Abstract
Background
Heart failure complicating acute myocardial infarction marks an ominous prognosis. Killip and Kimball's classification of heart failure remains a useful tool in these patients. Lung ultrasound can detect pulmonary congestion but its usefulness in this scenario is unknown.
Objective
To investigate the diagnostic accuracy of lung ultrasound to predict heart failure in patients with acute myocardial infarction.
Methods
Patients admitted with acute myocardial infarction and without heart failure were evaluated with a lung ultrasound. The presence of B-lines was recorded and counted. The presence of new heart failure (Killip Class B, C, or D) during hospitalization was evaluated by a cardiologist blinded to the results of lung ultrasound. A ROC curve analysis was done to evaluate the diagnostic accuracy of B-lines to predict heart failure.
Results
200 patients were included. Three patients were diagnosed with cardiogenic shock, 5 with acute pulmonary edema, and 17 with mild heart failure. Patients who develop heart failure had a median of 14 B-lines, however, patients who remained in Killip class A had a median of 2 (p = 0,0001). The area under the ROC curve of the sum of B-lines to predict any form of heart failure was 0,91 (CI95% 86–97). The best cut-off value was 5 B-lines, with a sensitivity of 88% (IC95% 68,8–97,5) and specificity of 81% (IC95% 73,9–86,2).
Conclusion
Lung ultrasound done at admission can help to predict heart failure In patients with acute myocardial infarction.
Keywords: Myocardial infarction, Heart failure, Ultrasound
Abbreviations: ROC, Receiver Operating Characteristic; GRACE, Global Registry of Acute Coronary Events; STARD, Standards for Reporting of Diagnostic Accuracy Studies; POC, Point of care; BSA, body surface area; BMI, body mass index; STEMI, ST-segment elevation myocardial infarction; LBBB, left bundle branch block; RBBB, right bundle branch block; OR, odds ratio
1. Background
Heart failure complicating acute myocardial infarction is a powerful predictor of fatal and non-fatal events.1
Since its original description by Killip in 19672 this classification has remained a cornerstone in the clinical armamentarium to evaluate a patient suffering an acute myocardial infarction. Patients with an acute myocardial infarction are categorized as Killip class A if they have no clinical signs of heart failure, class B as the presence of rales, third heart sound, and jugular venous distention, class C with acute pulmonary edema, and class D as cardiogenic shock. Even more recent, large, and well-designed registries as GRACE had confirmed its value in a changed scenario of modern strategies of primary percutaneous revascularization, stents, and antiplatelet therapy. Although cardiogenic shock marks the worst prognosis, even lesser grades of heart failure are associated with higher mortality. In the GRACE risk score model, a Killip class B had a twofold increase of risk of death as compared with no evidence of heart failure.3 Cardiogenic shock and acute pulmonary edema generally are distinctive syndromes, however, to detect signs of mild heart failure can be more challenging in certain patients.
Lung ultrasound is a simple tool that can be used to detect pulmonary congestion and was validated in several studies and meta-analysis.4, 5, 6, 7, 8, 9 Briefly, abnormal accumulation of lung water can be detected as “comet tails” artifacts using ultrasound in a bedside examination. These artifacts are called B-lines and the presence of more than 3 bilaterally are considered abnormal.
2. Objective
To investigate the diagnostic accuracy of lung ultrasound to predict heart failure in patients with acute myocardial infarction.
3. Methods
We studied patients admitted to the Emergency Department of our hospital with a diagnosis of suspected acute myocardial infarction (with or without ST-segment elevation).
Inclusion criteria were:
-
•
Male and female patients aged 18 years or more.
-
•
Suspected acute myocardial infarction, based on the presence of typical symptoms associated with ischemic abnormalities in the electrocardiogram.
-
•
Less than 24 h of symptoms onset.
Patients were excluded if they presented at admission with any form of heart failure.
The trial was approved by the local Investigation Committee. All patients gave informed consent to participate in the study.
The medical history, physical examination, and electrocardiogram were obtained and evaluated by a cardiologist. Myocardial infarction was suspected in the presence of typical symptoms and ischemic changes in the electrocardiogram.
A lung ultrasound examination was done according to a scanning protocol10 by cardiologists with the training of at least 10 studies under the direct supervision of an expert and a 1-h lecture about the method.
Anatomic zones to be scanned were defined by vertical lines through the sternum, anterior and posterior axillary lines, and by horizontal lines through the clavicle, third intercostal space, and the diaphragm. With this approach 8 thoracic lung zones were obtained, 4 anterior and 4 lateral. The examination was done bedside with the patient reclined at 45°. The study typically was completed in less than 5 min and did not generate any delay in the normal management of patients.
A commercially available portable ultrasound machine (SonoSite Titan, USA) with a curvilinear probe was used. The depth was set at 15 cm.
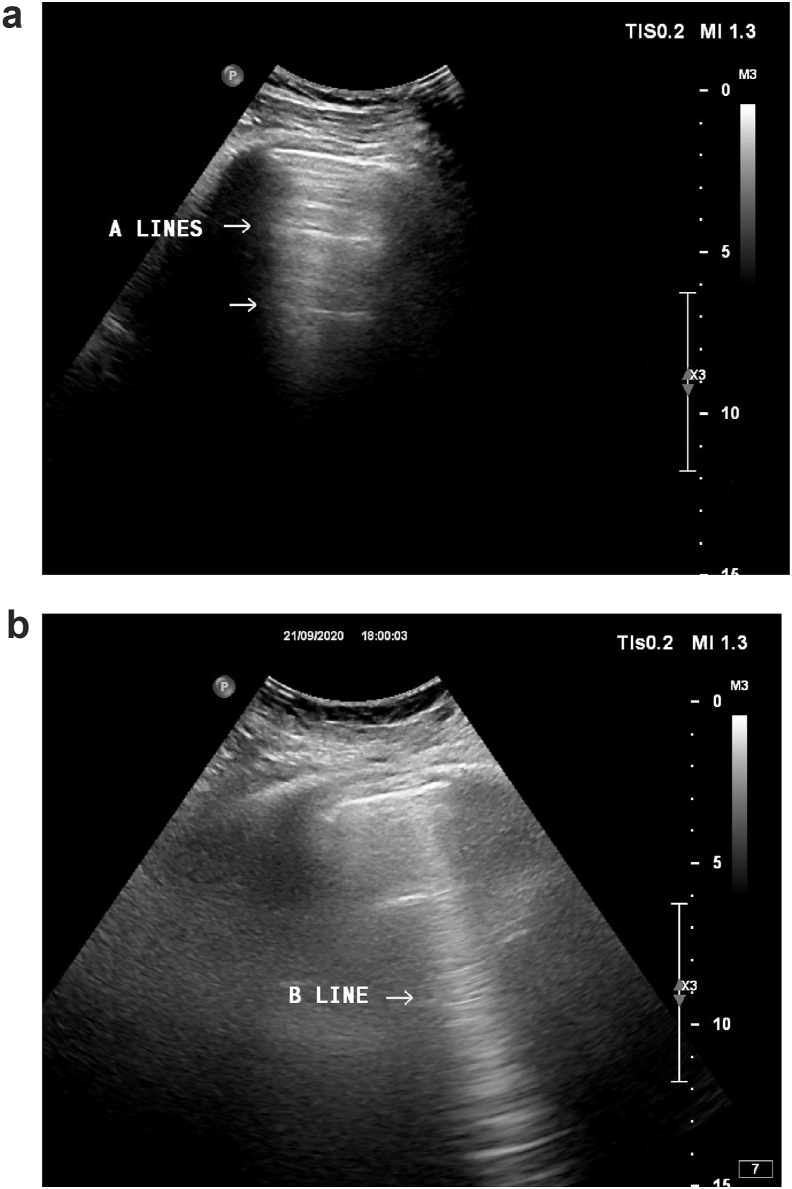
The presence of pleural and A-lines was considered a normal pattern. B-Lines were defined as hyperechogenic vertical lines arising from the pleural line and extending to the border of the screen. These “comet tail” artifacts move with the respiratory cycle. Fig. 1.
Fig. 1.
Typical patterns of lines A (a) and line B (b) in lung ultrasound scan.
B-lines were counted in each zone and added-up to obtain a total score. Patients were followed-up during hospitalizations until discharge or death. A cardiologist, unaware of the result of the lung ultrasound, evaluated the patients during hospitalization looking for the presence of heart failure. A chest x-ray was done at admission in all patients. The worst Killip class was recorded.
Myocardial infarction was diagnosed according to the criteria of the Fourth Universal definition of myocardial infarction.11 We used cardiac troponin T-high sensitive (Elecsys Roche).
Baseline characteristics, risk factors, vital signs, coronary angiography data, revascularization, and echocardiographic data were recorded.
3.1. Statistical analysis
The total number of B-lines were compared between the group of patients with and without incident heart failure. We used a non-parametric test (Mann–Whitney U test). A p-value of less than 0,05 was considered statistically significant.
We also evaluated the sensitivity, specificity, and the best cut-off value (estimated by the Youden index) of B-lines using ROC curve analysis. Assuming a heart failure rate of 10%, with a type I error level of 0,05 and statistical power of 80% a sample size of 198 was calculated to detect an area under the curve of at least 0,7.
A logistic regression model was constructed to evaluate if the presence of B-lines was an independent predictor of heart failure. Statistical analysis was performed using STATA 14 version software (StataCorp, College Station, TX).
We have tried to apply the STARD guidelines for reporting diagnostic accuracy studies.12
4. Results
From March 11, 2019, through November 27, 2019, we included 200 consecutive patients. Baseline characteristics are shown in Table 1. The mean age was 62 (SD11), 75,5% were male. Less than 5% of patients had a diagnosis of chronic pulmonary disease, and 85 were obese (BMI more than 30).
Table 1.
Baseline characteristics of patients, including demographics, clinical, electrocardiographic and angiographic data. Numerical variables are expressed as mean and ±standard deviation.
| Patient characteristics | N = 200 |
|---|---|
| Female | 49 (24,5%) |
| Age (years) | 62 (±12) |
| BSA (m2)b | 1,93 (±0,2) |
| BMI (kg/m2)a | 29 (±5) |
| Systolic blood pressure (mmHg) | 148 (±27) |
| Diastolic blood pressure (mmHg) | 83 (±14) |
| Pulse rate | 77 (±14) |
| Hypertension | 148 (74%) |
| Diabetes | 62 (31%) |
| Hyperlipidemia | 73 (36,5) |
| Smoking | 46 (23%) |
| STEMIe | 134 (67%) |
| Localization | |
| Anterior | 70 (35%) |
| Inferior | 60 (30%) |
| Undetermined | 70 (35%) |
| LBBBc | 18 (9%) |
| RBBBd | 18 (9%) |
| Number of vessels | |
| 0 | 18 (9%) |
| 1 | 116 (58%) |
| 2 | 36 (18%) |
| 3 | 29 (14,5%) |
| Primary angioplasty | 152 (76%) |
BMI: body mass index.
BSA: body surface area.
LBBB: left bundle branch block.
RBBB; right bundle branch block.
STEMI: ST-segment elevation myocardial infarction.
Seventy-six percent of patients received a primary coronary angioplasty. Typical medical treatment was administered including intravenous nitroglycerin in 50% of patients as anti-ischemic.
Three patients were diagnosed with cardiogenic shock, 5 with acute pulmonary edema, and 17 with mild heart failure after admission to the coronary care unit. All of them were treated with intravenous furosemide. The findings of lung ultrasound were not available to the attending physician; therefore, doses of the diuretic were chosen according to clinical criteria.
Five patients (2,5%) died during hospitalization. The median length of stay was 4 (IQR 3–6) days.
The events of heart failure were detected at a median of 1 day after admission.
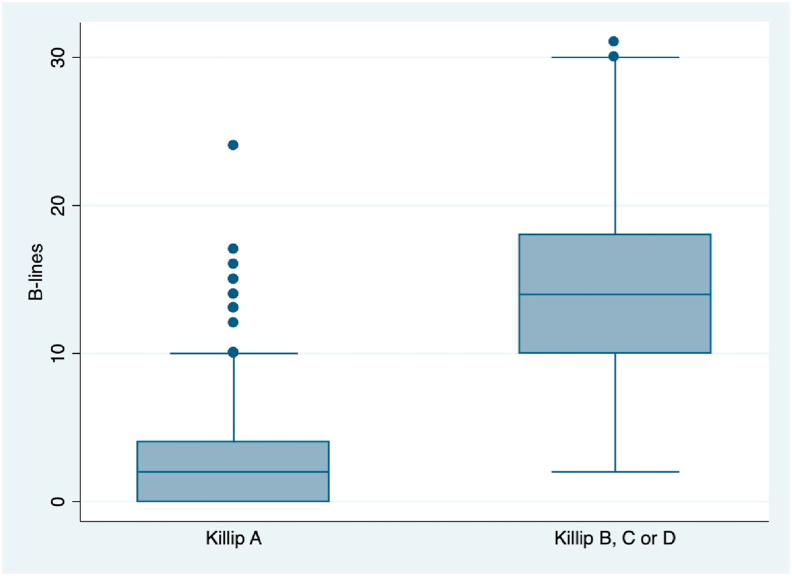
Patients who develop any form of heart failure during hospitalization had a median of 14 B-lines, however, patients who remained in Killip class A had a median of 2 (p = 0,0001). Fig. 2.
Fig. 2.
Box-plot of B-lines. Box-plot of number of B-lines in patients who remained without heart failure (Killip A) compared with patients who develop any form of heart failure (Killip B, C or D) during hospitalization.
A comprehensive Doppler echocardiography was done in 192 patients at a median of 1 day after admission. Patients who develop heart failure had a lower ejection fraction (47 vs. 58%) p = 0,0001, larger left atrial volume index (35 vs. 29 ml/m2) p = 0,008, higher E/e’ ratio (16 vs. 11) p = 0,001, tricuspid regurgitation velocity (291 vs. 235 cm/s) p = 0,003, estimated central venous pressure (7,6 vs. 3,8 mmHg) p = 0,001 and left ventricular mass index (139 vs. 119 gr/m2) p = 0,001.
Patients with heart failure had a higher value of cardiac troponin T (6303 vs. 2622 ng/L) p = 0,001). A comparison of other variables is shown in Table 2.
Table 2.
Comparison between patients with and without heart failure. Comparison of clinical and key echocardiographic variables between patients without (Killip A) and with heart failure (Killip B, C or D) developed during hospitalization.
| Killip A (n = 175) | Killip B, C, or D (n = 25) | P value | |
|---|---|---|---|
| Age (years) | 62 (±12) | 65 (±11) | 0,1 |
| STEMIa (count) | 111 (83%) | 23 (17%) | 0,004 |
| Non STEMIa (count) | 64 (97%) | 2 (3%) | |
| Onset of symptoms (hours) | 6,2 (±10) | 5,2 (±7) | 0,0001 |
| Cardiac troponin T (ng/l) | 2623 (±4763) | 6303 (±7253) | 0,001 |
| Systolic blood pressure (mmHg) | 150 (±26) | 134 (±28) | 0,01 |
| Diastolic blood pressure (mmHg) | 84 (±14) | 80 (±20) | 0,05 |
| Hemoglobin (g/dl) | 14,2 (±1,7) | 13,8 (±1,9) | 0,5 |
| White blood cells (mm3) | 10,353 (±3796) | 11,837 (±3356) | 0,01 |
| Blood glucose (mg/%) | 163 (±83) | 201 (±110) | 0,1 |
| Creatinine (mg/%) | 0,97 (±0,3) | 1,15 (±0,7) | 0,3 |
| Clearance (mL/min/1,73 m2) | 85,8 (±27) | 81 (±36) | 0,5 |
| Left ventricular mass index (gr/m2) | 118,7 (±34) | 139 (±28) | 0,01 |
| Ejection fraction (%) | 58 (±8) | 47 (±9) | 0,001 |
| Left atrial volume index (ml/m2) | 29,3 (±9) | 35 (±10) | 0,008 |
| E/e’ ratio | 10,8 (±3,8) | 15,9 (±7,6) | 0,001 |
| Tricuspid regurgitation velocity (cm/s) | 235 (±25) | 291 (±62) | 0,003 |
Numerical variables are expressed as mean and ±standard deviation.
STEMI: ST-segment elevation myocardial infarction.
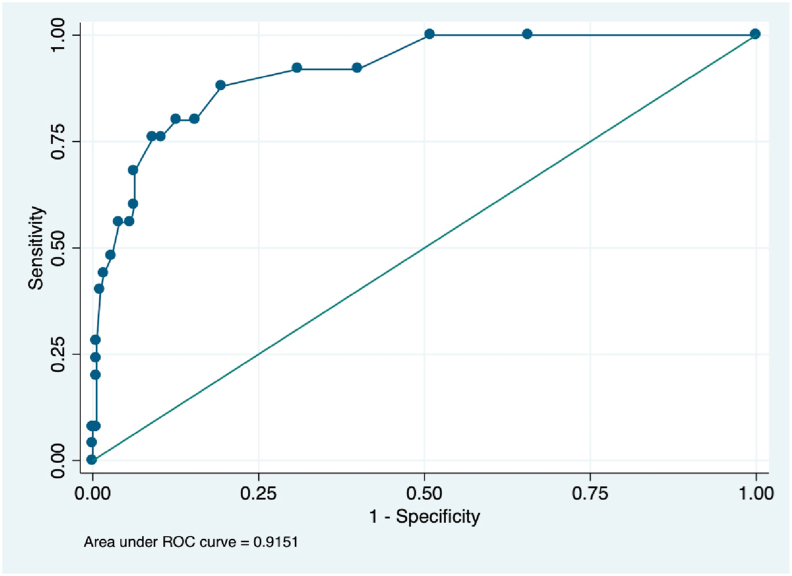
The area under the ROC curve of the sum of B-lines to predict any form of heart failure was 0,91 (CI95% 86–97). The best cut-off value was 5 B-lines, with a sensitivity of 88% (IC95% 68,8–97,5) and specificity of 81% (IC95% 73,9–86,2). Fig. 3.
Fig. 3.
ROC curve of B-lines to predict Killip B, C or D. The area under the ROC curve of the number of B-lines to predict any form of heart failure developed during hospitalization (Killip B, C or D). ROC: receiver operative characteristic.
Univariate analysis identified left bundle branch block, ST-segment elevation myocardial infarction, and systolic blood pressure at admission as variables associated with heart failure.
We constructed a logistic regression model to predict heart failure with these variables and the sum of B-lines as co-variables. The presence of B-lines remained statistically significant (p = 0,001) with OR 1,3 (IC95% 1,18–1,45). Table 3.
Table 3.
Multivariate analysis. Logistic regression model: variables statistically significant in univariate analysis of heart failure complicating acute myocardial infarction where included as co-variates. Dependent variable is heart failure (Killip B, C or D). Associated OR and p-values are shown.
| Variable | ORb (CI95%) | P value |
|---|---|---|
| B-lines (number) | 1,3 (1,18–1,45) | 0,0001 |
| LBBBa (yes/no) | 11,2 (2,1–59,4) | 0,004 |
| STEMIc (yes/no) | 3,1 (0,4–22,8) | 0,2 |
| Systolic blood pressure (mmHg) | 0,97 (0,94–0,99) | 0,027 |
LBBB: left bundle branch block.
OR: Odds ratio.
STEMI: ST-segment elevation myocardial infarction.
5. Discussion
In a recent article,13 the authors suggest adding a fifth pillar to the classic physical examination, e.g. insonation. The selective use of bedside ultrasound as a point of care (POC) tool has gained broad impulse in the last years. Ultrasound is safe, inexpensive, rapidly available, repeatable, and reproducible.14 Lung ultrasound, particularly, was extensively studied as a marker of lung congestion. Its usefulness was confirmed in different clinical scenarios. Helping in the diagnosis of heart failure in patients with acute dyspnea in the emergency department, monitoring treatment response in acute pulmonary edema,15, 16, 17 evaluating decompensation in patients with chronic heart failure in ambulatory care.18 Lung ultrasound also had shown value as a prognostic tool.19,20
In acute myocardial infarction, the detection of subtle or early phases of heart failure can be challenging. Several factors may be involved: elderly patients, obesity, chronic obstructive pulmonary disease makes detection of signs of mild heart failure difficult. Also, some evidence suggests that currently, the physical examination is used less and with worst proficiency.21
Recently, a “lung water cascade in heart failure”22 has been described. In these sequence of events, B-lines precedes rales and dyspnea. Therefore, this clinically silent interstitial pulmonary edema can be interpreted as an early stage in the path of heart failure complicating acute myocardial infarction. B-lines can be considered a biomarker of congestion allowing the physician to anticipate a worsening of Killip class.
In our study, half of the incident cases of heart failure (including cardiogenic shock) occurred within 24 h of admission. Lindholm et al23 published similar data, 59% of cases of cardiogenic shock developed within 48 h, 11% during days 3 and 4, and only 30% later than 4 days. The late shock was associated with a higher proportion of female sex, less use of revascularization, and a worse prognosis.
Our results show that lung ultrasound detecting B-lines can predict heart failure complicating acute myocardial infarction. Identifying patients at risk of heart failure offer an opportunity to tailor treatment, therefore the early use of revascularization in non-ST segment elevation myocardial infarction, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor blockers, or nitrates can be evaluated, and other therapies can be delayed or stopped: beta-blockers, calcium antagonists, etc.
Araujo et al24 published a study where the addition of lung ultrasound to Killip classification in patients with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention was more sensitive than physical examination to identify patients at high risk of for in-hospital mortality. Our data support and add new information: lung ultrasound may also detect patients at high risk to develop heart failure. Both studies suggest that more congestion, even subtle, is related to more myocardial injury, necrosis, remodeling, and finally mortality.
In the future, Killip class A could be reclassified as A (no lung ultrasound congestion) and A+ (with lung ultrasound congestion). However, further investigation is needed.
We think that the enduring use of the Killip classification can be enhanced by the use of newly available technology, therefore “standing on the shoulders of giants”.
5.1. Limitations
Patients included in our study showed baseline characteristics similar to those reported in other published registries and studies. However, the generalization of our results must be done cautiously in different clinical scenarios.
The specificity of lung ultrasound is affected by several causes. Acute and chronic lung diseases (infectious and not infectious) can produce B-lines not associated with heart failure. The correct classification of these patients warrants further investigations.
In our study, 42% of patients were obese. The sensitivity of lung ultrasound may be lower in these patients.
Killip classification is subjective, however, its value remained unaltered since its original description.
Some investigators had used N-terminal brain natriuretic peptide (NT-pro BNP) to identify patients at higher risk of adverse events after an acute myocardial infarction, specially infarct size and function.25,26 We did not include this biomarker in our investigation. However, our goal was to provide a point of care (POC) tool at patient admission. Obtain the NT -pro BNP results usually requires several minutes to hours to be available.
6. Conclusions
Lung ultrasound done at admission can help to predict heart failure in patients with acute myocardial infarction.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Bahit M.C., Kochar A., Granger C.B. Post-myocardial infarction heart failure. J Am Coll Cardiol HF. 2018 Mar;6(3):179–186. doi: 10.1016/j.jchf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Killip T 3rd, Kimball J.T. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967 Oct;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 3.Predictors of hospital mortality in the global registry of acute coronary events. Granger C.B., Goldberg R.J., Dabbous O. Global registry of acute coronary events investigators. Arch Intern Med. 2003 Oct 27;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 4.Staub L.J., Mazzali Biscaro R.R., Kaszubowski E. Lung ultrasound for the emergency diagnosis of pneumonia, acute heart failure, and exacerbations of chronic obstructive pulmonary disease/asthma in adults: a systematic review and meta-analysis. J Emerg Med. 2019 Jan;56(1):53–69. doi: 10.1016/j.jemermed.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Lian R., Zhang G.C., Yan S.T. Role of ultrasound lung comets in the diagnosis of acute heart failure in emergency department: a systematic review and meta-analysis. Biomed Environ Sci. 2018 Aug;31(8):596–607. doi: 10.3967/bes2018.081. [DOI] [PubMed] [Google Scholar]
- 6.McGivery K., Atkinson P., Lewis D. Emergency department ultrasound for the detection of B-lines in the early diagnosis of acute decompensated heart failure: a systematic review and meta-analysis. CJEM. 2018 May;20(3):343–352. doi: 10.1017/cem.2018.27. [DOI] [PubMed] [Google Scholar]
- 7.Al Deeb M., Barbic S., Featherstone R. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014 Aug;21(8):843–852. doi: 10.1111/acem.12435. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Shen Z., Lu X. Sensitivity and specificity of ultrasound for the diagnosis of acute pulmonary edema: a systematic review and meta-analysis. Med Ultrason. 2018 Feb 4;1(1):32–36. doi: 10.11152/mu-1223. [DOI] [PubMed] [Google Scholar]
- 9.Martindale J., Wakai A., Collins S. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016 Mar;23(3):223–242. doi: 10.1111/acem.12878. [DOI] [PubMed] [Google Scholar]
- 10.Volpicelli G., Mussa A., Garofalo G. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006 Oct;24(6):689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018 Oct 30;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J.F., Korevaar D.A., Altman D.G. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016 Nov 14;6(11) doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narula J., Chandrashekhar Y., Braunwald E. Time to add a fifth pillar to bedside physical examination inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol. 2018 Apr 1;3(4):346–350. doi: 10.1001/jamacardio.2018.0001. [DOI] [PubMed] [Google Scholar]
- 14.Nelson B.P., Narula S., Argulian E. Including insonation in undergraduate medical school curriculum. Ann Glob Health. 2019 Nov 12;85(1):135. doi: 10.5334/aogh.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martindale J.L. Resolution of sonographic B-lines as a measure of pulmonary decongestion in acute heart failure. Am J Emerg Med. 2016 Jun;34(6):1129–1132. doi: 10.1016/j.ajem.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Martindale J.L., Secko M., Kilpatrick J.F. Serial sonographic assessment of pulmonary edema in patients with hypertensive acute heart failure. J Ultrasound Med. 2018 Feb;37(2):337–345. doi: 10.1002/jum.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortellaro F., Ceriani E., Spinelli M. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern Emerg Med. 2017 Oct;12(7):1011–1017. doi: 10.1007/s11739-016-1510-y. [DOI] [PubMed] [Google Scholar]
- 18.Miglioranza M.H., Picano E., Badano L.P. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int J Cardiol. 2017 Aug 1;240:271–278. doi: 10.1016/j.ijcard.2017.02.150. [DOI] [PubMed] [Google Scholar]
- 19.Platz E., Merz A.A., Jhund P.S. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017 Sep;19(9):1154–1163. doi: 10.1002/ejhf.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platz E., Campbell R.T., Claggett B. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. J Am Coll Cardiol HF. 2019 Oct;7(10):849–858. doi: 10.1016/j.jchf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver C.M., Hunter S.A., Ikeda T. Junior doctor skill in the art of physical examination: a retrospective study of the medical admission note over four decades. BMJ Open. 2013 Apr 3;3(4) doi: 10.1136/bmjopen-2012-002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picano E., Scali M.C. The lung water cascade in heart failure. Echocardiography. 2017 Oct;34(10):1503–1507. doi: 10.1111/echo.13657. [DOI] [PubMed] [Google Scholar]
- 23.Lindholm M.G., Køber L., Boesgaard S. Cardiogenic shock complicating acute myocardial infarction: prognostic impact of early and late shock development. Eur Heart J. 2003 Feb;24(3):258–265. doi: 10.1016/s0195-668x(02)00429-3. [DOI] [PubMed] [Google Scholar]
- 24.Araujo G.N., Silveira A.D., Scolari F.L. Admission bedside lung ultrasound reclassifies mortality prediction in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imag. 2020 Jun;13(6) doi: 10.1161/CIRCIMAGING.119.010269. Epub 2020 Jun 15. [DOI] [PubMed] [Google Scholar]
- 25.Kleczyński P., Legutko J., Rakowski T. Predictive utility of NT-pro BNP for infarct size and left ventricle function after acute myocardial infarction in long-term follow-up. Dis Markers. 2013;34(3):199–204. doi: 10.3233/DMA-120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr A., Mair J., Schocke M. Predictive value of NT-pro BNP after acute myocardial infarction: relation with acute and chronic infarct size and myocardial function. Int J Cardiol. 2011 Feb 17;147(1):118–123. doi: 10.1016/j.ijcard.2009.09.537. [DOI] [PubMed] [Google Scholar]