Summary
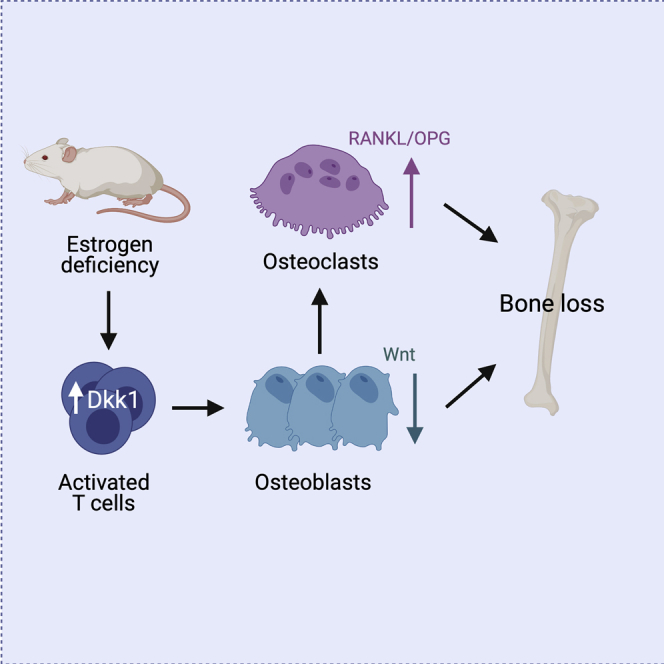
The Wnt inhibitor Dickkopf-1 (DKK1) is a negative regulator of bone formation and bone mass and is dysregulated in various bone diseases. How DKK1 contributes to postmenopausal osteoporosis, however, remains poorly understood. Here, we show that mice lacking DKK1 in T cells are protected from ovariectomy-induced bone loss. Ovariectomy activated CD4+ and CD8+ T cells and increased their production of DKK1. Co-culture of activated T cells with osteoblasts inhibited Wnt signaling in osteoblasts, leading to impaired differentiation. Importantly, DKK1 expression in T cells also controlled physiological bone remodeling. T-cell-deficient Dkk1 knock-out mice had a higher bone mass with an increased bone formation rate and decreased numbers of osteoclasts compared with controls, a phenotype that was rescued by adoptive transfer of wild-type T cells. Thus, these findings highlight that T cells control bone remodeling in health and disease via their expression of DKK1.
Subject areas: Molecular Physiology, Immunology, Cell Biology
Graphical abstract
Highlights
-
•
Lack of DKK1 in T cells leads to high bone mass due to increased bone formation
-
•
Activated T cells deficient of DKK1 suppress osteoblast marker expression
-
•
Mice with T cells deficient of DKK1 are protected from ovariectomy-induced bone loss
Molecular Physiology; Immunology; Cell Biology;
Introduction
Osteoporosis is a highly prevalent disease with significant socioeconomic burden, and its incidence will further increase due to a longer life expectancy and an aging population (Burge et al., 2007; Hernlund et al., 2013; US Department of Health and Human Services, 2004). Postmenopausal osteoporosis, which results from the decline in estrogen levels after menopause, is the most common form of osteoporosis (van Staa et al., 2001). The rapid and progressive loss of bone after menopause occurs as a result of an imbalanced bone turnover with bone resorption exceeding bone formation. Thus, anti-resorptive drugs are most commonly used to prevent bone loss and reduce fracture risk in affected patients (Rachner et al., 2011). However, besides directly affecting bone cells, estrogen deficiency also impacts lymphocyte differentiation and function, which may also contribute to pathogenesis of osteoporosis by negatively affecting the communication with osteoblasts and osteoclasts. In fact, estrogen deficiency leads to an expansion of B and T cell populations in mice (Cenci et al., 2003; Fujiwara et al., 2016; Garcia-Perez et al., 2005; Qiu et al., 2016). However, the role of B and T cells in estrogen-deficiency-induced bone loss remains controversial (Masuzawa et al., 1994; Miyaura et al., 1997). Although one study showed that B cells contribute to estrogen-deficiency-induced bone loss via their production of receptor activator of NF-κB ligand (RANKL) (Onal et al., 2012), another study completely depleted mature B cells and showed that these mice lost equal amounts of bone as their B cell replete controls (Li et al., 2007a). Similarly, although some studies show that both T-cell-deficient nude mice and T-cell-depleted wild-type mice fail to respond to ovariectomy with bone loss (8–12), another study using three different T-cell-deficient mouse models showed similar bone loss after ovariectomy as their corresponding controls (Lee et al., 2006). Of those studies that showed a critical role of lymphocytes in estrogen-deficiency-induced bone loss, the increased production of osteoclast stimulating factors such as RANKL, TNF-α, IL-6, and IL-17 by lymphocytes was proposed as a mechanism (Cenci et al., 2000, 2003; Roggia et al., 2001; Takayanagi, 2007). Mechanisms of T-cell-produced factors on osteoblasts, however, remain largely undefined, especially in the context of estrogen deficiency.
Previous evidence indicates that T cells can produce Wnt10b to amplify the osteo-anabolic effects of parathyroid hormone treatment (Bedi et al., 2012; Terauchi et al., 2009). T cells were recently also shown to express Dickkopf-1 (DKK1) (Chae et al., 2017), a potent Wnt inhibitor, which binds to the Wnt co-receptors LDL receptor–related protein 5 and 6 (LRP5/6) and thereby blocks further interactions with Wnt ligands (Bhat et al., 2007; Mao et al., 2001, 2002). By blocking Wnt signaling, DKK1 negatively regulates osteoblast differentiation and function, and it stimulates osteoclastogenesis indirectly via the increased production of RANKL and lower production of osteoprotegerin (OPG) in osteoblasts. Thus, DKK1 is a potent negative regulator of bone mass (Li et al., 2006; Morvan et al., 2006). DKK1 is expressed in various organs (bone, brain, reproductive system, kidney/urogenital tract) and by several cell types within bone (Christodoulides et al., 2006; Li et al., 2006; Pietilä et al., 2011), whereas osterix-expressing osteoprogenitors appear to be the main source of circulating DKK1 levels (Colditz et al., 2018). Interestingly, increased DKK1 expression is observed in various bone-related disorders, including rheumatoid arthritis (Diarra et al., 2007), glucocorticoid-induced osteoporosis (Rauner et al., 2013; Wang et al., 2008), and malignant bone disease (Rachner et al., 2014; Tian et al., 2003). Blocking DKK1 using monoclonal antibodies or genetic approaches protects mice from arthritis- (Diarra et al., 2007), GC-, and myeloma-induced bone loss (Colditz et al., 2019; Pozzi et al., 2013). Even though DKK1 levels are also increased in osteoporotic postmenopausal women (Ahmed et al., 2013), it has remained unclear whether DKK1 mediates bone loss due to estrogen deficiency and if so, whether its expression in T cells also contributes to this effect.
In this study, we generated global, osteoblast, osteocyte, and T-cell-specific Dkk1 knock-out mice to determine if and how DKK1 contributes to estrogen-deficiency-induced bone loss. Although global and T-cell-specific Dkk1 deletion protected mice from estrogen-deficiency-induced bone loss, lack of Dkk1 in the osteogenic lineage did not. Indirect co-culture experiments revealed that activated T cells suppressed osteoblast differentiation via inhibiting Wnt signaling. Importantly, lack of Dkk1 expression in T cells did not only mediate pathological bone loss but also controlled physiological bone remodeling. Thus, our findings underline the critical role of T cells in skeletal health and disease.
Results
Global loss of Dkk1 protects against estrogen-deficiency-induced bone loss
In order to assess the contribution of DKK1 in the pathogenesis of estrogen-deficiency-induced bone loss, we deleted Dkk1 in Dkk1fl/fl;Rosa26-CreERT2 mice with tamoxifen and induced bone loss by OVX. The success of the procedure was confirmed by weighing the uterine, which was significantly atrophied in the OVX groups (data not shown).
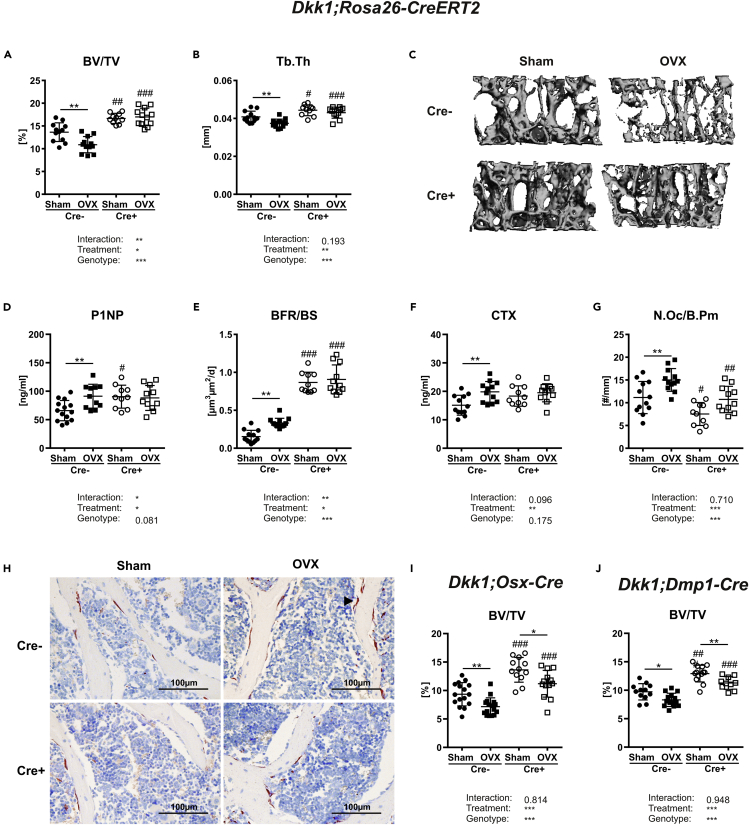
Dkk1-proficient Cre− mice showed a significant reduction of vertebral trabecular bone volume (−20%) and trabecular thickness (−8%) four weeks after OVX, whereas global Dkk1 conditional knock-out (cKO) mice were protected from OVX-induced bone loss (Figures 1A and 1B). Trabecular number and trabecular separation were not significantly altered in both genotypes (Table 1). Representative 3D reconstructions of the fourth vertebrae body are shown in Figure 1C. As DKK1 was recently shown to be upregulated in bone after OVX in rats and in serum from women suffering from postmenopausal osteoporosis (Ahmed et al., 2013; Tian et al., 2015), we analyzed skeletal Dkk1 mRNA levels in our mice. Dkk1 mRNA levels were higher in bone tissue from ovariectomized control mice, whereas Dkk1 was not detected in the bone tissue of cKO mice (Figure S1). The deletion of Dkk1 led to higher skeletal expression of the Wnt target genes Lef1 and Axin2, and both were decreased after OVX in both genotypes (Figure S1).
Figure 1.
Global, but not osteogenic, Dkk1 deletion protects against estrogen-deficiency-induced bone loss
The fourth vertebral body of 14-week-old female Sham operated or ovariectomized (OVX) Dkk1fl/fl;Rosa26-ERT2-Cre and respective Cre-negative control mice were analyzed by μCT.
(A and B) (A) Trabecular bone volume per total volume (BV/TV) and (B) trabecular thickness (Tb.Th) of fourth vertebral body.
(C) Representative 3D reconstruction of the analyzed fourth vertebral body.
(D and E) (D) Serum levels of the Wnt inhibitors dickkopf-1 (DKK1) and (E) bone formation marker procollagen type 1 amino-terminal propeptide (P1NP) were analyzed using commercially available ELISA.
(F) Histomorphometric analysis of calcein double staining was performed to determine the (F) bone formation rate/bone surface (BFR/BS).
(G) Serum level of bone resorption marker (G) carboxy-terminal collagen crosslinks (CTX) was measured using a commercially available ELISA.
(H) The fourth vertebral body was stained with tartrate-resistant acid phosphatase (TRAP) to determine the osteoclast number per bone parameter (N.Oc/B.Pm). Cre− Sham, n = 12; Cre− OVX, n = 12; Cre+ Sham, n = 10; Cre+ OVX, n = 12.
(I) Representative images (20x) of histological sections of fourth vertebral body stained with TRAP (arrow indicates osteoclast). The fourth vertebral body of 14-week-old female Sham operated or ovariectomized Dkk1fl/fl;Osx-Cre (Cre− Sham, n = 16; Cre− OVX, n = 16; Cre+ Sham, n = 12; Cre+ OVX, n = 13) and Dkk1fl/fl;Dmp1-Cre mice (Cre− Sham, n = 13; Cre− OVX, n = 15; Cre+ Sham, n = 13; Cre+ OVX, n = 10), and respective controls were analyzed to assess (J-M) trabecular bone volume per total volume (BV/TV) and serum levels of DKK1. Data represent the mean ± SD. Statistical analysis was performed by the two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus Sham or #p < 0.05, ##p < 0.01, ###p < 0.001 versus respective Cre-negative control.
Table 1.
Bone phenotype of third and fourth vertebral body from 14-week-old female sham versus ovariectomized (OVX) Dkk1fl/fl;Rosa26-CreERT2, Dkk1fl/fl;Osx-Cre and Dkk1fl/fl;Dmp1-Cre mice
| Spine | Cre− Sham | OVX | p value and % change | Cre+ Sham | OVX | p value and % change |
|---|---|---|---|---|---|---|
| Dkk1fl/fl;Rosa26-CreERT2 | n = 12 | n = 12 | n = 10 | n = 12 | ||
| μCT | ||||||
| Tb.N [1/mm] | 3.87 ± 0.35 | 3.61 ± 0.19 | 0.225–7% | 4.38 ± 0.32## | 4.17 ± 0.34### | 0.682–5% |
| Tb.Sp [mm] | 0.25 ± 0.03 | 0.27 ± 0.02 | 0.647 + 8% | 0.24 ± 0.04 | 0.26 ± 0.02 | 0.567 + 8% |
| Histomorphometry | ||||||
| MS/BS [%] | 17.7 ± 4.02 | 20.0 ± 2.75 | 0.787 + 13% | 18.6 ± 1.91 | 20.1 ± 4.51 | >0.999 + 8% |
| MAR [μm/day] | 1.26 ± 0.15 | 1.61 ± 0.25 | <0.05 + 28% | 1.69 ± 0.25## | 1.92 ± 0.34 | 0.351 + 13% |
| N.Ob/B.Pm [#/mm] | 11.9 ± 3.45 | 12.4 ± 3.39 | >0.999 + 4% | 13.1 ± 2.80 | 14.2 ± 4.05 | >0.999 + 8% |
| N.Ot/B.Ar [#/mm2] | 152 ± 49.2 | 184 ± 28.8 | 0.228 + 17% | 252 ± 34.4### | 244 ± 19.8## | >0.999–3% |
| Dkk1fl/fl;Osx-Cre | n = 16 | n = 16 | n = 12 | n = 13 | ||
| μCT | ||||||
| Tb.Th [mm] | 40.4 ± 2.28 | 36.7 ± 2.42 | <0.01–9% | 41.4 ± 3.01 | 38.2 ± 2.31 | <0.05–8% |
| Tb.N [1/mm] | 3.56 ± 0.41 | 3.22 ± 0.27 | <0.05–10% | 4.02 ± 0.24## | 3.67 ± 0.23## | <0.05–9% |
| Tb.Sp [mm] | 0.28 ± 0.03 | 0.31 ± 0.02 | <0.05 + 11% | 0.25 ± 0.02## | 0.27 ± 0.02## | <0.05 + 8% |
| Histomorphometry | ||||||
| MS/BS [%] | 25.2 ± 5.31 | 29.1 ± 4.60 | 0.246 + 15% | 28.2 ± 4.21 | 28.6 ± 3.72 | +1% |
| MAR [μm/day] | 1.49 ± 0.45 | 2.29 ± 0.55 | <0.05 + 54% | 2.44 ± 0.91## | 3.30 ± 0.90## | <0.05 + 35% |
| BFR/BS [μm3/μm2/day] | 0.40 ± 0.16 | 0.75 ± 0.33 | <0.05 + 88% | 0.78 ± 0.38# | 1.15 ± 0.30## | <0.05 + 47% |
| N.Ob/B.Pm [#/mm] | 16.2 ± 3.03 | 17.2 ± 2.56 | >0.999 + 6% | 16.4 ± 2.72 | 16.5 ± 3.54 | >0.999 + 1% |
| N.Oc/B.Pm [#/mm] | 7.06 ± 1.84 | 8.91 ± 1.77 | <0.05 + 26% | 4.39 ± 1.09### | 6.24 ± 1.67### | <0.05 + 42% |
| Dkk1fl/fl;Dmp1-Cre | n = 13 | n = 15 | n = 13 | n = 10 | ||
| μCT | ||||||
| Tb.Th [mm] | 41.0 ± 3.64 | 38.4 ± 2.85 | 0.087–6% | 42.7 ± 2.24 | 39.6 ± 1.70 | 0.066–7% |
| Tb.N [1/mm] | 3.70 ± 0.33 | 3.29 ± 0.32 | <0.05–11% | 4.10 ± 0.32# | 3.71 ± 0.33# | <0.05–10% |
| Tb.Sp [mm] | 0.28 ± 0.02 | 0.31 ± 0.03 | <0.01 + 11% | 0.25 ± 0.02# | 0.28 ± 0.03# | <0.05 + 12% |
| Histomorphometry | ||||||
| MS/BS [%] | 28.9 ± 3.60 | 30.1 ± 2.60 | >0.999 + 4% | 28.9 ± 3.57 | 29.9 ± 2.62 | >0.999 + 3% |
| MAR [μm/day] | 1.39 ± 0.23 | 1.95 ± 0.44 | <0.01 + 40% | 1.96 ± 0.36## | 2.42 ± 0.34# | <0.05 + 23% |
| BFR/BS [μm3/μm2/day] | 0.37 ± 0.17 | 0.62 ± 0.16 | <0.05 + 68% | 0.70 ± 0.24## | 1.03 ± 0.22### | <0.01 + 47% |
| N.Ob/B.Pm [#/mm] | 23.2 ± 3.36 | 23.7 ± 3.06 | >0.999 + 2% | 23.6 ± 4.51 | 24.6 ± 3.58 | >0.999 + 4% |
| N.Oc/B.Pm [#/mm] | 8.02 ± 2.00 | 11.2 ± 2.58 | <0.001 + 40% | 5.90 ± 1.28# | 8.47 ± 1.30## | <0.05 + 44% |
Tb.Th = trabecular thickness, Tb.N = trabecular number, Tb.Sp = trabecular separation, MS/BS = mineralizing surface/bone surface, MAR = mineral apposition rate, BFR/BS = bone formation rate/bone surface, N.Ob/B.Pm = number of osteoblasts, N.Oc/B.Pm = number of osteoclasts/bone perimeter, N.Ot/B.Ar = number of osteocytes/bone area. Data represent the mean ± SD. Statistical analysis was performed using the two-way ANOVA. #p < 0.05, ##p < 0.01, ###p < 0.001 versus respective Cre-negative control.
As a significant proportion of women with postmenopausal osteoporosis show enhanced bone turnover (Eastell et al., 2016), we determined bone turnover after OVX. As expected, OVX increased both bone formation and bone resorption parameters in Cre− wild-type mice. OVX increased P1NP serum levels (+39%), the mineral apposition rate (+28%), and the bone formation rate (+116%) in control mice, whereas these parameters were not altered in global Dkk1 cKO mice (Figures 1D and 1E, Table 1). Interestingly, estrogen withdrawal only enhanced osteoblast activity but not osteoblast and osteocyte numbers (Table 1). In addition, the bone resorption marker CTX, the number of osteoclasts, and Rankl expression in bone was increased only in ovariectomized control mice but were not significantly changed in cKO mice (Figures 1F, 1G, and S1). Opg expression was highly increased in the bones of cKO mice but did not change after OVX in either genotype (Figure S1). Representative images of TRAP-stained bone sections are depicted in Figure 1H. Taken together, Dkk1 plays a critical role in OVX-induced bone loss.
Lack of Dkk1 in osteolineage cells has no influence on estrogen-deficiency-induced bone loss
To determine whether osteoprogenitors or osteocytes contribute to OVX-induced bone loss via their production of DKK1, Dkk1fl/fl;Osx-Cre and Dkk1fl/fl;Dmp1-Cre mice were subjected to OVX. In contrast to Dkk1fl/fl;Rosa26-CreERT2 mice, Dkk1fl/fl;Osx-Cre and Dkk1fl/fl;Dmp1-Cre mice lost similar amounts of bone after OVX as their controls (Figures 1I and 1J). Moreover, all genotypes showed similar alterations in bone microarchitecture (Table 1). Finally, histomorphometric analysis confirmed that Dkk1fl/fl;Osx-Cre and Dkk1fl/fl;Dmp1-Cre mice showed similar alterations in bone turnover after OVX as their wild-type littermate controls (Table 1).
Estrogen deficiency is associated with increased proliferation and activation of T cells
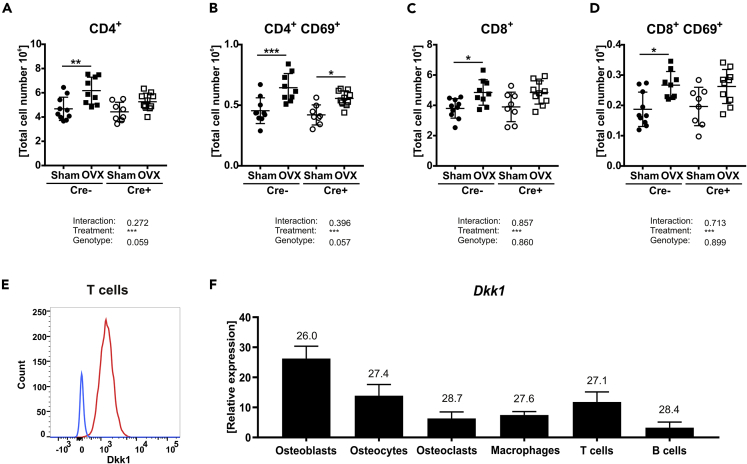
As estrogen deficiency is associated with an increased number of T cells and T cells have been reported to alter bone metabolism via their production of Wnt10b (Pacifici, 2012), we investigated whether T cells also use DKK1 to modulate bone turnover in estrogen deficiency. First, we assessed whether OVX also activates T cells in our model. Flow cytometric analyses revealed an increased number of total splenic T cells in all three mouse lines (ERT2-Cre, Osx-Cre, Dmp1-Cre) after OVX (Table S1). However, this increase was less pronounced in global cKO (Table S1). When we analyzed T lymphocyte subsets, global cKO mice failed to significantly increase CD4+ (T helper cells) and CD8+ (cytotoxic T lymphocyte) T cell numbers after OVX, whereas mice that lacked Dkk1 in osteogenic cells showed similar increase as their Cre− controls (Table S1). Furthermore, OVX led to an activation of CD4+ and to a lesser extent of CD8+ T cells in Cre-negative controls, which was apparent by the increase of the CD69+ cell fraction (Figures 2A–2D and Table S1) (Ziegler et al., 1994). Similar increases were found in Dkk1fl/fl;Osx-Cre and Dkk1fl/fl;Dmp1-Cre mice (Table S1). Surprisingly, global deletion of Dkk1 mitigated the OVX-induced activation of CD4+ and CD8+ T cells (Figures 2B and 2D). Apart from T cells, we also investigated the total number of B cells (CD45R+) in the spleen and myeloid-derived suppressor cells (CD11b+GR1+) in the bone marrow. However, total numbers of both cell populations were unaltered after OVX (Table S1).
Figure 2.
Estrogen-deficiency is associated with an increased number of activated T cells
Absolute numbers of splenic T lymphocyte populations of Sham versus OVX-operated 14-week-old Dkk1fl/fl;Rosa26-CreERT2 and control mice were determined using FACS analysis.
(A–D) (A) T helper cells, (B) activated T helper cells, (C) cytotoxic T cells, and (D) activated cytotoxic T cells were determined by their specific indicated surface markers. Cre− Sham, n = 10; Cre− OVX, n = 9; Cre+ Sham, n = 8; Cre+ OVX, n = 10.
(E) Intracellular DKK1 staining of wild-type-derived splenic T cells was measured using FACS analysis. n = 4 animals per group.
(F) Real-time PCR analysis was performed for Dkk1 mRNA expression in different cell types. n = 5 animals per group. Gene expression levels were normalized to β-Actin. Data represent the mean ± SD. Numbers in graph indicate threshold values at constant β-Actin. Statistical analysis was performed by the two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus Sham (Cre− or Cre+) and #p < 0.05, ##p < 0.01, ###p < 0.001 versus Cre-negative control.
Finally, we examined whether T cells express DKK1. Flow cytometric analysis revealed that wild-type T cells exhibit DKK1 expression (Figure 2E). In addition, qPCR analysis confirmed that T cells express Dkk1, albeit to a lesser degree than osteoblasts (Figure 2F).
T-cell-specific Dkk1 deletion increases bone mass
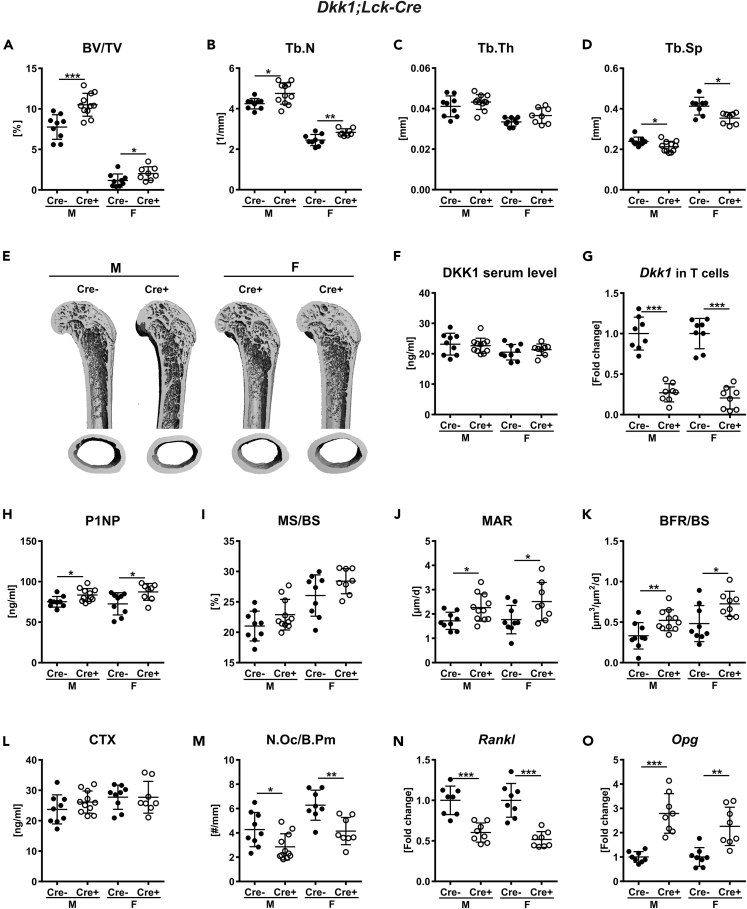
As T cells express DKK1, we next investigated whether T-cell-derived DKK1 can influence bone remodeling. To test this, we generated Dkk1fl/fl;Lck-Cre mice, which express Cre under the control of the Lck (lymphocyte protein tyrosine kinase) promoter, enabling T-cell-specific excision of Dkk1 (Baumann et al., 2005). Recombination of the floxed Dkk1 allele was only found in MACS-purified T cells from Dkk1fl/fl;Lck-Cre mice but not in any other organ investigated (Figure S2A). Dkk1fl/fl;Lck-Cre and Cre− control mice were born at a Mendelian ratio with no abnormalities in size and body weight (Figure S2B). Twelve-week-old male and female Dkk1fl/fl;Lck-Cre mice showed an increased bone volume at the fourth lumbar vertebrae and distal femur (Figure 3A and Table S2). At the structural level, the fourth vertebral body had a higher trabecular number (male: +11%, female: +10%), whereas trabecular thickness was unchanged and trabecular separation was significantly lower (male: −10%, female: −11%) (Figures 3B–3D). T-cell-specific Dkk1 deletion had no effect on the cortical compartment (BMD: male: Cre−: 923 ± 40.4, Cre+: 918 ± 37.8; female: Cre−: 923 ± 37.5, Cre+: 923 ± 44.3). The distal femur showed a similar pattern, and the data are summarized in Table S2. Representative 3D reconstructions of the femur are depicted in Figure 3E. Interestingly, DKK1 serum levels (Figure 3F) as well as mRNA expression in the femora of Dkk1fl/fl;Lck-Cre mice were not altered (bone: male: Cre−: 1.00 ± 0.09, Cre+: 0.97 ± 0.04; female: Cre−: 1.00 ± 0.05, Cre+: 1.07 ± 0.04). However, Dkk1 mRNA expression was reduced by 73%–79% in MACS-purified T cells (Figure 3G).
Figure 3.
Lack of Dkk1 in T cells (Lck-Cre) increases bone volume
The fourth vertebral body of 12-week-old male and female Dkk1fl/fl;Lck-Cre (Cre-positive and Cre-negative) mice were analyzed by μCT.
(A–D) (A) Trabecular bone volume per total volume (BV/TV), (B) trabecular number (Tb.N), (C) trabecular thickness (Tb.Th), and (D) trabecular separation (Tb.Sp) of the fourth vertebral body.
(E) Representative 3D reconstruction of the analyzed fourth vertebral body. Cre− male, n = 9; Cre+ male, n = 11; Cre−female, n = 9; Cre+ female, n = 8.
(F and G) (F) DKK1 Serum levels (Cre− male, n = 9; Cre+ male, n = 11; Cre− female, n = 9; Cre+ female, n = 8) were assessed using a commercially available ELISA, and (G) real-time PCR analysis was performed for Dkk1 mRNA expression in T cells (n = 8 animals per group). Histomorphometric and serum osteoblast and osteoclast parameters of 12-week-old male and female Dkk1fl/fl;Lck-Cre mice and littermate controls were analyzed.
(H–K) (H) Quantification of serum procollagen type 1 amino-terminal propeptide (P1NP) was performed by ELISA. Histomorphometric analysis of calcein double staining was performed to determine the (I) mineralizing surface/bone surface (MS/BS), (J) mineral apposition rate (MAR), and (K) bone formation rate/bone surface (BFR/BS).
(L) Serum carboxy-terminal collagen crosslinks (CTX) were measured using ELISA.
(M) Tartrate-resistant acid phosphatase (TRAP) staining was used to determine the number of osteoclast/bone parameter (N.Oc/B.Pm). Cre− male, n = 9; Cre+ male, n = 11; Cre− female, n = 9; Cre+ female, n = 8.
(N and O) (N) Receptor activator of nuclear factor-κB ligand (Rankl) and (O) osteoprotegerin (Opg) mRNA expression in femoral bone tissue were analyzed using real-time PCR analysis. n = 8 animals per group. Gene expression levels were normalized to β-Actin. Data represent the mean ± SD. Statistical analysis was performed by the Student's t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus Cre-negative control.
We next determined whether the high bone mass in Dkk1fl/fl;Lck-Cre is also due to increased bone formation as previously shown in cKO mice lacking Dkk1 globally or in osteogenic cells (Colditz et al., 2018). Lack of Dkk1 in T cells resulted in higher P1NP serum levels (male: +11%, female: +22%), an increased mineral apposition rate, and a higher bone formation rate at the vertebra in both sexes (Figures 3H–3K). Mineralizing surface was not altered in Dkk1fl/fl;Lck-Cre mice (Figure 3I). The femora of Cre-positive mice showed a similar increase in bone formation parameters (Table S2). Similar to mice lacking Dkk1 globally or in cells of the osteogenic lineage, T-cell-specific Dkk1 deletion had no effect on serum CTX levels, whereas the number of osteoclasts was significantly reduced in the axial and appendicular skeleton (Figures 3L-M, Table S2). However, the reduced number of osteoclasts was associated with a decrease in TRAP5b serum levels (male: Cre−: 9.92 ± 0.21, Cre+: 8.60 ± 0.44; female: Cre−: 9.70 ± 0.32, Cre+: 8.50 ± 0.44). In line with this, Rankl expression was decreased, whereas Opg expression was increased in the bones of Dkk1fl/fl;Lck-Cre mice (Figures 3N-O).
Because T-cell-derived DKK1 has been shown to modify T cell activation (Chae et al., 2017), which may impact on bone remodeling as well, and the Lck-cre has been shown to alter the T cell phenotype (Carow et al., 2016), we investigated the T cell phenotype in Dkk1fl/fl;Lck-Cre mice and Dkk1fl/fl;Rosa26-CreERT2 mice in more detail using FACS analysis (Table S3). Global or T-cell-specific Dkk1 deletion did not alter the cellularity of the thymus and spleen. Furthermore, CD69 expression and the number of major T cell subsets, including Treg cells, which have been shown to express high DKK1 levels (Chae et al., 2017), did not differ in Dkk1fl/fl;Lck-Cre mice. In case of Dkk1fl/fl;Rosa26-CreERT2 mice only the number of double-negative CD4−CD8− cells was significantly reduced. Hence alterations in the T cell phenotype are not the cause of the bone phenotype seen in Dkk1fl/fl;Lck-Cre mice.
Taken together, our data show that T-cell-specific deficiency of Dkk1 contributes to the maintenance of bone remodeling by controlling bone formation and osteoclast numbers in both sexes.
Transfer of wild-type T cells rescues the high bone mass of T-cell-specific Dkk1 cKO mice
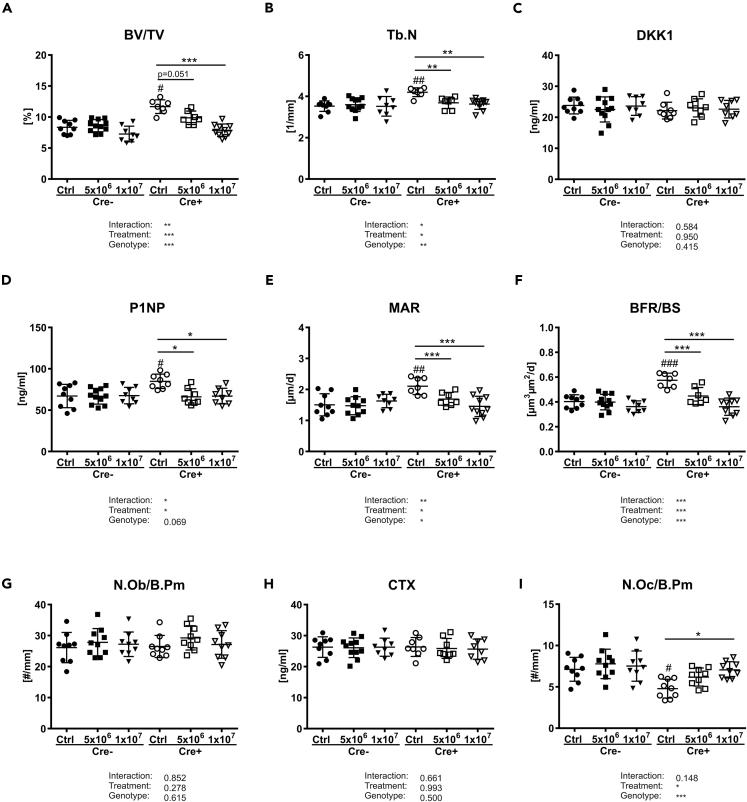
We next investigated whether the changes in bone remodeling in Dkk1fl/fl;Lck-Cre mice can be rescued by transplanting Dkk1-proficient wild-type T cells (Ly5.1 mice) into those mice. Therefore, MACS-purified T cells were transplanted into 9-week-old female Dkk1fl/fl;Lck-Cre mice and their littermate controls. All mice tolerated this procedure and showed no signs of immune response. After 3 weeks, bone parameters were assessed. In Cre− control mice, transplantation did not influence any of the investigated bone parameters, whereas Dkk1fl/fl;Lck-Cre mice, which were injected with 5 × 106 T cells, showed slightly reduced bone volume and trabecular number (Figures 4A and 4B). This effect on bone was even more pronounced when mice were injected with a higher number of T cells, resulting in a significant reduction of bone volume and trabecular number (Figures 4A and 4B). No alterations in DKK1 serum levels were detected after transplantation (Figure 4C). The reduced bone mass in transplanted Dkk1fl/fl;Lck-Cre mice was triggered by reduced bone formation, which is evident by reduced P1NP serum levels and decreased mineral apposition and bone formation rates, whereas number of osteoblasts was not altered (Figures 4D–4G). Even though T cell transfer did not affect CTX serum levels, it increased the number of osteoclasts in Dkk1fl/fl;Lck-Cre reaching similar numbers as their Cre− controls (Figures 4H and 4I). Comparable results were found when T cells were transplanted into male mice (date not shown). Thus, these results indicate an important role of alterations that occur as a result of T-cell-deficiency of DKK1 for physiologic bone remodeling.
Figure 4.
Transplantation of T cells abolishes the positive effect of T-cell-specific Dkk1 deletion on bone
The fourth vertebral bodies of 13-week-old female Dkk1fl/fl;Lck-Cre (Cre-positive and Cre-negative) mice were analyzed 3 weeks after transplantation of wild-type-derived T cells by μCT.
(A and B) (A) Trabecular bone volume per total volume (BV/TV) and (B) trabecular number (Tb.N) of the fourth vertebral body.
(C–F) Quantification of serum (C) DKK1 and (D) procollagen type 1 amino-terminal propeptide (P1NP) was performed by ELISA. Cre− Ctrl, n = 9; Cre− 5x106, n = 11; Cre− 1x107, n = 8; Cre+ Ctrl, n = 8; Cre+ 5x106, n = 8; Cre+ 1x107, n = 9. Histomorphometric analysis of calcein double staining was performed to determine bone mineralizing surface/bone surface (MS/BS) and (F) formation rate/bone surface (BFR/BS). Cre− Ctrl, n = 9; Cre− 5x106, n = 10; Cre− 1x107, n = 8; Cre+ Ctrl, n = 7; Cre+ 5x106, n = 7; Cre+ 1x107, n = 9.
(G) Tartrate-resistant acid phosphatase (TRAP) staining was used to determine the number of osteoblast/bone parameter (N.Ob/B.Pm).
(H and I) (H) Serum carboxy-terminal collagen crosslinks (CTX) were measured using ELISA, and (I) the number of osteoclast/bone parameter (N.Oc/B.Pm) was assessed using TRAP staining. Cre− Ctrl, n = 9; Cre− 5x106, n = 11; Cre− 1x107, n = 8; Cre+ Ctrl, n = 8; Cre+ 5x106, n = 8; Cre+ 1x107, n = 9. Data represent the mean ± SD. Statistical analysis was performed by the two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus untransplanted control (Cre− or Cre+) and #p < 0.05, ##p < 0.01, ###p < 0.001 versus untransplanted Cre− control.
T cells affect osteoblast differentiation depending on their activation status
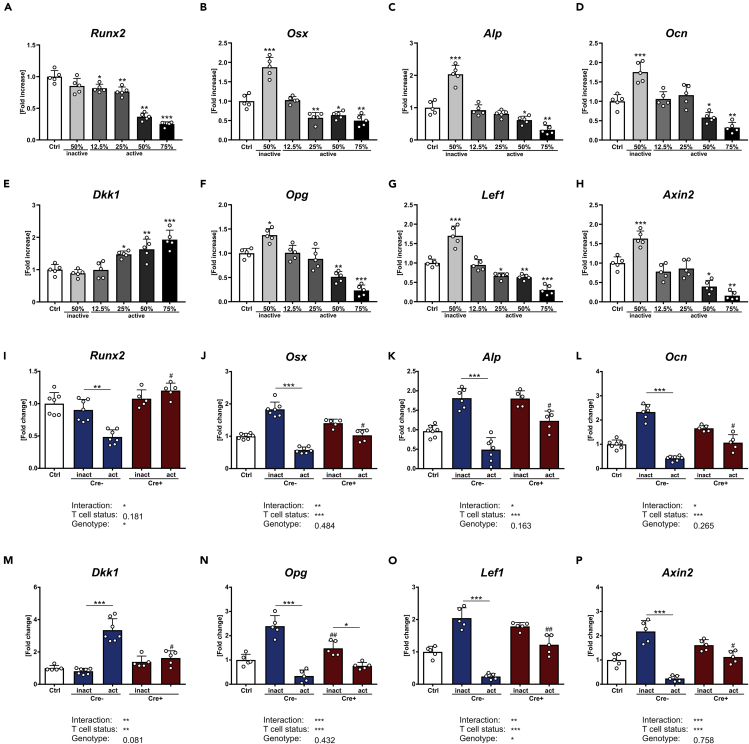
To explore how secreted proteins from T cells influence osteoblast function, conditioned medium (CM) was collected from T cells that were isolated from the spleen of wild-type mice and treated with anti-CD3 (active T cells) or without anti-CD3 (inactive T cells) for 72 h. Afterward CM was used for indirect co-culture experiments with primary osteoblasts. After 7 days of differentiation with different ratios of CM, osteoblasts exposed to CM from inactive T cells had a 2-fold higher expression of Osx, Alp, and Ocn, whereas Runx2 was not altered (Figures 5A–5D). In contrast, CM from activated T cells inhibited gene expression of Runx2, Osx, Alp, and Ocn in a dose-dependent manner, with 75% CM showing the highest inhibition. This suggests that depending on the activation status, T-cell-derived CM can influence osteogenic differentiation.
Figure 5.
T cells influence osteoblast differentiation dependent on their activation status
(A–H) Primary osteoblasts from 12-week-old wild-type mice were differentiated and treated with different percentages of CM from activated or inactivated T cells for a time period of 7 days.
Gene expression analysis of the osteogenic genes (A) runt-related transcription factor 2 (Runx2), (B) osterix (Osx), (C) alkaline phosphatase (Alp), and (D) osteocalcin (Ocn), as well as Dkk1, osteoprotegerin (Opg), lymphoid enhancer-binding factor 1 (Lef1), and axin-related protein 2 (Axin2) from wild-type-derived primary osteoblasts was performed using real-time PCR. Primary osteoblasts from 12-week-old wild-type (Cre-positive and Cre-negative) mice were differentiated and treated with CM from activated or inactivated T cells derived from 12-week-old Dkk1fl/fl;Rosa26-ERT2-Cre mice (Cre-positive: lack Dkk1 expression and Cre-negative: Dkk1-expressing T cells).
(I–P) Gene expression analysis of abovementioned genes was performed using real-time PCR. Gene expression levels were normalized to β-Actin. Data represent the mean ± SD. n = 5–7 individual mice per group. For A–H statistical analysis was performed using Student's t test for inactive versus untreated control and one-way ANOVA for dose dependency. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. For I–P statistical analysis was performed using two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01 versus inactive or #p < 0.05, ##p < 0.01, ###p < 0.001 versus respective Cre-negative control.
As estrogen deficiency is associated with an increased T cell activation, we determined whether the activation status of T cells influences Dkk1 gene expression in osteoblasts. CM from activated T cells increased Dkk1 expression in osteoblasts in a dose-dependent manner (Figure 5E). At the same time, expression levels of the Wnt target genes Opg, Lef1, and Axin2 were reduced (Figures 5F–5H). In line with that finding, CM from activated T cells also suppressed Wnt3a-induced TCF/LEF Wnt promoter activity (Figure S3). CM from inactive T cells led to an upregulation of Wnt target genes in osteoblasts and Wnt promoter activity (Figures 5F–5H, and Figure S3). Interestingly, activation of T cells also increased their own Dkk1 mRNA expression by 2-fold (inactive: 1.00 ± 0.02, active: 2.05 ± 0.42). These results indicate that T cells differentially influence osteoblast differentiation depending on their activation status.
Lack of Dkk1 in T cells diminishes their effect on osteoblast differentiation
To determine whether DKK1 mediates the negative effects of activated T cells on osteoblasts, we collected CM from T cells isolated from Dkk1fl/fl;Rosa26-ERT2-Cre and control mice 3 weeks after tamoxifen injections. Successful deletion of Dkk1 in T cells was confirmed by qPCR of purified T cells (Figure S4). Similar to wild-type-derived CM, osteoblasts that were treated with CM derived from inactive Dkk1-expressing T cells (Cre−) showed an increased expression of osteogenic genes (Osx, Alp, and Ocn), whereas CM from activated T cells had the opposite effect (Figures 5I–5L). However, when Dkk1 was deleted in T cells (Cre+) the positive effect of CM from inactive T cells persisted, whereas the negative effect of activated T cells on osteoblast differentiation was fully abolished (Figures 5I–5L). The same was true for Dkk1, which was only increased in osteoblasts that were treated with CM derived from activated Dkk1-expressing T cells (Figure 5M). Inhibited Wnt signaling, reflected by reduced expression of Opg, Lef1, and Axin2, only occurred when osteoblasts were treated with CM derived from activated Dkk1-expressing T cells, whereas Dkk1 deletion fully abolished this effect (Figures 5N–5P). Interestingly, the induction of Wnt target genes in osteoblasts cultured with CM from inactive T cells appeared blunted when Dkk1 in the CM was absent, even though a significantly increased expression was observed for Lef1 and Axin2. These data suggest that T cells deficient of Dkk1 show less inhibitory effects on osteoblast differentiation than T cells that express DKK1. To examine whether this is a direct effect of secreted DKK1 on osteoblasts we performed co-culture experiments with CM from activated WT T cells and osteoblasts with and without the addition of a DKK1 neutralizing antibody or isotype control. Neutralization of DKK1 in CM from activated T cells did not rescue the downregulation of the osteoblast markers Runx2, Osx, Alp, and Ocn (Figures S5A–5D), implying that other factors that secreted DKK1 produced by activated T cells inhibit osteoblast differentiation.
T-cell-specific deletion of Dkk1 protects against estrogen-deficiency-induced bone loss
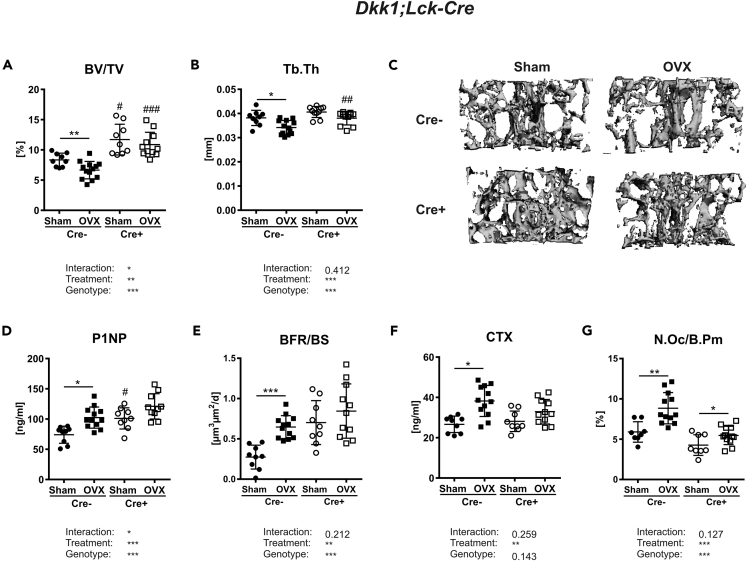
To finally address whether T cells contribute to the pathogenesis of OVX-induced bone loss via their expression of DKK1, we subjected Dkk1fl/fl;Lck-Cre mice to OVX. Estrogen withdrawal resulted in a reduced bone volume (−28%), trabecular number (−12%), and trabecular thickness (−11%) in Cre− controls, whereas Dkk1fl/fl;Lck-Cre mice did not lose a significant amount of bone mass (−7%) (Figures 6A and 6B and Table S4). Trabecular separation was only increased in Cre− control mice after OVX, but not in Cre+ mice (Table S4). Representative 3D reconstructions of the fourth vertebral body are shown in Figure 6C. Loss of DKK1 in T cells further mitigated the OVX-induced activation of CD4+ and CD8+ T cells (Table S5). At the skeletal level, OVX only induced Dkk1 expression in Cre− but not Cre+ mice (Figure S6). Thus, OVX-induced changes in the bone formation parameters P1NP (+38%) and the bone formation rate (+124%) were only detected in Cre− mice, but not in Cre+ mice (Figures 6D and 6E and Table S4). Along those lines, OVX led to a reduced expression of Lef1 and Axin2 in bone only in control mice, whereas Cre+ mice, which already had a higher baseline expression of Lef1 and Axin2, showed no suppression of those markers after OVX (Figure S1E). Furthermore, estrogen withdrawal in control mice resulted in an enhanced CTX serum level and higher number of osteoclasts, whereas these changes were blunted in T-cell-specific Dkk1 cKO mice (Figures 6F and 6G). Although OVX induced Rankl expression in the bone of Cre− mice, its expression was unaltered in Cre+ mice (Figure S6). Opg expression was significantly higher in Cre+ mice but was not changed after OVX (Figure S6). Whether the rescue in Dkk1fl/fl;Lck-Cre mice is directly mediated through T-cell-derived DKK1 or indirectly due to its influence on other cells and factors needs to be examined in more detail in future studies.
Figure 6.
T-cell-specific Dkk1 deletion protects against estrogen-deficiency-induced bone loss
The fourth vertebral body of 14-week-old female Sham operated or ovariectomized (OVX) Dkk1fl/fl;Lck-Cre and Cre-negative control mice were analyzed by μCT.
(A and B) (A) Trabecular bone volume per total volume (BV/TV) and (B) trabecular thickness (Tb.Th) of fourth vertebral body.
(C) Representative 3D reconstruction of the analyzed fourth vertebral body.
(D–F) (D) Serum levels of the Wnt inhibitors dickkopf-1 (DKK1) and (E) bone formation marker procollagen type 1 amino-terminal propeptide (P1NP) were analyzed using commercially available ELISA. Cre− Sham, n = 9; Cre− OVX, n = 12; Cre+ Sham, n = 9; Cre+ OVX, n = 10. Histomorphometric analysis of calcein double staining was performed to determine the (F) bone formation rate/bone surface (BFR/BS).
(G) Serum levels of the bone resorption marker (G) carboxy-terminal collagen crosslinks (CTX) were measured using a commercially available ELISA.
(H) The fourth vertebral body was stained with tartrate-resistant acid phosphatase (TRAP) to determine osteoclast number per bone parameter (N.Oc/B.Pm). Cre− Sham, n = 9; Cre− OVX, n = 13; Cre+ Sham, n = 9; Cre+ OVX, n = 11. Data represent the mean ± SD. Statistical analysis was performed by the two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus Sham or #p < 0.05, ##p < 0.01, ###p < 0.001 versus respective Cre-negative control.
Discussion
Whether and how T cells contribute to estrogen-deficiency-induced bone loss remains an important topic in osteoimmunology. Several studies argue for a role of T cells in ovariectomy-induced bone loss in mice (Gao et al., 2004, 2007; Grassi et al., 2007; Li et al., 2011b; Robbie-Ryan et al., 2006; Roggia et al., 2001), whereas earlier studies failed to confirm this (Anginot et al., 2007; Lee et al., 2006). In this study, we show experimental evidence underlining a role of T cells in estrogen-deficiency-induced bone loss, however, not via the well-known effects of activated T cells on osteoclasts (Cenci et al., 2000, 2003; Roggia et al., 2001; Takayanagi, 2007) but via their inhibition of Wnt signaling in osteoblasts in a paracrine fashion.
By globally deleting DKK1 in adolescent mice, we confirmed previous studies using DKK1 inhibition strategies with monoclonal antibodies or antisense oligonucleotides (Glantschnig et al., 2011; Wang et al., 2007) and show that DKK1 is a key mediator of estrogen-deficiency-induced bone loss. However, osteogenic cells, which appear to be the main producers of circulating DKK1 (Colditz et al., 2018), were not accountable for estrogen-deficiency-induced bone loss via DKK1 production, but T cells. Mechanistically, the protection against ovariectomy-induced bone loss in global Dkk1 cKO mice appears to be linked to the lack of induction of bone formation, rather than inhibition of bone resorption. Even though increased bone resorption is considered one of the key mechanisms of estrogen-deficiency-induced bone loss, an increased bone formation rate, i.e. increased bone turnover, is found in a significant proportion of postmenopausal women with osteoporosis (Garnero et al., 2000; Jabbar et al., 2011; Ross et al., 2000). This high rate of bone turnover was also appreciated in our ovariectomy mouse model. As bone resorption parameters were increased in all ovariectomized mice regardless of genotype, yet, only Cre-negative control mice and osteogenic Dkk1-deficient mice lost a significant amount of bone, these data suggest that DKK1 does not mediate estrogen-deficiency-induced bone loss by increasing bone resorption. This was somewhat surprising considering the well-established link between Wnt signaling in osteoblasts and the subsequent regulation of the RANKL/OPG system and osteoclastogenesis. Several studies have shown that canonical Wnt/β-catenin signaling in osteoblastic cells inhibits osteoclast differentiation by increasing Opg and decreasing Rankl expression in vitro and in vivo (Glass et al., 2005; Holmen et al., 2005; Shin et al., 2005; Spencer, 2006). Intriguingly, global and T-cell-specific loss of DKK1 prevented the ovariectomy-induced increase in the RANKL/OPG ratio in bone but failed to mitigate the increase in osteoclast numbers, suggesting that mechanisms other than the RANKL/OPG system may contribute to enhanced osteoclast formation during estrogen deficiency.
Importantly, estrogen withdrawal increased systemic and even more so local bone formation in control mice, Dkk1fl/fl;Osx-Cre, and Dkk1fl/fl;Dmp1-Cre mice but not in global or T-cell-specific Dkk1 cKO mice, suggesting that limiting the increase of bone formation is the primary mechanism in the prevention of ovariectomy-induced bone loss. This mechanism appears to be mediated via alterations occurring by the deficiency of DKK1 in activated T cells. In fact, co-cultures showed that activated T cells, as occurring during estrogen deficiency, expressed higher amounts of DKK1 and inhibited the expression of osteoblastic markers. This effect was limited to activated T cells, as naive T cells even increased osteoblastic gene expression. Differential effects of T cell subpopulations and/or activation states have also been observed in osteoclasts. Although Th1 and Th17 cells enhance osteoclastogenesis via their production of pro-inflammatory cytokines and RANKL, naive and regulatory T cells suppress osteoclastogenesis (John et al., 1996; Li et al., 2007b; Toraldo et al., 2003). Importantly, the negative effect of activated T cells on osteoblasts was not only found in vitro, but also in vivo, indicated by the high bone formation rate in mice lacking DKK1 specifically in T cells. Taken together, our study suggests that T cells express DKK1, as previously shown for human Foxp3+ Treg cells (Chae et al., 2017), and that T cells can modulate osteoblast activity not only through the production of Wnt10b as previously published (Hardiman et al., 1996; Ouji et al., 2006; Terauchi et al., 2009) but also via effects mediated via DKK1. Our findings are in contradiction to other studies that showed that activated human T cells promote osteoblast differentiation (Croes et al., 2016; Rifas et al., 2003). However, in another study using mouse cells, osteoblast markers were suppressed by activated, but not resting, T cells, which is in line with our data (Young et al., 2005). Although the number of major T cell subsets and the expression of CD69 were not altered in Dkk1fl/fl;Lck-Cre mice, in vitro neutralization of DKK1 had no effect on osteoblast differentiation. Therefore, we cannot rule out whether the effect of DKK1 from T cells on bone comes directly from DKK1 or indirectly through other factors that are changed in Dkk1fl/fl;Lck-Cre mice.
Besides elucidating the mechanisms of how T cells affect osteoblast function and bone mass, this study investigated changes in the immune cell status during estrogen deficiency, another somewhat controversially discussed topic. Our study shows that ovariectomy increases the proportion and activation of CD4+ and CD8+ T cells, whereas B cell numbers are not affected. Several studies, however, have shown that B lymphopoiesis is stimulated during estrogen deficiency (Mansoori et al., 2016; Masuzawa et al., 1994; Onal et al., 2012; Tyagi et al., 2011), whereas estrogen treatment inhibits B cell development (Erlandsson et al., 2003). Moreover, some studies suggest that B cells may contribute to ovariectomy-induced bone loss via their secretion of RANKL and other inflammatory factors (Miyaura et al., 1997). Importantly, most of the studies that found increased B cell numbers after ovariectomy analyzed B cells in the bone marrow, while we assessed the number of B cells in the spleen. In fact, our study is in line with a previous study showing an increased number of B cells in the bone marrow but not in the spleen of ovariectomized mice (Bernardi et al., 2014). Thus, estrogen deficiency may have more profound effects on B cells in the bone microenvironment: However, details about the differential regulation of B cells during estrogen deficiency need to be further delineated.
With regard to T cells, several studies show an increase in T cell numbers and activation status after estrogen withdrawal in mice (Gao et al., 2004, 2007; Grassi et al., 2007; Li et al., 2011a; Robbie-Ryan et al., 2006; Roggia et al., 2001). In addition, menopause has been shown to upregulate T cell activity and increased T cell production of cytokines (Adeel et al., 2013; D'Amelio et al., 2008), whereas hormone therapy decreases osteoclastogenic cytokine production in postmenopausal women (Rogers and Eastell, 2001). As both osteoblasts and osteoclasts have been shown to activate T cells via the secretion of cytokines and by presenting antigens (Li et al., 2010; Pacifici, 2013), these cells may contribute to T cell activation in estrogen deficiency, which is characterized by a state of high bone turnover. Furthermore, the increased T-cell-mediated secretion of TNFα has also been shown to synergize with RANKL, thereby upregulating bone resorption (Cenci et al., 2000). By suppressing the expansion of CD4+ T cells and TNFα production, bone loss is prevented in ovariectomized mice (Zhang et al., 2016), further underlining the importance of T cell activation in bone loss induced by estrogen deficiency. Besides pro-inflammatory cytokines, we now also show that increased expression of DKK1 in T cells after estrogen withdrawal seems to contribute to bone loss via modulating osteoblast function, likely through indirect mechanisms. Nonetheless, additional cell types may be involved in the actions of DKK1 in ovariectomy-induced bone loss, as mice with a T-cell-specific Dkk1 deletion still exhibited increased DKK1 serum levels after ovariectomy and a tendency of reduced bone mass, whereas global Dkk1 deletion fully abolished bone loss.
Finally, our study shows that T cell expansion and activation due to ovariectomy was inhibited in global and T-cell-specific Dkk1 knock-out mice, indicating that DKK1 may be an important autocrine activator of T cells during estrogen deficiency. Indeed, a critical role of the canonical Wnt pathway in T cell biology has been suggested in multiple experimental systems (Ding et al., 2008; Guo et al., 2007; vanLoosdregt et al., 2013; Xie et al., 2005). Moreover, an increasing body of evidence suggests direct immunomodulatory effects of DKK1. As such, recent studies showed that DKK1 is also present in Foxp3+ Treg cells, where it controls effector CD4+ T cell function and is an important regulator of immunological tolerance in colitis (Chae et al., 2017). Further, numerous studies suggest that DKK1 plays a pro-inflammatory role in various diseases including asthma, parasitic or viral infection, and cancer (Chae et al., 2016; D'Amico et al., 2016; Guo et al., 2015; Malladi et al., 2016). It is important to note that besides the classical Wnt ligands and LRP receptors, DKK1 may use different types of receptors to trigger other signaling pathways. Recent studies indicate formally unknown receptors such as cytoskeleton-associated protein-4 to transmit DKK1-mediated signaling events (Kimura et al., 2016; Moon and Gough, 2016). Additional studies are required to unravel how DKK1 promotes T cell activation and proliferation under pathological conditions.
Taken together, our study highlights the importance of T cells in the control of bone mass maintenance and pathological bone remodeling in states of estrogen deficiency and suggest that dysregulations of Wnt signaling may play a critical role therein.
Limitations of the study
When considering these results, it should be noticed that our study is limited by the use of Cre-lines, which are never completely specific, as well as the use of tamoxifen, which has intrinsic effects on bone. However, by using appropriate controls, these limitations are kept at a minimum and effects of tamoxifen were shown to be negligible as Cre-negative controls, which also received tamoxifen, showed reduced bone mass after ovariectomy. Moreover, we did not include a Dkk1+/+;Lck-Cre-positive line as additional control. However, the similar T cell phenotype in Dkk1fl/fl;Rosa26-CreERT2 and Dkk1fl/fl;Lck-Cre mice suggest no major effect of the Lck-Cre on T cells. Also, mice were ovariectomized at a relatively young age.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Martina Rauner (martina.rauner@ukdd.de).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed materials transfer agreement.
Data and code availability
No large datasets were generated within this study. Original data can be made available upon request.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We would like to thank our technicians Tina Dybek and Ina Gloe for their excellent work. This work was supported by grants from the German Research Foundation to TDR, LCH, MR, and ST. MR was supported by the Support-the-Best Initiative of the TUD-funded through the Excellence Initiative of the German Federal and State Governments. ST was supported by the Maria Reiche program of the Medical Faculty of the Technische Universität Dresden. Graphical abstract was created with Biorender.com.
Author contributions
Study Design: JL, ST, and MR. Data collection: JL, ST, and UB. Data analysis: JL, ST, UB, and MR. Data interpretation: JL, ST, UB, TDR, CN, LCH, and MR. Drafting Manuscript: JL, LCH, and MR. Revising and approving final version of manuscript: all authors. MR takes responsibility of the integrity of the data analysis.
Declaration of interests
The authors declare no competing interests.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102224.
Supplemental information
References
- Adeel S., Singh K., Vydareny K.H., Kumari M., Shah E., Weitzmann M.N., Tangpricha V. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J. Invest. Med. 2013;61:1178–1183. doi: 10.231/JIM.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.F., Fouda N., Abbas A.A. Serum dickkopf-1 level in postmenopausal females: correlation with bone mineral density and serum biochemical markers. J. Osteoporos. 2013;2013:e460210. doi: 10.1155/2013/460210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anginot A., Dacquin R., Mazzorana M., Jurdic P. Lymphocytes and the Dap12 adaptor are key regulators of osteoclast activation associated with gonadal failure. PLoS One. 2007;2:e585. doi: 10.1371/journal.pone.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S., Dostert A., Novae N., Bauer A., Schmid W., Fas S.C., Krueger A., Heinzel T., Kirchhoff S., Schütz G. Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer. Blood. 2005;106:617–625. doi: 10.1182/blood-2004-11-4390. [DOI] [PubMed] [Google Scholar]
- Bedi B., Li J.-Y., Tawfeek H., Baek K.-H., Adams J., Vangara S.S., Chang M.-K., Kneissel M., Weitzmann M.N., Pacifici R. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc. Natl. Acad. Sci. U S A. 2012;109:E725–E733. doi: 10.1073/pnas.1120735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A.I., Andersson A., Grahnemo L., Nurkkala-Karlsson M., Ohlsson C., Carlsten H., Islander U. Effects of lasofoxifene and bazedoxifene on B cell development and function. Immunity, Inflamm. Dis. 2014;2:214–225. doi: 10.1002/iid3.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B.M., Allen K.M., Liu W., Graham J., Morales A., Anisowicz A., Lam H.S., McCauley C., Coleburn V., Cain M. Structure-based mutation analysis shows the importance of LRP5 β-propeller 1 in modulating Dkk1-mediated inhibition of Wnt signaling. Gene. 2007;391:103–112. doi: 10.1016/j.gene.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J. Bone Miner. Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Carow B., Gao Y., Coquet J., Reilly M., Rottenberg M.E. Lck -driven cre expression alters T cell development in the thymus and the frequencies and functions of peripheral T cell subsets. J. Immunol. 2016;197:2261–2268. doi: 10.4049/jimmunol.1600827. [DOI] [PubMed] [Google Scholar]
- Cenci S., Weitzmann M.N., Roggia C., Namba N., Novack D., Woodring J., Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J. Clin. Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S., Toraldo G., Weitzmann M.N., Roggia C., Gao Y., Qian W.P., Sierra O., Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc. Natl. Acad. Sci. U S A. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae W.-J.J., Ehrlich A.K., Chan P.Y., Teixeira A.M., Henegariu O., Hao L., Shin J.H., Park J.-H.H., Tang W.H., Kim S.-T.T. The Wnt antagonist Dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity. 2016;44:246–258. doi: 10.1016/j.immuni.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae W.-J.J., Park J.-H.H., Henegariu O., Yilmaz S., Hao L., Bothwell A.L.M.M. Membrane-bound Dickkopf-1 in Foxp3+regulatory T cells suppresses T-cell-mediated autoimmune colitis. Immunology. 2017;152:265–275. doi: 10.1111/imm.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulides C., Laudes M., Cawthorn W.P., Schinner S., Soos M., O’Rahilly S., Sethi J.K., Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J. Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz J., Thiele S., Baschant U., Garbe A.I., Niehrs C., Hofbauer L.C., Rauner M. Osteogenic Dkk1 mediates glucocorticoid-induced, but not arthritis-induced bone loss. J. Bone Miner. Res. 2019;34:1314–1323. doi: 10.1002/jbmr.3702. [DOI] [PubMed] [Google Scholar]
- Colditz J., Thiele S., Baschant U., Niehrs C., Bonewald L.F., Hofbauer L.C., Rauner M. Postnatal skeletal deletion of Dickkopf-1 increases bone formation and bone volume in male and female mice, despite increased sclerostin expression. J. Bone Miner. Res. 2018;33:1698–1707. doi: 10.1002/jbmr.3463. [DOI] [PubMed] [Google Scholar]
- Croes M., Öner F.C., van Neerven D., Sabir E., Kruyt M.C., Blokhuis T.J., Dhert W.J.A., Alblas J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- D’Amelio P., Grimaldi A., Di Bella S., Brianza S.Z.M., Cristofaro M.A., Tamone C., Giribaldi G., Ulliers D., Pescarmona G.P., Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- D’Amico L., Mahajan S., Capietto A.-H., Yang Z., Zamani A., Ricci B., Bumpass D.B., Meyer M., Su X., Wang-Gillam A. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 2016;213:827–840. doi: 10.1084/jem.20150950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M.S., Dwyer D., Korb A., Smolen J., Hoffmann M., Scheinecker C. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Ding Y., Shen S., Lino A.C., Curotto De Lafaille M.A., Lafaille J.J. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- Eastell R., O’Neill T.W., Hofbauer L.C., Langdahl B., Reid I.R., Gold D.T., Cummings S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016;2:e16069. doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- Erlandsson M.C., Jonsson C.A., Islander U., Ohlsson C., Carlsten H. Oestrogen receptor specificity in oestradiol-mediated effects on B lymphopoiesis and immunoglobulin production in male mice. Immunology. 2003;108:346–351. doi: 10.1046/j.1365-2567.2003.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Piemontese M., Liu Y., Thostenson J.D., Xiong J., O’Brien C.A. RANKL (Receptor Activator of NFκB Ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J. Biol. Chem. 2016;291:24838–24850. doi: 10.1074/jbc.M116.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Qian W.-P.W.-P., Dark K., Toraldo G., Lin A.S.P., Guldberg R.E., Flavell R.A., Weitzmann M.N., Pacifici R. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc. Natl. Acad. Sci. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Grassi F., Ryan M.R., Terauchi M., Page K., Yang X., Weitzmann M.N., Pacifici R. IFN-γ stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez M.A., Noguera I., Hermenegildo C., Martínez-Romero A., Tarín J.J., Cano A. Alterations in the phenotype and function of immune cells in ovariectomy-induced osteopenic mice. Hum. Reprod. 2005;21:880–887. doi: 10.1093/humrep/dei413. [DOI] [PubMed] [Google Scholar]
- Garnero P., Sornay-Rendu E., Claustrat B., Delmas P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone Miner. Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- Glantschnig H., Scott K., Hampton R., Wei N., McCracken P., Nantermet P., Zhao J.Z., Vitelli S., Huang L., Haytko P. A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J. Pharmacol. Exp. Ther. 2011;338:568–578. doi: 10.1124/jpet.111.181404. [DOI] [PubMed] [Google Scholar]
- Glass D.A., Bialek P., Ahn J.D., Starbuck M., Patel M.S., Clevers H., Taketo M.M., Long F., McMahon A.P., Lang R.A. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Grassi F., Tell G., Robbie-Ryan M., Gao Y., Terauchi M., Yang X., Romanello M., Jones D.P., Weitzmann M.N., Pacifici R. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc. Natl. Acad. Sci. 2007;104:15087–15092. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Mishra A., Howland E., Zhao C., Shukla D., Weng T., Liu L. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood. 2015;126:2220–2229. doi: 10.1182/blood-2015-02-622233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Dose M., Kovalovsky D., Chang R., O’Neil J., Look A.T., Von Boehmer H., Khazaie K., Gounari F. β-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman G., Albright S., Tsunoda J.I., McClanahan T., Lee F. The mouse Wnt-10B gene isolated from helper T cells is widely expressed and a possible oncogene in BR6 mouse mammary tumorigenesis. Gene. 1996;172:199–205. doi: 10.1016/0378-1119(96)00109-6. [DOI] [PubMed] [Google Scholar]
- Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European union: medical management, epidemiology and economic burden: a report prepared in collaboration with the international osteoporosis foundation (IOF) and the European federation of pharmaceutical industry associations (EFPIA) Arch. Osteoporos. 2013;8:1–115. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen S.L., Zylstra C.R., Mukherjee A., Sigler R.E., Faugere M.C., Bouxsein M.L., Deng L., Clemens T.L., Williams B.O. Essential role of beta-catenin in postnatal bone acquisition. J. Biol. Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Jabbar S., Drury J., Fordham J.N., Datta H.K., Francis R.M., Tuck S.P. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J. Clin. Pathol. 2011;64:354–357. doi: 10.1136/jcp.2010.086595. [DOI] [PubMed] [Google Scholar]
- John V., Hock J.M., Short L.L., Glasebrook A.L., Galvin R.J. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996;137:2457–2463. doi: 10.1210/endo.137.6.8641199. [DOI] [PubMed] [Google Scholar]
- Kimura H., Fumoto K., Shojima K., Nojima S., Osugi Y., Tomihara H., Eguchi H., Shintani Y., Endo H., Inoue M. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J. Clin. Invest. 2016;126:2689–2705. doi: 10.1172/JCI84658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-K.K., Kadono Y., Okada F., Jacquin C., Koczon-Jaremko B., Gronowicz G., Adams D.J., Aguila H.L., Choi Y., Lorenzo J.A. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J. Bone Miner. Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- Li H., Hong S., Qian J., Zheng Y., Yang J., Yi Q. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+and CD8+T cells. Blood. 2010;116:210–217. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Tawfeek H., Bedi B., Yang X., Adams J., Gao K.Y., Zayzafoon M., Weitzmann M.N., Pacifici R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc. Natl. Acad. Sci. U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sarosi I., Cattley R.C., Pretorius J., Asuncion F., Grisanti M., Morony S., Adamu S., Geng Z., Qiu W. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Li X., Grisanti M., Fan W., Asuncion F.J., Tan H.L., Dwyer D., Han C.Y., Yu L., Lee J., Lee E. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J. Bone Miner. Res. 2011;26:2610–2612. doi: 10.1002/jbmr.472. [DOI] [PubMed] [Google Scholar]
- Li Y., Li A., Yang X., Weitzmann M.N. Ovariectomy-induced bone loss occurs independently of B cells. J. Cell. Biochem. 2007;100:1370–1375. doi: 10.1002/jcb.21121. [DOI] [PubMed] [Google Scholar]
- Li Y., Toraldo G., Li A., Yang X., Zhang H., Qian W.P., Weitzmann M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malladi S., MacAlinao D.G., Jin X., He L., Basnet H., Zou Y., De Stanchina E., Massagué J. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoori M.N., Shukla P., Kakaji M., Tyagi A.M., Srivastava K., Shukla M., Dixit M., Kureel J., Gupta S., Singh D. IL-18BP is decreased in osteoporotic women: prevents Inflammasome mediated IL-18 activation and reduces Th17 differentiation. Sci. Rep. 2016;6:33680. doi: 10.1038/srep33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Dellus H., Hoppe D., Stannek P., Walter C. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Masuzawa T., Miyaura C., Onoe Y., Kusano K., Ohta H., Nozawa S., Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J. Clin. Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C., Onoe Y., Inada M., Maki K., Ikuta K., Ito M., Suda T. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc. Natl. Acad. Sci. U S A. 1997;94:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.T., Gough N.R. Beyond canonical: the Wnt and β-catenin story. Sci. Signal. 2016;9:1–2. doi: 10.1126/scisignal.aaf6192. [DOI] [PubMed] [Google Scholar]
- Morvan F., Boulukos K., Clément-Lacroix P., Roman S.R., Suc-Royer I., Vayssière B., Ammann P., Martin P., Pinho S., Pognonec P. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- Onal M., Xiong J., Chen X., Thostenson J.D., Almeida M., Manolagas S.C., O’Brien C.A. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J. Biol. Chem. 2012;287:29851–29860. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouji Y., Yoshikawa M., Shiroi A., Ishizaka S. Wnt-10b secreted from lymphocytes promotes differentiation of skin epithelial cells. Biochem. Biophys. Res. Commun. 2006;342:1063–1069. doi: 10.1016/j.bbrc.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Pacifici R. Role of T cells in ovariectomy induced bone loss- revisited. J. Bone Miner. Res. 2012;27:231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- Pacifici R. Osteoimmunology and its implications for transplantation. Am. J. Transpl. 2013;13:2245–2254. doi: 10.1111/ajt.12380. [DOI] [PubMed] [Google Scholar]
- Pietilä I., Ellwanger K., Railo A., Jokela T., Barrantes I.del B., Shan J., Niehrs C., Vainio S.J. Secreted Wnt antagonist Dickkopf-1 controls kidney papilla development coordinated by Wnt-7b signalling. Dev. Biol. 2011;353:50–60. doi: 10.1016/j.ydbio.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Pozzi S., Fulciniti M., Yan H., Vallet S., Eda H., Patel K., Santo L., Cirstea D., Hideshima T., Schirtzinge L. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53:487–496. doi: 10.1016/j.bone.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Gui Y., Zhang N., Xu Y., Li D., Wang L. Effects of Bu-Shen-Ning-Xin Decoction on immune cells of the spleen and bone marrow in ovariectomized mice. Biosci. Trends. 2016;10:400–409. doi: 10.5582/bst.2016.01012. [DOI] [PubMed] [Google Scholar]
- Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner T.D., Göbel A., Thiele S., Rauner M., Benad-Mehner P., Hadji P., Bauer T., Muders M.H., Baretton G.B., Jakob F. Dickkopf-1 is regulated by the mevalonate pathway in breast cancer. Breast Cancer Res. 2014;16:R20. doi: 10.1186/bcr3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner M., Thiele S., Sinningen K., Winzer M., Salbach-Hirsch J., Gloe I., Peschke K., Haegeman G., Tuckermann J.P., Hofbauer L.C. Effects of the selective glucocorticoid receptor modulator compound A on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology. 2013;154:3719–3728. doi: 10.1210/en.2012-2221. [DOI] [PubMed] [Google Scholar]
- Rifas L., Arackal S., Weitzmann M.N. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J. Cell. Biochem. 2003;88:650–659. doi: 10.1002/jcb.10436. [DOI] [PubMed] [Google Scholar]
- Robbie-Ryan M., Pacifici R., Weitzmann M.N. IL-7 drives T cell-mediated bone loss following ovariectomy. Ann. N. Y. Acad. Sci. 2006;1068:348–351. doi: 10.1196/annals.1346.051. [DOI] [PubMed] [Google Scholar]
- Rogers A., Eastell R. The effect of 17β-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–34. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- Roggia C., Gao Y., Cenci S., Weitzmann M.N., Toraldo G., Isaia G., Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc. Natl. Acad. Sci. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P.D., Kress B.C., Parson R.E., Wasnich R.D., Armour K.A., Mizrahi I.A. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos. Int. 2000;11:76–82. doi: 10.1007/s001980050009. [DOI] [PubMed] [Google Scholar]
- Shin C.S., Her S.J., Kim J.G.J.A., Kim D.H., Kim S.W.S.Y., Kim S.W.S.Y., Kim H.S., Park K.H., Kim J.G.J.A., Kitazawa R. Dominant negative N-cadherin inhibits osteoclast differentiation by interfering with β-catenin regulation of RANKL, independent of cell-cell adhesion. J. Bone Miner. Res. 2005;20:2200–2212. doi: 10.1359/JBMR.050809. [DOI] [PubMed] [Google Scholar]
- Spencer G.J. Wnt signalling in osteoblasts regulates expression of the receptor activator of NF B ligand and inhibits osteoclastogenesis in vitro. J. Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- van Staa T.P., Dennison E.M., Leufkens H.G.G.M., Cooper C., Baron J., Karagas M., Barrett J., Kniffin W., Malenka D., Mayor M. Epidemiology of fractures in england and wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. 2007. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. [DOI] [PubMed] [Google Scholar]
- Terauchi M., Li J.Y., Bedi B., Baek K.H., Tawfeek H., Galley S., Gilbert L., Nanes M.S., Zayzafoon M., Guldberg R. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10:229–240. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E., Zhan F., Walker R., Rasmussen E., Ma Y., Barlogie B., Shaugnessey J.D. The role of the wnt-signalling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2438–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Tian J., Xu X.J., Shen L., Yang Y.P., Zhu R., Shuai B., Zhu X.W., Li C.G., Ma C., Lv L. Association of serum Dkk-1 levels with β-catenin in patients with postmenopausal osteoporosis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2015;35:212–218. doi: 10.1007/s11596-015-1413-6. [DOI] [PubMed] [Google Scholar]
- Toraldo G., Roggia C., Qian W.-P., Pacifici R., Weitzmann M.N. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc. Natl. Acad. Sci. U S A. 2003;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A.M., Srivastava K., Sharan K., Yadav D., Maurya R., Singh D. Daidzein prevents the increase in CD4+CD28null T cells and B lymphopoesis in ovariectomized mice: a key mechanism for anti-osteoclastogenic effect. PLoS One. 2011;6:e21216. doi: 10.1371/journal.pone.0021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services Bone health and osteoporosis: a report of the Surgeon General. Off. Surg. Gen. 2004:1–404. [PubMed] [Google Scholar]
- vanLoosdregt J., Fleskens V., Tiemessen M.M., Mokry M., VanBoxtel R., Meerding J., Pals C.E.G.M., Kurek D., Baert M.R.M., Delemarre E.M. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39:298–310. doi: 10.1016/j.immuni.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Wang F.S., Ko J.Y., Lin C.L., Wu H.L., Ke H.J., Tai P.J. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone. 2007;40:485–492. doi: 10.1016/j.bone.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wang F.S., Ko J.Y., Yeh D.W., Ke H.C., Wu H.L. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology. 2008;149:1793–1801. doi: 10.1210/en.2007-0910. [DOI] [PubMed] [Google Scholar]
- Xie H., Huang Z., Sadim M.S., Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J. Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- Young N., Mikhalkevich N., Yan Y., Chen D., Zheng W. Differential regulation of osteoblast activity by Th cell subsets mediated by parathyroid hormone and IFN-γ. J. Immunol. 2005;175:8287–8295. doi: 10.4049/jimmunol.175.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Gui Y., Qiu X., Tang W., Li L., Gober H.-J., Li D., Wang L. DHEA prevents bone loss by suppressing the expansion of CD4(+) T cells and TNFa production in the OVX-mouse model for postmenopausal osteoporosis. Biosci. Trends. 2016;10:277–287. doi: 10.5582/bst.2016.01081. [DOI] [PubMed] [Google Scholar]
- Ziegler S.F., Ramsdell F., Alderson M.R. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets were generated within this study. Original data can be made available upon request.