Abstract
Background
Sympathetic activation is a primary mechanism mediating increased blood pressure (BP) in obstructive sleep apnea (OSA). However, the relationships between overweight/obesity, sympathetic activation and BP in OSA are not well understood. We hypothesized that increased sympathetic drive is associated with increased BP in normal weight, but not in overweight/obese males with OSA. We therefore examined the effects of body mass index (BMI) on the association between sympathetic activation and BP in males with OSA.
Methods
We studied 115 males with OSA recruited consecutively from clinic. Twenty-four-hour urinary norepinephrine was used to assess sympathetic activation. Blood pressure was measured both in the evening and in the morning. Hypertension was defined based on either BP measurements or an existing diagnosis. Linear and logistic regressions were conducted to examine the associations between sympathetic activation and both BP and risk of hypertension.
Results
We found 24-hour urinary norepinephrine levels were associated with systolic and diastolic BP (SBP, β=0.157, p=0.082; DBP, β=0.212, p=0.023) and mean arterial pressure (MAP, β=0.198, p=0.032) after adjusting for confounders. Interestingly, these associations were modified by overweight/obesity. After adjusting for confounders, increased 24-hour urinary norepinephrine levels were significantly associated with elevated SBP (β=0.454, p=0.012), DBP (β=0.399, p=0.041), and MAP (β=0.432, p=0.023) in normal weight, but not in overweight/obese patients (all p>0.2). Similar findings were observed in the associations between 24-hour urinary norepinephrine levels and hypertension.
Conclusion
Sympathetic activation is associated with elevated BP in normal weight but not in overweight/obese males with OSA, suggesting that BMI may moderate the association between sympathetic activation and BP in males with OSA.
Keywords: obesity, overweight, sympathetic activation, blood pressure, hypertension, obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is highly prevalent in the general population, affecting approximately 20–30% of males and 10–15% of females.1 The association of OSA with cardiovascular comorbidities, such as hypertension, is well established. Approximately 50% of patients with OSA are hypertensive2 and a dose–response relationship between OSA severity and the prevalence of hypertension has been observed.3
Sympathetic activation, independent of obesity,4 has been reported as the primary mechanisms for acute increases in blood pressure in patients with OSA.5 Increased sympathetic outflow, measured as muscle sympathetic nerve activity (MSNA) and urinary and plasma catecholamines, may also contribute importantly to longer term increases in blood pressure. Increases in both nocturnal (apneic)5 and diurnal (non-apneic) sympathetic activity6 are evident in patients with OSA. Acute sympathetic activation during apneic sleep leads to blood pressure surges at the end of apneic events5 and chronic persistent sympathetic activation during normoxic wakefulness is accompanied by sustained increases in blood pressure and in likelihood of hypertension.6 Conversely, heightened nocturnal and diurnal sympathetic activation, as well as increased blood pressure, were attenuated by treatment of OSA using nasal continuous positive airway pressure (CPAP).5
Obesity and sympathetic activation are closely linked and both conditions are associated with increased risk for hypertension.3,5,7,8 However, previous findings have suggested that sympathetic activity differs in normal weight and obese essential hypertension patients.7,9,10 In normal weight essential hypertension, the total spillover of norepinephrine to plasma is increased and higher than in obesity hypertension.7,9 Furthermore, it has been reported that body mass index (BMI) moderated the association between urinary norepinephrine and blood pressure in women without OSA.11 Weber et al reported that the cardiovascular characteristics of lean hypertensive patients were more dependent on catecholamines compared to obese patients.10 Taken together, it appears that obese hypertensive patients might be spared the toxic effects of sympathetic activation. OSA and obesity are highly associated1 and sympathetic activation has been proposed as an underlying mechanism for hypertension in OSA.5 However, whether heightened sympathetic activity has more striking pressor consequences in normal weight versus overweight/obese patients with OSA remains unknown.
We therefore sought to examine the effects of BMI on the association between sympathetic activation and blood pressure in males with OSA. Specifically, we hypothesized that increased sympathetic drive is associated with increased blood pressure in normal weight, but not in overweight/obese males with OSA.
Materials and Methods
Subjects
We studied 115 male adults (age≥18 years) who had a diagnosis of OSA. The subjects were consecutively recruited from the Sleep Disorders Clinic. All subjects were interviewed following a comprehensive questionnaire, which provided a history of sleep complaints, general health, and medication use. Obstructive sleep apnea was defined as an apnea-hypopnea index (AHI) ≥5 events/hour based on an overnight polysomnography (PSG) recording.12 Exclusion criteria included a history of type 2 diabetes mellitus, coronary artery disease, other sleep disorders (ie, insomnia, narcolepsy, periodic limb movement disorder, and circadian disorder), and/or psychiatric disorders (ie, major depression, panic disorder, generalized anxiety disorder or substance abuse), and use of medications or supplements that may affect sympathetic activity (ie, steroids, serotonin-norepinephrine reuptake inhibitors and amphetamines, etc.). Patients were also excluded if they had previously received treatment of OSA such as CPAP and oral appliances. Because this study did not involve any potential harmful examination or intervention, a verbal informed consent was conducted instead of in a written form. Prior to the enrollment, a verbal informed consent was obtained from each participant and the information was documented in each patient’s medical record by researchers. The study and verbal informed consent were approved by the Institutional Review Board of Shantou University Mental Health Center (SUMHC 20190604). This study complied with the Declaration of Helsinki.
Power calculation revealed that with a sample size of 29 normal weight and 86 overweight/obese patients, there was 72% statistical power to reject the null hypothesis and detect a significant difference of regression coefficients of 24-hour urinary norepinephrine on blood pressure between normal weight and overweight/obese patients with alpha set at 0.05 (PASS 15.0.5, NCSS, Kaysville, Utah).
Sleep Laboratory
All potential participants were evaluated for an overnight PSG in the sleep laboratory in sound-attenuated, light- and temperature-controlled rooms. During this evaluation, subjects were allowed to sleep ad libitum based on their habitual sleep time, with the recording time ranging from 22:00–23:00 to 6:00–7:00. Subjects were continuously monitored with 16-channel polygraphs, including the electroencephalogram, electrooculogram, electromyogram, and electrocardiogram. All polysomnography measures were analyzed and scored according to the international criteria of the American Academy of Sleep Medicine.13 Mild, moderate and severe OSAs were defined based on AHI=5–14.9/hour, 15–29.9/hour and ≥30/hour, respectively.12 Body mass index was calculated as weight (kg) divided by the square of height (m), which were measured by a nurse as part of the physical examination. Overweight/obesity was defined as BMI≥25 kg/m2 and normal weight was defined as BMI=18.5–24.9 kg/m2.14 Epworth Sleepiness Scale (ESS) was used to assess daytime sleepiness.15
Twenty-Four-Hour Urinary Norepinephrine
Twenty-four-hour urinary norepinephrine was used to assess sympathetic activity. From 3 days prior to urine collection till its completion, participants were instructed to avoid vigorous exercise, smoking and certain foods (ie, coffee, tea, chocolate, bananas) which could affect norepinephrine levels. On the day of PSG recording, participants were asked to collect complete 24-hour urine specimens. Urine samples were collected in polyethylene bottles containing 25 mL of 0.6 mol/L hydrochloric acid. After the total volume of 24-hour urine collection was measured, 20-mL aliquots were frozen at −80°C until being assayed. Urine volume and creatinine were measured to confirm the completeness of each collection. To exclude demonstrable inaccuracy of norepinephrine measures, samples were excluded if their 24-hour urine volume was <400 mL. Urinary norepinephrine was measured by radioimmunoassay.16 Higher levels of 24-hour urinary norepinephrine indicate higher levels of sympathetic activity during a 24-hour period. The sensitivity for 24-hour urinary norepinephrine was ±1.1 ug.
Blood Pressure Measurement
Blood pressure was measured at 2 time points: in the evening prior to the PSG recording (20:00 to 21:00) and in the morning right after the end of PSG recording (6:00 to 7:00). After at least 10 minutes of rest in the supine position, blood pressure was recorded as the average of 3 consecutive readings during a 5-minute period. An electronic sphygmomanometer (Omron, HEM-7136) with the accuracy of ±3 mm Hg, which was checked against a mercury sphygmomanometer at least once a month, was used for blood pressure measurements. Mean arterial pressure (MAP) was calculated as (systolic blood pressure [SBP] + 2* diastolic blood pressure [DBP])/3.17 The mean values of SBP, DBP, and MAP were calculated as (evening BP values + morning BP values)/2 and were used for statistical analyses. Hypertension was defined as: 1) the mean value of evening and morning DBP ≥90 mm Hg or the mean value of evening and morning SBP ≥140 mm Hg,18 or 2) a self-report of history of a hypertension diagnosis by a physician or using antihypertensive medication.
Statistical Analysis
Data are presented as the mean±standard deviation for continuous variables and percentage for categorical variables. Bivariate comparisons between groups were performed using independent t-test or Mann–Whitney U-test for normally and non-normally distributed continuous variables, respectively, or using χ2 test for categorical variables.
In order to examine the association between 24-hour urinary norepinephrine and blood pressure, we conducted 3 linear regression models with 24-hour urinary norepinephrine values as predictors and mean values of SBP, DBP and MAP at evening and morning as outcomes, respectively. Given that there are clear associations between blood pressures and age, overweight/obesity, and use of antihypertensive medications, age, overweight/obesity, and use of antihypertensive medications were always entered in the models as covariates using a forced entry method, while other relevant demographic, nighttime sleep and daytime sleepiness covariates (p-value of the comparison between subjects with and without hypertension ≤0.1, Table 1) were entered using a backward conditional method. As the severity of OSA may affect the association between 24-hour urinary norepinephrine levels and blood pressure, we further adjusted for AHI as sensitivity analyses. Due to the strong correlation between AHI and minimum oxygen saturation (min SaO2, r=−0.604, p<0.001), we did not include AHI and min SaO2 in regression models simultaneously. Thus, we first adjusted min SaO2 and then conducted sensitivity analyses with AHI as a covariate in regression models to control for the severity of OSA. To assess the potential BMI effects on the association between 24-hour norepinephrine levels and blood pressure in OSA, we further conducted linear regression models to examine these associations in normal weight (BMI<25 kg/m2) and overweight/obese patients with OSA (BMI≥25 kg/m2) after adjusting for potential confounders, respectively. Logistic regression models with 24-hour urinary norepinephrine levels as predictors and hypertension as outcome were provided as secondary analyses to examine BMI effects on the association between 24-hour urinary norepinephrine levels and hypertension in normal weight and overweight/obese patients with OSA, respectively. Similar to linear regression models, age was always entered in these models as a covariate using a forced entry method, while other relevant demographic, nighttime sleep and daytime sleepiness covariates (p-value of the comparison between subjects with and without hypertension ≤0.1, Table 1) were entered in the models using a backward conditional method. Furthermore, binary correlation analyses were conducted to examine the correlations between 24-hour urinary norepinephrine levels and sleep parameters, including sleep onset latency, total sleep time, sleep efficiency, wake time after sleep onset, percentages of non-rapid eye movement (NREM) sleep stage 1, stage 2, stage 3, and rapid eye movement (REM) sleep, AHI, and min SaO2. The level of p<0.05 was used to determine statistical significance. All analyses were conducted using SPSS 23.0 (IBM Corp., Armonk, NY).
Table 1.
Demographic, Clinical and Sleep Characteristics of Study Sample
| Overall N=115 | Normal-Weight N=29 | Overweight/Obesity N=86 | p | No Hypertension N=49 | Hypertension N=66 | p | |
|---|---|---|---|---|---|---|---|
| Age (year) | 43.72±8.80 | 43.38±8.89 | 43.84±8.82 | 0.810 | 41.79±7.96 | 46.33±9.29 | 0.006 |
| BMI (kg/m2) | 27.32±3.06 | 23.46±1.36 | 28.62±2.26 | <0.001 | 27.27±3.22 | 27.38±2.84 | 0.845 |
| SBP (mm Hg) | 126.39±15.27 | 124.23±17.68 | 127.11±14.40 | 0.560 | 117.88±9.12 | 138.10±14.27 | <0.001 |
| DBP (mm Hg) | 84.10±11.74 | 81.17±13.21 | 85.10±11.10 | 0.130 | 77.83±7.01 | 92.72±11.50 | <0.001 |
| MAP (mm Hg) | 98.20±12.44 | 95.55±14.32 | 99.10±11.69 | 0.221 | 91.18±7.13 | 107.85±11.74 | <0.001 |
| NM-SBP (mm Hg) * | 124.47±13.93 | 122.29±16.74 | 125.22±12.87 | 0.618 | 117.88±9.12 | 140.02±10.53 | <0.001 |
| NM-DBP (mm Hg) * | 83.28±11.09 | 79.96±11.94 | 84.42±10.64 | 0.163 | 77.83±7.01 | 96.13±8.87 | <0.001 |
| NM-MAP (mm Hg) * | 91.01±11.61 | 94.07±13.16 | 98.02±10.95 | 0.261 | 91.18±7.13 | 110.76±7.92 | <0.001 |
| Hypertension (n, %) | 66 (57.3) | 16 (55.2) | 50 (58.1) | 0.780 | 0 (0) | 66 (100) | <0.001 |
| Smoke (n, %) | 64 (55.7) | 18 (62.1) | 46 (53.5) | 0.421 | 28 (57.1) | 36 (54.5) | 0.782 |
| Sleep onset latency (min) | 12.56±14.38 | 12.95±10.51 | 12.43±15.52 | 0.146 | 12.81±15.60 | 12.22±12.72 | 0.830 |
| Total sleep time (min) | 436.48±61.62 | 425.10±51.68 | 440.32±64.44 | 0.252 | 439.17±57.07 | 432.86±67.70 | 0.589 |
| Sleep efficiency (%) | 85.26±9.84 | 82.81±8.15 | 86.09±10.25 | 0.017 | 85.67±8.97 | 84.72±10.97 | 0.611 |
| NREM sleep stage 1 (%) | 44.14±21.09 | 42.87±19.70 | 44.57±21.64 | 0.807 | 41.94±20.73 | 47.11±21.63 | 0.209 |
| NREM sleep stage 2 (%) | 35.18±17.26 | 36.37±16.54 | 34.78±17.57 | 0.668 | 37.69±17.36 | 31.79±16.70 | 0.054 |
| NREM sleep stage 3 (%) | 5.33±5.70 | 5.69±5.51 | 5.21±5.80 | 0.624 | 5.50±5.79 | 5.11±5.63 | 0.582 |
| REM sleep (%) | 15.34±6.13 | 15.05±5.99 | 15.44±6.20 | 0.770 | 14.86±6.01 | 15.98±6.29 | 0.568 |
| Wake time after sleep onset (min) | 63.29±48.96 | 75.53±40.86 | 59.16±50.96 | 0.016 | 61.32±46.69 | 65.94±52.24 | 0.619 |
| AHI (event/hour) | 56.82±23.93 | 48.71±23.35 | 59.56±23.64 | 0.019 | 55.29±24.09 | 58.88±23.81 | 0.428 |
| Minimum SaO2 (%) | 63.00±18.66 | 68.10±19.61 | 61.28±18.13 | 0.025 | 66.47±15.32 | 58.33±21.69 | 0.077 |
| ESS scores | 9.72±5.42 | 7.31±4.90 | 10.53±5.37 | 0.007 | 8.76±5.05 | 11.02±5.67 | 0.026 |
| 24-hour urinary norepinephrine (ug/day) | 50.02±26.24 | 43.77±28.99 | 52.13±25.08 | 0.024 | 46.46±22.20 | 54.81±30.45 | 0.127 |
Notes: Data are presented as mean ±standard deviation for continuous variables, and sample size (percentage) for categorical variables. *There are 94 subjects not on antihypertensive medication, including 70 normal-weight and 24 overweight/obese patients, and 66 non-hypertensive and 28 hypertensive patients. Overweight/obesity was defined as a body mass index≥25 kg/m2.
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; MAP, mean arterial pressure; NM-DBP, diastolic blood pressure in subjects not on antihypertensive medication; NM-SBP, systolic blood pressure in subjects not on antihypertensive medication; NM-MAP, mean arterial pressure in subjects not on antihypertensive medication; NREM, non-rapid eye movement; REM, rapid eye movement; SaO2, oxygen saturation; SBP, systolic blood pressure.
Results
A total of 115 male patients with OSA with a mean age of 43.72 ± 8.80 years (range: 21 to 64) and mean BMI of 27.32 ± 3.06 kg/m2 (range: 18.52 to 36.23) were included. Twenty-nine patients were normal-weight and 86 patients were overweight/obese. Of these, 7 patients had mild, 16 patients had moderate, and 94 patients had severe OSA, respectively. Demographic, clinical, sleep, and 24-hour urinary norepinephrine measures of the whole study sample, as well as the overweight/obese and hypertension subgroups, are presented in Table 1.
In linear regression models, increased levels of 24-hour urinary norepinephrine were significantly associated with higher DBP (β=0.212, p=0.023) and MAP (β=0.198, p=0.032) after adjusting for age, antihypertensive medication use, overweight/obesity, min SaO2, percentage of NREM sleep stage 2 and total scores of ESS (Table 2). The association between 24-hour urinary norepinephrine and SBP was marginally significant (β=0.157, p=0.082, Table 2). Sensitivity analyses showed that findings were similar and in the same direction after adjusting AHI instead of min SaO2.
Table 2.
Association Between 24-Hour Norepinephrine Levels and Blood Pressure in Males with Obstructive Sleep Apnea
| Overall* | Normal-Weight† | Overweight/Obesity† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | β | p | Interaction p‡ | B | β | p | B | β | p | |
| SBP (mm Hg) | ||||||||||
| 24-hour urinary norepinephrine (ug) | 0.093 | 0.157 | 0.082 | 0.009 | 0.277 | 0.454 | 0.012 | 0.007 | 0.012 | 0.909 |
| DBP (mm Hg) | ||||||||||
| 24-hour urinary norepinephrine (ug) | 0.096 | 0.212 | 0.023 | 0.140 | 0.182 | 0.399 | 0.041 | 0.060 | 0.133 | 0.223 |
| MAP (mm Hg) | ||||||||||
| 24-hour urinary norepinephrine (ug) | 0.095 | 0.198 | 0.032 | 0.046 | 0.213 | 0.432 | 0.023 | 0.042 | 0.089 | 0.407 |
Notes: *The linear regression models in overall samples were adjusted for age, overweight/obesity, antihypertensive medications use, minimum oxygen saturation, Epworth Sleepiness Scale scores, and percentage of non-rapid eye movement sleep stage 2. †The linear regression models in normal weight and overweight patients with OSA were adjusted for age, antihypertensive medications use, minimum oxygen saturation, Epworth Sleepiness Scale scores, and percentage of non-rapid eye movement sleep stage 2. ‡The p values of interaction between 24-hour urinary norepinephrine and overweight/obesity. Overweight/obesity was defined as a body mass index≥25 kg/m2.
Abbreviations: B, unstandardized coefficients; β, standardized coefficients; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
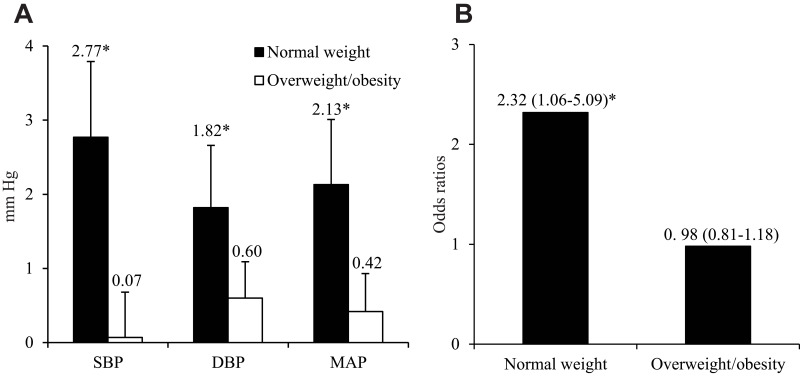
We further examined the effects of BMI on the association between 24-hour urinary norepinephrine and blood pressure. Interestingly, we found that the association between 24-hour urinary norepinephrine and blood pressure was modified by overweight/obesity. Interactions between 24-hour urinary norepinephrine and overweight/obesity on SBP (interaction-p=0.009) and MAP (interaction-p=0.046), as well as DBP (interaction-p=0.140) were significant and marginally significant, respectively. As showed in Table 2, after adjusting for age, antihypertensive medication use, min SaO2, percentage of NREM sleep stage 2, and total scores of ESS, increased 24-hour urinary norepinephrine was significantly associated with higher SBP (β=0.454, p=0.012), DBP (β=0.399, p=0.041), and MAP (β=0.432, p=0.023) in normal-weight male patients with OSA, but not in overweight/obese patients (all p>0.2). As shown in Figure 1A, each 10 ug rise in 24-hour urinary norepinephrine was associated with 2.77 and 0.07 mm Hg rise in SBP (p=0.009), 1.82 and 0.60 mm Hg rise in DBP (p=0.140), and 2.13 and 0.42 mm Hg rise in MAP (p=0.046) in normal-weight and overweight/obese male patients with OSA, respectively. Sensitivity analyses showed that findings were similar and in the same direction after adjusting AHI instead of min SaO2.
Figure 1.
(A) Blood pressure increase for each 10 ug rise in 24-hour urinary norepinephrine levels in normal-weight and overweight/obese male patients with obstructive sleep apnea. (B) Odds ratios of hypertension for each 10 ug rise in 24-hour urinary norepinephrine levels in normal-weight and overweight/obese male patients with obstructive sleep apnea.
Notes: *P<0.05. Error bars indicate standard error. Overweight/obesity was defined as a body mass index≥25 kg/m2. All data were adjusted for age, antihypertensive medication use, minimum oxygen saturation, percentage of non-rapid eye movement sleep stage 2, and total scores of Epworth Sleepiness Scale.
Abbreviations: DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
In secondary analyses, we used logistic regression models to examine BMI effects on the association between 24-hour urinary norepinephrine and hypertension in normal-weight and overweight/obese male patients with OSA, respectively. As shown in Figure 1B, after adjusting for age, min SaO2, percentage of NREM sleep stage 2, and total scores of ESS, each 10 ug rise in 24-hour urinary norepinephrine was significantly associated with 2.32-fold increased odds for hypertension in normal-weight male patients with OSA (adjusted OR=2.33, 95% CI=1.06 to 5.09, p=0.034), but not in overweight/obese patients (adjusted OR=0.98, 95% CI=0.81 to 1.18, p=0.849). Interaction between 24-hour urinary norepinephrine and overweight/obesity on hypertension was significant (interaction-p=0.022). Sensitivity analyses showed that these findings were similar and in the same direction after adjusting for AHI instead of min SaO2.
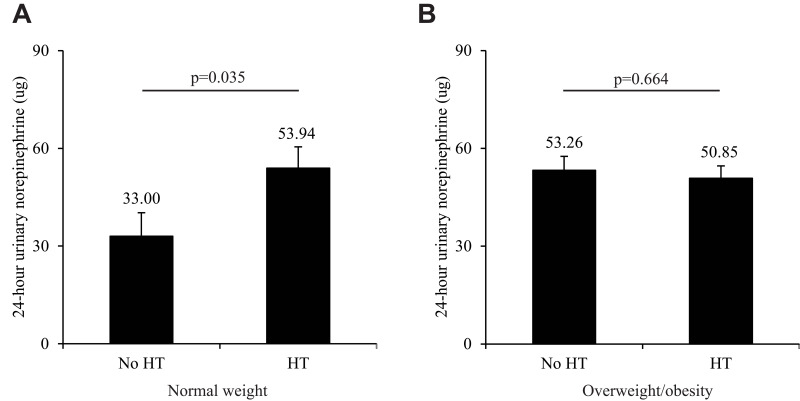
As shown in Figure 2A, 24-hour urinary norepinephrine levels were significantly higher in normal-weight male patients with OSA and hypertension compared to those without hypertension (p=0.035) after adjusting for age, min SaO2, percentage of NREM sleep stage 2 and total scores of ESS. However, among those overweight/obese patients with OSA, no significant difference in 24-hour urinary norepinephrine levels was observed between patients with and without hypertension (Figure 2B, p=0.664).
Figure 2.
(A) Twenty-four-hour urinary norepinephrine levels in normal-weight male OSA patients with and without hypertension. (B) Twenty-four-hour urinary norepinephrine levels in overweight/obese male OSA patients with and without hypertension.
Notes: Error bars indicate standard error. Overweight/obesity was defined as a body mass index ≥25 kg/m2. HT=hypertension; OSA=obstructive sleep apnea. All data were adjusted for age, minimum oxygen saturation, percentage of non-rapid eye movement sleep stage 2, and total scores of Epworth Sleepiness Scale.
As shown in Supplemental Table 1, among overweight/obese OSA patients, correlations between levels of 24-hour urinary norepinephrine and percentage of REM sleep (r=0.242, p=0.025) and min SaO2 (r=−0.211, p=0.051) were significant, while no significant correlation was observed between levels of 24-hour urinary norepinephrine and other sleep parameters (all p-values>0.1). No significant correlation was observed between levels of 24-hour urinary norepinephrine and any sleep parameters in normal-weight OSA patients (all p-values>0.07).
Discussion
To our knowledge, this is the first study examining moderating effects of BMI on the association between sympathetic activation and blood pressure in males with OSA. Our data suggest that sympathetic activation is differentially associated with elevated blood pressure in OSA, with this association only evident in normal-weight, but not in overweight/obese males with OSA.
Sympathetic activation has been proposed as one of the primary pathways of OSA related hypertension.5 In contrast to normal sleep, sympathetic activity as well as blood pressure does not fall during sleep in patients with OSA due to repetitive episodes of hypoxia and arousal induced by respiratory events during sleep.19,20 These high levels of sympathetic activity are sustained into wakefulness.21 In the current study, we found increased 24-hour urinary norepinephrine was independently and significantly associated with elevated blood pressure in patients with OSA, which was consistent with previous findings.22 However, this relationship was evident only in normal-weight, but not in overweight/obese patients with OSA.
Although obesity is a major risk factor for OSA and hypertension, up to 36–74% patients with OSA may not be obese.23–26 Evidence from previous studies showed a dose–response relationship between the severity of untreated OSA and risk of hypertension, and this association was independent of obesity.27,28 Similarly, heightened sympathetic drive is present in patients with OSA independent of obesity.29 Our data show that increased sympathetic activity was associated with elevated blood pressure and increased risk of hypertension in normal-weight, but not overweight/obese patients with OSA. These findings suggest that sympathetic activation is likely to be a key mechanism mediating OSA-related hypertension in normal-weight patients with OSA.
The mechanisms mediating the more potent pressor effect of sympathetic activation on blood pressure in normal-weight patients with OSA are unclear. It has been demonstrated that the patterns of sympathetic activation differ in normal-weight and obesity-related hypertension.30 The firing rate of single vasoconstrictor nerve fibers is increased in normal-weight essential hypertension subjects, whereas it is normal in obese subjects regardless of their prevailing blood pressure despite the total multiunit MSNA being elevated.30 It appears that previously silent nerve fibers are recruited in obesity, but they do not subserve vasoconstriction. Furthermore, sustained elevation of MSNA in obesity might lead to downregulation of α-adrenergic receptors and reduction of sympathetic vascular tone.31 In support of this, it has been reported that the reflex sympathetic responses during baroreceptor stimulation and deactivation induced by intravenous infusions of phenylephrine and nitroprusside were preserved in normal-weight hypertensive subjects but reduced in obese hypertensive subjects.32 As for obesity-related hypertension, it may be related to several collective mechanisms other than sympathetic drive, such as systemic inflammation,33 insulin resistance,34 increased levels of leptin,35 and activation of the renin-angiotensin-aldosterone system.36 In addition, there may be a “ceiling effect”, where the rise in blood pressure is finite, and addition of further pressor mechanisms above and beyond a certain level, does not result in further substantial blood pressure increase. Thus, given the multifactorial contribution to elevated blood pressure in obese patients with OSA, the redundancy in mechanisms driving higher blood pressure may blur any individual contribution of sympathetic activation. Indeed, compared to normal-weight patients with OSA, overweight/obese patients are reported to have greater likelihood of objective daytime sleepiness.37 We recently found that OSA with objective daytime sleepiness was associated with systemic inflammation38 and increased risk for hypertension.39 However, we did not assess measures of systemic inflammation in the current study to further examine this hypothesis.
In a recent meta-analysis, sympathetic activity was evaluated in 1438 obese or overweight subjects without hypertension and metabolic syndrome, and MSNA was noted to be significantly greater in overweight and obese individuals compared to normal weight subjects.40 Consistent with the findings of this meta-analysis, we found that overweight/obese patients had higher 24-hour urinary norepinephrine compared to normal weight patients, independent of blood pressure status. The increased sympathetic activation in obese subjects might conceivably be mediated by hyperinsulinemia,41 hyperleptinemia42 and/or baroreflex impairment43 in obesity. In another study in 10 obese normotensive OSA patients, Marrone et al reported that one night CPAP therapy had significant effects on normalizing blood pressure oscillations whereas it did not have any effect on decreasing nocturnal urinary norepinephrine levels.20 Taken together, it appears that the association of sympathetic overactivation and elevated blood pressure may be only present in normotensive, normal weight OSA patients. Future studies should be conducted to examine this hypothesis.
Our findings have several important clinical implications, suggesting that the underlying mechanisms driving OSA-related hypertension in normal-weight and overweight/obese subjects might be different. Thus, in order to prevent and improve OSA-related hypertension, treatments for normal-weight patients with OSA should target reductions in sympathetic activity levels (ie, CPAP or renal sympathetic denervation),44 while weight loss interventions should be emphasized in addition to CPAP treatment for overweight/obese patients with OSA. Indeed, regarding the former, prior studies strongly suggest that the blood pressure lowering effects of renal sympathetic denervation are especially evident in resistant hypertensive patients with co-morbid OSA.44,45 Our results also provide support for individualized antihypertensive medication in patients with OSA-related hypertension. For example, central sympathetic inhibitors and/or β-adrenergic blockade combined with CPAP treatment might provide selective cardiovascular benefit in normal-weight patients with OSA-related hypertension. As for overweight/obese hypertensive OSA patients, diuretics and RAAS blockers combined with CPAP treatment might possibly produce greater antihypertensive effects.46
Strengths of our study include the measurement of sympathetic drive using 24-hour urinary norepinephrine. Because the norepinephrine levels are easily influenced by stress, and their moment-to-moment variation is great due to the very short half-life in plasma (only 2 to 3 minutes),22,47 we used 24-hour urinary norepinephrine to assess overall systemic sympathetic drive, rather than use a single measurement of plasma norepinephrine or the direct measurement of sympathetic nerve traffic via microneurography. Furthermore, compared to the direct measurement of sympathetic nerve traffic via microneurography, 24-hour urinary norepinephrine is a noninvasive, convenient and easy operative measure in clinical practice. However, several limitations should be acknowledged. First, we only included male patients with OSA in this study and our findings cannot be generalized to females with OSA. However, gender differences in sympathetic activation48 and OSA-related cardiometabolic outcomes49 have been well established. Further studies should be conducted to examine the BMI effects on the association between sympathetic activation and blood pressure in females with OSA. Second, we did not examine daytime and nighttime urinary norepinephrine separately. However, 24-hour urinary norepinephrine is a valid surrogate marker of 24-hour sympathetic activity. Third, the lack of data regarding adherence and types of antihypertensive medications prevented further analyses of their impact on blood pressure. In mitigation, this may have negligible effects on the results because our statistical models have been adjusted for “presence or absence of antihypertensive medications” and the findings remained similar after excluding 21 patients on antihypertensive medications. Finally, we did not perform ambulatory blood pressure monitoring or examine CPAP treatment effects on sympathetic activity and blood pressure in normal-weight and overweight/obese males with OSA. Future randomized controlled trials using ambulatory blood pressure monitoring should be conducted.
Conclusions
In conclusion, sympathetic activation was associated with increased blood pressure as well as higher odds for hypertension in normal-weight, but not in overweight/obese male patients with OSA. These findings suggest that BMI may moderate the association between sympathetic activation and blood pressure in males with OSA.
Acknowledgments
The authors thank the sleep technicians and staff of the Sleep Medicine Center at the Shantou University Medical College for their support with this project.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No.81970087), Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program and Department of Educational of Guangdong Province (2017KTSCX065) and 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG05B).
Abbreviations
AHI, apnea-hypopnea index; BMI, body mass index; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; HT, hypertension; MAP, mean arterial pressure; Min SaO2, minimum oxygen saturation; MSNA, muscle sympathetic nerve activity; NM-DBP, diastolic blood pressure in patients not on antihypertensive medication; NM-SBP, systolic blood pressure in patients not on antihypertensive medication; NM-MAP, mean arterial pressure in patients not on antihypertensive medication; NREM, non-rapid eye movement; OR, odds ratio; OSA, obstructive sleep apnea.
Disclosure
All authors have seen and approved the manuscript. Dr. Somers has served as a consultant for Respicardia, Sleep Number, Baker Tilly and Jazz Pharmaceuticals. Dr Somers also reports grants from NIH, outside of the submitted work. Drs. Li, Chen and Tang have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in the article. The authors report no other conflicts of interest in this work.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American heart association/American college of cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 4.Grassi G, Facchini A, Trevano FQ, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46(2):321–325. doi: 10.1161/01.HYP.0000174243.39897.6c [DOI] [PubMed] [Google Scholar]
- 5.Venkataraman S, Vungarala S, Covassin N, Somers VK. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med. 2020;9(2):591. doi: 10.3390/jcm9020591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97(10):943–945. doi: 10.1161/01.CIR.97.10.943 [DOI] [PubMed] [Google Scholar]
- 7.Rumantir MS, Vaz M, Jennings GL, et al. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17(8):1125–1133. doi: 10.1097/00004872-199917080-00012 [DOI] [PubMed] [Google Scholar]
- 8.Landsberg L, Troisi R, Parker D, Young JB, Weiss ST. Obesity, blood pressure, and the sympathetic nervous system. Ann Epidemiol. 1991;1(4):295–303. doi: 10.1016/1047-2797(91)90040-J [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Lambert G, Schlaich M, Dixon J, Sari CI, Lambert E. Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension takes a benign form? Hypertension. 2018;71(1):22–33. doi: 10.1161/HYPERTENSIONAHA.117.09790 [DOI] [PubMed] [Google Scholar]
- 10.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37(1):169–174. doi: 10.1016/S0735-1097(00)01103-7 [DOI] [PubMed] [Google Scholar]
- 11.van Berge-landry HM, Bovbjerg DH, James GD. Relationship between waking-sleep blood pressure and catecholamine changes in African-American and European-American women. Blood Press Monit. 2008;13(5):257–262. doi: 10.1097/MBP.0b013e3283078f45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 13.Berry RB, Albertario CL, Harding SM, for the American Academy of Sleep Medicine, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. Darien, IL: American Academy of Sleep Medicine;2018. [Google Scholar]
- 14.World Health Organization. Definition of obesity. Available from: https://www.who.int/topics/obesity/en/. Accessed March1, 2020.
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 16.Raum WJ. Methods of plasma catecholamine measurement including radioimmunoassay. Am J Physiol. 1984;247(1 Pt 1):E4–E12. doi: 10.1152/ajpendo.1984.247.1.E4 [DOI] [PubMed] [Google Scholar]
- 17.Hallisey SD, Greenwood JC. Beyond mean arterial pressure and lactate: perfusion end points for managing the shocked patient. Emerg Med Clin North Am. 2019;37(3):395–408. doi: 10.1016/j.emc.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 18.Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. [PubMed] [Google Scholar]
- 19.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103(3):722–727. doi: 10.1378/chest.103.3.722 [DOI] [PubMed] [Google Scholar]
- 21.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens. 1997;15(12 Pt 2):1613–1619. doi: 10.1097/00004872-199715120-00062 [DOI] [PubMed] [Google Scholar]
- 22.Vardhan V, Shanmuganandan K. Hypertension and catecholamine levels in sleep apnoea. Med J Armed Forces India. 2012;68(1):33–38. doi: 10.1016/S0377-1237(11)60128-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Li Y, He F, et al. Mild-to-moderate sleep apnea is associated with incident hypertension: age effect. Sleep. 2019;42(4). doi: 10.1093/sleep/zsy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan SF, O’Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11(3):149–157. doi: 10.1007/s11325-006-0095-5 [DOI] [PubMed] [Google Scholar]
- 25.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 26.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065 [DOI] [PubMed] [Google Scholar]
- 27.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160(15):2289–2295. doi: 10.1001/archinte.160.15.2289 [DOI] [PubMed] [Google Scholar]
- 28.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. doi: 10.1001/archinte.1997.00440360178019 [DOI] [PubMed] [Google Scholar]
- 29.Henderson LA, Macefield VG. Obstructive sleep apnoea and hypertension: the role of the central nervous system. Curr Hypertens Rep. 2016;18(7):59. doi: 10.1007/s11906-016-0665-2 [DOI] [PubMed] [Google Scholar]
- 30.Lambert E, Straznicky N, Schlaich M, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50(5):862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649 [DOI] [PubMed] [Google Scholar]
- 31.Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension. 2008;52(4):687–695. doi: 10.1161/HYPERTENSIONAHA.107.109603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36(4):538–542. doi: 10.1161/01.HYP.36.4.538 [DOI] [PubMed] [Google Scholar]
- 33.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309(7):H1101–H1111. doi: 10.1152/ajpheart.00094.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30(3 Pt 2):619–623. doi: 10.1161/01.HYP.30.3.619 [DOI] [PubMed] [Google Scholar]
- 36.Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol. 2016;13(4):333–343. doi: 10.11909/j.issn.1671-5411.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083(1):329–344. doi: 10.1196/annals.1367.023 [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Objective, but not subjective, sleepiness is associated with inflammation in sleep apnea. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren R, Covassin N, Yang L, et al. Objective but not subjective short sleep duration is associated with hypertension in obstructive sleep apnea. Hypertension. 2018;72(3):610–617. doi: 10.1161/HYPERTENSIONAHA.118.11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grassi G, Biffi A, Seravalle G, et al. Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74(2):349–358. doi: 10.1161/HYPERTENSIONAHA.119.12885 [DOI] [PubMed] [Google Scholar]
- 41.Da SA, Do CJ, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–682. doi: 10.1016/j.cjca.2020.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234 [DOI] [PubMed] [Google Scholar]
- 43.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32(6):1039–1043. doi: 10.1161/01.HYP.32.6.1039 [DOI] [PubMed] [Google Scholar]
- 44.Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase II trial. Hypertension. 2018;72(2):381–390. doi: 10.1161/HYPERTENSIONAHA.118.11180 [DOI] [PubMed] [Google Scholar]
- 45.Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58(4):559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799 [DOI] [PubMed] [Google Scholar]
- 46.Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35(1):4–16. doi: 10.1038/hr.2011.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenberg E, Zimlichman R, Lavie P. Plasma norepinephrine levels in patients with sleep apnea syndrome. N Engl J Med. 1990;322(13):932–933. [DOI] [PubMed] [Google Scholar]
- 48.Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26(2):122–126. doi: 10.1046/j.1440-1681.1999.02995.x [DOI] [PubMed] [Google Scholar]
- 49.Cano-Pumarega I, Barbe F, Esteban A, Martinez-Alonso M, Egea C, Duran-Cantolla J. Sleep apnea and hypertension: are there sex differences? The Vitoria sleep cohort. Chest. 2017;152(4):742–750. doi: 10.1016/j.chest.2017.03.008 [DOI] [PubMed] [Google Scholar]