 2-[4'-(iodoacetamido)anilino]naphthalene-6-sulfonic acid (59, 60, 68, 69) or reporters such as F27W (51, 70) can be used to quantify this binding interaction. At 21 °C, bovine F27W cTnC had a Kd of ∼5 μM, and 2-[4'-(iodoacetamido)anilino]naphthalene-6-sulfonic acid-labeled C35S cTnC had a Kd of ∼7 μM (20, 71). Through fluorescence-based measurement, the Kd of N-cTnC for Ca2+ was previously reported to be between 11.3 and 12.3 μM (70, 72). These parameters agree with our measured Ca2+ binding to apo-state N-cTnC and apo-state cTnC (Table S2), deviating only slightly, likely because of, different buffer and temperature conditions.
Normally, cytosolic [Mg2+]free is maintained around ∼0.5 to 1 mM (73). At these concentrations, Mg2+ is known to compete with Ca2+ for sites III and IV. Circular dichroism has been used to show that Ca2+ binding to sites III/IV increases the α-helical content of cTnC, from 19 to 41% (27, 74) and causes conformational changes that remove nonpolar amino acids from the solvent-exposed environment (19). This contrasts with NMR-based visualization of N-cTnC, in which the apo-state and Ca2+-bound forms showed minimal structural deviation (30).
Ca2+ has many times greater polarizability than Mg2+ and lower hydration energy (75). The bare ion radius of Mg2+ is smaller than Ca2+ (0.65 versus 0.99 Å) (76); conversely, in its hydrated form, Mg2+ is larger than Ca2+ (4.3 versus 4.1 Å) (77). In other Ca2+-binding proteins such as calmodulin (CaM), metals with similar ionic radii are able to substitute for this cation (78, 79). Mg2+ is able to bind to CaM but does not induce the conformational change associated with Ca2+ binding; a phenomenon that is commonly observed in cell biology and is expected in cTnC (80, 81).
Normally, six oxygen atoms arranged in an octahedral geometry are thought to coordinate Mg2+ (82). This is one less oxygen than needed to coordinate Ca2+ through a pentagonal bipyramid (83). However, Ca2+ can be coordinated by six to eight coordinating residues (but also by as many as 12) at a distance that can vary greatly (2.3–2.7 Å) compared with a much smaller variance for Mg2+ coordination (2.0–2.2 Å) (84).
Ca2+ and Mg2+ are most often coordinated by oxygen atoms, and this is usually accomplished by a hydroxyl group for Mg2+ and a carboxyl group for Ca2+ (85). Ca2+ is most frequently coordinated by side chains of aspartic acid, glutamic acid, asparagine, followed by serine/threonine, whereas Mg2+ is most frequently coordinated by aspartic acid, glutamic acid, histidine, threonine, serine, or asparagine (86). EF-hand–containing proteins have also been shown to bind Mg2+ when there are appropriately placed negatively charged amino residues (especially in the +z and −z positions) (54, 87, 88). In site II of mammalian cTnC, there is a polar serine at the +z position (residue 69) and a negatively charged glutamic acid at the −z position (residue 76) (Fig. S1).
Data from earlier studies suggested that Mg2+ binds exclusively at sites III and IV of TnC (4). Shortly thereafter, a limited series of equilibrium dialysis experiments did not show competition between Mg2+ and Ca2+ for the N-terminal sites of cTnC; instead, other binding sites were suggested (89). Later still, enthalpic titrations were unable to visualize a discernable change in Mg2+ binding to the low-affinity sites of sTnC (50, 90). However, assuming competitive binding, fluorescence assays at room temperature determined the Kd associated with Mg2+ binding to be about 4 mM (91). Moreover, Ca2+ sensitivity of the actomyosin ATPase and force production of skinned rat cardiac cells were unaltered when Mg2+ was increased from 1 to 8 mM (92). However, these findings were brought into question by studies that utilized metallochromic indicators to deduce sufficiently high Mg2+ affinity at the regulatory sites of sTnC (42).
The observation of Mg2+ binding to the low-affinity site of N-cTnC has led to the suggestion that differences in affinity may be due, at least in part, to Ca2+ buffering, and thus, the free concentration of the ion in these experiments. Given the kinetic rates associated with these interactions, it is difficult to have confidence in EGTA-determined rates of binding (93). Moreover, the temperature sensitivity of cTnC alone can alter experimental outcomes by orders of magnitude (53, 94). Change in sensitivity in the face of altered temperature has been suggested to result mostly from binding to the low-affinity sites and possibly through interactions with other members of the cTn complex (95, 96, 97).
Experiments testing the effects of alterations in free Mg2+ on Ca2+ activation of isolated myofibrils and skinned fiber bundles from different laboratories provide corroborative findings supporting the credibility of our postulate of a role for cytosolic Mg2+ as a controller of cTnC function at the N lobe. Fabiato and Fabiato (98) showed that increasing the concentration of free Mg2+ decreases myofilament Ca2+ sensitivity of skinned cardiomyocytes. [Mg2+] affects the Ca2+ sensitivity of the myofibrillar ATPase as well as actomyosin tension development in both skeletal and cardiac muscle preparations (10, 39, 44, 99, 100, 101, 102, 103).
Mg2+ affinity of sites III/IV alone is not sufficient to fully explain the change in the force–negative log of Ca2+ concentration relationship caused by Mg2+ in skinned skeletal muscle fibers (104). In rabbit fast skeletal muscle, Mg2+ competes with Ca2+ for low-affinity binding sites of TnC, where it binds with an affinity of 1.9 ∗ 102 M−1 (much lower than the 6.2 ∗ 106 M−1 seen for Ca2+). The KA associated with sites III and IV was measured to be 1.2 ∗ 106 M−1 for Ca2+ and 1.1 ∗ 102 M−1 for Mg2+ in canine ventricular skinned myocytes (105).
In isolated cTnC, Mg2+ was found to interact with site II with an apparent binding constant of 5.2 ∗ 102 M−1. This was only slightly lower than the constant associated with Mg2+ binding to sites III/IV (∼103 M−1), Ca2+ binding to sites III/IV (∼106 M−1), and Ca2+ binding to site II (∼104 M−1) (42).
Fluorescent probes were used to measure the Mg2+ affinity of site II at 15 °C (∼1.2–1.9 mM) (20). In the presence of 3 mM Mg2+, the Kd associated with binding of Ca2+ to site II of full-length cTnC was increased from 7 μM in the apo-state to 24 μM (20). Moreover, a system containing cTnC–cTnI had 2.5-fold lower Ca2+ affinity in the presence of 3 mM Mg2+ (106). Given these affinities, Tikunova and Davis (20) hypothesized that site II would be 33 to 44% saturated by 1 mM cytosolic Mg2+ at diastolic Ca2+ concentrations.
In a recent ITC study, the Mg2+-binding affinity of site II in lobster TnC isoforms, which are similar in sequence to human variants, was explored. Mg2+ affinity of site II was a single order of magnitude lower than that of Ca2+, such that the cations would compete for binding under physiological conditions (62).
In our experiments on N-cTnC and full-length cTnC, site II-binding affinity of Mg2+ was an order of magnitude lower than seen for Ca2+ (Fig. 2 and 7 and Tables S2 and S3). At these affinities and given the relatively high cytosolic [Mg2+]free (79, 82), this cation would compete for binding to site II of cTnC (107). Competition experiments were also in agreement (Figs. 1 and 2) as were experiments that utilized a double mutant removing coordinating residues in site II (Figs. 4 and 5). Competition experiments were also analyzed using the model available on Origin, and the thermodynamic parameters obtained from this alternative method were comparable and within the range of errors obtained Fig. 3 and Table S2).
In order to further validate the ITC data, we also performed TI to calculate absolute binding affinities computationally. We performed these calculations for both Ca2+ and Mg2+ binding separately for both WT N-cTnC and D67A/D73A N-cTnC. For both sets of simulations, the structure of Ca2+-bound N-cTnC (Protein Data Bank [PDB]: 1AP4) was used as the starting parameter and restrained throughout the simulation. ITC measures the thermodynamically quantifiable closed-to-open transition of the N-cTnC molecule. TI does not allow for such a transition, rather, it quantifies only the binding interaction. In the future, the closed structure of N-cTnC (PDB: 1SPY) can be simulated to quantify the presumably lower affinity it has for each of Ca2+ and Mg2+. The difference between these sets of simulations could then be used to better corroborate the ITC data.
For Ca2+ binding, our TI results agreed very well with the binding affinities from ITC. For Mg2+ binding, the calculated absolute binding affinities were consistently underestimated by about 4 kcal ∗ mol−1 but showed the same relative trends. Mg2+ was calculated to bind more weakly than Ca2+ in the WT and D67A/D73A mutant, in agreement with the ITC results. The Mg2+ absolute binding affinities were likely underestimated for multiple reasons. First, the crystal structure of WT N-cTnC (1AP4) was bound by Ca2+, and no structure of Mg2+-bound WT N-cTnC is available. We attempted to correct for this issue by minimizing the structure with Mg2+ bound WT N-cTnC. Because of the lack of an exact starting structure and restraints chosen, there is still likely some error. In addition, while we did try to choose the most accurate Mg2+ parameters for binding affinity calculations, there are well-documented difficulties in free energy calculations for Mg2+, most notably that the free energy of solvation (ΔGsolvation) is consistently underestimated (108, 109). Even when using the same Mg2+ force field, solvation ΔG values are also known to have large variations for Mg2+ depending on the exact simulation parameters used. For example, both Panteva et al. (109) and Li et al. tried to reproduce Mg2+ salvation-free energy using the same parameters as Åqvist but saw variations on the order of 20 kcal ∗ mol−1 (110). While this may be an extreme example, it illustrates the difficulty in the calculation of free-energy changes with Mg2+ ions involved. Given these potential errors in TI for ΔGsolvation of Mg2+, the fact that we still see relatively good agreement with the ITC data for absolute binding affinity of Mg2+ helps further validate the in vitro results.
The experiments outlined previously were designed with the intent to test the hypothesis that both Ca2+ and Mg2+ interact with all the functional EF-hand motifs in cTnC. The interaction with sites III/IV has been established for some time (91), but site II may also bind Mg2+. Interestingly, a hypothesis that is reconcilable with our own was initially put forth; that of six binding sites (4). In this scenario, there were two Ca2+-specific sites, 2 Mg2+-specific sites, and two sites that can bind both cations. During these experiments, only the absence of Mg2+ allowed for the binding sites in cTnC to be separated into low-affinity sites (∼105 M−1) and high-affinity sites (∼107 M−1) (4). That the presence of Mg2+ affects the affinity of Ca2+ binding to TnC is also evident in a more recent study in which a fluorescence protein–based Ca2+ sensor was utilized to show the reorientation of both N and C domains of TnC upon Mg2+ binding at sites III and IV (111). In addition, studies using the ATPase activity on the strong-binding myosin heads also demonstrates the opening of more TF active sites upon Mg2+ binding to C-terminal domain, supporting the notion that Mg2+ binding causes structural changes in TnC (112).
Binding of Mg2+ to site II is not expected to induce significant structural changes in N-cTnC based on previous molecular dynamics simulation data (30, 61, 64). Therefore, it is likely that the favorable ΔS associated with the interaction is due to increased degrees of freedom for water molecules that would result when stabilizing hydrogen bonds are transferred from the positively charged metal cation and the negatively charged amino acid side chains in the binding site II to the buffered environment (61).
Given that the binding of Ca2+ to site II of cTnC at systolic Ca2+ levels (0.5–1.2 μM) strengthens the interaction with cTnI and the rest of the cTn complex and the orders of magnitude differ between binding affinity at varying levels of filament complexity (4, 60, 72, 113), care must be taken when translating observations at the level of cTnC to more complex systems. We suggest that other proteins in the TF, particularly cTnI, may play a central role in the mechanism discussed here. Moreover, a further limitation may be highlighted in our approach, concerning the double mutant D67A/D73A. This mutation was able to reduce the binding of both Ca2+ (11.9-fold) and Mg2+ (1.8-fold) to site II of N-cTnC; however, the impact on binding might be expected to be greater. It is possible that the effect of this double mutant is to reduce the binding of these cations, especially Mg2+ through allosteric interactions. In CaM, mutation of Ca2+ coordinating residues within the EF-hand can have structural consequences leading to altered binding kinetics (114); this is conceivable in our double mutant. Similarly, it is possible that the competition observed between Ca2+ and Mg2+ for binding to site II of cTnC occurs through structural perturbations, which follow binding of Mg2+ to an allosteric site. Exploration of these limitations in future studies may shed light on the true nature of these interactions.
Our ITC results strongly suggest that Mg2+ binds to site III/IV and competes with Ca2+ for binding to site II. The amount of Mg2+ that binds the regulatory site II is likely to be highly dependent on the technique, biological system, and buffer conditions. In N-cTnC, occupation of site II by Mg2+ was again seen to reduce the amount of Ca2+, which was able to bind this protein, at concentrations that may have physiologically relevant consequences under normal conditions and even more so in the face of diseases that alter the Ca2+ sensitivity of contraction.
Moreover, increases in cAMP in the cell through α- and β-adrenergic stimulation elicit extrusion of Mg2+ from the cell in mammalian tissues (115, 116, 117) including cardiomyocytes (118, 119). If shown in the heart, both Na+-dependent and Na+-independent removal of Mg2+ from the cytosol under stressful conditions would lower cytosolic presence of this cation. Despite this, free Mg2+ does not fluctuate greatly under such stimulation, suggesting that buffered Mg2+ is removed from the cell (120). Nevertheless, this altered Mg2+ pool may affect the subset of ions available to compete with Ca2+ for binding to troponin.
Based on our binding experiments and given the previous studies cited herein, Mg2+ may also compete with Ca2+ in binding to the regulatory site II. Free Ca2+ is tightly regulated at rest (∼0.1 μM) despite relatively high total cytosolic concentrations (2.1–2.6 mM) (84). Mg2+ is also abundant in the cell but is less tightly controlled. Binding of both Ca2+ and Mg2+ to site II is endothermic and thus driven by entropy. Relative to Ca2+, Mg2+ binds site II with lower affinity; however, at physiological concentrations or with elevation of free Mg2+, which accompanies states of energy depletion, it may reduce Ca2+ binding, leading to structural perturbations that modify the contractile function of the myofilament. Conversely, Mg2+ can be altered by diseased states such as secondary hyperparathyroidism, which results in hypomagnesia and could potentially impact cardiac contractility (121).
Conclusions
Our study provides insights regarding the thermodynamics of metal cation binding to cTnC. The interaction of Ca2+ and Mg2+ with cTnC is characterized by differences consistent with dissimilar ionic radius, number of required coordinating residues, as well as the energic cost of exposing hydrophobic amino acids to an aqueous environment. In the cell, these differences are functionally necessitated by dissimilar free cytosolic concentrations of each cation. Cellular Mg2+ is not necessarily prevalent enough to directly regulate contraction and is not thought to cause a conformational change upon binding to cTnC. However, given the affinities we have observed, its occupation of the binding site may restrict Ca2+ binding, disable key interactions with components of the cTn complex, such as cTnI, and prevent the subsequent conformational changes necessary for rigor-state formation. This competition for binding likely favors Ca2+ and is well tolerated; however, elevation of free Mg2+, which may accompany states of ATP depletion, for example, during ischemic stress, could have relatively significant functional consequence for cardiac force production.
Experimental procedures
Construct preparation and protein expression
The human TNNC1 gene (Uniprot ID: P63316) had previously been cloned into pET21a(+) vector and had a stop codon inserted at residue 90 to create the human N-cTnC construct using the Phusion site-directed mutagenesis protocol (Thermo Scientific). This construct was transformed into the BL21(DE3) Escherichia coli expression strain. The double-mutant D76A/D73A construct was made using site-directed mutagenesis carried out by GenScript. Expression and purification of all constructs were carried out as described previously (63, 64). In brief, 100 ml of lysogeny broth was supplemented with 50 μg/ml ampicillin and a glycerol stock stab and grown overnight at a shaking speed of 250 rpm and 37 °C. The next day, the overnight grown culture was used to inoculate each liter of lysogeny broth with 1:100 back dilution supplemented with 50 μg/ml ampicillin. Cell cultures were grown for ∼3 h until an absorbance reached to 0.8 to 1.0 at 600 nm followed by induction with 1 mM IPTG. After 3 h, the cells were harvested by centrifugation at 6000g for 6 min, and the collected cell pellets were stored at −80 °C.
Protein purification
The cell pellet was thawed and suspended in buffer A (50 mM Tris–HCl at pH 8.0 and 5 mM EDTA) and sonicated on ice at 50% amplitude with 30 s on and 30 s off for 5 min, or until there was no visible viscosity of the lysate solution. After sonication, the lysate was centrifuged at 30,000g for 15 min at 4 °C, and the supernatant was obtained. This centrifugation process was performed twice to ensure the removal of all cell debris before loading onto a fast-flow (FF) Q-Sepharose column (GE Healthcare). The FF Q-Sepharose column was connected with an AKTA FPLC system (GE Healthcare) and pre-equilibrated with buffer A with the addition of 1 mM DTT. After applying the clear supernatant onto the column, the solution was run at 5 ml/min with a gradual gradient mixing with buffer A and buffer B (buffer A with 0.5 M NaCl), starting from 0% buffer B up to 100% buffer B by the end of the run. Following analysis by 12% SDS-PAGE, the fractions containing purified N-cTnC were pooled and concentrated using Amicon ultracentrifugal filter device (Millipore) with a 3-kDa molecular weight cutoff.
The full-length TnC was purified using the same protocol described previously, with the addition of 30% ammonium sulphate precipitation following the sonication step. After the addition of 30% ammonium sulphate, the solution was stirred on ice for 30 min and subsequently centrifuged at 28,900g for 30 min at 4 °C. The supernatant was obtained and dialyzed overnight against 4 l of buffer C (50 mM Tris–HCl at pH 8.0 and 100 mM NaCl).
After purification using the FF Q-Sepharose column, the fractions containing partially pure cTnC were concentrated to 3 ml using an Amicon ultracentrifugal filter device (Millipore) with a 10-kDa molecular weight cutoff. The concentrated protein sample was further purified by a HiPrep 26/60 Sephacryl S-100 column size-exclusion chromatography (GE Healthcare), which was equilibrated with buffer C. After confirming the purity of the protein on a 12% SDS-PAGE gel, all fractions containing the purified cTnC were combined, aliquoted, and stored at −80 °C prior to pre-ITC dialysis.
Protein dialysis
To generate the apo-state protein, TnC was first dialyzed against 2 l of 50 mM Hepes at pH 7.2, 150 mM KCl, 2 mM EDTA, and 15 mM β-mercaptoethanol, followed by another dialysis against the same buffer with no EDTA added. Each of these dialysis steps was completed at 4 °C for a minimum of 4 h. A third dialysis was performed for a minimum of 16 h overnight against 2 l of 50 mM Hepes at pH 7.2, 150 mM KCl, and 2 mM β-mercaptoethanol. An extinction coefficient of 1490 and 4595 M−1 cm−1 and a molecular weight of 10.1 and 18.4 kDa was used to determine protein concentration for the N-cTnC and full-length cTnC constructs, respectively, by 280 nm UV–visible spectroscopy using a NanoDrop 2000 spectrophotometer (Thermo Scientific). The final dialysis buffer was used to dilute the protein samples to a final concentration of 200 μM for the N-terminal construct and 100 μM for full-length cTnC as described previously (64).
Standard 1.0 M CaCl2 and MgCl2 stock solutions (Sigma) were serially diluted in the final dialysis buffer to produce 6 mM Ca2+ and 40 mM Mg2+, respectively. The same standards were used to produce 4 mM Ca2+ and 20 mM Mg2+ titrants for the N-cTnC experiments. Given the key role of protein concentration in determination of affinity, we aimed to ensure consistency and did so through dilution of the protein from stock solutions with care taken to minimize human and pipetting error to fall at the minimum possible using recently calibrated instrumentation.
Isothermal titration calorimetry
The ITC experiments were carried out in a MicroCal ITC200 instrument (Malvern). Repeat titrations were used to ensure reproducibility. The sample cell was set at 25 °C, 200 μl of the protein was loaded, and the experiment was carried out at the same temperature. For the N-cTnC, 19 injections of the titrant were used with the first being a dummy injection of 0.8 μl and the subsequent 18 injections, 2 μl each. For these experiments, 4 mM Ca2+ was titrated into 200 μl of 200 μM apo-state N-cTnC as the baseline condition. For the full-length cTnC, 6 mM Ca2+ was titrated into 200 μl of 100 μM apo-state full-length cTnC with a dummy injection of 0.5 μl and 38 injections of 1 μl. The time interval between injections was 120 s, and stirring speed was set at 1000 rpm throughout each experiment.
Analysis of results
Titration data were analyzed using MicroCal2000 for ITC through Origin 8.0 (OriginLab). Raw heats were integrated and fit by a least-squares algorithm using a “single-binding site” model for the N-cTnC titrations and a “two sets-of-binding sites” model for the full-length cTnC titrations to calculate the thermodynamic parameters. As a point of comparison, the “competition” model on Origin was also used to study the binding of Ca2+ and Mg2+ to the apo-state N-cTnC as well as in the presence of 1 mM of the counter ion (Fig. 3) (122). For the N-cTnC constructs (apart from the Ca2+ into apo-state N-cTnC condition), the N (number of binding sites) associated with each interaction was necessarily constrained to equal 1.00 to facilitate curve fitting without altering protein concentration. The baseline condition was repeated daily, and the consistency of the thermodynamic parameters in these sets of titrations indicates protein quality and function throughout the set of experiments. If multiple ligands were simultaneously present in the reaction mixture, an “apparent affinity” was determined for the injected titrant.
JMP 14.0 software package was used for statistical analysis. ANOVA was used to identify differences in each studied thermodynamic parameter from the N-cTnC and full-length cTnC titrations: in the apo-state and competition experiments, including both the WT and double-mutant proteins (N-cTnC only). Tukey's post hoc test was subsequently used to explore where the differences lie with p < 0.05 considered the threshold for statistical significance.
Thermodynamic integration
Starting from the representative model of PDB: 1AP4 (30), which contains N-cTnC with a single Ca2+ ion bound, the system was solvated with a 12 Å-padded transferable intermolecular potential 3P water box and neutralized with Na+ in Amber16 (123). The system was also prepared similarly for only the Ca2+ ion in a 12 Å-padded transferable intermolecular potential 3P water box. The alchemical thermodynamic cycle used for ligand binding was described in detail previously (124). In short, TI was performed using the following three steps for Ca2+ in protein: turn on restraints, turn off charge, and turn off van der Waals forces. The specific distance restraints used in all systems can be found in Table S1. In addition, TI was performed for the following two steps for Ca2+ in water: turn off charge and turn off van der Waals forces. Each step of the thermodynamic cycle was performed with the coupling parameter (λ) ranging from 0.0 to 1.0 in increments of 0.1. For each simulation, the system was minimized (2000 cycles) and heated (0.5 ns) before the 5 ns production run at 300 K using the ff14SB force field (125). These calculations were also performed on the D67A/D73A mutated system. The mutations were imposed on the 1AP4 representative model using PyMOL (126).
For the calculation of Mg2+-binding affinity, Ca2+ was replaced with Mg2+ in the 1AP4 representative model since no Mg2+-bound N-cTnC structure was available in the PDB. In order to generate more accurate restraints and starting coordinates for the TI calculations, a minimization was performed on the structure in Amber (2000 cycles). Following the minimization, TI simulations were run similarly as for Ca2+. However, because of previously documented errors in the default Mg2+ parameters, the ΔGsolvation-optimized Mg2+ parameters from Li et al. were used (109, 127). These calculations were also performed on the D67A/D73A mutated system.
To calculate absolute binding affinities for the ions, the change in free energy (ΔG) was calculated for each step in the thermodynamic cycle by integrating the potential energy with respect to the coupling parameter, λ (128). Two corrections were made to these calculated ΔG values. The first correction was necessary because of the introduction of the distance restraints (as described in the study by Boresch et al. (129)), which quantified the free energy cost of restraining the ion to the binding site. The second correction was performed to correct the charged system (as described in the study by Rocklin et al. (130)) to revise the free energy for the fact that the system is charged during the disappearance of the charged ions. The overall ΔG of binding was the change in free energy between the ions in complex with the protein (ion in protein steps 1, 2, and 3) and the ions in water (ion in water steps 1 and 2). For each system, five independent runs were performed, and results were averaged.
Data availability
All data presented in this article are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
K. R. and A. Y. L. preliminary experiments; K. R., J. S., A. Y. L., J. P. D., A. M. S., F. V. P., S. L., and G. F. T. experimental design; K. R. and J. S. data collection; K. R., J. S., A. M. S., and S. L. data analysis; K. R., J. S., A. M. S., F. V. P., S. L., and G. F. T. manuscript preparation; K. R., A. Y. L., J. P. D., A. M. S., F. V. P., R. J. S., S. L., and G. F. T. manuscript review.
Funding and additional information
This work was primarily supported by a grant from the Canadian Institutes of Health Research (PJT—148964) to G. F. T. K. R. was supported by the National Sciences and Engineering Research Council Canada Graduate Scholarship—Doctoral fellowship.
Edited by Enrique De La Cruz
Supporting information
References
- 1.Parmacek M.S., Solaro R.J. Biology of the troponin complex in cardiac myocytes. Prog. Cardiovasc. Dis. 2004;47:159–176. doi: 10.1016/j.pcad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki Y., van Eerd J.-P. The effect of Mg2+ on the conformation of the Ca2+-binding component of troponin. Biochem. Biophys. Res. Commun. 1972;49:898–905. doi: 10.1016/0006-291x(72)90297-5. [DOI] [PubMed] [Google Scholar]
- 3.Murray A.C., Kay C.M. Hydrodynamic and optical properties of troponin A. Demonstration of a conformational change upon binding calcium ion. Biochemistry. 1972;11:2622–2627. doi: 10.1021/bi00764a012. [DOI] [PubMed] [Google Scholar]
- 4.Potter J.D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1975;250:4628–4633. [PubMed] [Google Scholar]
- 5.Filatov V.L., Katrukha A.G., Bulargina T.V., Gusev N.B. Troponin: Structure, properties, and mechanism of functioning. Biochemistry (Mosc) 1999;64:969–985. [PubMed] [Google Scholar]
- 6.Strynadka N.C., James M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 7.Yap K.L., Ames J.B., Swindells M.B., Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499–507. doi: 10.1002/(sici)1097-0134(19991115)37:3<499::aid-prot17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Lewit-Bentley A., Rety S. EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 9.Seamon K.B., Hartshorne D.J., Bothner-By A.A. Ca2+ and Mg2+ dependent conformations of troponin C as determined by 1H and 19F nuclear magnetic resonance. Biochemistry. 1977;16:4039–4046. doi: 10.1021/bi00637a016. [DOI] [PubMed] [Google Scholar]
- 10.Ebashi S., Nonomura Y., Kohama K., Kitazawa T., Mikawa T. Regulation of muscle contraction by Ca ion. Mol. Biol. Biochem. Biophys. 1980;32:183–194. doi: 10.1007/978-3-642-81503-4_14. [DOI] [PubMed] [Google Scholar]
- 11.van Eerd J.P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976;15:1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]
- 12.Farah C.S., Reinach F.C. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- 13.Schober T., Huke S., Venkataraman R., Gryshchenko O., Kryshtal D., Hwang H.S., Baudenbacher F.J., Knollmann B.C. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ. Res. 2012;111:170–179. doi: 10.1161/CIRCRESAHA.112.270041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J.D., Charlton S.C., Potter J.D. A fluorescence stopped flow analysis of Ca2+ exchange with troponin C. J. Biol. Chem. 1979;254:3497–3502. [PubMed] [Google Scholar]
- 15.Johnson J.D., Nakkula R.J., Vasulka C., Smillie L.B. Modulation of Ca2+ exchange with the Ca2+-specific regulatory sites of troponin C. J. Biol. Chem. 1994;269:8919–8923. [PubMed] [Google Scholar]
- 16.Bers D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 17.Leavis P., Kraft E.L. Calcium binding to cardiac troponin C. Arch. Biochem. Biophys. 1978;186:411–415. doi: 10.1016/0003-9861(78)90453-8. [DOI] [PubMed] [Google Scholar]
- 18.Robertson S., Johnson J.D., Potter J. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+ Biophys. J. 1981;34:559. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturtevant J.M. Heat capacity and entropy changes in processes involving proteins. Proc. Natl. Acad. Sci. U. S. A. 1977;74:2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tikunova S.B., Davis J.P. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J. Biol. Chem. 2004;279:35341–35352. doi: 10.1074/jbc.M405413200. [DOI] [PubMed] [Google Scholar]
- 21.Sundaralingam M., Bergstrom R., Strasburg G., Rao S.T., Roychowdhury P., Greaser M., Wang B.C. Molecular structure of troponin C from chicken skeletal muscle at 3-angstrom resolution. Science. 1985;227:945–948. doi: 10.1126/science.3969570. [DOI] [PubMed] [Google Scholar]
- 22.Sia S.K., Li M.X., Spyracopoulos L., Gagné S.M., Liu W., Putkey J.A., Sykes B.D. Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J. Biol. Chem. 1997;272:18216–18221. doi: 10.1074/jbc.272.29.18216. [DOI] [PubMed] [Google Scholar]
- 23.Cheung J.Y., Tillotson D.L., Yelamarty R., Scaduto R. Cytosolic free calcium concentration in individual cardiac myocytes in primary culture. Am. J. Physiol. Cell Physiol. 1989;256:C1120–C1130. doi: 10.1152/ajpcell.1989.256.6.C1120. [DOI] [PubMed] [Google Scholar]
- 24.Kirschenlohr H.L., Grace A.A., Vandenberg J.I., Metcalfe J.C., Smith G.A. Estimation of systolic and diastolic free intracellular Ca2+ by titration of Ca2+ buffering in the ferret heart. Biochem. J. 2000;346 Pt 2:385–391. [PMC free article] [PubMed] [Google Scholar]
- 25.Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix–loop–helix EF-hand motifs. Biochem. J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 26.Bowman J.D., Lindert S. Molecular dynamics and umbrella sampling simulations elucidate differences in troponin C isoform and mutant hydrophobic patch exposure. J. Phys. Chem. B. 2018;122:7874–7883. doi: 10.1021/acs.jpcb.8b05435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzberg O., James M.N. Structure of the Calcium Regulatory Muscle Protein Troponin-C at 2.8 Å Resolution. Nature. 1985;313:653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- 28.Slupsky C.M., Sykes B.D. NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry. 1995;34:15953–15964. doi: 10.1021/bi00049a010. [DOI] [PubMed] [Google Scholar]
- 29.Houdusse A., Love M.L., Dominguez R., Grabarek Z., Cohen C. Structures of four Ca2+-bound troponin C at 2.0 A resolution: Further insights into the Ca2+-switch in the calmodulin superfamily. Structure. 1997;5:1695–1711. doi: 10.1016/s0969-2126(97)00315-8. [DOI] [PubMed] [Google Scholar]
- 30.Spyracopoulos L., Li M.X., Sia S.K., Gagné S.M., Chandra M., Solaro R.J., Sykes B.D. Calcium-induced structural transition in the regulatory domain of human cardiac troponin C. Biochemistry. 1997;36:12138–12146. doi: 10.1021/bi971223d. [DOI] [PubMed] [Google Scholar]
- 31.Li M.X., Spyracopoulos L., Sykes B.D. Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999;38:8289–8298. doi: 10.1021/bi9901679. [DOI] [PubMed] [Google Scholar]
- 32.Dai L.J., Friedman P.A., Quamme G.A. Cellular mechanisms of chlorothiazide and cellular potassium depletion on Mg2+ uptake in mouse distal convoluted tubule cells. Kidney Int. 1997;51:1008–1017. doi: 10.1038/ki.1997.141. [DOI] [PubMed] [Google Scholar]
- 33.Romani A., Scarpa A. Regulation of cell magnesium. Arch. Biochem. Biophys. 1992;298:1–12. doi: 10.1016/0003-9861(92)90086-c. [DOI] [PubMed] [Google Scholar]
- 34.Tessman P.A., Romani A. Acute effect of EtOH on Mg2+ homeostasis in liver cells: Evidence for the activation of an Na+/Mg2+ exchanger. Am. J. Physiol. 1998;275:G1106–G1116. doi: 10.1152/ajpgi.1998.275.5.G1106. [DOI] [PubMed] [Google Scholar]
- 35.Hongo K., Konishi M., Kurihara S. Cytoplasmic free Mg2+ in rat ventricular myocytes studied with the fluorescent indicator furaptra. Jpn. J. Physiol. 1994;44:357–378. doi: 10.2170/jjphysiol.44.357. [DOI] [PubMed] [Google Scholar]
- 36.Laires M.J., Monteiro C.P., Bicho M. Role of cellular magnesium in health and human disease. Front. Biosci. 2004;9:262–276. doi: 10.2741/1223. [DOI] [PubMed] [Google Scholar]
- 37.Godt R.E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J. Gen. Physiol. 1974;63:722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godt R.E., Morgan J.L. Contractile responses to MgATP and pH in a thick filament regulated muscle: Studies with skinned scallop fibers. Adv. Exp. Med. Biol. 1984;170:569–572. doi: 10.1007/978-1-4684-4703-3_51. [DOI] [PubMed] [Google Scholar]
- 39.Best P.M., Donaldson S.K., Kerrick W.G. Tension in mechanically disrupted mammalian cardiac cells: Effects of magnesium adenosine triphosphate. J. Physiol. 1977;265:1–17. doi: 10.1113/jphysiol.1977.sp011702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M.X., Gagne S.M., Tsuda S., Kay C.M., Smillie L.B., Sykes B.D. Calcium binding to the regulatory N-domain of skeletal muscle troponin C occurs in a stepwise manner. Biochemistry. 1995;34:8330–8340. doi: 10.1021/bi00026a014. [DOI] [PubMed] [Google Scholar]
- 41.Potter J.D., Robertson S.P., Johnson J.D. Magnesium and the regulation of muscle contraction. Fed. Proc. 1981;40:2653–2656. [PubMed] [Google Scholar]
- 42.Ogawa Y. Calcium binding to troponin C and troponin: Effects of Mg2+, ionic strength and pH. J. Biochem. 1985;97:1011–1023. doi: 10.1093/oxfordjournals.jbchem.a135143. [DOI] [PubMed] [Google Scholar]
- 43.Zot A.S., Potter J.D. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu. Rev. Biophys. Biophys. Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- 44.Morimoto S. Effect of myosin cross-bridge interaction with actin on the Ca2+-binding properties of troponin C in fast skeletal myofibrils. J. Biochem. 1991;109:120–126. doi: 10.1093/oxfordjournals.jbchem.a123331. [DOI] [PubMed] [Google Scholar]
- 45.Francois J.M., Gerday C., Prendergast F.G., Potter J.D. Determination of the Ca2+ and Mg2+ affinity constants of troponin C from eel skeletal muscle and positioning of the single tryptophan in the primary structure. J. Muscle Res. Cell Motil. 1993;14:585–593. doi: 10.1007/BF00141555. [DOI] [PubMed] [Google Scholar]
- 46.She M., Dong W.J., Umeda P.K., Cheung H.C. Tryptophan mutants of troponin C from skeletal muscle: An optical probe of the regulatory domain. Eur. J. Biochem. 1998;252:600–607. doi: 10.1046/j.1432-1327.1998.2520600.x. [DOI] [PubMed] [Google Scholar]
- 47.Allen T.S., Yates L.D., Gordon A.M. Ca2+-dependence of structural changes in troponin-C in demembranated fibers of rabbit psoas muscle. Biophys. J. 1992;61:399–409. doi: 10.1016/S0006-3495(92)81846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada K. The enthalpy titration of troponin C with calcium. Biochim. Biophys. Acta. 1978;535:342–347. doi: 10.1016/0005-2795(78)90100-9. [DOI] [PubMed] [Google Scholar]
- 49.Kometani K., Yamada K. Enthalpy, entropy and heat capacity changes induced by binding of calcium ions to cardiac troponin C. Biochem. Biophys. Res. Commun. 1983;114:162–167. doi: 10.1016/0006-291x(83)91608-x. [DOI] [PubMed] [Google Scholar]
- 50.Yamada K., Kometani K. The changes in heat capacity and entropy of troponin C induced by calcium binding. J. Biochem. 1982;92:1505–1517. doi: 10.1093/oxfordjournals.jbchem.a134075. [DOI] [PubMed] [Google Scholar]
- 51.Gillis T.E., Blumenschein T.M., Sykes B.D., Tibbits G.F. Effect of temperature and the F27W mutation on the Ca2+ activated structural transition of trout cardiac troponin C. Biochemistry. 2003;42:6418–6426. doi: 10.1021/bi0340494. [DOI] [PubMed] [Google Scholar]
- 52.Gillis T.E., Liang B., Chung F., Tibbits G.F. Increasing mammalian cardiomyocyte contractility with residues identified in trout troponin C. Physiol. Genomics. 2005;22:1–7. doi: 10.1152/physiolgenomics.00007.2005. [DOI] [PubMed] [Google Scholar]
- 53.Gillis T.E., Moyes C.D., Tibbits G.F. Sequence mutations in teleost cardiac troponin C that are permissive of high Ca2+ affinity of site II. Am. J. Physiol. Cell Physiol. 2003;284:C1176–C1184. doi: 10.1152/ajpcell.00339.2002. [DOI] [PubMed] [Google Scholar]
- 54.Davis J.P., Rall J.A., Reiser P.J., Smillie L.B., Tikunova S.B. Engineering competitive magnesium binding into the first EF-hand of skeletal troponin C. J. Biol. Chem. 2002;277:49716–49726. doi: 10.1074/jbc.M208488200. [DOI] [PubMed] [Google Scholar]
- 55.Yamada K. Calcium binding to troponin C as a primary step of the regulation of contraction. A microcalorimetric approach. Adv. Exp. Med. Biol. 2003;538:203–212. doi: 10.1007/978-1-4419-9029-7_19. discussion 213. [DOI] [PubMed] [Google Scholar]
- 56.Wilcox D.E. Isothermal titration calorimetry of metal ions binding to proteins: An overview of recent studies. Inorg. Chim. Acta. 2008;361:857–867. [Google Scholar]
- 57.Grossoehme N.E., Spuches A.M., Wilcox D.E. Application of isothermal titration calorimetry in bioinorganic chemistry. J. Biol. Inorg. Chem. 2010;15:1183–1191. doi: 10.1007/s00775-010-0693-3. [DOI] [PubMed] [Google Scholar]
- 58.Sacco C., Skowronsky R.A., Gade S., Kenney J.M., Spuches A.M. Calorimetric investigation of copper(II) binding to Abeta peptides: Thermodynamics of coordination plasticity. J. Biol. Inorg. Chem. 2012;17:531–541. doi: 10.1007/s00775-012-0874-3. [DOI] [PubMed] [Google Scholar]
- 59.Davis J.P., Norman C., Kobayashi T., Solaro R.J., Swartz D.R., Tikunova S.B. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li A.Y., Stevens C.M., Liang B., Rayani K., Little S., Davis J., Tibbits G.F. Familial Hypertrophic Cardiomyopathy Related Cardiac Troponin C L29Q Mutation Alters Length-dependent Activation and Functional Effects of Phosphomimetic Troponin I∗. PLoS One. 2013;8:e79363. doi: 10.1371/journal.pone.0079363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skowronsky R.A., Schroeter M., Baxley T., Li Y., Chalovich J.M., Spuches A.M. Thermodynamics and molecular dynamics simulations of calcium binding to the regulatory site of human cardiac troponin C: Evidence for communication with the structural calcium binding sites. J. Biol. Inorg. Chem. 2013;18:49–58. doi: 10.1007/s00775-012-0948-2. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka H., Takahashi H., Ojima T. Ca2+-binding properties and regulatory roles of lobster troponin C sites II and IV. FEBS Lett. 2013;587:2612–2616. doi: 10.1016/j.febslet.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 63.Stevens C.M., Rayani K., Genge C.E., Singh G., Liang B., Roller J.M., Li C., Li A.Y., Tieleman D.P., van Petegem F. Characterization of zebrafish cardiac and slow skeletal troponin C paralogs by MD simulation and ITC. Biophys. J. 2016;111:38–49. doi: 10.1016/j.bpj.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens C.M., Rayani K., Singh G., Lotfalisalmasi B., Tieleman D.P., Tibbits G.F. Changes in the dynamics of the cardiac troponin C molecule explain the effects of Ca2+-sensitizing mutations. J. Biol. Chem. 2017;292:11915–11926. doi: 10.1074/jbc.M116.770776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson R.A., Fulcher L.M., Vang K., Palmer C.D., Grossoehme N.E., Spuches A.M. In depth, thermodynamic analysis of Ca2+ binding to human cardiac troponin C: Extracting buffer-independent binding parameters. Biochim. Biophys. Acta. 2019;1867:359–366. doi: 10.1016/j.bbapap.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Kolte D., Vijayaraghavan K., Khera S., Sica D.A., Frishman W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014;22:182–192. doi: 10.1097/CRD.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 67.Smith D.W. Ionic hydration enthalpies. J. Chem. Edu. 1977;54:540. [Google Scholar]
- 68.Wang S.-Q., Huang Y.-H., Liu K.-S., Zhou Z.-Q. Dependence of myocardial hypothermia tolerance on sources of activator calcium. Cryobiology. 1997;35:193–200. doi: 10.1006/cryo.1997.2040. [DOI] [PubMed] [Google Scholar]
- 69.Hazard A.L., Kohout S.C., Stricker N.L., Putkey J.A., Falke J.J. The kinetic cycle of cardiac troponin C: Calcium binding and dissociation at site II trigger slow conformational rearrangements. Protein Sci. 1998;7:2451–2459. doi: 10.1002/pro.5560071123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang B., Chung F., Qu Y., Pavlov D., Gillis T.E., Tikunova S.B., Davis J.P., Tibbits G.F. Familial hypertrophic cardiomyopathy-related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol. Genomics. 2008;33:257–266. doi: 10.1152/physiolgenomics.00154.2007. [DOI] [PubMed] [Google Scholar]
- 71.Gillis T.E., Marshall C.R., Xue X.-H., Borgford T.J., Tibbits G.F. Ca2+ binding to cardiac troponin C: Effects of temperature and pH on mammalian and salmonid isoforms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1707–R1715. doi: 10.1152/ajpregu.2000.279.5.R1707. [DOI] [PubMed] [Google Scholar]
- 72.Pinto J.R., Parvatiyar M.S., Jones M.A., Liang J., Ackerman M.J., Potter J.D. A functional and structural study of troponin C mutations related to hypertrophic cardiomyopathy. J. Biol. Chem. 2009;284:19090–19100. doi: 10.1074/jbc.M109.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romani A.M.P. Intracellular magnesium homeostasis. In: Vink R., Nechifor M., editors. Magnesium in the Central Nervous System. University of Adelaide Press (c) 2011 The Authors.; Adelaide, AU: 2011. pp. 13–58. [Google Scholar]
- 74.Yumoto F., Nara M., Kagi H., Iwasaki W., Ojima T., Nishita K., Nagata K., Tanokura M. Coordination structures of Ca2+ and Mg2+ in Akazara scallop troponin C in solution. FTIR spectroscopy of side-chain COO- groups. Eur. J. Biochem. 2001;268:6284–6290. doi: 10.1046/j.1432-1327.2001.02583.x. [DOI] [PubMed] [Google Scholar]
- 75.Carafoli E., Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lockless S.W., Zhou M., MacKinnon R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maguire M.E. Magnesium transporters: Properties, regulation and structure. Front. Biosci. 2006;11:3149–3163. doi: 10.2741/2039. [DOI] [PubMed] [Google Scholar]
- 78.Chao S.H., Suzuki Y., Zysk J.R., Cheung W.Y. Activation of calmodulin by various metal cations as a function of ionic radius. Mol. Pharmacol. 1984;26:75–82. [PubMed] [Google Scholar]
- 79.Malmendal A., Linse S., Evenas J., Forsen S., Drakenberg T. Battle for the EF-hands: Magnesium-calcium interference in calmodulin. Biochemistry. 1999;38:11844–11850. doi: 10.1021/bi9909288. [DOI] [PubMed] [Google Scholar]
- 80.Follenius A., Gerard D. Fluorescence investigations of calmodulin hydrophobic sites. Biochem. Biophys. Res. Commun. 1984;119:1154–1160. doi: 10.1016/0006-291x(84)90896-9. [DOI] [PubMed] [Google Scholar]
- 81.Gilli R., Lafitte D., Lopez C., Kilhoffer M., Makarov A., Briand C., Haiech J. Thermodynamic analysis of calcium and magnesium binding to calmodulin. Biochemistry. 1998;37:5450–5456. doi: 10.1021/bi972083a. [DOI] [PubMed] [Google Scholar]
- 82.Linse S., Forsen S. Determinants that govern high-affinity calcium binding. Adv. Second Messenger Phosphoprotein Res. 1995;30:89–151. doi: 10.1016/s1040-7952(05)80005-9. [DOI] [PubMed] [Google Scholar]
- 83.Cates M.S., Berry M.B., Ho E.L., Li Q., Potter J.D., Phillips G.N., Jr. Metal-ion affinity and specificity in EF-hand proteins: Coordination geometry and domain plasticity in parvalbumin. Structure. 1999;7:1269–1278. doi: 10.1016/s0969-2126(00)80060-x. [DOI] [PubMed] [Google Scholar]
- 84.Brini M., Cali T., Ottolini D., Carafoli E. Calcium pumps: Why so many? Compr. Physiol. 2012;2:1045–1060. doi: 10.1002/cphy.c110034. [DOI] [PubMed] [Google Scholar]
- 85.Harding M.M. Metal-ligand geometry relevant to proteins and in proteins: Sodium and potassium. Acta Crystallogr. D Biol. Crystallogr. 2002;58:872–874. doi: 10.1107/s0907444902003712. [DOI] [PubMed] [Google Scholar]
- 86.Dokmanic I., Sikic M., Tomic S. Metals in proteins: Correlation between the metal-ion type, coordination number and the amino-acid residues involved in the coordination. Acta Crystallogr. D Biol. Crystallogr. 2008;64:257–263. doi: 10.1107/S090744490706595X. [DOI] [PubMed] [Google Scholar]
- 87.Reid R.E., Procyshyn R.M. Engineering magnesium selectivity in the helix-loop-helix calcium-binding motif. Arch. Biochem. Biophys. 1995;323:115–119. doi: 10.1006/abbi.1995.0016. [DOI] [PubMed] [Google Scholar]
- 88.Tikunova S.B., Black D.J., Johnson J.D., Davis J.P. Modifying Mg2+ binding and exchange with the N-terminal of calmodulin. Biochemistry. 2001;40:3348–3353. doi: 10.1021/bi0021333. [DOI] [PubMed] [Google Scholar]
- 89.Holroyde M., Robertson S., Johnson J., Solaro R., Potter J. The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1980;255:11688–11693. [PubMed] [Google Scholar]
- 90.Li M.X., Chandra M., Pearlstone J.R., Racher K.I., Trigo-Gonzalez G., Borgford T., Kay C.M., Smillie L.B. Properties of isolated recombinant N and C domains of chicken troponin C. Biochemistry. 1994;33:917–925. doi: 10.1021/bi00170a010. [DOI] [PubMed] [Google Scholar]
- 91.Johnson J.D., Collins J.H., Robertson S.P., Potter J.D. A fluorescent probe study of Ca2+ binding to the Ca2+-specific sites of cardiac troponin and troponin C. J. Biol. Chem. 1980;255:9635–9640. [PubMed] [Google Scholar]
- 92.Allen K., Xu Y.Y., Kerrick W.G. Ca2+measurements in skinned cardiac fibers: Effects of Mg2+ on Ca2+ activation of force and fiber ATPase. J. Appl. Physiol. (1985) 2000;88:180–185. doi: 10.1152/jappl.2000.88.1.180. [DOI] [PubMed] [Google Scholar]
- 93.Ebashi S., Ogawa Y. Ca2+ in contractile processes. Biophys. Chem. 1988;29:137–143. doi: 10.1016/0301-4622(88)87033-9. [DOI] [PubMed] [Google Scholar]
- 94.Kohama K. Role of the high affinity Ca binding sites of cardiac and fast skeletal troponins. J. Biochem. 1980;88:591–599. doi: 10.1093/oxfordjournals.jbchem.a133007. [DOI] [PubMed] [Google Scholar]
- 95.Stephenson D.G., Williams D.A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J. Physiol. 1982;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Godt R.E., Lindley B.D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J. Gen. Physiol. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wnuk W., Schoechlin M., Stein E.A. Regulation of actomyosin ATPase by a single calcium-binding site on troponin C from crayfish. J. Biol. Chem. 1984;259:9017–9023. [PubMed] [Google Scholar]
- 98.Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J. Physiol. 1975;249:497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donaldson S.K., Kerrick W.G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J. Gen. Physiol. 1975;66:427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kerrick W.G.L., Donaldson S.K.B. The comparative effects of [Ca2+] and [Mg2+] on tension generation in the fibers of skinned frog skeletal muscle and mechanically disrupted rat ventricular cardiac muscle. Pflügers Arch. 1975;358:195–201. doi: 10.1007/BF00587216. [DOI] [PubMed] [Google Scholar]
- 101.Solaro R.J., Shiner J.S. Modulation of Ca2+ control of dog and rabbit cardiac myofibrils by Mg2+. Comparison with rabbit skeletal myofibrils. Circ. Res. 1976;39:8–14. doi: 10.1161/01.res.39.1.8. [DOI] [PubMed] [Google Scholar]
- 102.Ashley C.C., Moisescu D.G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J. Physiol. 1977;270:627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Donaldson S.K., Best P.M., Kerrick G.L. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned rat cardiac fibers. J. Gen. Physiol. 1978;71:645–655. doi: 10.1085/jgp.71.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ebashi S., Endo M. Calcium ion and muscle contraction. Prog. Biophys. Mol. Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- 105.Pan B.S., Solaro R.J. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J. Biol. Chem. 1987;262:7839–7849. [PubMed] [Google Scholar]
- 106.Siddiqui J.K., Tikunova S.B., Walton S.D., Liu B., Meyer M., de Tombe P.P., Neilson N., Kekenes-Huskey P.M., Salhi H.E., Janssen P.M., Biesiadecki B.J., Davis J.P. Myofilament calcium sensitivity: Consequences of the effective concentration of troponin I. Front. Physiol. 2016;7:632. doi: 10.3389/fphys.2016.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nara M., Morii H., Tanokura M. Infrared study of synthetic peptide analogues of the calcium-binding site III of troponin C: The role of helix F of an EF-hand motif. Biopolymers. 2013;99:342–347. doi: 10.1002/bip.22176. [DOI] [PubMed] [Google Scholar]
- 108.Steinbrecher T., Joung I., Case D.A. Soft-core potentials in thermodynamic integration: Comparing one- and two-step transformations. J. Comput. Chem. 2011;32:3253–3263. doi: 10.1002/jcc.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Panteva M.T., Giambasu G.M., York D.M. Comparison of structural, thermodynamic, kinetic and mass transport properties of Mg(2+) ion models commonly used in biomolecular simulations. J. Comput. Chem. 2015;36:970–982. doi: 10.1002/jcc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ȧqvist J. Ion-water interaction potentials derived from free energy perturbation simulations. J. Phys. Chem. 1990;94:8021–8024. [Google Scholar]
- 111.Badr M.A., Pinto J.R., Davidson M.W., Chase P.B. Fluorescent protein-based Ca2+ sensor reveals global, divalent cation-dependent conformational changes in cardiac troponin C. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fuchs F., Grabarek Z. The Ca2+/Mg2+ sites of troponin C modulate crossbridge-mediated thin filament activation in cardiac myofibrils. Biochem. Biophys. Res. Commun. 2011;408:697–700. doi: 10.1016/j.bbrc.2011.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramos C.H. Mapping subdomains in the C-terminal region of troponin I involved in its binding to troponin C and to thin filament. J. Biol. Chem. 1999;274:18189–18195. doi: 10.1074/jbc.274.26.18189. [DOI] [PubMed] [Google Scholar]
- 114.Wang K., Brohus M., Holt C., Overgaard M.T., Wimmer R., Van Petegem F. Arrhythmia mutations in calmodulin can disrupt cooperativity of Ca2+ binding and cause misfolding. J. Physiol. 2020;598:1169–1186. doi: 10.1113/JP279307. [DOI] [PubMed] [Google Scholar]
- 115.Cefaratti C., Romani A.M. Functional characterization of two distinct Mg2+ extrusion mechanisms in cardiac sarcolemmal vesicles. Mol. Cell. Biochem. 2007;303:63–72. doi: 10.1007/s11010-007-9456-z. [DOI] [PubMed] [Google Scholar]
- 116.Fagan T.E., Romani A. α1-Adrenoceptor-induced Mg2+ extrusion from rat hepatocytes occurs via Na+-dependent transport mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1145–G1156. doi: 10.1152/ajpgi.2001.280.6.G1145. [DOI] [PubMed] [Google Scholar]
- 117.Wolf F.I., Di Francesco A., Covacci V., Corda D., Cittadini A. Regulation of intracellular magnesium in ascites cells: Involvement of different regulatory pathways. Arch. Biochem. Biophys. 1996;331:194–200. doi: 10.1006/abbi.1996.0298. [DOI] [PubMed] [Google Scholar]
- 118.Howarth F., Waring J., Hustler B., Singh J. Effects of extracellular magnesium and beta adrenergic stimulation on contractile force and magnesium mobilization in the isolated rat heart. Magnes. Res. 1994;7:187–197. [PubMed] [Google Scholar]
- 119.Vormann J., Günther T. Amiloride-sensitive net Mg2+ efflux from isolated perfused rat hearts. Magnesium. 1987;6:220–224. [PubMed] [Google Scholar]
- 120.Amano T., Matsubara T., Watanabe J., Nakayama S., Hotta N. Insulin modulation of intracellular free magnesium in heart: Involvement of protein kinase C. Br. J. Pharmacol. 2000;130:731–738. doi: 10.1038/sj.bjp.0703361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morsy M.S., Dishmon D.A., Garg N., Weber K.T. Secondary hyperparathyroidism in heart failure. Am. J. Med. Sci. 2017;354:335–338. doi: 10.1016/j.amjms.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 122.Sigurskjold B.W. Exact analysis of competition ligand binding by displacement isothermal titration calorimetry. Anal. Biochem. 2000;277:260–266. doi: 10.1006/abio.1999.4402. [DOI] [PubMed] [Google Scholar]
- 123.Case D.A., Betz R., Cerutti D., Cheatham T., Darden T., Duke R., Giese T., Gohlke H., Goetz A., Homeyer N. University of California; San Francisco, CA: 2016. AMBER 2016 Reference Manual; pp. 1–923. [Google Scholar]
- 124.Leelananda S.P., Lindert S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016;12:2694–2718. doi: 10.3762/bjoc.12.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maier J.A., Martinez C., Kasavajhala K., Wickstrom L., Hauser K.E., Simmerling C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theor. Comput. 2015;11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.L DeLano W. DeLano Scientific; Palo Alto, CA: 2002. The PyMOL Molecular Graphics System (2002) [Google Scholar]
- 127.Li P., Roberts B.P., Chakravorty D.K., Merz K.M., Jr. Rational design of particle mesh ewald compatible Lennard-Jones parameters for +2 metal cations in explicit solvent. J. Chem. Theor. Comput. 2013;9:2733–2748. doi: 10.1021/ct400146w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shirts M.R., Mobley D.L., Brown S.P. Free-energy calculations in structure-based drug design. In: Merz K.M., Ringe D., Reynolds C.H., editors. Drug Design: Structure-and Ligand-Based Approaches. Cambridge University Press; Cambridge, England: 2010. pp. 61–86. [Google Scholar]
- 129.Boresch S., Tettinger F., Leitgeb M., Karplus M. Absolute binding free energies: A quantitative approach for their calculation. J. Phys. Chem. B. 2003;107:9535–9551. [Google Scholar]
- 130.Rocklin G.J., Mobley D.L., Dill K.A., Hunenberger P.H. Calculating the binding free energies of charged species based on explicit-solvent simulations employing lattice-sum methods: An accurate correction scheme for electrostatic finite-size effects. J. Chem. Phys. 2013;139:184103. doi: 10.1063/1.4826261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this article are contained within the article.